Mammalian Life History: Weaning and Tooth Emergence in a Seasonal World
Abstract
Simple Summary
Abstract
1. Introduction
2. Background
3. Materials and Methods
| Birth Status | Weight | Timed Events in Life History (Years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primates Family Genus Species | Category 2 | No. Decid. Birth | ♀ Body (kg) | Body (kg) | Brain (g) | Gest. Length | Weaning | Complete Deciduous Dentition | M1 Emergence | M2 Emergence |
| Cheirogaleidae | ||||||||||
| Cheirogaleus medius [93] | Tr | 12 | 0.182 | 0.197 | 2.60 | 0.169 | 0.167 | 0.030 | 0.070 | 0.10 |
| Lemuridae | ||||||||||
| Lemur catta | Tr | 7 | 2.205 | 1.960 | 22.90 | 0.370 | 0.290 | 0.115 | 0.340 | 0.57 |
| Eulemur fulvus | Tr | 2 | 1.775 | 2.292 | 25.77 | 0.323 | 0.370 | 0.212 | 0.420 | 0.61 |
| Varecia variegata | Tr | 2 | 3.700 | 3.575 | 32.12 | 0.269 | 0.300 | 0.213 | 0.480 | 0.69 |
| Indriidae | ||||||||||
| Propithecus verreauxi | Tr | 16 + V | 3.150 | 2.955 | 26.21 | 0.406 | 0.490 | prenatal | 0.220 | - |
| Galagidae | ||||||||||
| Galago senegalensis [94] | Tr | 12 | 0.199 | 0.213 | 3.96 | 0.345 | 0.233 | 0.030 | 0.100 | - |
| Tarsiidae | ||||||||||
| Tarsius bancanus [87,95] | Tr | 16 + V | 0.114 | 0.126 | 3.16 | 0.488 | 0.220 | 0.011 | 0.037 | - |
| Callitrichidae | ||||||||||
| Callithrix jacchus | Tr | 0 | 0.322 | 0.320 | 7.24 | 0.395 | 0.167 | 0.104 | 0.310 | 0.59 |
| Saguinas fuscicollis [96] | Tr | ? | 0.320 | 0.401 | 7.94 | 0.403 | 0.250 | 0.125 | 0.380 | 0.58 |
| Cebidae | ||||||||||
| Cebus apella | Tr | 8 | 2.350 | 2.936 | 66.63 | 0.425 | 0.740 | 0.393 | 1.100 | 2.17 |
| Saimiri sciureus | Tr | 2 | 0.950 | 0.852 | 24.14 | 0.466 | 0.480 | 0.118 | 0.370 | 0.59 |
| Aotus trivirgatus | Tr | 0 | 0.736 | 0.989 | 16.85 | 0.389 | 0.210 | 0.108 | 0.360 | 0.53 |
| Cercopithecidae | ||||||||||
| Macaca mulatta | Tr | 0 | 5.340 | 6.793 | 88.98 | 0.460 | 0.803 | 0.437 | 1.350 | 3.50 |
| Macaca nemestrina | Tr | 0 | 5.940 | 8.821 | 105.59 | 0.469 | 1.000 | 0.410 | 1.370 | 3.34 |
| Papio cynocephalus | Tr | 0 | 11.900 | 17.150 | 163.19 | 0.488 | 0.993 | 0.596 | 1.665 | 3.79 |
| Chlorocebus aethiops | Tr | 0 | 3.200 | 3.720 | 65.00 | 0.447 | 0.710 | - | 0.830 | 1.83 |
| Trachypithecus vetulus | Tr | 0 | 6.525 | 6.237 | 61.29 | 0.556 | 0.625 | 0.203 | - | - |
| Hylobatidae | ||||||||||
| Hylobates agilis [94,97] | Tr | 2 | 5.82 | 5.850 | 91.16 | 0.562 | 2.000 | 0.450 | 1.36 | 2.68 |
| Hominidae | ||||||||||
| Pongo pygmaeus 3 [98] | Tr | 0 | 35.80 | 57.150 | 377.38 | 0.682 | 6.500 | 1.160 | ≅3.5 | ≅6 |
| Gorilla gorilla | Tr | 0 | 71.50 | 120.950 | 490.41 | 0.701 | 3.800 | 0.994 | 3.50 | 6.58 |
| Pan troglodytes [99,100] | Tr | 0 | 39.70 | 44.967 | 368.35 | 0.658 | 4.710 | 1.120 | 3.33 | 6.45 |
| Homo sapiens [101,102] | Tr | 0 | 42.00 | 49.500 | 1290.00 | 0.767 | 2.800 | 2.333 | 5.75 | 10.73 |
| Australopithecus africanus [100,103,104] | - | 30.20 | 35.500 | 433.95 | - | - | - | 3.20 | - | |
| Megaladapidae | ||||||||||
| Megaladapis edwardsi 4 [33] | - | - | 88.00 | 137.00 | >0.54 | - | - | 0.92 | >1.39 | |
| Birth Status | Weight | Timed Events in Life History (Years) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Order Family Genus Species | Category 2 | No. Decid. Birth | Body (kg) | Brain (g) | Gest. Length | Weaning | Complete Deciduous Dentition | M1 Emergence | M2 Emergence |
