Effect of Exercise on Regulating miRNA Expression in Brain Health and Diseases
Simple Summary
Abstract
1. Introduction
2. How Does Exercise Affect the Biogenesis of miRNAs?
3. Effect of Exercise on Circulatory miRNAs in Brain Health
4. Exercise-Mediated Molecular Signaling on miRNA Expression to Reverse Brain Physiopathology
4.1. Aerobic Exercise-Mediated Molecular Signaling on miRNA Expression
4.2. Resistance Exercise-Mediated Molecular Signaling on miRNA Expression
4.3. Effect of Different Exercise Intensities on miRNA Expression
5. Exercise-Mediated miRNAs on Oxidative-Related Stress in Brain Pathophysiology
6. Limitations of Using miRNAs as Drugs in Treating Brain Diseases
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAP2K3 | Dual specificity mitogen-activated protein kinase kinase 3 |
| STAT3 | Signal transducer and activator of transcription 3 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| JAK | Janus kinase |
| PARK2 | Parkin |
| ITGB8 | Integrin beta-8 |
| HMBOX1 | Homeobox containing 1 |
| HDAC4 | Histone deacetylase 4 |
| Mef2 | Myocyte enhancer factor-2 |
| BDNF | Brain-derived neurotrophic factor |
| IGFBP-5 | Insulin-like growth factor-binding protein 5 |
| ITM2A | Integral membrane protein 2A |
| NNAT | Neuronatin |
| SERCA | Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase |
| AMPK | 5′ AMP-activated protein kinase |
| mTOR | The mammalian target of rapamycin |
| GSK3β | Glycogen synthase kinase-3 beta, |
| MMP15 | Matrix metalloproteinase 15 |
| IRAK-1 | Interleukin-1 receptor-associated kinase 1 |
| TRAF6 | TNF receptor associated factor |
| PTEN | Phosphatase and tensin homolog |
| IL-6 | Interleukin 6 |
| TLR4 | Toll-like receptor 4 |
| BACE1 | Beta-secretase 1 |
| ANRIL | Antisense non-coding RNA in the INK4 locus |
| Atg3 | Autophagy related 3 |
| EFNA3 | Ephrin A3 |
| Cav1.2 | Calcium channel, voltage-dependent, L type, alpha 1C subunit |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| Limk1 | LIM domain kinase 1 |
| CREB | cAMP response element-binding protein |
| NF1 | Neurofibromin |
References
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 3, 402. [Google Scholar] [CrossRef] [PubMed]
- Makarova, J.A.; Shkurnikov, M.U.; Wicklein, D.; Lange, T.; Samatov, T.R.; Turchinovich, A.A.; Tonevitsky, A.G. Intracellular and extracellular microRNA: An update on localization and biological role. Prog. Histochem. Cytochem. 2016, 51, 33–49. [Google Scholar] [CrossRef]
- Moretti, F.; Thermann, R.; Hentze, M.W. Mechanism of translational regulation by miR-2 from sites in the 5′ untranslated region or the open reading frame. RNA 2010, 16, 2493–2502. [Google Scholar] [CrossRef]
- Jackstadt, R.; Hermeking, H. MicroRNAs as regulators and mediators of c-MYC function. Biochim. Biophys. Acta 2015, 1849, 544–553. [Google Scholar] [CrossRef]
- Jones, R.G., 3rd; von Walden, F.; Murach, K.A. Exercise-Induced MYC as an Epigenetic Reprogramming Factor That Combats Skeletal Muscle Aging. Exerc. Sport Sci. Rev. 2024, 52, 63–67. [Google Scholar] [CrossRef]
- Improta Caria, A.C.; Nonaka, C.K.V.; Pereira, C.S.; Soares, M.B.P.; Macambira, S.G.; Souza, B.S.F. Exercise Training-Induced Changes in MicroRNAs: Beneficial Regulatory Effects in Hypertension, Type 2 Diabetes, and Obesity. Int. J. Mol. Sci. 2018, 15, 3608. [Google Scholar] [CrossRef]
- Poy, M.N.; Eliasson, L.; Krutzfeldt, J.; Kuwajima, S.; Ma, X.; Macdonald, P.E.; Pfeffer, S.; Tuschl, T.; Rajewsky, N.; Rorsman, P.; et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004, 432, 226–230. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Liang, Y.; Liu, S.; Xiao, H.; Li, F.; Cheng, H.; Fu, Z. miR-375 enhances palmitate-induced lipoapoptosis in insulin-secreting NIT-1 cells by repressing myotrophin (V1) protein expression. Int. J. Clin. Exp. Pathol. 2010, 3, 254–264. [Google Scholar]
- Krek, A.; Grün, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Son, G.; Na, Y.; Kim, Y.; Son, J.H.; Clemenson, G.D.; Schafer, S.T.; Yoo, J.Y.; Parylak, S.L.; Paquola, A.; Do, H.; et al. miR-124 coordinates metabolic regulators acting at early stages of human neurogenesis. Commun. Biol. 2024, 25, 1393. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Fehrenbach, E.; Niess, A.M. Regulation of immediate early gene expression by exercise: Short cuts for the adaptation of immune function. Exerc. Immunol. Rev. 2006, 12, 112–131. [Google Scholar] [PubMed]
- Baskerville, S.; Bartel, D.P. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 2005, 11, 241–247. [Google Scholar] [CrossRef]
- Witvrouwen, I.; Gevaert, A.B.; Possemiers, N.; Ectors, B.; Stoop, T.; Goovaerts, I.; Boeren, E.; Hens, W.; Beckers, P.J.; Vorlat, A.; et al. Plasma-Derived microRNAs Are Influenced by Acute and Chronic Exercise in Patients with Heart Failure with Reduced Ejection Fraction. Front. Physiol. 2021, 12, 736494. [Google Scholar] [CrossRef]
- Kirby, T.J.; McCarthy, J.J. MicroRNAs in skeletal muscle biology and exercise adaptation. Free Radic. Biol. Med. 2013, 64, 95–105. [Google Scholar] [CrossRef]
