Effects of Dietary Gracilaria lichenoides and Bacillus amyloliquefaciens on Growth Performance, Antioxidant Capacity, and Intestinal Health of Penaeus monodon
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Diets Preparation and Nutritional Composition
2.3. Shrimp and Culture System
2.4. Sampling and Preservation
2.5. Measurement and Analysis
2.5.1. Growth Performance
2.5.2. Activity of Physiological and Biochemical Indexes
2.5.3. RNA Extraction and qPCR Measurement
2.5.4. HE Stain of the Intestinal Tissue
2.5.5. Intestinal Microbiome Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Whole-Body Composition
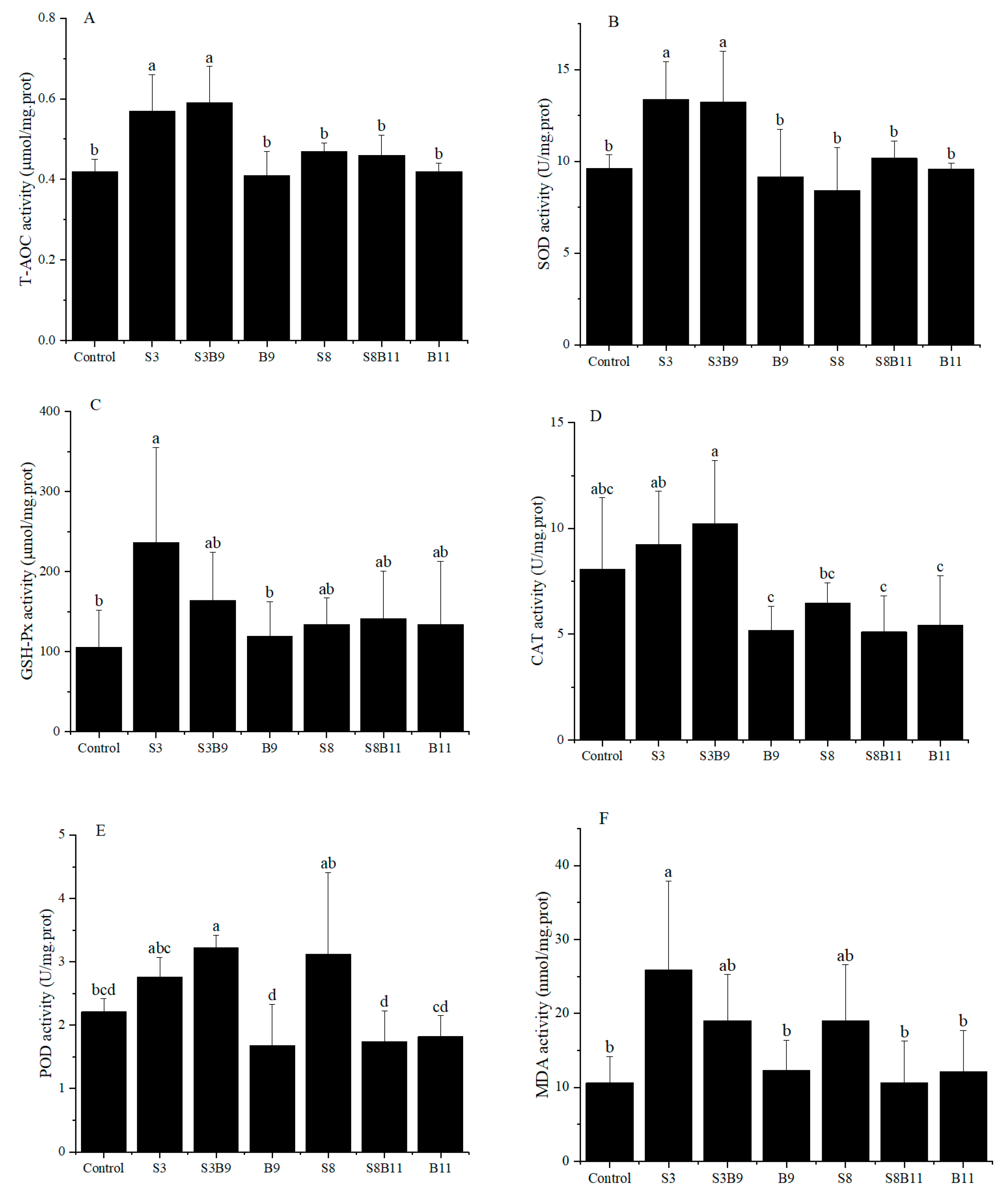