| Scandentia | |||||||||
| Tupaiidae | |||||||||
| Tupaia glis 3 [76] | A | 0 | 0.170 | 3.20 | 0.123 | 0.099 | 0.066 | ≅0.085 | - |
| Rodentia | |||||||||
| Cricetidae | |||||||||
| Myodes glareolus [112] | A | 0 | 0.022 | 0.52 | 0.058 | 0.060 | - | 0.027 | 0.041 |
| Hystricidae | |||||||||
| Hystrix africaeaustralis [113] | Lj | 4 | 17.667 | 22.42 | 0.256 | 0.277 | 0.038 | 0.188 | 0.458 |
| Sciuridae | |||||||||
| Sciurus carolinensis [114] | A | 0 | 0.503 | 7.41 | 0.121 | 0.178 | - | 0.134 | - |
| Tamiasciurus hudsonicus [115] | A | 0.202 | 3.67 | 0.093 | 0.137 | - | 0.128 | - | |
| Thryonomyidae | |||||||||
| Thryonomys swinderianus [116] | P | 4 | 3.580 | 13.28 | 0.425 | 0.083 | - | 0.058 | 0.334 |
| Perissodactyla | |||||||||
| Equidae | |||||||||
| Equus burchelli [117] | P | 0 | 227.600 | 612.00 | 1.00 | 0.750 | 0.420 | 0.875 | 1.750 |
| Tapiridae | |||||||||
| Tapirus terrestris 4 [118] | P | 0 | 158.489 | 181.97 | 1.09 | 0.792 | - | ≈1.2 | - |
| Rhinocerotidae | |||||||||
| Ceratotherium simum [119,120] | P | 0 | 1396.000 | 642.00 | 1.315 | 1.00 | 0.384 | 2.75 | - |
| Diceros bicornis [120,121] | P | 0 | 954.993 | 616.60 | 1.299 | 1.66 | 0.384 | 2.0 | 4.0 |
| Artiodactyla | |||||||||
| Antilocapridae | |||||||||
| Antilocapra americana [122] | P | ≥4 | 46.750 | 125.60 | 0.690 | 0.250 | 0.058 | 0.167 | 0.458 |
| Bovidae | |||||||||
| Aepyceros melampus [123] | P | 8 | 47.000 | 181.70 | 0.559 | 0.460 | 0.125 | 0.330 | 0.792 |
| Antidorcas marsupialis [124] | P | 20 | 36.518 | 135.00 | 0.468 | 0.329 | - | 0.083 | 0.625 |
| Bison bonasus [125,126] | P | ≥18 | 900.000 | 665.142 | 0.740 | 0.550 | 0.104 | 0.542 | 1.417 |
| Connochaetes taurinus [127] | P | 6 | 154.667 | 365.60 | 0.690 | 0.667 | - | 0.458 | 1.167 |
| Hemitragus jemlahicus [128] | P | ≅14 | 70.000 | 166.00 | 0.575 | 0.414 | 0.019 | 0.208 | 0.958 |
| Saiga tatarica [129] | P | 8 | 40.000 | 96.67 | 0.416 | 0.243 | - | 0.125 | 0.292 |
| Sylvicapra grimmia [130,131] | P | 8 | 12.625 | 77.20 | 0.452 | 0.287 | 0.043 | 0.229 | 0.790 |
| Taurotragus oryx [132,133] | P | 20 | 531.50 | 578.00 | 0.729 | 0.500 | prenatal | 0.528 | 1.50 |
| Cervidae | |||||||||
| Alces alces [134] | P | ≥8 | 272.160 | 406.50 | 0.666 | 0.274 | 0.067 | 0.231 | 0.666 |
| Cervus elaphus [135,136] | P | 8 | 120.177 | 379.20 | 0.671 | 0.333 | 0.019 | 0.330 | 1.125 |
| Muntiacus reevesi [137] | P | 22 | 13.500 | 70.17 | 0.545 | 0.166 | prenatal | 0.207 | 0.577 |
| Odocoileus virginianus [138] | P | ≥8 | 65.000 | 210.00 | 0.559 | 0.330 | 0.058 | 0.180 | 0.656 |
| Rangifer tarandus [139] | P | ≅22 | 106.360 | 299.00 | 0.625 | 0.330 | prenatal? | 0.170 | 0.833 |
| Giraffa camelopardalis [140] | P | 18 | 828.000 | 678.30 | 1.252 | 1.0 | 0.040 | 0.917 | 2.0 |
| Hippopotamidae | |||||||||
| Hippopotamus amphibius [141] | P | 6+ | 1553.000 | 651.00 | 0.641 | 0.934 | 0.75 | ≈1.5 | 8? |
| Suidae | |||||||||
| Potamochoerus porcus [142] | Lj | 8 | 130.000 | 155.60 | 0.334 | 0.250 | 0.265 | 0.438 | 1.212 |
| Sus scrofa [143] | Lj | 8 | 117.000 | 188.20 | 0.315 | 0.290 | 0.261 | 0.472 | 1.055 |
| Tayassuidae | |||||||||