- Castaño, C.; Mirasierra, M.; Vallejo, M.; Novials, A.; Párrizas, M. Delivery of muscle-derived exosomal miRNAs induced by HIIT improves insulin sensitivity through down-regulation of hepatic FoxO1 in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 30335–30343. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Kargarfard, M.; Braune, S.; Jung, F.; Naderi, M. Long-term aerobic exercise training in type two diabetic patients alters the expression of miRNA-223 and its corresponding target, the P2RY12 receptor, attenuating platelet function. Clin. Hemorheol. Microcirc. 2022, 80, 107–116. [Google Scholar] [CrossRef]
- Masuzawa, R.; Konno, R.; Ohsawa, I.; Watanabe, A.; Kawano, F. Muscle type-specific RNA polymerase II recruitment during PGC-1α gene transcription after acute exercise in adult rats. J. Appl. Physiol. 2018, 125, 1238–1245. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Zhang, M.; Feng, T.; Chen, R.; Shen, J.; Li, R.; Wang, Z.; Xie, Y.; Wang, D.; et al. Histone deacetylase inhibition enhances extracellular vesicles from muscle to promote osteogenesis via miR-873-3p. Sig. Transduct. Target Ther. 2024, 9, 256. [Google Scholar] [CrossRef]
- Rdw, K.; Kr, S.; A, C.; Lc, J.; Sw, H.; Sm, C. Histone Deacetylases (HDACs) maintain expression of the pluripotent gene network via recruitment of RNA polymerase II to coding and non-coding loci. bioRxiv 2023. [Google Scholar] [CrossRef]
- Yang, A.J.; Hayward, G.C.; MacPherson, R.E.K. Acute exercise and brain BACE1 protein content: A time course study. Physiol. Rep. 2019, 7, 14084. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.P.; Lamon, S.; Boon, H.; Wada, S.; Güller, I.; Brown, E.L.; Chibalin, A.V.; Zierath, J.R.; Snow, R.J.; Stepto, N.; et al. Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short-term endurance training. J. Physiol. 2013, 591, 4637–4653. [Google Scholar] [CrossRef]
- Swahari, V.; Nakamura, A.; Hollville, E.; Stroud, H.; Simon, J.M.; Ptacek, T.S.; Beck, M.V.; Flowers, C.; Guo, J.; Plestant, C.; et al. MicroRNA-29 is an essential regulator of brain maturation through regulation of CH methylation. Cell Rep. 2021, 35, 108946. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.; Marques, F.Z.; O’Brien, B.J.; Charchar, F.J. Exercise: Putting action into our epigenome. Sports Med. 2014, 44, 189–209. [Google Scholar] [CrossRef]
- Assali, A.; Harrington, A.J.; Cowan, C.W. Emerging roles for MEF2 in brain development and mental disorders. Curr. Opin. Neurobiol. 2019, 59, 49–58. [Google Scholar] [CrossRef]
- Lee, Q.Y.; Mall, M.; Chanda, S.; Zhou, B.; Sharma, K.S.; Schaukowitch, K.; Adrian-Segarra, J.M.; Grieder, S.D.; Kareta, M.S.; Wapinski, O.L.; et al. Pro-neuronal activity of Myod1 due to promiscuous binding to neuronal genes. Nat. Cell Biol. 2020, 22, 401–411. [Google Scholar] [CrossRef]
- Mercer, H.M.; Nair, A.M.; Ridgel, A.; Piontkivska, H. Alterations in RNA editing in skeletal muscle following exercise training in individuals with Parkinson’s disease. PLoS ONE 2023, 18, e0287078. [Google Scholar] [CrossRef]
- Heale, B.S.; Keegan, L.P.; O’Connell, M.A. The Effect of RNA Editing and ADARs on miRNA Biogenesis and Function. Adv. Exp. Med. Biol. 2011, 700, 76–84. [Google Scholar]
- Hao, X.; Li, Y.; Huang, G.; Zeng, Y. Role of the N6-methyladenosine regulatory factor in reducing the risk of cardiovascular disease: Subtype diagnosis following aerobic exercise-assisted weight loss. Am. J. Transl. Res. 2022, 14, 5363–5378. [Google Scholar]
- Ha, M.; Kim, V. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Dorner, H.; Htun, P.; Lensche, H.; Platt, D.; Erdmann, E. Effects of exercise on myocardial adenylate cyclase and Gi alpha expression in senescence. Am. J. Physiol. 1993, 264, H805–H814. [Google Scholar] [CrossRef]
- Silva, F.C.D.; Iop, R.D.R.; Andrade, A.; Costa, V.P.; Gutierres Filho, P.J.B.; Silva, R.D. Effects of Physical Exercise on the Expression of MicroRNAs: A Systematic Review. J Strength Cond. Res. 2020, 34, 270–280, Erratum in: J. Strength Cond. Res. 2020, 34, e273. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Yu, P.; Feng, J.; Xu, G.E.; Zhao, X.; Wang, T.; Lehmann, H.I.; Li, G.; Sluijter, J.P.G.; et al. METTL14 is required for exercise-induced cardiac hypertrophy and protects against myocardial ischemia-reperfusion injury. Nat. Commun. 2022, 13, 6762. [Google Scholar] [CrossRef]
- Safdar, A.; Tarnopolsky, M.A. Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029827. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Wang, B. Momeni, M.R. Exosomes and microRNAs as mediators of the exercise. Eur. J. Med. Res. 2025, 30, 38. [Google Scholar] [CrossRef]
- Hourigan, S.T.; Solly, E.L.; Nankivell, V.A.; Ridiandries, A.; Weimann, B.M.; Henriquez, R.; Tepper, E.R.; Zhang, J.Q.J.; Tsatralis, T.; Clayton, Z.E.; et al. The regulation of miRNAs by reconstituted high-density lipoproteins in diabetes-impaired angiogenesis. Sci. Rep. 2018, 11, 13596. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.J.; Hu, J.; Hu, Z.; Kraemer, F.B.; Azhar, S. Scavenger receptor class B type I (SR-BI): A versatile receptor with multiple functions and actions. Metabolism 2014, 63, 875–886. [Google Scholar] [CrossRef]
- Canfrán-Duque, A.; Lin, C.S.; Goedeke, L.; Suárez, Y.; Fernández-Hernando, C. Micro-RNAs and High-Density Lipoprotein Metabolism. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1076–1084. [Google Scholar] [CrossRef]
- Tabet, F.; Vickers, K.; Cuesta Torres, L.; Wiese, C.B.; Shoucri, B.M.; Lambert, G.; Catherinet, C.; Prado-Lourenco, L.; Levin, M.G.; Thacker, S.; et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 2014, 5, 3292. [Google Scholar] [CrossRef]