3.3. Change of Antioxidant Enzyme Activities in the Hepatopancreas
3.4. The Relative Expression of Antioxidant Genes in the Hepatopancreas of Shrimp
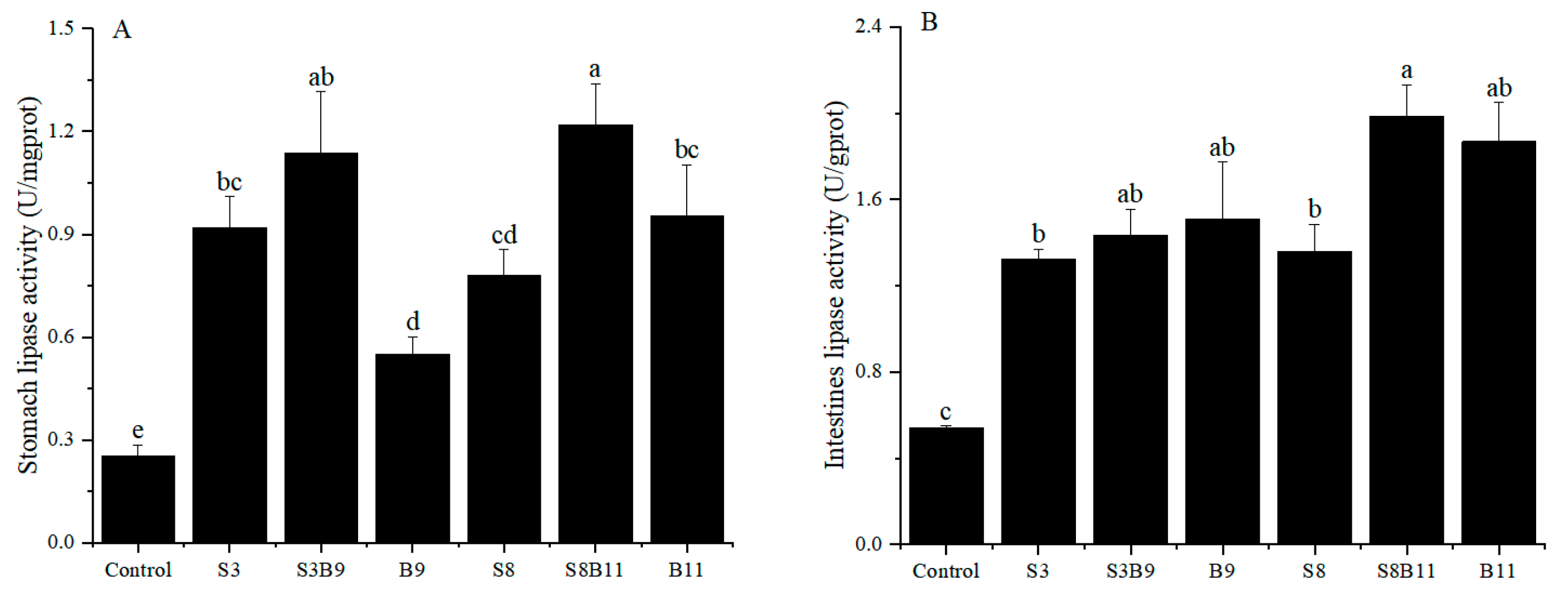
3.5. Lipase Activity
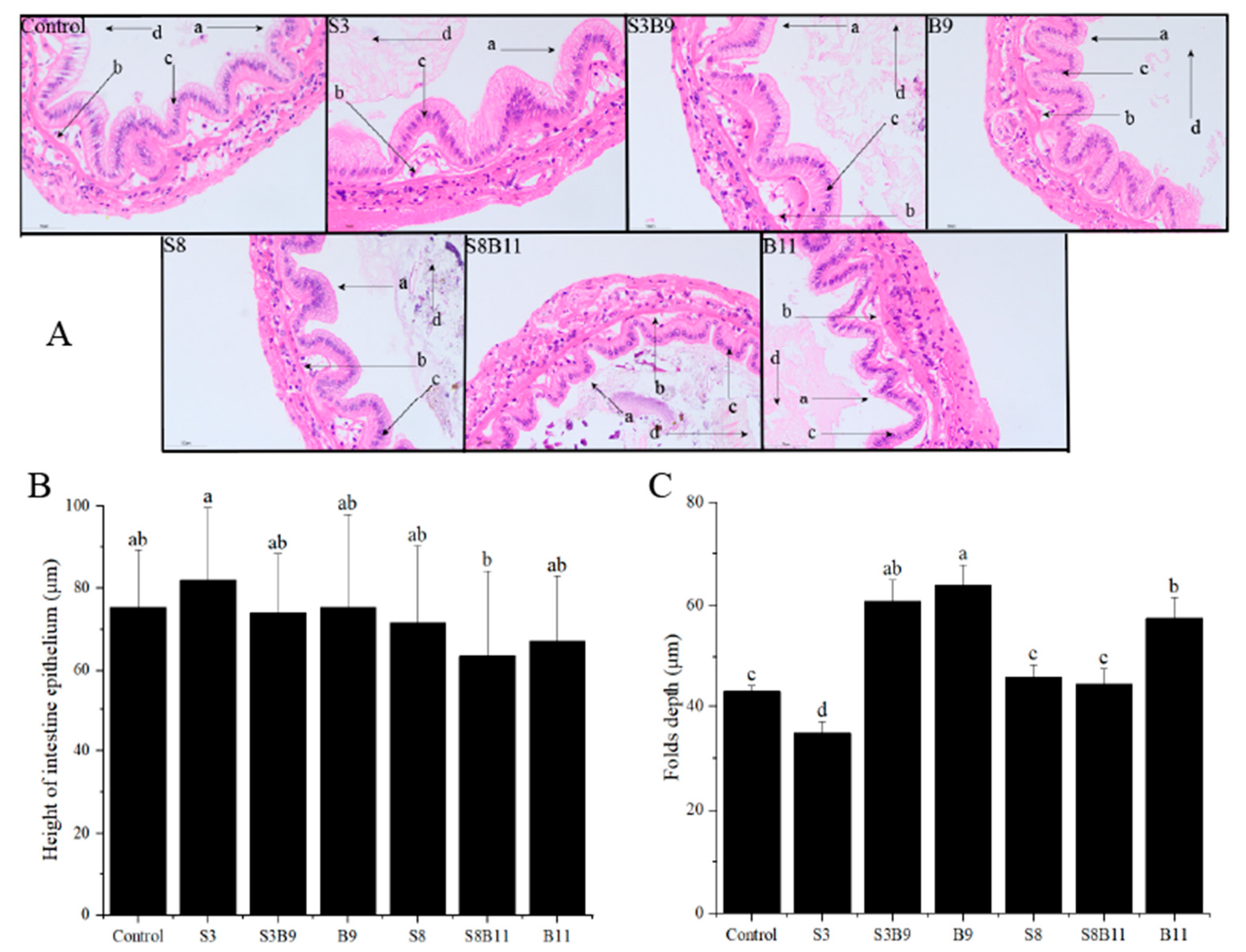
3.6. Intestinal Tissue Structure
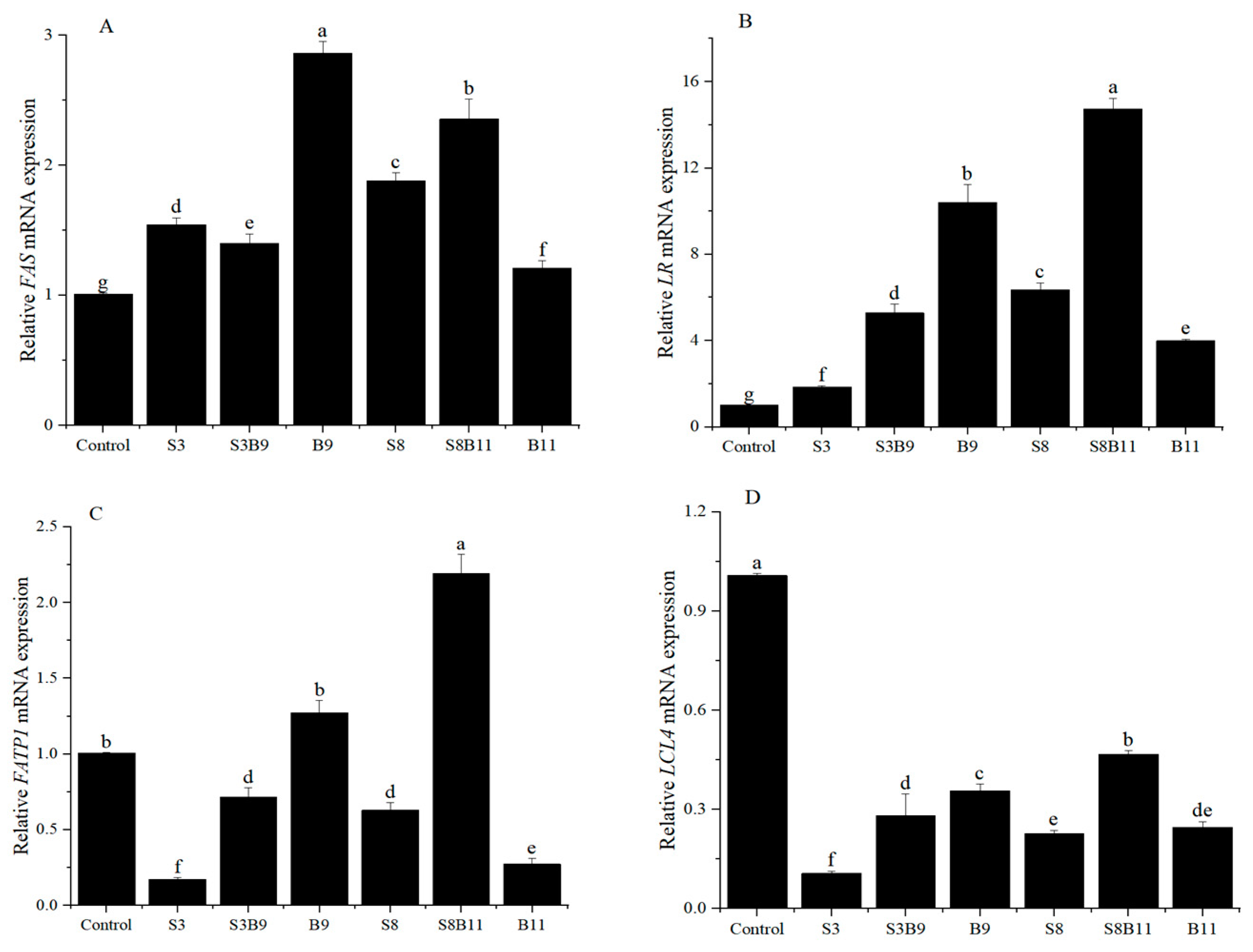