| Pecari tajacu [144] | Lj | 6 | 8.430 | 66.00 | 0.397 | 0.130 | 0.165 | 0.400 | 0.819 |
| Tragulidae | |||||||||
| Hyemoschus aquaticus [42] | P | - | 10.800 | 25.20 | 0.479 | 0.493 | - | 0.417 | 0.830 |
| Carnivora | |||||||||
| Canidae | |||||||||
| Canis mesomelas [67] | A | 0 | 8.500 | 51.42 | 0.173 | 0.164 | 0.053 | 0.356 | 0.389 |
| Cerdocyon thous [145] | A | 0 | 5.240 | 41.80 | 0.151 | 0.250 | 0.089 | - | - |
| Vulpes vulpes [68] | A | 0 | 8.000 | 43.38 | 0.143 | 0.131 | 0.079 | 0.327 | 0.356 |
| Felidae | |||||||||
| Lynx rufus [146] | A | 0 | 8.900 | 57.97 | 0.178 | 0.238 | 0.135 | 0.442 | x 5 |
| Panthera leo [69] | A | 0 | 161.500 | 223.63 | 0.299 | 0.602 | 0.208 | 1.042 | x |
| Hyaenidae | |||||||||
| Crocuta crocuta [147,148,149] | Lj | 16 | 63.000 | 144.03 | 0.301 | 0.726 | 0.164 | 0.833 | x |
| Mustelidae | |||||||||
| Eira barbera [150] | A | 0 | 3.910 | 35.87 | 0.181 | 0.226 | 0.242 | 0.533 | 0.568 |
| Enhydra lutris [151] | ? | 10 + V | 23.500 | 125.21 | 0.384 | 0.481 | 0.060 | 0.580 | - |
| Mephitis mephitis [152] | A | V | 2.090 | 10.28 | 0.173 | 0.164 | prenatal | 0.134 | 0.138 |
| Neovison vison [153] | A | 0 | 0.645 | 7.00 | 0.115 | 0.133 | 0.110 | 0.157 | 0.171 |
| Procyonidae | |||||||||
| Procyon lotor [154] | A | 0 | 5.530 | 40.04 | 0.173 | 0.246 | 0.135 | 0.214 | - |
| Ursidae | |||||||||
| Ailuropoda melanoleuca [155] | A | 0 | 108.400 | 235.10 | 0.369 | 0.885 | 0.384 | 1.0 | - |
| Ursus arctos 6 [156] | A | 0 | 172.720 | 338.30 | 0.282 | 0.584 | 0.315 | 0.372 | 0.567 |
| Pinnepedia (sub-order) | |||||||||
| Phocidae | |||||||||
| Mirounga leonina [157] | P | V | 2470.625 | 700.88 | 0.603 | 0.058 | prenatal | 0.074 | x |
| Hyracoidea | |||||||||
| Procaviidae | |||||||||
| Procavia capensis [158] | P | 3.090 | 20.07 | 0.589 | 0.317 | - | 0.542 | 1.167 | |
| Probosciodea Elephantidae | |||||||||
| Loxodonta africana [159,160] | P | 4 | 4353.167 | 5806.67 | 1.836 | 3.500 | - | 6.0 | 14.50 |
| Macroscelidea | |||||||||
| Macroscelididae | |||||||||
| Macroscelides proboscideus [108,161] | P | 0.039 | 1.34 | 0.178 | 0.052 | - | 0.095 | 0.233 | |
4. Results
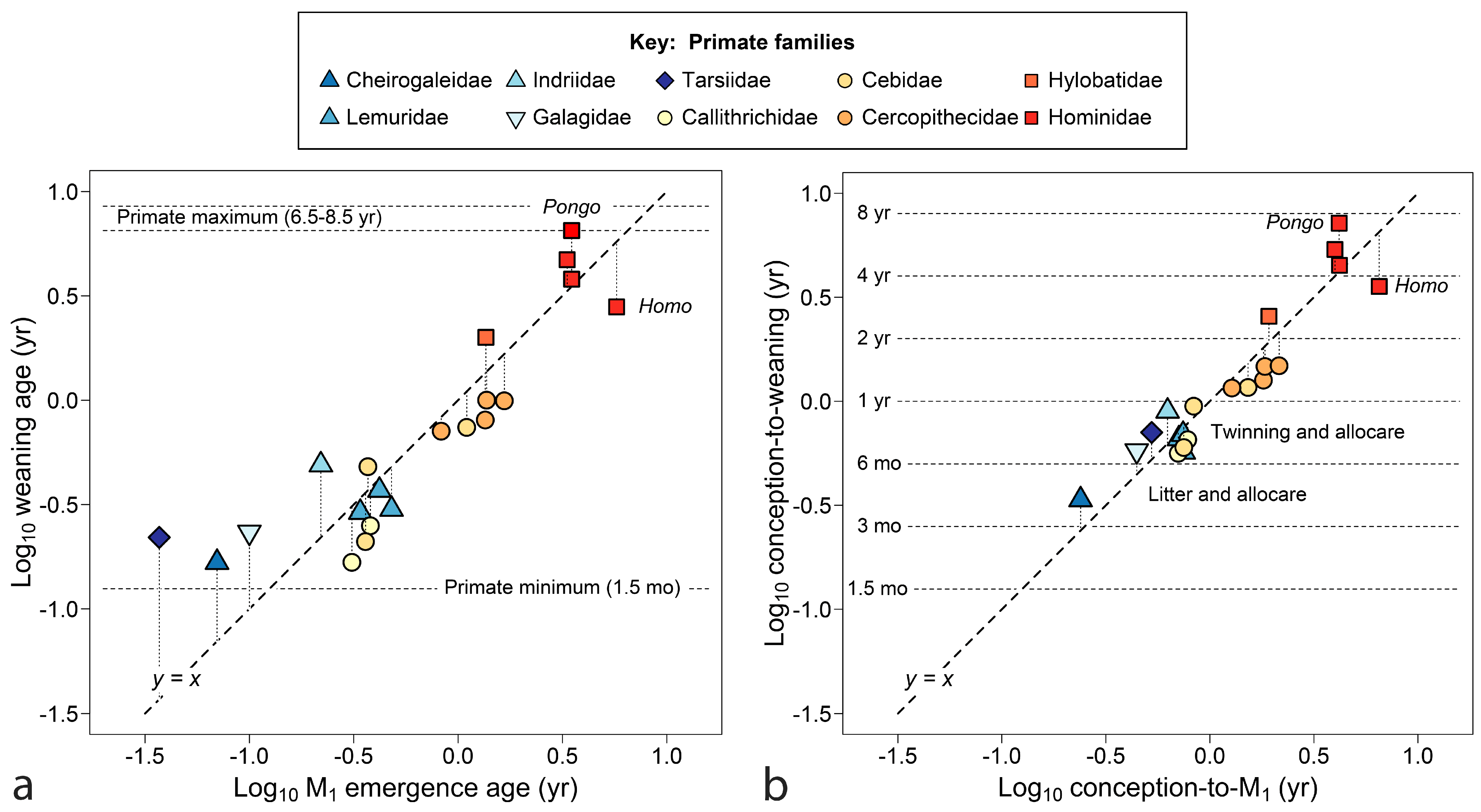
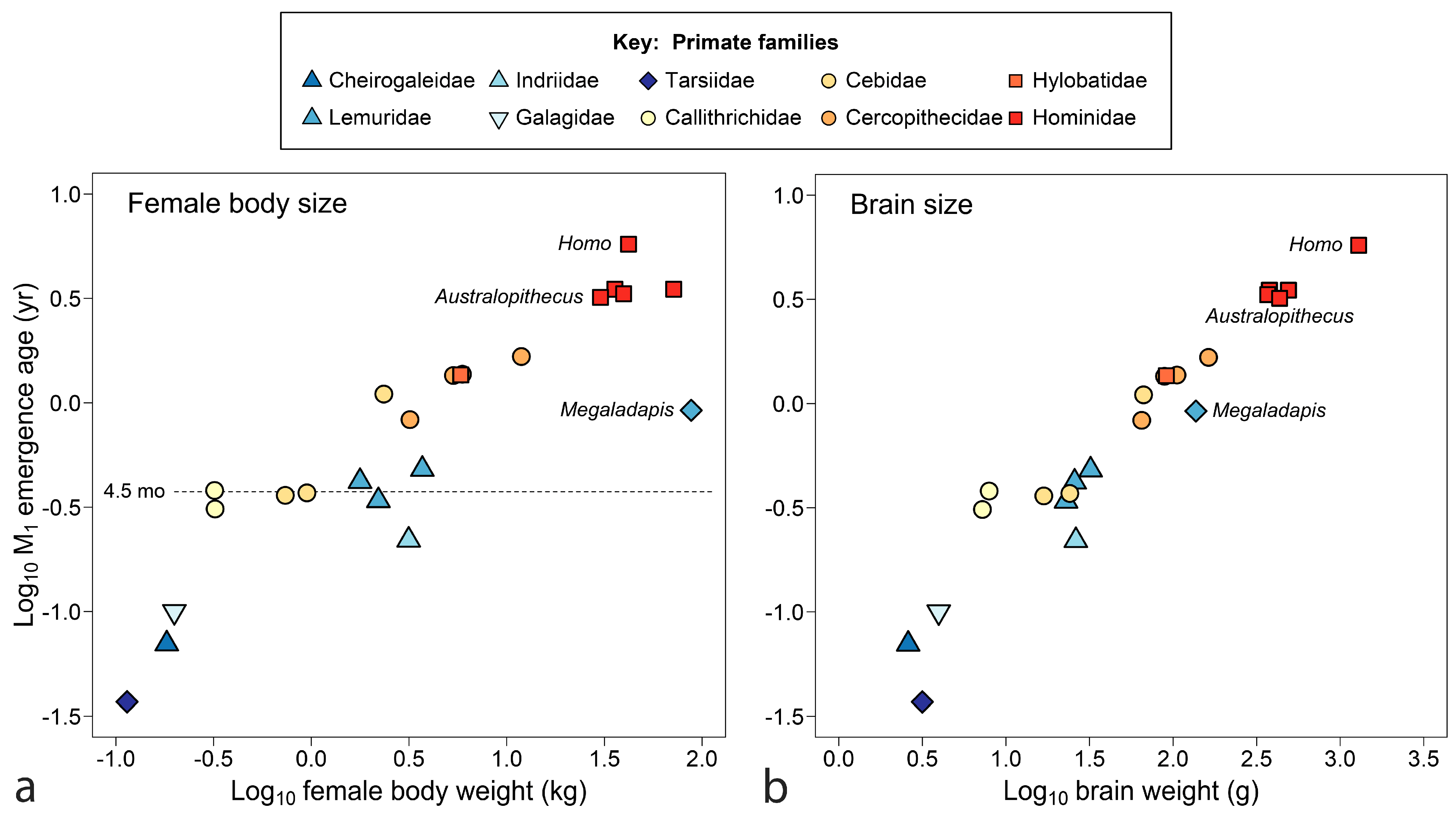
4.1. Primates
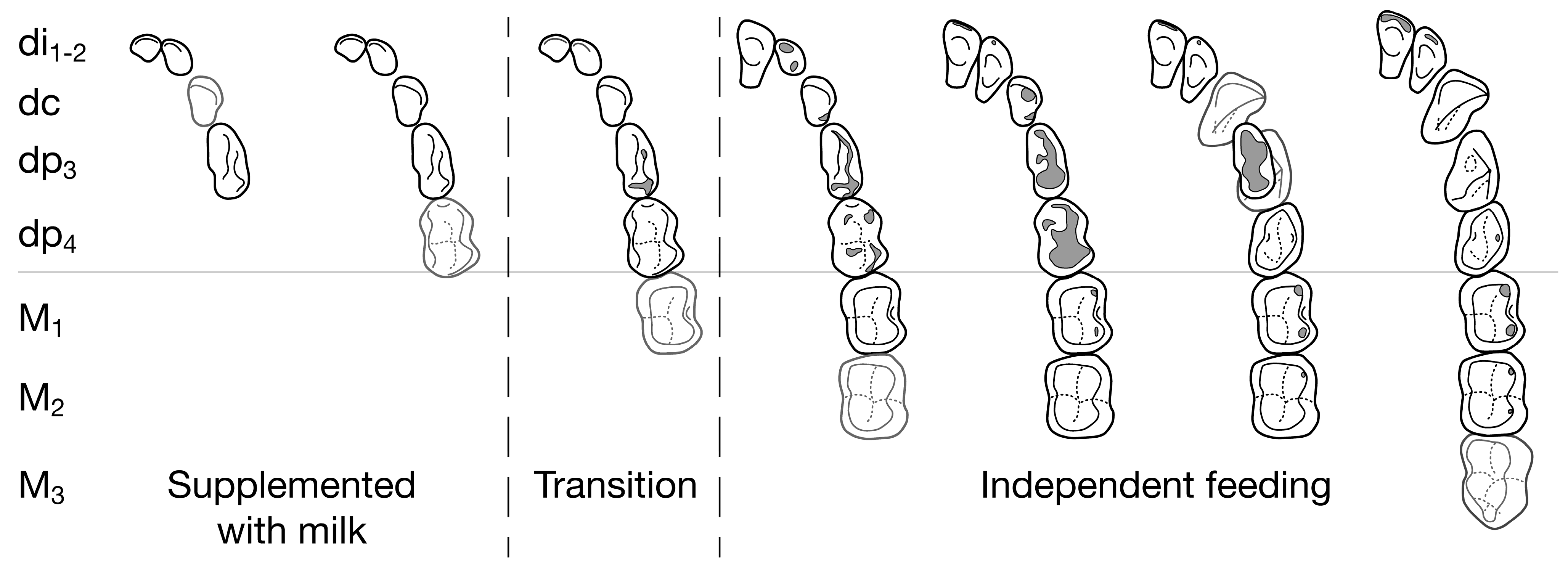
4.1.1. The Dentition at Birth
4.1.2. Maternal Investment
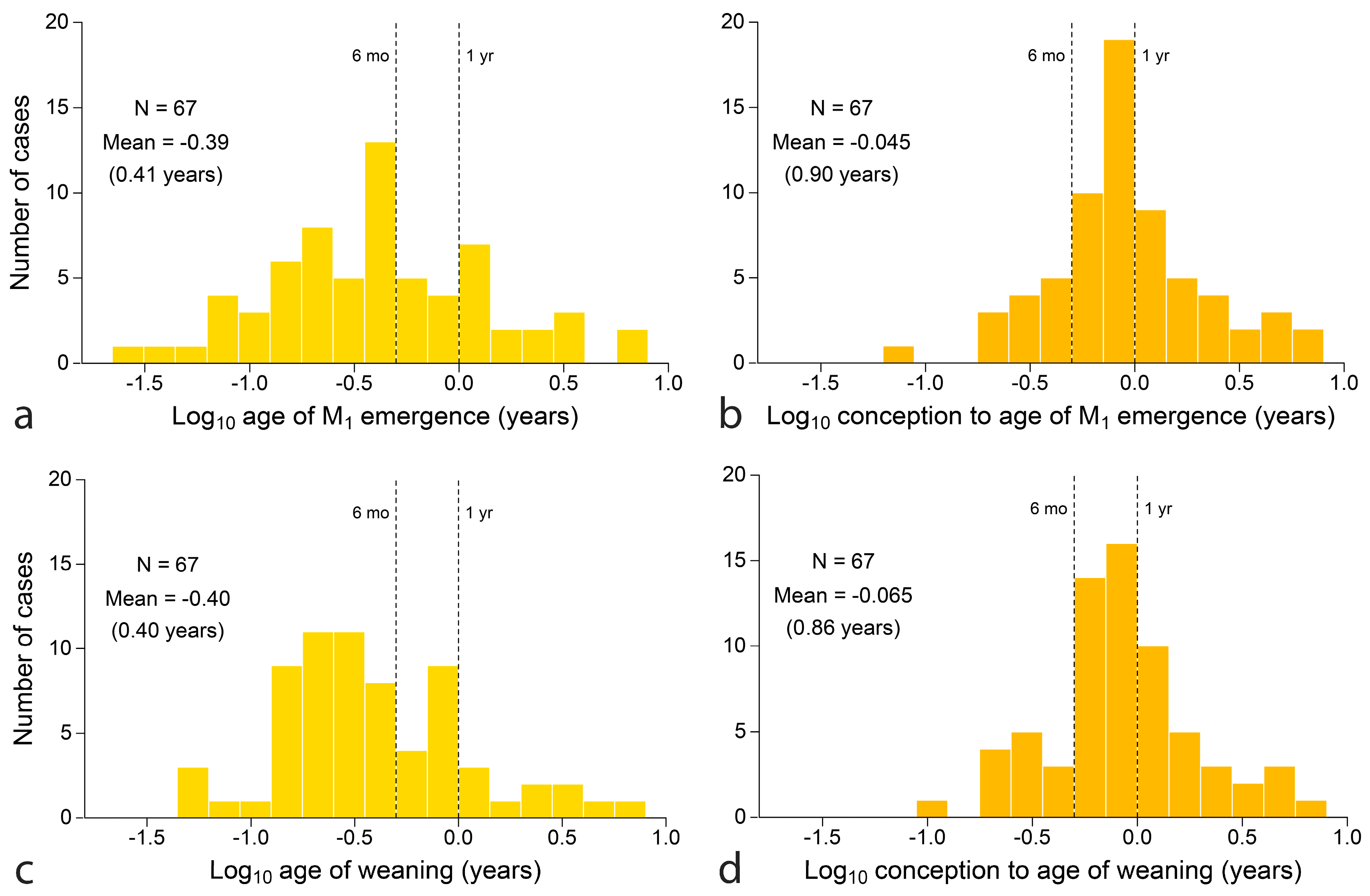
4.1.3. Mapping Weaning onto Tooth Emergence
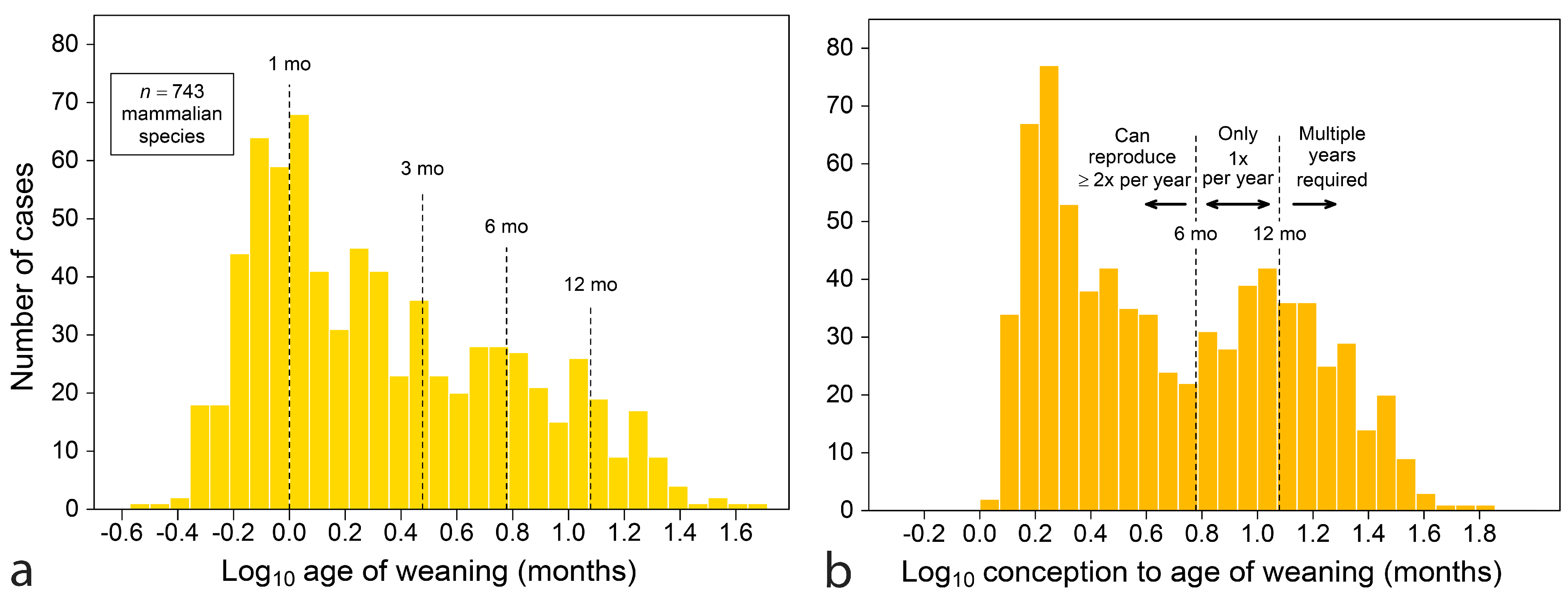
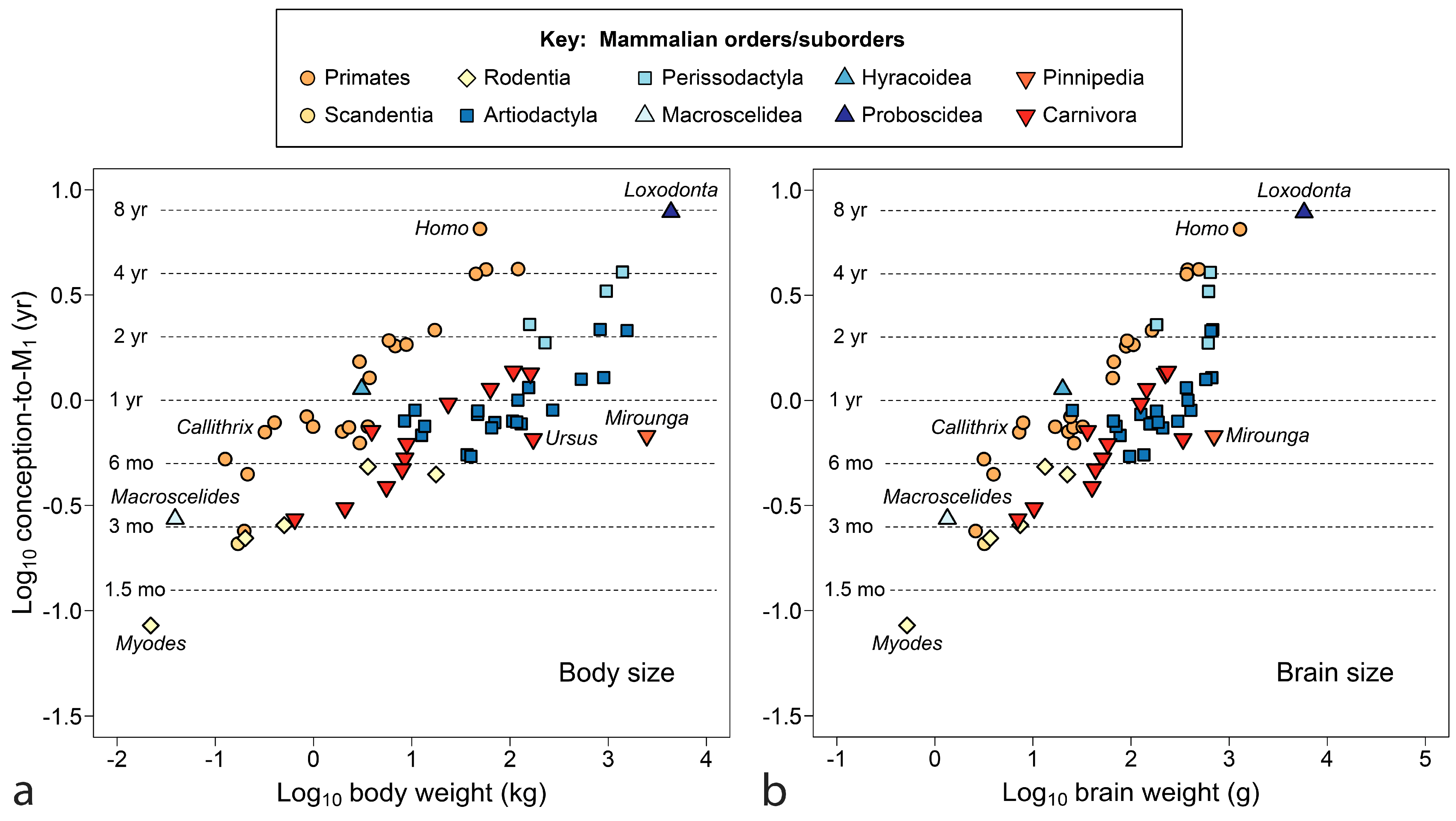
4.2. All Mammals
4.2.1. The Dentition at Birth
4.2.2. Maternal Investment
4.2.3. Mapping Weaning onto Tooth Emergence
5. Discussion
6. Conclusions
- Broadly, just as seasonal reproduction shapes infant growth, it also shapes timing of emergence of teeth necessary to wean infants onto adult foods. Emergence of the first permanent molar and age of weaning are closely related measures of the time to raise young to independent feeding.
- The proportion of the dentition formed [79,96] or erupted at birth [26] is one measure of relative infant precociality, although birth status has more than one dimension [81]. Because the proportion of the dentition formed at birth is recorded in hard tissues as the neonatal line, infant precociality has a fossil record.