- Palazón-Bru, A.; Hernández-Lozano, D.; Gil-Guillén, V.F. Which Physical Exercise Interventions Increase HDL-Cholesterol Levels? A Systematic Review of Meta-analyses of Randomized Controlled Trials. Sports Med. 2021, 51, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Caruso Bavisotto, C.; Bucchieri, F.; Cappello, F. The unexplored potential of exosomes in the muscle-brain axis. Proc. Natl. Acad. Sci. USA 2025, 122, e2420766121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, O.; Li, N.; Sha, Z.; Zhao, Z.; Xu, J. Association between the BDNF Val66Met polymorphism and major depressive disorder: A systematic review and meta-analysis. Front. Psychiatry 2023, 14, 1143833. [Google Scholar] [CrossRef]
- Toader, C.; Serban, M.; Munteanu, O.; Covache-Busuioc, R.A.; Enyedi, M.; Ciurea, A.V.; Tataru, C.P. From Synaptic Plasticity to Neurodegeneration: BDNF as a Transformative Target in Medicine. Int. J. Mol. Sci. 2025, 26, 4271. [Google Scholar] [CrossRef]
- Alexandrov, P.N.; Dua, P.; Hill, J.M.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. microRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF). Int. J. Biochem. Mol. Biol. 2012, 3, 365–373. [Google Scholar]
- Polakovičová, M.; Musil, P.; Laczo, E.; Hamar, D.; Kyselovič, J. Circulating MicroRNAs as Potential Biomarkers of Exercise Response. Int. J. Mol. Sci. 2016, 17, 1553. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.M.J.; Krauskopf, J.; Ramaekers, J.G.; Kleinjans, J.C.S.; Prickaerts, J.; Briedé, J.J. Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Prog. Neurobiol. 2020, 185, 101732. [Google Scholar] [CrossRef]
- De Miguel, Z.; Khoury, N.; Betley, M.J.; Lehallier, B.; Willoughby, D.; Olsson, N.; Yang, A.C.; Hahn, O.; Lu, N.; Vest, R.T.; et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 2021, 600, 494–499. [Google Scholar] [CrossRef]
- Chen, S.L.; Cai, G.X.; Ding, H.G.; Liu, X.Q.; Wang, Z.H.; Jing, Y.W.; Han, Y.L.; Jiang, W.Q.; Wen, M.Y. JAK/STAT signaling pathway-mediated microRNA-181b promoted blood-brain barrier impairment by targeting sphingosine-1-phosphate receptor 1 in septic rats. Ann. Transl. Med. 2020, 8, 1458. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, X.; Wu, W.; Tang, Y.; Meng, X.; Peng, M.; Tang, C.; Zheng, L.; Liu, W. The Potential Related Genes and Mechanisms Involved in Improving the Treadmill Exercise Ability of APP/PS1 Mice. Int. J. Mol. Sci. 2024, 25, 10244. [Google Scholar] [CrossRef]
- Li, M.; Mo, J.; Wu, D.; He, H.; Hu, P. Treadmill training improves neural function recovery in rats with spinal cord injury via JAK2/STAT3 signaling pathway and attenuating apoptosis. Neuroreport 2024, 35, 811–821. [Google Scholar] [CrossRef]
- Valenti, M.T.; Deiana, M.; Cheri, S.; Dotta, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Dalle Carbonare, L.; Mottes, M. Physical Exercise Modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p Expression in Progenitor Cells Promoting Osteogenesis. Cells 2019, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Guo, C.; Zou, Y.Y.; Feng, C.; Yang, D.X.; Sun, C.C.; Wen, W.; Jian, Z.J.; Zhao, Z.; Xiao, Q.; et al. Aerobic exercise enhances mitochondrial homeostasis to counteract D-galactose-induced sarcopenia in zebrafish. Exp. Gerontol. 2023, 180, 112265. [Google Scholar] [CrossRef]
- Papagiannakopoulos, T.; Friedmann-Morvinski, D.; Neveu, P.; Dugas, J.C.; Gill, R.M.; Huillard, E.; Liu, C.; Zong, H.; Rowitch, D.H.; Barres, B.A.; et al. Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene 2012, 31, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- Shvarts-Serebro, I.; Sheinin, A.; Gottfried, I.; Adler, L.; Schottlender, N.; Ashery, U.; Barak, B. miR-128 as a Regulator of Synaptic Properties in 5xFAD Mice Hippocampal Neurons. J. Mol. Neurosci. 2021, 71, 2593–2607. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Pang, J.C.; Ching, A.K.; Wong, C.K.; Kong, X.; Wang, Y.; Zhou, L.; Chen, Z.; Ng, H.K. miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Hum. Pathol. 2009, 40, 1234–1243. [Google Scholar] [CrossRef]
- Mojtahedi, S.; Shabkhiz, F.; Ravasi, A.A.; Rosenkranz, S.; Soori, R.; Soleimani, M.; Tavakoli, R. Voluntary wheel running promotes improvements in biomarkers associated with neurogenic activity in adult male rats. Biochem. Biophys. Res. Commun. 2020, 533, 1505–1511. [Google Scholar] [CrossRef]
- Song, W.; Teng, L.; Wang, H.; Pang, R.; Liang, R.; Zhu, L. Exercise preconditioning increases circulating exosome miR-124 expression and alleviates apoptosis in rats with cerebral ischemia-reperfusion injury. Brain Res. 2025, 1851, 149457. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Zhan, Z.; Wang, X. MicroRNA-132 is associated with the cognition improvement following voluntary exercise in SAMP8 mice. Brain Res. Bull. 2018, 140, 80–87. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, J.; Sun, X.; Ma, G.; Luo, G.; Miao, Z.; Song, L. miR-132 improves the cognitive function of rats with Alzheimer’s disease by inhibiting the MAPK1 signal pathway. Exp. Ther. Med. 2020, 20, 159. [Google Scholar] [CrossRef]
- Carbonell, T.; Gomes, A.V. MicroRNAs in the regulation of cellular redox status and its implications in myocardial ischemia-reperfusion injury. Redox Biol. 2020, 36, 101607. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Koltai, E.; Bori, Z.; Osvath, P.; Ihasz, F.; Peter, S.; Toth, G.; Degens, H.; Rittweger, J.; Boldogh, I.; Radak, Z. Master athletes have higher miR-7, SIRT3 and SOD2 expression in skeletal muscle than age-matched sedentary controls. Redox Biol. 2018, 19, 46–51. [Google Scholar] [CrossRef]
- Kim, T.; Mehta, S.L.; Morris-Blanco, K.C.; Chokkalla, A.K.; Chelluboina, B.; Lopez, M.; Sullivan, R.; Kim, H.T.; Cook, T.D.; Kim, J.Y.; et al. The microRNA miR-7a-5p ameliorates ischemic brain damage by repressing α-synuclein. Sci. Signal. 2018, 11, eaat4285. [Google Scholar] [CrossRef]
- Yamagata, K.; Sanders, L.K.; Kaufmann, W.E.; Yee, W.; Barnes, C.A.; Nathans, D.; Worley, P.F. rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J. Biol. Chem. 1994, 269, 16333–16339. [Google Scholar] [CrossRef]
- Srivastava, K.; Tripathi, R.; Mishra, R. Age-dependent alterations in expression and co-localization of Pax6 and Ras-GAP in brain of aging mice. J. Chem. Neuroanat. 2018, 92, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Greco, F.; Quinzi, F.; Scionti, F.; Maurotti, S.; Montalcini, T.; Mancini, A.; Buono, P.; Emerenziani, G.P. The Effect of Physical Activity/Exercise on miRNA Expression and Function in Non-Communicable Diseases-A Systematic Review. Int. J. Mol. Sci. 2024, 25, 6813. [Google Scholar] [CrossRef]
- Mitchelson, K.R.; Qin, W.Y. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J. Biol. Chem. 2015, 6, 162–208. [Google Scholar] [CrossRef]
- Bao, H.; Mao, S.; Hu, X.; Li, L.; Tao, H.; Zhou, J.; Xu, L.; Fang, Y.; Zhang, Y.; Chu, L. Exosomal miR-486 derived from bone marrow mesenchymal stem cells promotes angiogenesis following cerebral ischemic injury by regulating the PTEN/Akt pathway. Sci. Rep. 2024, 14, 18086. [Google Scholar] [CrossRef]
- Gomes, C.P.; Oliveira, G.P., Jr.; Madrid, B.; Almeida, J.A.; Franco, O.L.; Pereira, R.W. Circulating miR-1, miR-133a, and miR-206 levels are increased after a half-marathon run. Biomarkers 2014, 19, 585–589. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.Y.; Wang, L.; Zhao, F.F.; Yang, S.; Ning, F.B. MiR-133a Promoted cerebral ischemia/reperfusion injury by targeting brain-derived neurotrophic factor. J. Biol. Regul. Homeost. Agents 2020, 34, 1419–1422. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.B.; Jin, X. Evidence for the Contribution of the miR-206/BDNF Pathway in the Pathophysiology of Depression. Int. J. Neuropsychopharmacol. 2024, 27, pyae039. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.C.; Rode, M.P.; Vietta, G.G.; Iop, R.D.R.; Creczynski-Pasa, T.B.; Martin, A.S.; Da Silva, R. Expression levels of specific microRNAs are increased after exercise and are associated with cognitive improvement in Parkinson’s disease. Mol. Med. Rep. 2021, 24, 618. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Esser, K.A. MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J. Appl. Physiol. 2007, 102, 306–313. [Google Scholar] [CrossRef]
- Bondy, C.; Cheng, C.; Zhong, J.; Lee, W. IGF-1 in Brain Growth and Repair Processes. In Handbook of Neurochemistry and Molecular Neurobiology; Lajtha, A., Lim, R., Eds.; Springer: Boston, MA, USA, 2006. [Google Scholar]
- McNay, E.C.; Pearson-Leary, J. GluT4: A central player in hippocampal memory and brain insulin resistance. Exp. Neurol. 2020, 323, 113076. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, C.; Zhang, A.; Cai, H.; Price, S.R.; Wang, X.H. MicroRNA-23a and MicroRNA-27a Mimic Exercise by Ameliorating CKD-Induced Muscle Atrophy. J. Am. Soc. Nephrol. 2017, 28, 2631–2640. [Google Scholar] [CrossRef]
- Liu, L.; Bai, J.; Liu, F.; Xu, Y.; Zhao, M.; Zhao, C.; Zhou, Z. Cross-Talking Pathways of Forkhead Box O1 (FOXO1) Are Involved in the Pathogenesis of Alzheimer’s Disease and Huntington’s Disease. Oxid. Med. Cell. Longev. 2022, 2022, 7619255. [Google Scholar] [CrossRef] [PubMed]
- Gazova, A.; Samakova, A.; Laczo, E.; Hamar, D.; Polakovicova, M.; Jurikova, M.; Kyselovic, J. Clinical utility of miRNA-1, miRNA-29g and miRNA-133s plasma levels in prostate cancer patients with high-intensity training after androgen-deprivation therapy. Physiol. Res. 2019, 68 (Suppl. S2), S139–S147. [Google Scholar] [CrossRef]
- Li, Y.; Yao, M.; Zhou, Q.; Cheng, Y.; Che, L.; Xu, J.; Xiao, J.; Shen, Z.; Bei, Y. Dynamic Regulation of Circulating microRNAs During Acute Exercise and Long-Term Exercise Training in Basketball Athletes. Front. Physiol. 2018, 26, 282. [Google Scholar] [CrossRef]
- Su, Z.F.; Sun, Z.W.; Zhang, Y.; Wang, S.; Yu, Q.G.; Wu, Z.B. Regulatory effects of miR-146a/b on the function of endothelial progenitor cells in acute ischemic stroke in mice. Kaohsiung J. Med. Sci. 2017, 33, 369–378. [Google Scholar] [CrossRef]
- Kim, S.J.; Russell, A.E.; Wang, W.; Gemoets, D.E.; Sarkar, S.N.; Simpkins, J.W.; Brown, C.M. miR-146a Dysregulates Energy Metabolism During Neuroinflammation. J. Neuroimmune Pharmacol. 2022, 17, 228–241. [Google Scholar] [CrossRef]