- ‘Milk’ teeth are aptly named because the deciduous dentition is typically complete when infants transit fully to an adult diet in nearly all cases examined (47/50). Given a dentition, we can predict a typical feeding stage from teeth erupted, understand the direction of likely exceptions (e.g., highly precocious species) and sort fossil and skeletal collections into meaningful age categories. The emergence of an M1 is a reasonable proxy for independent feeding for species in the fossil record.
- Ages of emergence of the last deciduous and first permanent teeth reveal another layer of meaning when developmental time is counted from conception, because the total time to produce offspring feeding independently comes up against seasonal boundaries that are costly to cross for reproductive fitness. The effect shows strongly for species committed to producing one independently feeding offspring per year.
- Age of M1 emergence is not well explained by future adult body size, although relationships to neonatal and weaning weight [7] remain to be investigated. Brain weight, established much earlier than final adult body weight, appears to be a better proxy for energetics at the boundary of infancy/juvenility, see [256,257]. With adult brain weight as the independent variable, the extremely late emergence of M1 in humans is finally explained, although not all clusters of data resolve.
- The emergence of M1 is a useful standard for assessing weaning as early or late across mammals compared to a standard point in growth and development, a comparison that may separate phylogenetic groups, identify dietary guilds and highlight species on the edge of extinction. The orangutan stands as the most divergent mammal in this regard across known species in nine orders, with extremely late weaning in relation to offspring development and tooth emergence.
- The dental fossil record contains information close to the fundamentals of reproductive effort beyond inferences that can be made from size alone.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pond, C.M. The significance of lactation in the evolution of mammals. Evolution 1977, 31, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Vernon, R.G.; Pond, C.M. Adaptations of maternal adipose tissue to lactation. J. Mammary Gland Biol. Neoplasia 1997, 2, 231–241. [Google Scholar] [CrossRef]
- Langer, P. Lactation, weaning period, food quality, and digestive tract differentiations in Eutheria. Evolution 2003, 57, 1196–1215. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, L.G.; Brown, G.R. Social influences on foraging behavior in young nonhuman primates: Learning what, where, and how to eat. Evol. Anthropol. 2008, 17, 189–201. [Google Scholar] [CrossRef]
- Preuschoft, S.; Marshall, A.J.; Scott, L.; Badriyah, S.N.; Purba, M.D.T.; Yuliani, E.; Corbi, P.; Yassir, I.; Wibawanto, M.A.; Kalcher-Sommersguter, E. The development of feeding competence in rehabilitant orphaned orangutans and how to measure it. Animals 2023, 13, 2111. [Google Scholar] [CrossRef] [PubMed]
- Langer, P. The phases of maternal investment in eutherian mammals. Zoology 2008, 111, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Majluf, P.; Gordon, I.J. Growth, weaning and maternal investment from a comparative perspective. J. Zool. 1991, 225, 99–114. [Google Scholar] [CrossRef]
- Trivers, R.L. Parent-offspring conflict. Am. Zool. 1974, 14, 249–264. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H. The Evolution of Parental Care; Princeton University Press: Princeton, NJ, USA, 1991; 352p. [Google Scholar]
- Janson, C.H.; Verdolin, J. Seasonality of Primate Births in Relation to Climate. In Seasonality in Primates; Brockman, D.K., Van Schaik, C.P., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 307–350. [Google Scholar]
- Diskin, M.; Evans, A. Preface: Reproductive cycles of animals. Anim. Reprod. Sci. 2011, 124, 127. [Google Scholar] [CrossRef]
- van Rosmalen, L.; van Dalum, J.; Appenroth, D.; Roodenrijs, R.T.M.; de Wit, L.; Hazlerigg, D.G.; Hut, R.A. Mechanisms of temperature modulation in mammalian seasonal timing. FASEB J. 2021, 35, 1–12. [Google Scholar] [CrossRef]
- Cerrito, P.; Hu, B.; Kalisher, R.; Bailey, S.E.; Bromage, T.G. Life history in primate teeth is revealed by changes in major and minor element concentrations measured via field-emission SEM-EDS analysis. Biol. Lett. 2023, 19, 20220438. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.C.; Scandrett, A.E. The relation between long-period incremental markings in dentine and daily cross-striations in enamel in human teeth. Arch. Oral Biol. 1996, 41, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Bromage, T.G.; Janal, M.N. The Havers-Halberg oscillation regulates primate tissue and organ masses across the life-history continuum. Biol. J. Linn. Soc. 2014, 112, 649–656. [Google Scholar] [CrossRef]
- Rountrey, A.N.; Fisher, D.C.; Tikhonov, A.; Kosintsev, P.; Lazarev, P.A.; Boeskorov, G.; Buigues, B. Early tooth development, gestation, and season of birth in mammoths. Quat. Int. 2012, 255, 196–205. [Google Scholar] [CrossRef]
- Smith, B.H. Dental development as a measure of life history in primates. Evolution 1989, 43, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, H.; Schwartz, G.T. A biomechanical perspective on molar emergence and primate life history. Sci. Adv. 2021, 7, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, L.R.; Samonds, K.E.; Jungers, W.L.; Sutherland, M.R. Teeth, brain, and primate life histories. Am. J. Phys. Anthropol. 2001, 114, 192–214. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, L.R.; Samonds, K.E.; Jungers, W.L.; Sutherland, M.R. Dental Development and Primate Life Histories. In Primate Life Histories and Socioecology; Kappeler, P.M., Pereira, M.E., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 177–203. [Google Scholar]
- Smith, B.H. Age of weaning approximates age of emergence of the first permanent molar in nonhuman primates. Am. J. Phys. Anthropol. 1991, 34, 163–164. [Google Scholar]
- Smith, B.H. Life history and the evolution of human maturation. Evol. Anthropol. 1992, 1, 134–142. [Google Scholar] [CrossRef]
- Kelley, J.; Smith, T.M. Age at first molar emergence in early Miocene Afropithecus turkanensis and life-history evolution in the Hominoidea. J. Hum. Evol. 2003, 44, 307–329. [Google Scholar] [CrossRef]
- Kelley, J.; Schwartz, G.T. Life-history inference in the early hominins Australopithecus and Paranthropus. Int. J. Primatol. 2012, 33, 1332–1363. [Google Scholar] [CrossRef]
- Smith, T.M. Teeth and human life-history evolution. Annu. Rev. Anthropol. 2013, 42, 191–208. [Google Scholar] [CrossRef]
- Smith, B.H.; Crummett, T.L.; Brandt, K.L. Ages of eruption of primate teeth: A compendium for aging individuals and comparing life histories. Yearb. Phys. Anthropol. 1994, 37, 177–231. [Google Scholar] [CrossRef]
- Rowe, N.; Myers, M. (Eds.) All the World’s Primates; Pogonias Press: Charleston, RI, USA, 2016; 777p. [Google Scholar]
- Zhao, L.; Lu, Q.; Zhang, W. Age at first molar emergence in Lufengpithecus lufengensis and its implications for life-history evolution. J. Hum. Evol. 2008, 54, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bromage, T.G.; Dean, M.C. Re-evaluation of the age at death of immature fossil hominids. Nature 1985, 317, 525–527. [Google Scholar] [CrossRef]
- Dean, M.C.; Beynon, A.D.; Thackeray, J.F.; Macho, G.A. Histological reconstruction of dental development and age at death of a juvenile Paranthropus robustus specimen, SK 63, from Swartkrans, South Africa. Am. J. Phys. Anthropol. 1993, 91, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Beynon, A.D.; Clayton, C.B.; Ramirez Rozzi, F.; Reid, D.J. Radiographic and histological methodologies in estmating the chronology of crown development in modern humans and great apes: A review, with some applications for studies on juvenile hominids. J. Hum. Evol. 1998, 35, 351–370. [Google Scholar] [CrossRef]
- Nargolwalla, M.C.; Begun, D.R.; Dean, M.C.; Reid, D.J.; Kordos, L. Dental development and life history in Anapithecus hernyaki. J. Hum. Evol. 2005, 49, 99–121. [Google Scholar] [CrossRef]
- Schwartz, G.T.; Mahoney, P.; Godfrey, L.R.; Cuozzo, F.P.; Jungers, W.L.; Randria, G.F.N. Dental development in Megaladapis edwardsi (Primates, Lemuriformes): Implications for understanding life history variation in subfossil lemurs. J. Hum. Evol. 2005, 49, 702–721. [Google Scholar] [CrossRef]
- Smith, T.M.; Olejniczak, A.J.; Reid, D.J.; Bailey, S.E.; Glantz, M.; Viola, B.; Hublin, J.-J. Dental development and age at death in a Middle Paleolithic juvenile hominin from Obi-Rakhmat Grotto, Uzbekistan. In Continuity and Discontinuity in the Peopling of Europe: One Hundred Years of Neanderthal Study; Condemi, S., Weniger, G.-C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 155–163. ISBN 978-94-007-0491-6. [Google Scholar]
- Lefèvre, C.M.; Sharp, J.A.; Nicholas, K.R. Evolution of lactation: Ancient origin and extreme adaptations of the lactation system. Annu. Rev. Genom. Hum. Genet. 2010, 11, 219–238. [Google Scholar] [CrossRef]
- Glazier, D.S. Reproductive efficiency and the timing of gestation and lactation in rodents. Am. Nat. 1990, 135, 269–277. [Google Scholar] [CrossRef]
- Bercovitch, F.B. Female weight and reproductive condition in a population of olive baboons (Papio anubis). Am. J. Primatol. 1987, 12, 189–195. [Google Scholar] [CrossRef]
- Oftedal, O.T. Pregnancy and lactation. In Bioenergetics of Wild Herbivores; Hudson, R.J., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 215–238. [Google Scholar]
- Ellison, P.T. On Fertile Ground: A Natural History of Human Reproduction; Harvard University Press: Cambridge, UK, 2001; 346p. [Google Scholar]
- Valeggia, C.; Ellison, P.T. Interactions between metabolic and reproductive functions in the resumption of postpartum fecundity. Am. J. Hum. Biol. 2009, 21, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Wade, G.N.; Schneider, J.E. Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 1992, 16, 235–272. [Google Scholar] [CrossRef] [PubMed]
- Dubost, G. Reproductive characteristics of the water chevrotain, Hyemoschus aquaticus. Mammalia 2016, 80, 601–611. [Google Scholar] [CrossRef]
- Deacon, F.; Nel, P.J.; Bercovitch, F.B. Concurrent pregnancy and lactation in wild giraffes (Giraffa camelopardalis). Afr. Zool. 2015, 50, 331–334. [Google Scholar] [CrossRef]
- Bartošová, J.; Komárková, M.; Dubcová, J.; Bartoš, L.; Pluháček, J. Concurrent lactation and pregnancy: Pregnant domestic horse mares do not increase mother-offspring conflict during intensive lactation. PLoS ONE 2011, 6, 6–9. [Google Scholar] [CrossRef]
- Ross, A.C.; Porter, L.M.; Power, M.L.; Sodaro, V. Maternal care and infant development in Callimico goeldii and Callithrix jacchus. Primates 2010, 51, 315–325. [Google Scholar] [CrossRef]
- Morano, S.; Stewart, K.M.; Sedinger, J.S.; Nicolai, C.A.; Vavra, M. Life-history strategies of North American elk: Trade-offs associated with reproduction and survival. J. Mammal. 2013, 94, 162–172. [Google Scholar] [CrossRef]
- Parvathi, S.; Rao, M.; Kumar, V.; Unapathy, G. Observations on reproductive performance of Indian mouse deer (Moschiola indica) in captivity. Curr. Sci. 2014, 106, 439–442. [Google Scholar]
- Bielby, J.; Mace, G.M.; Bininda-Emonds, O.R.P.; Cardillo, M.; Gittleman, J.L.; Jones, K.E.; Purvis, A.; Orme, C.D.L.; Purvis, A. The fast-slow continuum in mammalian life history: An empirical reevaluation. Am. Nat. 2007, 169, 748–757. [Google Scholar] [CrossRef]
- Ewer, R.F. The Carnivores; Comstock Publishing: Sacramento, CA, USA, 1973; 494p. [Google Scholar]
- Hill, K. Altruistic cooperation during foraging by the Ache, and the evolved human predisposition to cooperate. Hum. Nat. 2002, 13, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Feistner, A.T.C.; Mcgrew, W.C. Food-sharing in primates: A critical review. In Perspectives in Primate Biology; Seth, P.K., Seth, S., Eds.; Today & Tomorrow’s Printers and Publishers: New Delhi, India, 1989; Volume 3, pp. 21–36. [Google Scholar]
- Lovegrove, B.G. The metabolism of social subterranean rodents: Adaptation to aridity. Oecologia 1986, 69, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Tučková, V.; Šumbera, R.; Čížková, B. Alloparental behaviour in Sinai spiny mice Acomys dimidiatus: A case of misdirected parental care? Behav. Ecol. Sociobiol. 2016, 70, 437–447. [Google Scholar] [CrossRef]
- Kaplan, H.; Hill, K.; Cadeliña, R.V.; Hayden, B.; Hyndman, D.C.; Preston, R.J.; Smith, E.A.; Stuart, D.E.; Yesner, D.R. Food sharing among Ache foragers: Tests of explanatory hypotheses [and Comments and Reply]. Curr. Anthropol. 1985, 26, 223–246. [Google Scholar] [CrossRef]
- Rosenbaum, S.; Gettler, L.T. With a little help from her friends (and family) part II: Non-maternal caregiving behavior and physiology in mammals. Physiol. Behav. 2018, 193, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.L.; Veile, A. Infant allocare in traditional societies. Physiol. Behav. 2018, 193, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, K.; O’Connell, J.F.; Blurton Jones, N.G.; Alvarez, H.; Charnov, E.L. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl. Acad. Sci. USA 1998, 95, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.F. Mammals: Comparisons and Contrasts. In Ecology and Evolution of Cooperative Breeding in Birds; Koenig, W.D., Dickinson, J.L., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 210–227. [Google Scholar]
- Ernest, S.K.M. Life history characteristics of placental nonvolant mammals. Ecology 2003, 84, 3402. [Google Scholar] [CrossRef]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Ooë, T. Human Tooth and Dental Arch Development; Ishiyaku, Ed.; Ishiyaku EuroAmerica, Inc.: Osaka, Japan, 1981; 217p. [Google Scholar]
- Ooë, T. Development of human first and second permanent molar, with special reference to the distal portion of the dental lamina. Anat. Embryol. 1979, 155, 221–240. [Google Scholar] [CrossRef]
- Smith, B.H. “Schultz’s rule” and the Evolution of Tooth Emergence and Replacement Patterns in Primates and Ungulates. In Development, Function and Evolution of Teeth; Teaford, M.F., Smith, M.M., Ferguson, M.W.J., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 212–227. ISBN 0521570115. [Google Scholar]
- Rose, K.D.; Smith, B.H. Dental anomaly in the early Eocene condylarth Ectocion. J. Paleontol. 1979, 53, 756–760. [Google Scholar]
- Rose, K.D.; Holbrook, L.T.; Luckett, W.P. Deciduous premolars of Eocene Equidae and their phylogenetic significance. Hist. Biol. 2018, 30, 89–118. [Google Scholar] [CrossRef]
- Rodrigues, H.G.; Lihoreau, F.; Orliac, M.; Thewissen, J.G.M.; Boisserie, J.R. Unexpected evolutionary patterns of dental ontogenetic traits in cetartiodactyl mammals. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182417. [Google Scholar] [CrossRef] [PubMed]
- Lombaard, L.J. Age determination and growth curves in the black-backed jackal Canis mesomelas Shreber, 1775 (Carnivora: Canidae). Ann. Transvaal Museum 1971, 27, 135–169. [Google Scholar]
- Linhart, S.B. Dentition and pelage in the juvenile red fox (Vulpes vulpes). J. Mammal. 1968, 49, 526–528. [Google Scholar] [CrossRef]
- Smuts, G.L.; Anderson, J.L.; Austin, J.C. Age determination of the African lion (Panthera leo). J. Zool. 1978, 185, 115–146. [Google Scholar] [CrossRef]
- Scheffer, V.B.; Kraus, B.S. Dentition of the northern fur seal. Fish. Bull. 1964, 63, 293–315. [Google Scholar]
- Veitschegger, K.; Sánchez-Villagra, M.R. Tooth eruption sequences in cervids and the effect of morphology, life history, and phylogeny. J. Mamm. Evol. 2016, 23, 251–263. [Google Scholar] [CrossRef]
- Monson, T.A.; Hlusko, L.J. The evolution of dental eruption sequence in artiodactyls. J. Mamm. Evol. 2016, 25, 15–26. [Google Scholar] [CrossRef]
- Konigsberg, L.W.; Frankenberg, S.R.; Liversidge, H. Optimal trait scoring for age estimation. Am. J. Phys. Anthropol. 2016, 159, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Phillips-Conroy, J.E.; Jolly, C.J. Dental eruption schedules of wild and captive baboons. Am. J. Primatol. 1988, 15, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, A.A.; Menegaz-Bock, R.M. Emergence of the permanent teeth in Pima Indian children. J. Dent. Res. 1958, 37, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Hertenstein, B.; Zimmermann, E.; Rahmann, H. Zur Reproduktion und ontogenetischen Entwicklung von Spitzhörnchen. Z. Des Kölner Zoo 1987, 30, 119–133. [Google Scholar]
- Setchell, J.M.; Wickings, E.J. Sequences and timing of dental eruption in semi-free-ranging mandrills (Mandrillus sphinx). Folia Primatol. 2004, 75, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Hemming, J.E. Cemental deposition, tooth succession, and horn development as criteria of age in dall sheep. J. Wildl. Manage. 1969, 33, 552–558. [Google Scholar] [CrossRef]
- Gingerich, P.D.; Ul-Haq, M.; von Koenigswald, W.; Sanders, W.J.; Smith, B.H.; Zalmout, I.; Von Koenigswald, W. New protocetid whale from the middle Eocene of Pakistan: Birth on land, precocial development, and sexual dimorphism. PLoS ONE 2009, 4, e4366. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, J.F. The Mammalian Radiations; University of Chicago Press: Chicago, IL, USA, 1981; 610p. [Google Scholar]
- Derrickson, E.M. Comparative reproductive strategies of altricial and precocial eutherian mammals. Funct. Ecol. 1992, 6, 57–65. [Google Scholar] [CrossRef]
- Starck, J.M.; Ricklefs, R.E. (Eds.) Patterns of Development: The Altricial-Precocial Spectrum. In Avian Growth and Development: Evolution within the Altricial-Precocial Spectrum; Oxford University Press: Oxford, UK, 1998; pp. 3–30. [Google Scholar]
- Bivin, W.S.; McClure, R.C. Deciduous tooth chronology in the mandible of the domestic pig. J. Dent. Res. 1976, 55, 591–597. [Google Scholar] [CrossRef]
- Luckett, W.P. An Ontogenetic Assessment of Dental Homologies in Therian Mammals. In Mammal Phylogeny: Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials; Szalay, F.S., Novacek, M.J., McKenna, M.C., Eds.; Springer: New York, NY, USA, 1993; pp. 182–204. ISBN 1461392519. [Google Scholar]
- Gingerich, P.D. Dentition of Adapis parisiensis and the evolution of lemuriform primates. In Lemur Biology; Tattersall, I., Sussman, R.W., Eds.; Taylor & Francis, Ltd.: London, UK, 1975; pp. 65–80. [Google Scholar]
- van Nievelt, A.F.H.; Smith, K.K. To replace or not to replace: The significance of reduced functional tooth replacement in marsupial and placental mammals. Paleobiology 2005, 31, 324–346. [Google Scholar] [CrossRef]
- Luckett, W.P.; Maier, W. Development of deciduous and permanent dentition in Tarsius and its phylogenetic significance. Folia Primatol. 1982, 37, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, L.R.; Samonds, K.E.; Jungers, W.L.; Sutherland, M.R.; Irwin, M.T. Ontogenetic correlates of diet in Malagasy lemurs. Am. J. Phys. Anthropol. 2004, 123, 250–276. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, P.D. Homologies of the anterior teeth in Indriidae. Am. J. Phys. Anthropol. 1979, 51, 283–285. [Google Scholar] [CrossRef][Green Version]
- Sanders, W.J. Horizontal tooth displacement and premolar occurrence in elephants and other elephantiform proboscideans. Hist. Biol. 2018, 30, 137–156. [Google Scholar] [CrossRef]
- Lillegraven, J.A.; Thompson, S.D.; McNab, B.K.; Patton, J.L. The origin of eutherian mammals. Biol. J. Linn. Soc. 1987, 32, 281–336. [Google Scholar] [CrossRef]
- van Nievelt, A.F.H.; Smith, K.K. Tooth eruption in Monodelphis domestica and its significance for phylogeny and natural history. J. Mammal. 2005, 86, 333–341. [Google Scholar] [CrossRef]
- Müller, A.E. Aspects of social life in the fat-tailed dwarf lemur (Cheirogaleus medius): Inferences from body weights and trapping data. Am. J. Primatol. 1999, 49, 265–280. [Google Scholar] [CrossRef]
- Smith, R.J.; Jungers, W.L. Body mass in comparative primatology. J. Hum. Evol. 1997, 32, 523–559. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M. Growth, development, and parental care in the western tarsier (Tarsius bancanus) in captivity: Evidence for a “slow” life-history and nonmonogamous mating system. Int. J. Primatol. 1994, 15, 1–28. [Google Scholar] [CrossRef]
- Smith, T.D.; Muchlinski, M.N.; Jankord, K.D.; Progar, A.J.; Bonar, C.J.; Evans, S.; Williams, L.E.; Vinyard, C.J.; Deleon, V.B. Dental maturation, eruption, and gingival emergence in the upper jaw of newborn primates. Anat. Rec. 2015, 298, 2098–2131. [Google Scholar] [CrossRef]
- Uchikoshi, M.; Matsuzawa, T. Tooth eruption in two agile gibbons (Hylobates agilis). Gibbon J. 2007, 3, 66–73. [Google Scholar]
- van Noordwijk, M.A.; Willems, E.P.; Utami Atmoko, S.S.; Kuzawa, C.W.; van Schaik, C.P. Multi-year lactation and its consequences in Bornean orangutans (Pongo pygmaeus wurmbii). Behav. Ecol. Sociobiol. 2013, 67, 805–814. [Google Scholar] [CrossRef]
- Lonsdorf, E.V.; Stanton, M.A.; Pusey, A.E.; Murray, C.M. Sources of variation in weaned age among wild chimpanzees in Gombe National Park, Tanzania. Am. J. Phys. Anthropol. 2020, 171, 419–429. [Google Scholar] [CrossRef]
- McHenry, H.M. Sexual dimorphism in fossil hominids and its socioecological implications. In The Archaeology of Human Ancestry: Power, Sex and Tradition; Steele, J., Shennan, S., Eds.; Routledge: Oxon, MS, USA; New York, NY, USA, 1996; pp. 91–109. ISBN 978-0-415-11862-0. [Google Scholar]
- Brown, T.; Barrett, M.J. Increase in average weight of Australian aborigines. Med. J. Aust. 1973, 2, 25–28. [Google Scholar] [CrossRef]
- Friedlaender, J.S.; Bailit, H.L. Eruption time of the deciduous and permanent teeth of natives on Bougainville Island, Territory of New Guinea: A study of racial variation. Hum. Biol. 1969, 41, 51–65. [Google Scholar]
- Kelley, J.; Schwartz, G.T. New ages at first molar emergence in extant great apes and a reassessment of early hominin first molar emergence ages. Am. J. Phys. Anthropol. 2009, 138, 164. [Google Scholar]
- Ruff, C.B.; Burgess, M.L.; Squyres, N.; Junno, J.A.; Trinkaus, E. Lower limb articular scaling and body mass estimation in Pliocene and Pleistocene hominins. J. Hum. Evol. 2018, 115, 85–111. [Google Scholar] [CrossRef]
- Smaers, J.B.; Rothman, R.S.; Hudson, D.R.; Balanoff, A.M.; Beatty, B.; Dechmann, D.K.N.; Dunn, J.C.; Fleagle, J.G.; Gilbert, C.C.; Goswami, A.; et al. The evolution of mammalian brain size. Sci. Adv. 2021, 7, eabe2101. [Google Scholar] [CrossRef]
- De Magalhães, J.P.; Costa, J. A database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 2009, 22, 1770–1774. [Google Scholar] [CrossRef]
- Myers, P.; Espinosa, R.; Parr, C.S.; Jones, T.; Hammond, G.S.; Dewey, T.A. The Animal Diversity Web. Available online: https://animaldiversity.org/ (accessed on 30 September 2023).