- Törpel, A.; Herold, F.; Hamacher, D.; Müller, N.G.; Schega, L. Strengthening the Brain-Is Resistance Training with Blood Flow Restriction an Effective Strategy for Cognitive Improvement? J. Clin. Med. 2018, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Delfan, M.; Amadeh Juybari, R.; Gorgani-Firuzjaee, S.; Høiriis Nielsen, J.; Delfan, N.; Laher, I.; Saeidi, A.; Granacher, U.; Zouhal, H. High-Intensity Interval Training Improves Cardiac Function by miR-206 Dependent HSP60 Induction in Diabetic Rats. Front. Cardiovasc Med. 2022, 9, 927956. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, A.; Wang, Y.; Hu, S.; Zhang, R.; Qian, S. Genome-wide identification of brain miRNAs in response to high-intensity intermittent swimming training in Rattus norvegicus by deep sequencing. BMC Mol. Biol. 2019, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.D., Jr.; Fernandes, T.; Soci, U.P.; Monteiro, A.W.; Phillips, M.I.; De Oliveira, E.M. Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med. Sci. Sports Exerc. 2012, 44, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Telles, G.D.; Libardi, C.A.; Conceição, M.S.; Vechin, F.C.; Lixandrão, M.E.; Andrade, A.L.L.; Guedes, D.N.; Ugrinowitsch, C.; Camera, D.M. Time Course of Skeletal Muscle miRNA Expression after Resistance, High-Intensity Interval, and Concurrent Exercise. Med. Sci. Sports Exerc. 2021, 53, 1708–1718. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, Y.L.; Yin, X.; Xu, S.J.; Tian, D.; Zhang, C.Y.; Wang, S.; Ma, J.Z. Excessive Treadmill Training Enhances Brain-Specific MicroRNA-34a in the Mouse Hippocampus. Front. Mol. Neurosci. 2020, 13, 7. [Google Scholar] [CrossRef]
- Farsani, Z.H.; Banitalebi, E.; Faramarzi, M.; Bigham-Sadegh, A. Effects of different intensities of strength and endurance training on some osteometabolic miRNAs, Runx2 and PPARγ in bone marrow of old male wistar rats. Mol. Biol. Rep. 2019, 46, 2513–2521. [Google Scholar] [CrossRef]
- Torma, F.; Gombos, Z.; Jokai, M.; Berkes, I.; Takeda, M.; Mimura, T.; Radak, Z.; Gyori, F. The roles of microRNA in redox metabolism and exercise-mediated adaptation. J. Sport Health Sci. 2020, 9, 405–414. [Google Scholar] [CrossRef]
- Chen, Y.; Melton, D.W.; Gelfond, J.A.; McManus, L.M.; Shireman, P.K. MiR-351 transiently increases during muscle regeneration and promotes progenitor cell proliferation and survival upon differentiation. Physiol. Genom. 2012, 44, 1042–1051. [Google Scholar] [CrossRef]
- Hu, Y.; Mao, Z.; Xu, L.; Yin, L.; Tao, X.; Tang, Z.; Qi, Y.; Sun, P.; Peng, J. Protective effect of dioscin against intestinal ischemia/reperfusion injury via adjusting miR-351-5p-mediated oxidative stress. Pharmacol. Res. 2018, 137, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Cao, K.; Xu, C.; Sun, A.; Lu, W.; Zheng, Y.; Li, H.; Hong, G.; Wu, B.; Qiu, Q. Crosstalk between Mitochondrial Fission and Oxidative Stress in Paraquat-Induced Apoptosis in Mouse Alveolar Type II Cells. Int. J. Biol. Sci. 2017, 13, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.N.; Park, S.; Kim, H.L.; Jung, M.K.; Pack, C.G.; Park, J.; Cho, Y.; Jo, D.G.; Kim, D.K.; Mook-Jung, I.; et al. miR-351-5p/Miro2 axis contributes to hippocampal neural progenitor cell death via unbalanced mitochondrial fission. Mol. Ther. Nucleic Acids 2020, 23, 643–656. [Google Scholar] [CrossRef]
- Wu, C.; Du, M.; Yu, R.; Cheng, Y.; Wu, B.; Fu, J.; Tan, W.; Zhou, Q.; Balawi, E.; Liao, Z.B. A novel mechanism linking ferroptosis and endoplasmic reticulum stress via the circPtpn14/miR-351-5p/5-LOX signaling in melatonin-mediated treatment of traumatic brain injury. Free Radic. Biol. Med. 2022, 178, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Bai, M.; Xu, J.; Zhu, L.; Liu, C.; Duan, R. Long-Term Exercise Alters the Profiles of Circulating Micro-RNAs in the Plasma of Young Women. Front. Physiol. 2020, 11, 372. [Google Scholar] [CrossRef]
- Dungan, C.M.; Valentino, T.; Vechetti, I.J., Jr.; Zdunek, C.J.; Murphy, M.P.; Lin, A.L.; McCarthy, J.J.; Peterson, C.A. Exercise-mediated alteration of hippocampal Dicer mRNA and miRNAs is associated with lower BACE1 gene expression and Aβ1-42 in female 3xTg-AD mice. J. Neurophysiol. 2020, 124, 1571–1577. [Google Scholar] [CrossRef]
- Huang, X.; Ding, L.; Bennewith, K.L.; Tong, R.T.; Welford, S.M.; Ang, K.K.; Story, M.; Le, Q.T.; Giaccia, A.J. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol. Cell 2009, 35, 856–867. [Google Scholar] [CrossRef]
- Ye, F.; Lu, X.; van Neck, R.; Jones, D.L.; Feng, Q. Novel circRNA-miRNA-mRNA networks regulated by maternal exercise in fetal hearts of pregestational diabetes. Life Sci. 2023, 314, 121308. [Google Scholar] [CrossRef]
- Kelly, T.J.; Souza, A.L.; Clish, C.B.; Puigserver, P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol. Cell. Biol. 2011, 31, 2696–2706. [Google Scholar] [CrossRef]
- Huang, L.; Ma, Q.; Li, Y.; Li, B.; Zhang, L. Inhibition of microRNA-210 suppresses pro-inflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp. Neurol. 2018, 300, 41–50. [Google Scholar] [CrossRef]
- Bartoszewska, S.; Kochan, K.; Piotrowski, A.; Kamysz, W.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1α expression in human endothelial cells through a negative feedback loop. FASEB J. 2015, 29, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Duan, Y.; Xiao, J. Exercise Improves the Function of Endothelial Cells by MicroRNA. J. Cardiovasc. Transl. Res. 2019, 12, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Gao, J.; Ding, S.L.; Wang, K.; Jiao, J.Q.; Wang, Y.; Sun, T.; Zhou, L.Y.; Long, B.; Zhang, X.; et al. Oxidative Modification of miR-184 Enables It to Target Bcl-xL and Bcl-w. Mol. Cell 2015, 59, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, H.Z.; Li, X.; Wu, Z.; Han, Y.; Li, Y.; Chen, G.; Xie, X.; Huang, Y.; Du, Z.; et al. MicroRNA-184 inhibits cell proliferation and invasion, and specifically targets TNFAIP2 in Glioma. J. Exp. Clin. Cancer Res. 2015, 34, 27. [Google Scholar] [CrossRef]