- Herberstein, M.E.; McLean, D.J.; Lowe, E.; Wolff, J.O.; Khan, M.K.; Smith, K.; Allen, A.P.; Bulbert, M.; Buzatto, B.A.; Eldridge, M.D.B.; et al. AnimalTraits—A curated animal trait database for body mass, metabolic rate and brain size. Nature 2022, 9, 265. [Google Scholar] [CrossRef]
- Burger, J.R.; George, M.A.; Leadbetter, C.; Shaikh, F. The allometry of brain size in mammals. J. Mammal. 2019, 100, 276–283. [Google Scholar] [CrossRef]
- Kappeler, P.M.; Pereira, M.E. (Eds.) A Primate Life History Database. In Primate Life Histories and Socioecology; University of Chicago Press: Chicago, IL, USA, 2003; pp. 313–330. [Google Scholar]
- Fooden, J.; Izor, R. Growth curves, dental emergence norms, and supplementary morphological observations in known-age captive orangutans. Am. J. Primatol. 1983, 5, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Mazák, V. Notes on the dentition in Clethrionomys glareolus Schreber, 1780 in the course of postnatal life. Saugetierk. Mitt. 1963, 11, 1–11. [Google Scholar]
- van Aarde, R.J. Age determination of cape porcupines, Hystrix africaeaustralis. S. Afr. J. Zool. 1985, 20, 232–236. [Google Scholar] [CrossRef]
- Shorten, M. Squirrels; Collins: London, UK, 1954; 212p. [Google Scholar]
- Layne, J.N. The biology of the red squirrel, Tamia hudsonicus loquax (Bangs), in central New York. Ecol. Monogr. 1954, 24, 227–268. [Google Scholar] [CrossRef]
- Van der Merwe, M. Tooth succession in the greater cane rat Thryonomys swinderianus (Temminck, 1827). J. Zool. 2000, 251, 541–545. [Google Scholar] [CrossRef]
- Smuts, G.L. Age determination in Burchell’s zebra (Equus burchelli antiquorum) from the Kruger National Park. J. S. Afr. Wildl. Manag. Assoc. 1974, 4, 103–115. [Google Scholar]
- Simpson, G.G. Notes on Pleistocene and recent tapirs. Bull. Am. Museum Nat. Hist. 1945, 86, 37–81. [Google Scholar]
- Hillman-Smith, A.K.; Owen-Smith, N.; Anderson, J.L.; Hall-Martin, A.; Selaladi, J.P. Age estimation of the white rhinoceros (Ceratotherium simum). J. Zool. 1986, 210, 355–379. [Google Scholar] [CrossRef]
- Dittrich, L. Birth and growth of a male white rhinoceros Ceratotherium simum simum at Hanover Zoo. Int. Zoo 1971, 210, 122–125. [Google Scholar]
- Goddard, J. Age criteria and vital statistics of a black rhinoceros population. East Afr. Wildl. J. 1970, 8, 105–121. [Google Scholar] [CrossRef]
- Hoover, R.L.; Till, C.E.; Ogilvie, S. The Antelope of Colorado: A Research and Management Study; Colorado Department of Game and Fish: Denver, CO, USA, 1959; 110p. [Google Scholar]
- Roettcher, D.; Hofmann, R.R. The ageing of impala from a population in the Kenya Rift Valley. Afr. J. Ecol. 1970, 8, 37–42. [Google Scholar] [CrossRef]
- Rautenbach, I.L. Ageing criteria in the springbok, Antidorcas marsupialis (Zimmerman, 1780) (Artiodactyla: Bovidae). Ann. Transvaal Museum 1971, 27, 83–133. [Google Scholar] [CrossRef]
- Węgrzyn, M.; Serwatka, S. Teeth eruption in the European bison. Mammal Res. 1984, 29, 111–121. [Google Scholar]
- Kopperud, B.T. Artiodactyl Brain-Size Evolution—A Phylogenetic Comparative Study of Brain-Size Adaptation. Master Thesis, Universitetet i Oslo, Oslo, Norway, 2017. [Google Scholar]
- Attwell, C.A.M. Age determination of the blue wildebeest Connochaetes taurinus in Zululand. S. Afr. J. Zool. 1980, 15, 121–130. [Google Scholar] [CrossRef]
- Caughley, G. Horn rings and tooth eruption as criteria of age in the Himalayan thar, Hemitragus jemlahicus. N. Z. J. Sci. 1965, 8, 333–351. [Google Scholar]
- Bannikov, A.G.; Zhirnov, V.; Lebedeva, L.S.; Fabndeev, A.A. Biology of the Saiga Antelope; Translated from Russion by M. Fleischmann; Program for Scientific Translation: Jerusalem, Israel, 1967; 252p. [Google Scholar]
- Wilson, V.J.; Schmidt, J.L.; Hanks, J. Age determination and body growth of the common duiker Sylvicapra grimmia (Mammalia). J. Zool. 1984, 202, 283–297. [Google Scholar] [CrossRef]
- Furstenburg, D. Focus on the Common Grey Duiker (Sylvicapra grimmia). Available online: https://www.researchgate.net/publication/316167446_Focus_on_the_Common_Grey_Duiker_Sylvicapra_grimmia (accessed on 26 September 2023).
- Jeffery, R.C.V.; Hanks, J. Age determination of eland Taurotragus oryx (Pallas, 1766) in the Natal Highveld. S. Afr. J. Zool. 1981, 16, 113–122. [Google Scholar] [CrossRef]
- Pérez-Barbería, F.J.; Gordon, I.J. Gregariousness increases brain size in ungulates. Oecologia 2005, 145, 41–52. [Google Scholar] [CrossRef]
- Peterson, R. North American Moose; University of Toronto Press: Toronto, ON, Canada, 1955; 280p. [Google Scholar]
- Quimby, D.C.; Gaab, J.E. Mandibular dentition as an age indicator in Rocky Mountain elk. J. Wildl. Manag. 2007, 21, 435–451. [Google Scholar] [CrossRef]
- Murie, O.J. The Elk of North America; Stackpole Books: Harrisburg, PA, USA, 1979; 376p. [Google Scholar]
- Chapman, D.I.; Chapman, N.G. Tooth eruption in Reeves’muntjac (Muntiacus reevesi) and its use a a method of age estimation (Mammalia: Cervidae). J. Zool. 1985, 205, 205–221. [Google Scholar] [CrossRef]
- Severinghaus, C.W. Tooth development and wear as criteria of age in white-tailed deer. J. Wildl. Manag. 1949, 13, 195–216. [Google Scholar] [CrossRef]
- Miller, F.L. Eruption and attrition of mandibular teeth in barren-ground caribou. J. Vet. Med. Sci. 1972, 36, 606–612. [Google Scholar] [CrossRef]
- Hall-Martin, A.J. Dentition and age determination of the giraffe Giraffa camelopardalis. J. Zool. 1976, 180, 263–289. [Google Scholar] [CrossRef]
- Laws, R.M. Dentition and aging of the hippopotamus. East Afr. Wildl. J. 1968, 52, 19–52. [Google Scholar] [CrossRef]
- Sowls, L.K.; Phelps, R.J. Observations on the African bushpig Potamochoerus porcus Linn. in Rhodesia. Zool. Sci. Contrib. N. Y. Zool. Soc. 1968, 53, 75–84. [Google Scholar] [CrossRef]
- Matschke, G.H. Aging european wild hogs by dentition. J. Wildl. Manag. 1967, 31, 109–113. [Google Scholar] [CrossRef]
- Kirkpatrick, R.D.; Sowls, L.K. Age determination of the collared peccary by the tooth-replacement pattern. J. Wildl. Manag. 1962, 26, 214–217. [Google Scholar] [CrossRef]
- Brady, C.A. Reproduction, growth and parental care in Crab-eating foxes Cerdocyon thous at the National Zoological Part, Washington. Int. Zoo Yearb. 1978, 18, 130–134. [Google Scholar] [CrossRef]
- Jackson, D.L.; Gluesing, E.A.; Jacobson, H.A. Dental eruption in bobcats. J. Wildl. Manage. 1988, 52, 515–517. [Google Scholar] [CrossRef]
- Pournelle, G.H. Observations on birth and early development of the spotted hyena. J. Mammal. 1965, 46, 3–4. [Google Scholar] [CrossRef]
- Van Horn, R.C.; McElhinny, T.L.; Holekamp, K.E. Age estimation and dispersal in the spotted hyena (Crocuta crocuta). J. Mammal. 2003, 84, 1019–1030. [Google Scholar] [CrossRef]
- Tanner, J.B.; Smale, L.; Holekamp, K.E. Ontogenetic variation in the play behavior of spotted hyenas. J. Dev. Process. 2007, 2, 5–30. [Google Scholar]
- Poglayen-Neuwall, I. Breeding, rearing and notes on the behaviour of tayras Eira barbara in captivity. Int. Zoo Yearb. 1978, 18, 134–140. [Google Scholar] [CrossRef]
- Kenyon, K. The Sea Otter in the Eastern Pacific Ocean; US Department of the Interior: Washington DC, USA, 1969; Volume 68, 352p, Available online: https://digital.library.unt.edu/ark:/67531/metadc700973/ (accessed on 5 August 2024).
- Verts, B.J. The Biology of the Striped Skunk; University of Illinois Press: Urbana, IL, USA, 1967; 218p. [Google Scholar]
- Aulerich, R.J.; Swindler, D.R. The dentition of the mink (Mustela vison). J. Mammal. 1968, 49, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, G.G. Tooth eruption in preweaned raccoons. J. Wildl. Manag. 1964, 28, 582–584. [Google Scholar] [CrossRef]
- Peng, J.; Jiang, Z.; Liu, W.; Huang, S.; Zhang, J.; Wang, W. Growth and development of giant panda (Ailuropoda melanoleuca) cubs at Beijing Zoo. J. Zool. 2001, 254, 261–266. [Google Scholar] [CrossRef]
- Dittrich, L. Milchgebissentwicklung und Zahnwechsel beim Braunbären (Ursus arctos L.) und anderen Ursiden. Gegenbaurs Morphol. Jahrb. 1961, 101, 2–141. [Google Scholar]
- Laws, R.M. The Elephant Seal (Mirounga leonina Linn.) I. In Growth and Age; Falkland Island Dependencies Survey Scientific Reports, 8; HMSO: London, UK, 1953; 62p. [Google Scholar]
- Steyn, D.; Hanks, J. Age determination and growth in the hyrax Procavia capensis (Mammalia: Procaviidae). J. Zool. 1983, 201, 247–257. [Google Scholar] [CrossRef]
- Laws, R.M. Age criteria for the African elephant, Loxodonta a. africana. Afr. J. Ecol. 1966, 4, 1–37. [Google Scholar] [CrossRef]
- Lee, P.C.; Moss, C.J. Early maternal investment in male and female African elephant calves. Behav. Ecol. Sociobiol. 1986, 18, 353–361. [Google Scholar] [CrossRef]
- Asher, R.J.; Olbricht, G. Dental ontogeny in Macroscelides proboscideus (Afrotheria) and Erinaceus europaeus (Lipotyphla). J. Mamm. Evol. 2009, 16, 99–115. [Google Scholar] [CrossRef]
- Sellen, D.W. Evolution of infant and young child feeding: Implications for contemporary public health. Annu. Rev. Nutr. 2007, 27, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Sellen, D.W.; Smay, D.B. Relationship between subsistence and age at weaning in “preindustrial” societies. Hum. Nat. 2001, 12, 47–87. [Google Scholar] [CrossRef] [PubMed]
- Beals, K.L.; Courtland, L.S.; Dodd, S.M. Brain size, cranial morphology, climate, and time machines. Curr. Anthropol. 1984, 25, 301–330. [Google Scholar] [CrossRef]
- Dahle, B.; Swenson, J.E. Factors influencing length of maternal care in brown bears (Ursus arctos) and its effect on offspring. Behav. Ecol. Sociobiol. 2003, 54, 352–358. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Guinness, F.E.; Albon, S.D. The costs of reproduction to red deer hinds. J. Anim. Ecol. 1983, 52, 367–383. [Google Scholar] [CrossRef]
- Piasecke, J.R.; Bender, L.C.; Schmitt, S.M. Factors affecting pregnancy in free-ranging elk, Cervus elaphus nelsoni, in Michigan. Can. Field-Nat. 2009, 123, 230–235. [Google Scholar] [CrossRef]
- Wich, S.A.; Utami-Atmoko, S.S.; Setia, T.M.; Rijksen, H.D.; Schürmann, C.; van Hooff, J.A.R.A.M.; van Schaik, C.P. Life history of wild Sumatran orangutans (Pongo abelii). J. Hum. Evol. 2004, 47, 385–398. [Google Scholar] [CrossRef]
- Marshall, A.J.; Lacy, R.; Ancrenaz, M.; Byers, O.; Husson, S.J.; Leighton, M.; Meijaard, E.; Rosen, N.; Singleton, I.; Stephens, S.; et al. Perspectives from Population Viability Analysis Models. In Orangutan Population Biology, Life History, and Conservation; Wich, S.A., Utami Atmoko, S.S., Setia, T.M., van Schaik, C.P., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 311–326. ISBN 9780191707568. [Google Scholar]
- Harvey, P.H.; Pagel, M.D. The Comparative Method in Evolutionary Biology; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Gingerich, P.D. Rates of Evolution: A Quantitative Synthesis; Cambridge University Press: Cambridge, UK, 2019; 381p, ISBN 9781316711644. [Google Scholar]
- Clutton-Brock, T.H.; Albon, S.D.; Guinness, F.E. Fitness costs of gestation and lactation in wild mammals. Nature 1989, 337, 260–2262. [Google Scholar] [CrossRef]
- Paddock, K.; Zeigler, L.; Harvey, B.; Prufrock, K.A.; Liptak, J.M.; Ficorilli, C.M.; Hogg, R.T.; Bonar, C.J.; Evans, S.; Williams, L.; et al. Comparative dental anatomy in newborn primates: Cusp mineralization. Anat. Rec. 2020, 303, 2415–2475. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, L.R.; Schwartz, G.T.; Samonds, K.E.; Jungers, W.L.; Catlett, K.K. The secrets of lemur teeth. Evol. Anthropol. 2006, 15, 142–154. [Google Scholar] [CrossRef]
- Mitani, J.C.; Watts, D. The evolution of non-maternal caretaking among anthropoid primates: Do helpers help? Behav. Ecol. Sociobiol. 1997, 40, 213–220. [Google Scholar] [CrossRef]
- Di Bitetti, M.S.; Janson, C.H. When will the stork arrive? Patterns of birth seasonality in neotropical primates. Am. J. Primatol. 2000, 50, 109–130. [Google Scholar] [CrossRef]
- Schinz, H.R. Ossifikationsstudien beim neugeborenen Schwein und beim neugeborenen Tapir. Vierteljahrsschr. Der Naturforschenden Ges. Zürich 1937, 82, 21–44. [Google Scholar]
- Padilla, M.; Dowler, R.C. Tapirus terrestris. Mamm. Species 1994, 481, 1–8. [Google Scholar] [CrossRef]
- Teaford, M.F.; Walker, A. Prenatal jaw movements in the guinea pig, Cavia porcellus; Evidence from patterns of tooth wear. J. Mammal. 1983, 64, 534–536. [Google Scholar] [CrossRef]
- Aeschbach, M.; Carrillo, J.D.; Sánchez-Villagra, M.R. On the growth of the largest living rodent: Postnatal skull and dental shape changes in capybara species (Hydrochoerus spp.). Mamm. Biol. 2016, 81, 558–570. [Google Scholar] [CrossRef]
- Bowen, W.D.; Oftedal, O.T.; Boness, D.J. Birth to weaning in 4 days: Remarkable growth in the hooded seal, Cystophora cristata. Can. J. Zool. 1985, 63, 2841–2846. [Google Scholar] [CrossRef]
- Hayssen, V. Empirical and theoretical constraints on the evolution of lactation. J. Dairy Sci. 1993, 76, 3213–3233. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.G.; Glickman, S.E.; Licht, P. Fatal sibling aggression, precocial development, and androgens in neonatal spotted hyenas. Science 1991, 252, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, K. Life History Theory and Human Evolution. In The Evolution of Human Life History; Hawkes, K., Paine, R.R., Eds.; School of American Research: Santa Fe, NM, USA, 2006; pp. 45–93. [Google Scholar]
- Kuykendall, K.L.; Mahoney, C.J.; Conroy, G.C. Probit and survival analysis of tooth emergence ages in a mixed-longitudinal sample of chimpanzees (Pan troglodytes). Am. J. Phys. Anthropol. 1992, 89, 379–399. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.C.; Montgomery, C.; Dean, M.C. The natural history of deciduous tooth attrition in hominoids. J. Hum. Evol. 1991, 21, 397–412. [Google Scholar] [CrossRef]
- Nowell, A.A.; Fletcher, A.W. The development of feeding behaviour in wild western lowland gorillas (Gorilla gorilla gorilla). Behaviour 2008, 145, 171–193. [Google Scholar]
- Machanda, Z.; Brazeau, N.F.; Bernard, A.B.; Donovan, R.M.; Papakyrikos, A.M.; Wrangham, R.W.; Smith, T.M. Dental eruption in east African wild chimpanzees. J. Hum. Evol. 2015, 82, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bădescu, I.; Watts, D.P.; Curteanu, C.; Desruelle, K.J.; Sellen, D.W. Effects of infant age and sex, and maternal parity on the interaction of lactation with infant feeding development in chimpanzees. PLoS ONE 2022, 17, e0272139. [Google Scholar] [CrossRef]
- Ingicco, T.; Moigne, A.M.; Gommery, D. A deciduous and permanent dental wear stage system for assessing the age of Trachypithecus sp. specimens (Colobinae, Primates). J. Archaeol. Sci. 2012, 39, 421–427. [Google Scholar] [CrossRef]
- Catlett, K.K.; Schwartz, G.T.; Godfrey, L.R.; Jungers, W.L. “Life history space”: A multivariate analysis of life history variation in extant and extinct malagasy lemurs. Am. J. Phys. Anthropol. 2010, 142, 391–404. [Google Scholar] [CrossRef]
- Schultz, A.H. Age Changes in Primates and their Modification in Man. In Human Growth; Tanner, J.M., Ed.; Pergamon: Oxford, UK, 1960; pp. 1–20. [Google Scholar]
- Asher, R.J.; Lehmann, T. Dental eruption in afrotherian mammals. BMC Biol. 2008, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kiltie, R.A. Gestation as a Constraint on the Evolution of Seasonal Breeding in Mammals. In Evolution of Life Histories of Mammals: Theory and Pattern; Boyce, M.S., Ed.; Yale University Press: New Haven, CT, USA, 1988; pp. 257–289. [Google Scholar]
- Monson, T.A.; Hlusko, L.J. Breaking the rules: Phylogeny, not life history, explains dental eruption sequences in primates. Am. J. Phys. Anthropol. 2018, 167, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Brockman, D.K.; van Schaik, C.P. (Eds.) Seasonality in Primates: Studies of Living and Extinct Human and Non-Human Primates; Cambridge University Press: Cambridge, UK, 2005; 590p. [Google Scholar]
- Garn, S.M.; Lewis, A.B.; Kerewsky, R.S. Genetic, nutritional and maturational correlates of dental development. J. Dent. Res. 1965, 44, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.H.; Boesch, C. Mortality and the magnitude of the “wild effect” in chimpanzee tooth emergence. J. Hum. Evol. 2011, 60, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.L.; Widowski, T.M. Normal profiles for deciduous dental eruption in domestic piglets: Effect of sow, litter, and piglet characteristics. J. Anim. Sci. 2009, 87, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Hanegraef, H.; Spoor, F. Maxillary morphology of chimpanzees: Captive versus wild environments. J. Anat. 2024, 244, 977–994. [Google Scholar] [CrossRef] [PubMed]
- McCance, R.A.; Ford, E.H.R.; Brown, W.A.B. Severe undernutrition in growing and adult animals 7. Development of the skull, jaws and teeth in pigs. Br. J. Nutr. 1961, 15, 213–224. [Google Scholar] [CrossRef]
- Kelley, J.; Schwartz, G.T. Dental development and life history in living African and Asian apes. Proc. Natl. Acad. Sci. USA 2010, 107, 1035–1040. [Google Scholar] [CrossRef]
- Smith, T.M. Dental development in living and fossil orangutans. J. Hum. Evol. 2016, 94, 92–105. [Google Scholar] [CrossRef]
- Dickerson, J.W.T.; Widdowson, E.M. Some effects of accelerating growth. II. Skeletal development. Proc. R. Soc. B 1960, 152, 207–217. [Google Scholar]
- Tonge, C.H.; McCance, R.A. Severe undernutrition in growing and adult animals. 15. The mouth, jaws and teeth of pigs. Br. J. Nutr. 1965, 19, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Yerkes, R. Chimpanzees: A Laboratory Colony; Yale University Press: New Haven, CT, USA, 1943; 321p. [Google Scholar]
- Kraemer, H.C.; Horvat, J.R.; Doering, C.; McGinnis, P.R. Male chimpanzee development focusing on adolescence: Integration of behavioral with physiological changes. Primates 1982, 23, 393–405. [Google Scholar] [CrossRef]
- Hitchins, P.M.; Anderson, J.L. Reproduction, population characteristics and management of the black rhinoceros Diceros bicornis minor in the Hluhluwe/Corridor/Umfolozi game reserve complex. S. Afr. J. Wildl. Res. 1983, 13, 78–85. [Google Scholar]