- Song, J.; Saeman, M.R.; Baer, L.A.; Cai, A.R.; Wade, C.E.; Wolf, S.E. Exercise Altered the Skeletal Muscle MicroRNAs and Gene Expression Profiles in Burn Rats with Hindlimb Unloading. J. Burn. Care Res. 2017, 38, 11–19. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Y.; Zhang, M.; Yan, Y.; Yu, H.; Ge, L. microRNA-451 protects neurons against ischemia/reperfusion injury-induced cell death by targeting CELF2. Neuropsychiatr. Dis. Treat. 2018, 14, 2773–2782. [Google Scholar] [CrossRef]
- Gaál, Z.; Fodor, J.; Oláh, A.; Radovits, T.; Merkely, B.; Magyar, J.; Csernoch, L. Evaluation of muscle-specific and metabolism regulating microRNAs in a chronic swimming rat model. J. Muscle Res. Cell Motil. 2022, 43, 21–33. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Y.; Zhang, D. microRNA-21 Confers neuroprotection against cerebral ischemia-reperfusion injury and alleviates blood-brain barrier disruption in rats via the MAPK signaling pathway. J. Mol. Neurosci. 2018, 65, 43–53. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Li, B.; Sun, C.; Zhang, P. miR-21-5p protects hippocampal neurons of epileptic rats via inhibiting STAT3 expression. Adv. Clin. Exp. Med. 2020, 29, 793–801. [Google Scholar] [CrossRef]
- Morais, G.S., Jr.; Souza, V.C.; Machado-Silva, W.; Henriques, A.D.; Alves, A.M.; Morais, D.B.; Nóbrega, O.T.; Brito, C.J.; Silva, R.J.D.S. Acute strength training promotes responses in whole blood circulating levels of miR-146a among older adults with type 2 diabetes mellitus. Clin. Interv. Aging 2017, 12, 1443–1450. [Google Scholar]
- Zhao, W.; Spiers, J.G.; Vassileff, N.; Khadka, A.; Jaehne, E.J.; van den Buuse, M.; Hill, A.F. microRNA-146a modulates behavioural activity, neuroinflammation, and oxidative stress in adult mice. Mol. Cell Neurosci. 2023, 124, 103820. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, L.; Guo, Q.; Shi, H.; Gan, Y.; Wang, W.; Yang, X.; Zhou, Y. Aerobic exercise improves inflammation and insulin resistance in skeletal muscle by regulating miR-221-3p via JAK/STAT signaling pathway. Front. Physiol. 2025, 25, 1534911. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Deng, N.; Lu, K.; Liao, Q.; Long, X.; Gou, D.; Bi, F.; Zhou, J. Elevated plasma miR-133b and miR-221-3p as biomarkers for early Parkinson’s disease. Sci. Rep. 2021, 11, 15268. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, J.; Zhu, H.; Wei, X.; Platt, C.; Damilano, F.; Xiao, C.; Bezzerides, V.; Boström, P.; Che, L.; et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015, 21, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.Y.; Niu, J.Z. miR-222 regulates brain injury and inflammation following intracerebral hemorrhage by targeting ITGB8. Mol. Med. Rep. 2020, 21, 1145–1153. [Google Scholar] [CrossRef]
- Habibi, P.; Alihemmati, A.; NourAzar, A.; Yousefi, H.; Mortazavi, S.; Ahmadiasl, N. Expression of the Mir-133 and Bcl-2 could be affected by swimming training in the heart of ovariectomized rats. Iran J. Basic Med. Sci. 2016, 19, 381–387. [Google Scholar]
- Bahrami, F.; Fathi, M.; Ahmadvand, H.; Pajohi, N. Endurance training changes the expression of miR-1 and miR-133 and predicted genes in slow and fast twitch muscles. Arch. Gerontol. Geriatr. 2023, 108, 104929. [Google Scholar] [CrossRef]
- Fernandes, J.; Vieira, A.S.; Kramer-Soares, J.C.; Da Silva, E.A.; Lee, K.S.; Lopes-Cendes, I.; Arida, R.M. Hippocampal microRNA-mRNA regulatory network is affected by physical exercise. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1711–1720. [Google Scholar] [CrossRef]
- Vogel, J.; Niederer, D.; Engeroff, T.; Vogt, L.; Troidl, C.; Schmitz-Rixen, T.; Banzer, W.; Troidl, K. Effects on the Profile of Circulating miRNAs after Single Bouts of Resistance Training with and without Blood Flow Restriction—A Three-Arm, Randomized Crossover Trial. Int. J. Mol. Sci. 2019, 20, 3249. [Google Scholar] [CrossRef]
- Gilardi, C.; Martins, H.C.; Levone, B.R.; Bianco, A.L.; Bicker, S.; Germain, P.L.; Gross, F.; Sungur, A.Ö.; Kisko, T.M.; Stein, F.; et al. miR-708-5p is elevated in bipolar patients and can induce mood disorder-associated behavior in mice. EMBO Rep. 2025, 10, 2025. [Google Scholar] [CrossRef]
- de Gonzalo-Calvo, D.; Dávalos, A.; Montero, A.; García-González, Á.; Tyshkovska, I.; González-Medina, A.; Soares, S.M.; Martínez-Camblor, P.; Casas-Agustench, P.; Rabadán, M.; et al. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J. Appl. Physiol. 2015, 119, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tao, X.; Sun, R.; Han, C.; Li, X.; Zhu, Z.; Li, W.; Huang, P.; Gong, W. Cognitive-exercise dual-task intervention ameliorates cognitive decline in natural aging rats via inhibiting the promotion of LncRNA NEAT1/miR-124-3p on caveolin-1-PI3K/Akt/GSK3β Pathway. Brain Res. Bull. 2023, 202, 110761. [Google Scholar] [CrossRef]
- Sieland, J.; Niederer, D.; Engeroff, T.; Vogt, L.; Troidl, C.; Schmitz-Rixen, T.; Banzer, W.; Troidl, K. Effects of single bouts of different endurance exercises with different intensities on microRNA biomarkers with and without blood flow restriction: A three-arm, randomized crossover trial. Eur. J. Appl. Physiol. 2021, 121, 3243–3255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, W.; Jiang, Z.L.; Chen, Z.H.; Zhang, H. Intervention effects of miR-125b-5p on cognitive dysfunction induced by traumatic brain injury in rats and its mechanisms. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2022, 38, 424–429. [Google Scholar]
- Schmitz, B.; Schelleckes, K.; Nedele, J.; Thorwesten, L.; Klose, A.; Lenders, M.; Krüger, M.; Brand, E.; Brand, S.M. Dose-Response of High-Intensity Training (HIT) on Atheroprotective miRNA-126 Levels. Front. Physiol. 2017, 30, 349. [Google Scholar] [CrossRef]
- Ebrahimi, V.; Rastegar-Moghaddam, S.H.; Mohammadipour, A. Therapeutic Potentials of MicroRNA-126 in Cerebral Ischemia. Mol. Neurobiol. 2023, 60, 2062–2069. [Google Scholar] [CrossRef]
- Oghbaei, H.; Asl, N.A.; Sheikhzadeh, F. Can regular moderate exercise lead to changes in miRNA-146a and its adapter proteins in the kidney of streptozotocin-induced diabetic male rats? Endocr. Regul. 2017, 51, 145–152. [Google Scholar] [CrossRef]
- Fan, W.; Liang, C.; Ou, M.; Zou, T.; Sun, F.; Zhou, H.; Cui, L. MicroRNA-146a Is a Wide-Reaching Neuroinflammatory Regulator and Potential Treatment Target in Neurological Diseases. Front. Mol. Neurosci. 2020, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.Z.; Cirino, M.L.A.; Porsani, L.B.; Neto, F.S.L.; Novais, P.C.; Nunes, M.J.; Lellis, J.R.; Da Silva, J.P.; Tazima, M.F.G.S.; de Toledo Durand, M.; et al. Expression of microRNAs miR-126, miR-133b and miR-221 associated with apoptosis in rats submitted to focal cerebral ischemia and physical exercise. Int. J. Morphol. 2022, 40, 1518–1523. [Google Scholar] [CrossRef]
- Long, Y.F.; Chow, S.K.; Cui, C.; Wong, R.M.Y.; Zhang, N.; Qin, L.; Law, S.W.; Cheung, W.H. Does exercise influence skeletal muscle by modulating mitochondrial functions via regulating MicroRNAs? A systematic review. Ageing Res. Rev. 2023, 91, 102048. [Google Scholar] [CrossRef]
- Horak, M.; Zlamal, F.; Iliev, R.; Kucera, J.; Cacek, J.; Svobodova, L.; Hlavonova, Z.; Kalina, T.; Slaby, O.; Bienertova-Vasku, J. Exercise-induced circulating microRNA changes in athletes in various training scenarios. PLoS ONE 2018, 13, e0191060. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.F.; Markworth, J.F.; Aasen, K.M.M.; Zeng, N.; Cameron-Smith, D.; Mitchell, C.J. Acute resistance exercise modulates microRNA expression profiles: Combined tissue and circulatory targeted analyses. PLoS ONE 2017, 12, e0181594. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Wang, H.; Liu, Y.; Su, Z.; Li, X.; Zhu, Y.; Zhang, Z.; Yin, M.; Chen, C.; Li, L.; et al. Exercise-Induced miR-210 Promotes Cardiomyocyte Proliferation and Survival and Mediates Exercise-Induced Cardiac Protection against Ischemia/Reperfusion Injury. Research 2024, 26, 0327. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Ichikawa, H.; Mune, K.; Tanimura, Y.; Mizushima, K.; Naito, Y.; Yoshikawa, T. Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front. Physiol. 2013, 11, 80. [Google Scholar] [CrossRef]
- Jee, M.K.; Jung, J.S.; Choi, J.I.; Jang, J.A.; Kang, K.S.; Im, Y.B.; Kang, S.K. MicroRNA 486 is a potentially novel target for the treatment of spinal cord injury. Brain 2012, 135, 1237–1252. [Google Scholar] [CrossRef]
- Banzet, S.; Chennaoui, M.; Girard, O.; Racinais, S.; Drogou, C.; Chalabi, H.; Koulmann, N. Changes in circulating microRNAs levels with exercise modality. J. Appl. Physiol. 2013, 115, 1237–1244. [Google Scholar] [CrossRef]
- Adlakha, Y.K.; Saini, N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128. Mol. Cancer 2014, 13, 33. [Google Scholar] [CrossRef]
- Israni, D.K.; Patel, M.L.; Dodiya, R.K. Exploring the versatility of miRNA-128: A comprehensive review on its role as a biomarker and therapeutic target in clinical pathways. Mol. Biol. Rep. 2024, 51, 860. [Google Scholar] [CrossRef]
- Pan, J.Y.; Zhang, F.; Sun, C.C.; Li, S.J.; Li, G.; Gong, F.Y.; Bo, T.; He, J.; Hua, R.X.; Hu, W.D.; et al. miR-134: A Human Cancer Suppressor? Mol. Ther. Nucleic Acids 2017, 6, 140–149. [Google Scholar] [CrossRef]
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell. Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef]
- Chen, X.; Yang, F.; Zhang, T.; Wang, W.; Xi, W.; Li, Y.; Zhang, D.; Huo, Y.; Zhang, J.; Yang, A.; et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J. Exp. Clin. Cancer Res. 2019, 38, 99. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Armugam, A.; Jeyaseelan, K. MicroRNAs in Neurotoxicity. J. Toxicol. 2012, 2012, 870150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rabinovsky, R.; Wei, Z.; El Fatimy, R.; Deforzh, E.; Luan, B.; Peshkin, L.; Uhlmann, E.J.; Krichevsky, A.M. Secreted PGK1 and IGFBP2 contribute to the bystander effect of miR-10b gene editing in glioma. Mol. Ther. Nucleic Acids 2023, 31, 265–275. [Google Scholar] [CrossRef]
- Nies, Y.H.; Mohamad Najib, N.H.; Lim, W.L.; Kamaruzzaman, M.A.; Yahaya, M.F.; Teoh, S.L. MicroRNA Dysregulation in Parkinson’s Disease: A Narrative Review. Front. Neurosci. 2021, 15, 660379. [Google Scholar]
- Sarkar, S.; Jun, S.; Rellick, S.; Quintana, D.D.; Cavendish, J.Z.; Simpkins, J.W. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016, 1646, 139–151. [Google Scholar] [CrossRef]