- Emslie, R. Diceros bicornis. ICUN Red List Threat. Species 2020, e.T6557A152728945. [Google Scholar] [CrossRef]
- Gadgil, M.; Bossert, W.H. Life historical consequences of natural selection. Am. Nat. 1970, 104, 1–24. [Google Scholar] [CrossRef]
- Lovari, S.; Lorenzini, R.; Masseti, M.; Pereladova, O.; Carden, R.F.; Brook, S.M.; Mattioli, S. Cervus elaphus . ICUN Red List Threat. Species 2018, 2018, e.T55997072A142404453. [Google Scholar]
- Smith, T.M.; Machanda, Z.; Bernard, A.B.; Donovan, R.M.; Papakyrikos, A.M.; Muller, M.N.; Wrangham, R.W. First molar eruption, weaning, and life history in living wild chimpanzees. Proc. Natl. Acad. Sci. USA 2013, 110, 2787–2791. [Google Scholar] [CrossRef]
- Matsumoto, T. Developmental changes in feeding behaviors of infant chimpanzees at Mahale, Tanzania: Implications for nutritional independence long before cessation of nipple contact. Am. J. Phys. Anthropol. 2017, 163, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ancrenaz, M.; Gurnal, M.; Marshall, A.J.; Meijaard, E.; Wich, S.A.; Husson, S. Pongo pygmaeus. ICUN Red List. Threat. Species 2023, 2023, e.T17975A247631797. [Google Scholar] [CrossRef]
- Schuppli, C.; Forss, S.I.F.; Meulman, E.J.M.; Zweifel, N.; Lee, K.C.; Rukmana, E.; Vogel, E.R.; van Noordwijk, M.A.; van Schaik, C.P. Development of foraging skills in two orangutan populations: Needing to learn or needing to grow? Front. Zool. 2016, 13, 3–5. [Google Scholar] [CrossRef]
- Pontzer, H.; Raichlen, D.A.; Schumaker, R.W.; Ocobock, C.; Wich, S.A. Metabolic adaptation for low energy throughput in orangutans. Proc. Natl. Acad. Sci. USA 2009, 107, 14048–14052. [Google Scholar] [CrossRef]
- Fisher, D.C. Season of Death, Growth Rates, and Life History of North American Mammoths. In Proceedings of the Mammoth Site Studies: Proceedings of the First International Comference on Mammoth Site Studies; West, D.L., Ed.; Publications in Anthropology, University of Kansas: Lawrence, KS, USA, 2001; pp. 121–135. [Google Scholar]
- Bromage, T.G. Enamel incremental periodicity in the pig-tailed macaque: A polychrome fluorescent labeling study of dental hard tissues. Am. J. Phys. Anthropol. 1991, 86, 205–214. [Google Scholar] [CrossRef]
- Rosas, A.; Ríos, L.; Estalrrich, A.; Liversidge, H.; García-Tabernero, A.; Hugeut, R.; Cardoso, H.; Bastir, M.; Lalueza-Fox, C.; de la Rasilla, M.; et al. The growth pattern of Neanderthals reconstructed from a juvenile skeleton from El Sídron (Spain). Science 2017, 357, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.T.; Reid, D.J.; Dean, M.C.; Zihlman, A.L. A faithful record of stressful life events recorded in the dental developmental record of a juvenile gorilla. Int. J. Primatol. 2006, 27, 1201–1219. [Google Scholar] [CrossRef]
- Smith, T.M.; Tafforeau, P.; Le Cabec, A.; Bonnin, A.; Houssaye, A.; Pouech, J.; Moggi-Cecchi, J.; Manthi, F.K.; Ward, C.; Makaremi, M.; et al. Dental ontogeny in Pliocene and early Pleistocene hominins. PLoS ONE 2015, 10, e0118118. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.M.; Cook, L.; Dirks, W.; Green, D.R.; Austin, C. Teeth reveal juvenile diet, health and neurotoxicant exposure retrospectively: What biological rhythms and chemical records tell us. BioEssays 2021, 43, 2000298. [Google Scholar] [CrossRef] [PubMed]
- Le Cabec, A.; Tang, N.; Tafforeau, P. Accessing developmental information of fossil hominin teeth using new synchrotron microtomography-based visualization techniques of dental surfaces and interfaces. PLoS ONE 2015, 10, e0123019. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.H. Dental development in Australopithecus and early Homo. Nature 1986, 323, 327–330. [Google Scholar] [CrossRef]
- Kuykendall, K.L. Reconstructing Australopithecine Growth and Development: What do we think we know? In Patterns of Growth and Development in the Genus Homo; Krovitz, G.E., Nelson, A.J., Thompson, J.L., Eds.; Cambridge University Press: Cambridge, UK, 2003; pp. 191–218. [Google Scholar]
- Dean, M.C.; Lucas, V.S. Dental and skeletal growth in early fossil hominins. Ann. Hum. Biol. 2009, 36, 545–561. [Google Scholar] [CrossRef]
- Bermúdez de Castro, J.M.; Ramírez Rozzi, F.; Martinón-Torres, M.; Sarmiento Pérez, S.; Rosas, A. Patterns of dental development in Lower and Middle Pleistocene hominins from Atapuerca (Spain). In Patterns of Growth and Development in the Genus Homo; Thompson, J.L., Krovitz, G.E., Nelson, A.J., Eds.; Cambridge University Press: Cambridge, UK, 2003; pp. 246–270. [Google Scholar]
- Tacail, T.; Thivichon-Prince, B.; Martin, J.E.; Charles, C.; Viriot, L.; Balter, V. Assessing human weaning practices with calcium isotopes in tooth enamel. Proc. Natl. Acad. Sci. USA 2017, 114, 6268–6273. [Google Scholar] [CrossRef]
- Humphrey, L.T.; Dean, M.C.; Jeffries, T.E.; Penn, M. Unlocking evidence of early diet from tooth enamel. Proc. Natl. Acad. Sci. USA 2008, 105, 6834–6839. [Google Scholar] [CrossRef]
- Rountrey, A.N.; Fisher, D.C.; Vartanyan, S.; Fox, D.L. Carbon and nitrogen isotope analyses of a juvenile woolly mammoth tusk: Evidence of weaning. Quat. Int. 2007, 169–170, 166–173. [Google Scholar] [CrossRef]
- Franz-Odendaal, T.A.; Lee-Thorp, J.A.; Chinsamy, A. Insights from stable light isotopes on enamel defects and weaning in Pliocene herbivores. J. Biosci. 2003, 28, 765–773. [Google Scholar] [CrossRef]
- Beaumont, J.; Montgomery, J.; Buckberry, J.; Jay, M. Infant mortality and isotopic complexity: New approaches to stress, maternal health, and weaning. Am. J. Phys. Anthropol. 2015, 157, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villagra, M.R. Developmental palaeontology in synapsids: The fossil record of ontogeny in mammals and their closest relatives. Proc. R. Soc. B Biol. Sci. 2010, 277, 1139–1147. [Google Scholar] [CrossRef]
- Dean, M.C.; Spiers, K.M.; Garrevoet, J.; Le Cabec, A. Synchrotron X-ray fluorescence mapping of Ca, Sr and Zn at the neonatal line in human deciduous teeth reflects changing perinatal physiology. Arch. Oral Biol. 2019, 104, 90–102. [Google Scholar] [CrossRef]
- Berkovitz, B.K.B. Tooth development in the albino ferret (Mustela putorius) with special reference to the permanent carnassial. Arch. Oral Biol. 1973, 18, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Dirks, W.; Anemone, R.L.; Beard, K.C.; Nachmann, B.A.; Tafforeau, P.T. Enamel microstructure and molar development in Leptadapis magnus. Am. J. Phys. Anthropol. 2011, 144, 127. Available online: https://www.researchgate.net/publication/297880768 (accessed on 5 August 2024).
- Heldstab, S.A.; Isler, K.; Schuppli, C.; van Schaik, C.P. When ontogeny recapitulates phylogeny: Fixed neurodevelopmental sequence of manipulative skills among primates. Sci. Adv. 2020, 6, eabb4685. [Google Scholar] [CrossRef]
- Schwartz, G.T.; Samonds, K.E.; Godfrey, L.R.; Jungers, W.L.; Simons, E.L. Dental microstructure and life history in subfossil Malagasy lemurs. Proc. Natl. Acad. Sci. USA 2002, 99, 6124–6129. [Google Scholar] [CrossRef]
- Sacher, G.A.; Staffeldt, E.F. Relation of gestation time to brain weight for placental mammals: Implications for the theory of vertebrate growth. Am. Nat. 1974, 108, 593–615. [Google Scholar] [CrossRef]
- Štĕrba, O. Staging and ageing of mammalian embryos and fetuses. Acta Vet. Brno 1995, 64, 83–89. [Google Scholar] [CrossRef]
- Martin, R.D.; MacLarnon, A.M. Gestation period, neonatal size and maternal investment in placental mammals. Nature 1985, 313, 220–223. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, H.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Jablonski, N.G. Primate Diversity and Environmental Seasonality in Historical Perspective. In Seasonality in Primates: Studies of Living and Extinct Human and Non-human Primates; Brockman, D.K., van Schaik, C., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 465–486. [Google Scholar]
- Kaplan, H.; Hill, K.; Lancaster, J.; Hurtado, A.M. A theory of human life history evolution: Diet, intelligence, and longevity. Evol. Anthropol. 2000, 9, 156–185. [Google Scholar] [CrossRef]
- Humphrey, L.T. Weaning behaviour in human evolution. Semin. Cell Dev. Biol. 2010, 21, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.L. Cooperative breeding and its significance to the demographic success of humans. Annu. Rev. Anthropol. 2010, 39, 417–436. [Google Scholar] [CrossRef]
- Kaplan, H. The Evolution of the Human Life Course. In Between Zeus and the Salmon; National Academy Press: Washington, DC, 1997; pp. 175–211. ISBN 0309057876. [Google Scholar]
- Koch, P.L. Isotopic Study of the Biology of Modern and Fossil Vertebrates. In Stable Isotopes in Ecology and Environmental Science; Michener, R., Lajtha, K., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; pp. 99–154. [Google Scholar]
- Tsutaya, T.; Yoneda, M. Reconstruction of breastfeeding and weaning practices using stable isotope and trace element analyses: A review. Am. J. Phys. Anthropol. 2015, 156, 2–21. [Google Scholar] [CrossRef]
- Smith, T.M.; Austin, C.; Ávila, J.N.; Dirks, W.; Green, D.R.; Williams, I.S.; Arora, M. Permanent signatures of birth and nursing initiation are chemically recorded in teeth. J. Archaeol. Sci. 2022, 140, 2–21. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Koch, P.L.; Feranec, R.S.; Wing, S.L.; Shabel, A.B. Assessing the causes of late Pleistocene extinctions on the continents. Science 2004, 306, 70–75. [Google Scholar] [CrossRef]
- Charnov, E.L. Evolution of mammal life histories. Evol. Ecol. Res. 2001, 3, 521–535. [Google Scholar] [CrossRef]
- Jukic, A.M.; Baird, D.D.; Weinberg, C.R.; Mcconnaughey, D.R.; Wilcox, A.J. Length of human pregnancy and contributors to its natural variation. Hum. Reprod. 2013, 20, 281–282. [Google Scholar] [CrossRef]
- Lande, R. On comparing coefficients of variation. Syst. Zool. 1977, 26, 214–217. [Google Scholar] [CrossRef]
- Isler, K.; van Schaik, C.P. The expensive brain: A framework for explaining evolutionary changes in brain size. J. Hum. Evol. 2009, 57, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, K.; Finlay, B. Mammalian brain development and our grandmothering life history. Physiol. Behav. 2018, 193, 55–68. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, B.H. Mammalian Life History: Weaning and Tooth Emergence in a Seasonal World. Biology 2024, 13, 612. https://doi.org/10.3390/biology13080612
Smith BH. Mammalian Life History: Weaning and Tooth Emergence in a Seasonal World. Biology. 2024; 13(8):612. https://doi.org/10.3390/biology13080612
Chicago/Turabian StyleSmith, B. Holly. 2024. "Mammalian Life History: Weaning and Tooth Emergence in a Seasonal World" Biology 13, no. 8: 612. https://doi.org/10.3390/biology13080612
APA StyleSmith, B. H. (2024). Mammalian Life History: Weaning and Tooth Emergence in a Seasonal World. Biology, 13(8), 612. https://doi.org/10.3390/biology13080612