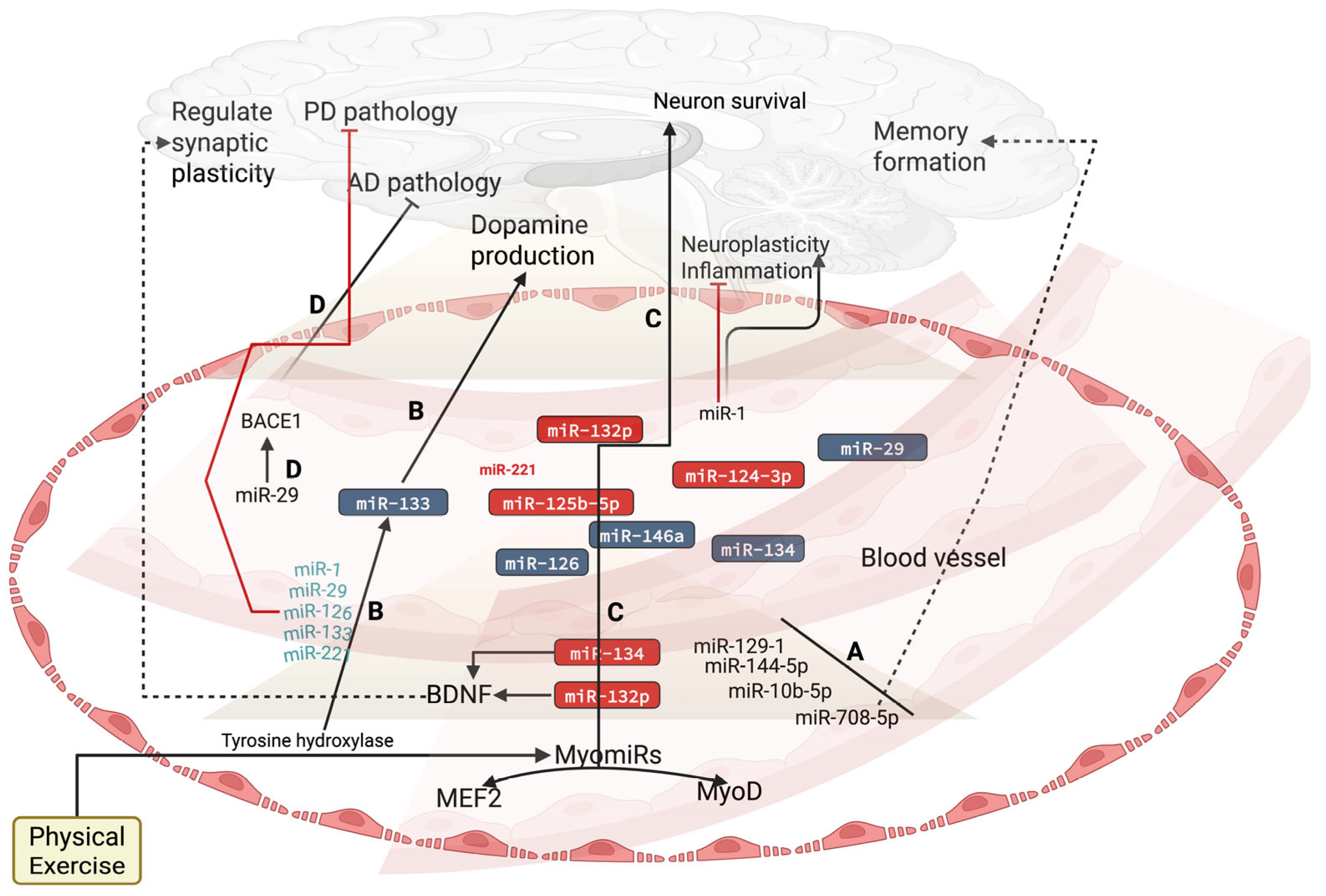
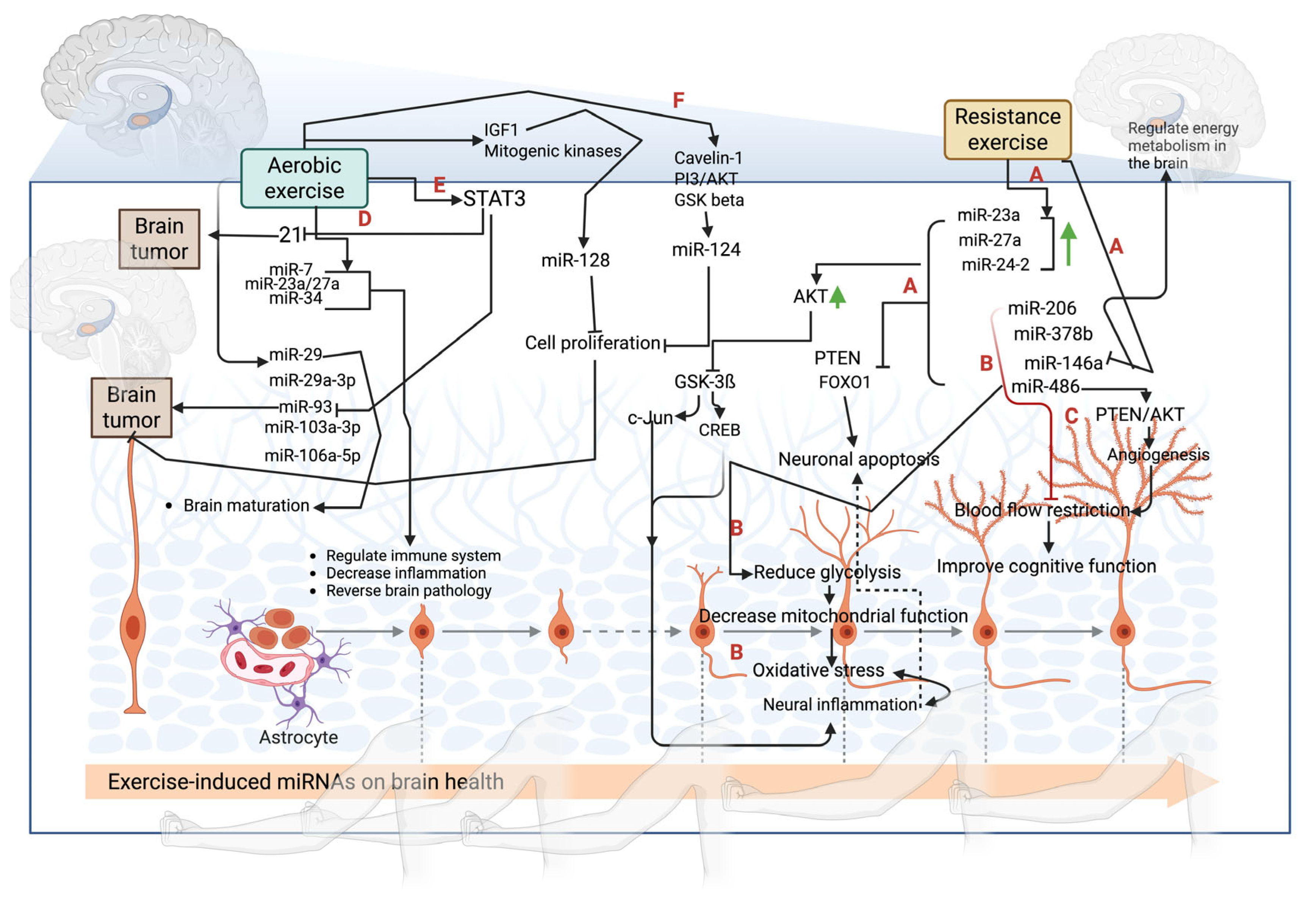
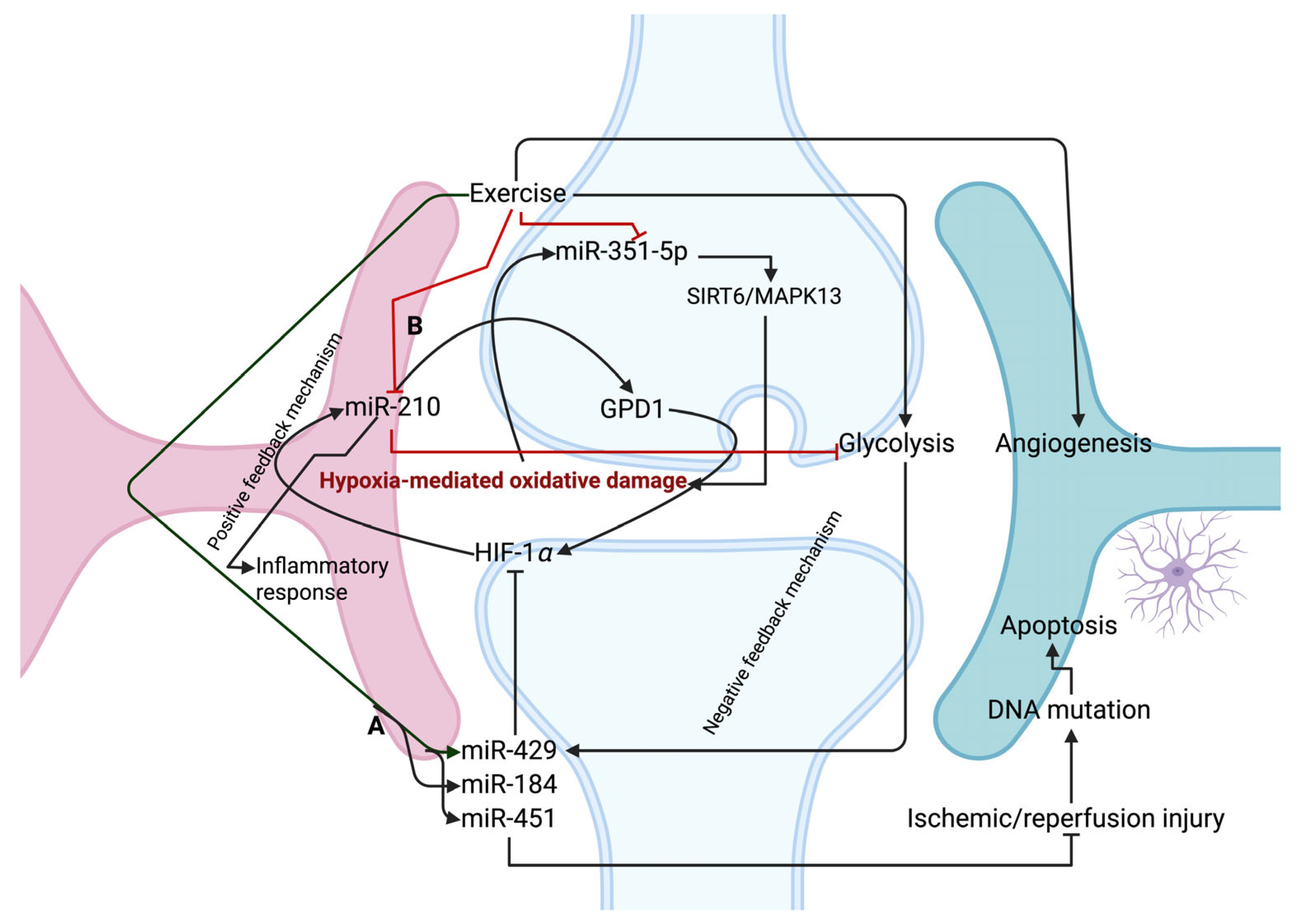
| miRNAs | Exercise Protocols | Possible Targeting Pathways | Effect of Exercise-Induced miRs on Brain Health | References |
|---|---|---|---|---|
| miR-21 (↑) | Running wheel exercise for 2 weeks | MAP2K3 and STAT3 | Improves cognitive function, decreases cerebral edema, increases BBB permeability, and decreases STAT3 expression, reducing neuronal death | [109,110] |
| miR-146-a (↑) | Resistance training (warm up for 5 min, 30 min of circuit, and cool down 5 min). | NF-κB signaling | Reduces neuroinflammation by repressing NF-κB | [111,112] |
| miR-221-3p (↑) | Treadmill exercise | JAK, STAT, SNCA, and PARK2 | Affects neural apoptosis and cell proliferation, and regulates autophagy and oxidative process | [113,114] |
| miR-222 (↑) | Two exercise protocols were performed. (1) Mice performed a forced swimming exercise for 4 weeks twice a day for 90 min for 7 days. (2) As voluntary exercise, mice performed a cage wheel exercise for 4 weeks. | ITGB8 and HMBOX1 | Decreases inflammation and brain injury | [115,116] |
| miR-133 (↑) | Swimming exercise for 8 weeks and treadmill training for 8 weeks, 50 min, 23 m/min. | Tyrosine hydroxylase, HDAC4, MEFC2, and BDNF | Dopamine production, neuronal plasticity, and neurological recovery | [117,118] |
| miR-129-1-3p (↑) | 4 weeks of treadmill running exercise | IGFBP-5 and ITM2A | Improves memory formation | [119] |
| miR-144-5p (↑) | 4 weeks of treadmill running exercise | IGFBP-5 and ITM2A | Improves memory formation | [119] |
| miR-10b-5p (↑) | Resistance training at 70% of the 1RM | BDNF and HOX | Survival and differentiation of neurons | [120] |
| miR-708-5p (↑) | 4 weeks of treadmill running exercise | BDNF, HOX, NNAT and SERCA | Perturbs calcium re-uptake into the ER and increases the leakage of calcium in the cytoplasm to induce bipolar disorder | [119,121] |
| 132-3p (↑) | Running exercise for 10 km race | FOXO3, NFAT and HDAC3 | Neuronal protection. Increases hippocampal cells | [122] |
| 124-3p (↑) | Cognitive-exercise dual-task intervention | AMPK/mTOR pathway, caveolin-1 and PI3K/AKT and GSK3β pathways. | Inhibits neuronal apoptosis and increases neuronal development and cognitive functions | [123] |
| miR-125b-5p (↑) | Treadmill running exercise for 20 min with 80% | BDNF pathway and MMP-15 | Improves cognitive dysfunction by inhibiting neuroinflammation | [124,125] |
| miR-126 (↔) | High-intensity running exercise at maximum speed for 4 × 30 s | Zonula occludens-1 and claudin-5 and occludin | Promotes angiogenesis and neurogenesis in cerebral ischemia | [126,127] |
| 146a (↑) | Treadmill exercise for 5 days up to 60 min/day with 22 m/min speed for 60 days | IRAK1, TRAF6, and NF-kB | Decreases inflammation and apoptosis | [128,129] |
| miR-221 (↔) | Treadmill exercise for a total period of 4 weeks with a total speed of running 18 m/min. | PTEN/PI3K/pathway | Modulates endothelial function and decrease apoptosis in cerebral ischemia | [130] |
| miR-128 (↑) | Swimming exercise (a total period of 12 weeks, 5 days a week for 200 min) | IGF-1 signaling pathway and mitogenic kinases and PHF6 | Regulates neuronal migration and neuronal development | [131] |
| miR-93 (↑) | 8 weeks of HIIT as follows: 5 min warm-up; 5 min standard stretching at low intensity; then, 30 min running at an intensity of 75% | IL-1β, TNF, IL-6, TLR4, and STAT3 | Axogenesis, inflammation, and metabolism of the brain | [132] |
| miR-29a-3p (↑) | Cycling ergometer exercise for 8-week period (30 min/3 times a week) | BACE1 | Improves cognitive function | [73] |
| miR-23a/27a and 34 (↑) | Resistance training | AKT, PTEN, FOXO1, PI3, and JNK/C-Jun | Regulate immune system, inflammation, and amyloid formation | [77] |
| miR-378b (↑) | Acute resistance training as follows: leg press (50–70% of 1RM) for 45 min | ANRIL and ATG3 | Decrease hypoxic–ischemic brain injury | [133] |
| miR-210 (↑) | Aerobic exercise (3 times per week, 8-week duration) | CDK10 and EFNA3 | Improves angiogenesis and metabolism | [134] |
| miR-486 (↓) | Aerobic exercise for 60 min at 70% VO2 max | Decreases glutathione peroxidase 3 and Thioredoxin-like-1 | Neurodegeneration | [135,136] |
| miR-499-5p (↑) | Treadmill exercise | Cav1.2 | Regulates neuroplasticity | [137] |
| miR- miR-451 | Swimming exercise | PGC-1α | Improves angiogenesis and decreases apoptosis | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Gu, F.; Thirupathi, A. Effect of Exercise on Regulating miRNA Expression in Brain Health and Diseases. Biology 2025, 14, 729. https://doi.org/10.3390/biology14060729
Zhang J, Gu F, Thirupathi A. Effect of Exercise on Regulating miRNA Expression in Brain Health and Diseases. Biology. 2025; 14(6):729. https://doi.org/10.3390/biology14060729
Chicago/Turabian StyleZhang, Jian, Fengmei Gu, and Anand Thirupathi. 2025. "Effect of Exercise on Regulating miRNA Expression in Brain Health and Diseases" Biology 14, no. 6: 729. https://doi.org/10.3390/biology14060729
APA StyleZhang, J., Gu, F., & Thirupathi, A. (2025). Effect of Exercise on Regulating miRNA Expression in Brain Health and Diseases. Biology, 14(6), 729. https://doi.org/10.3390/biology14060729