3.7. Intestinal Lipid Metabolism Genes Expression
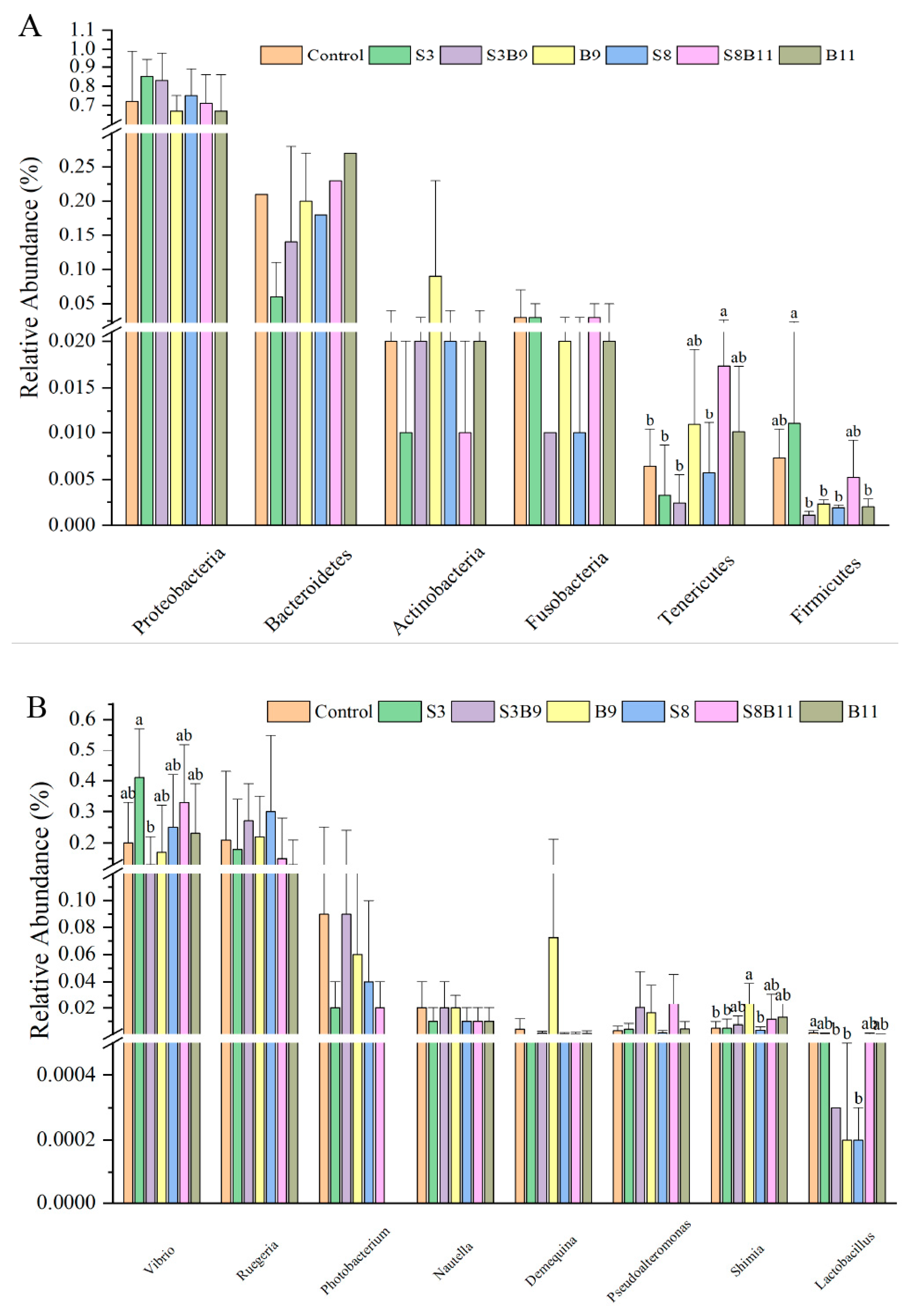
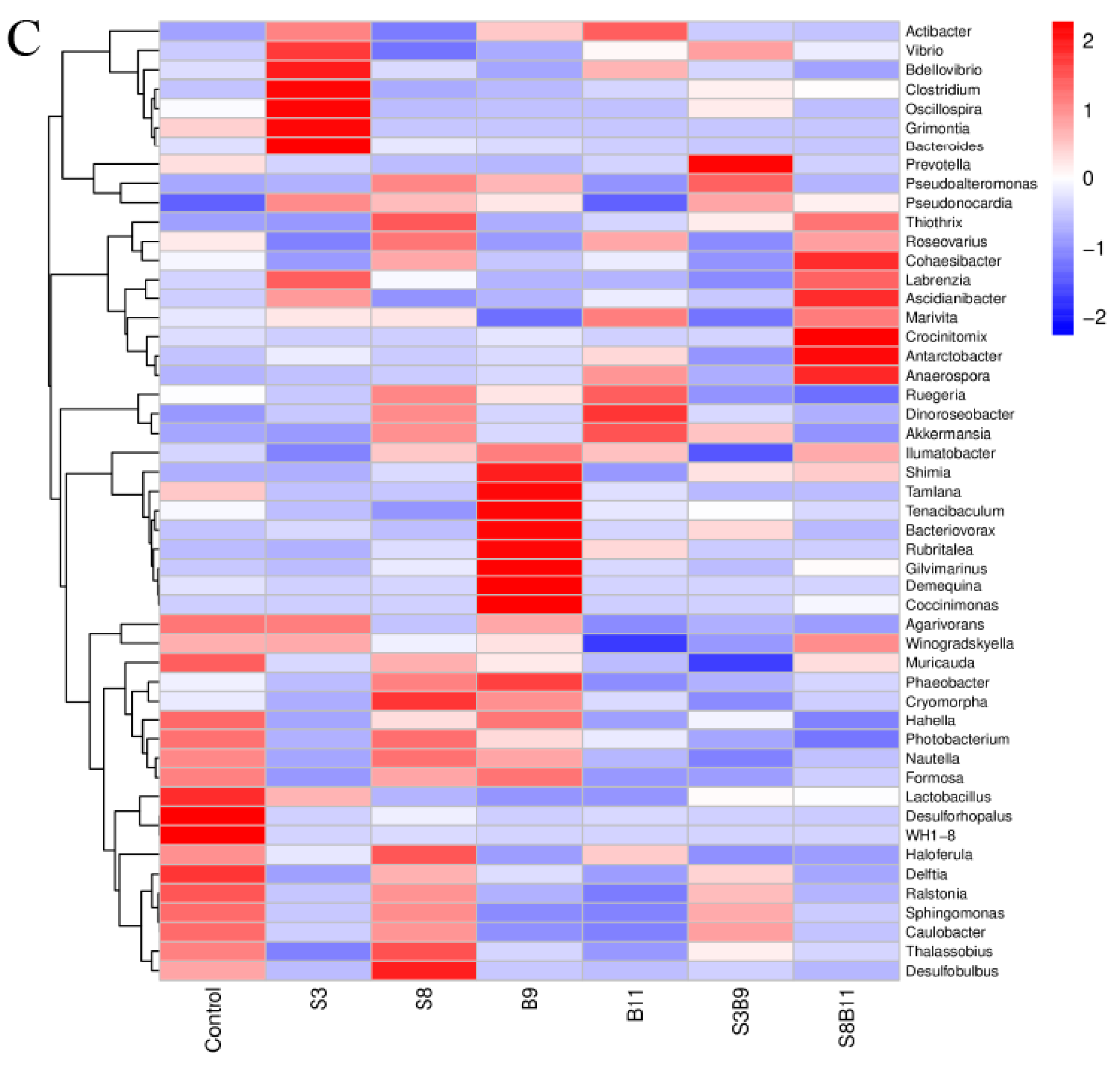
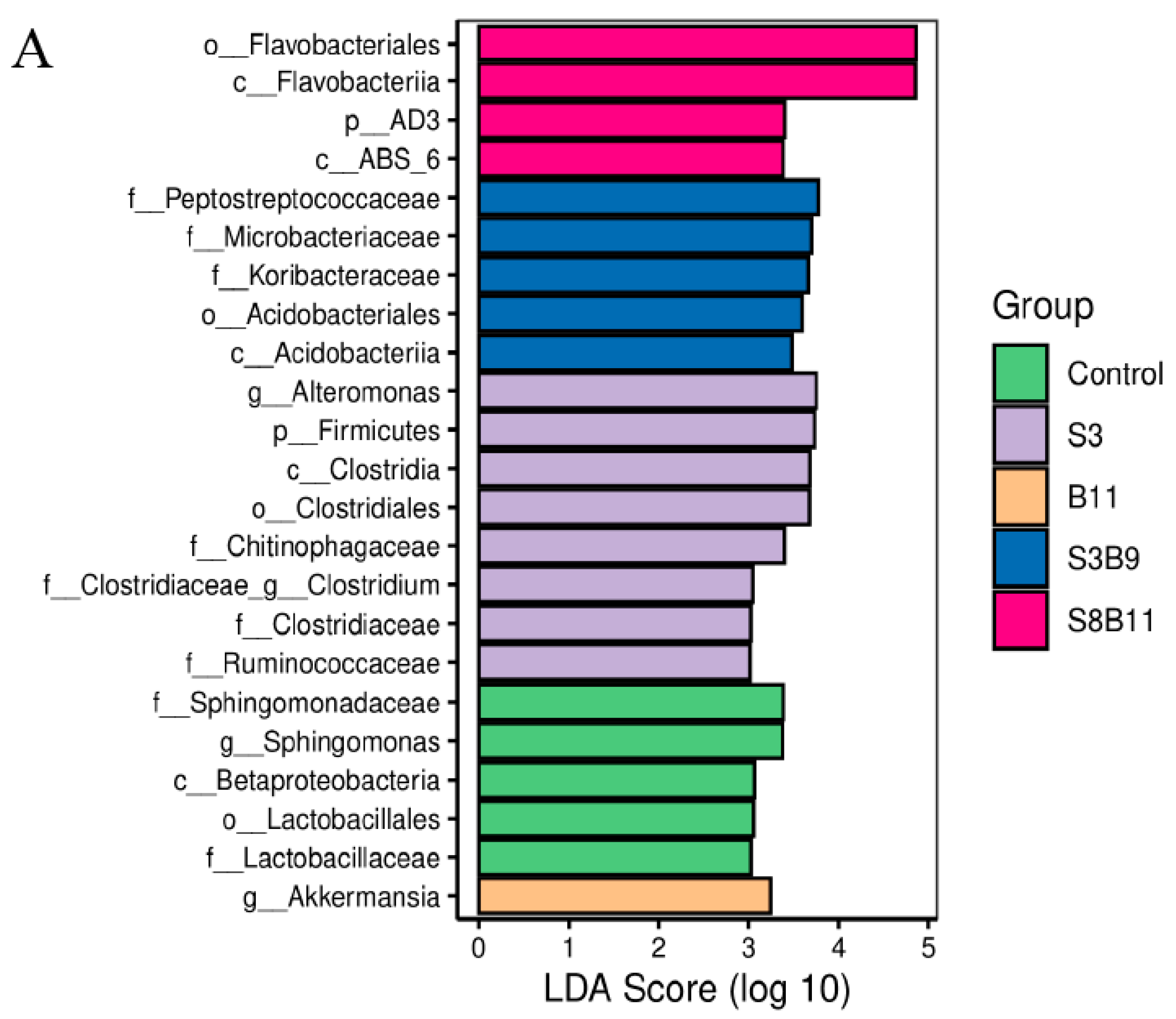
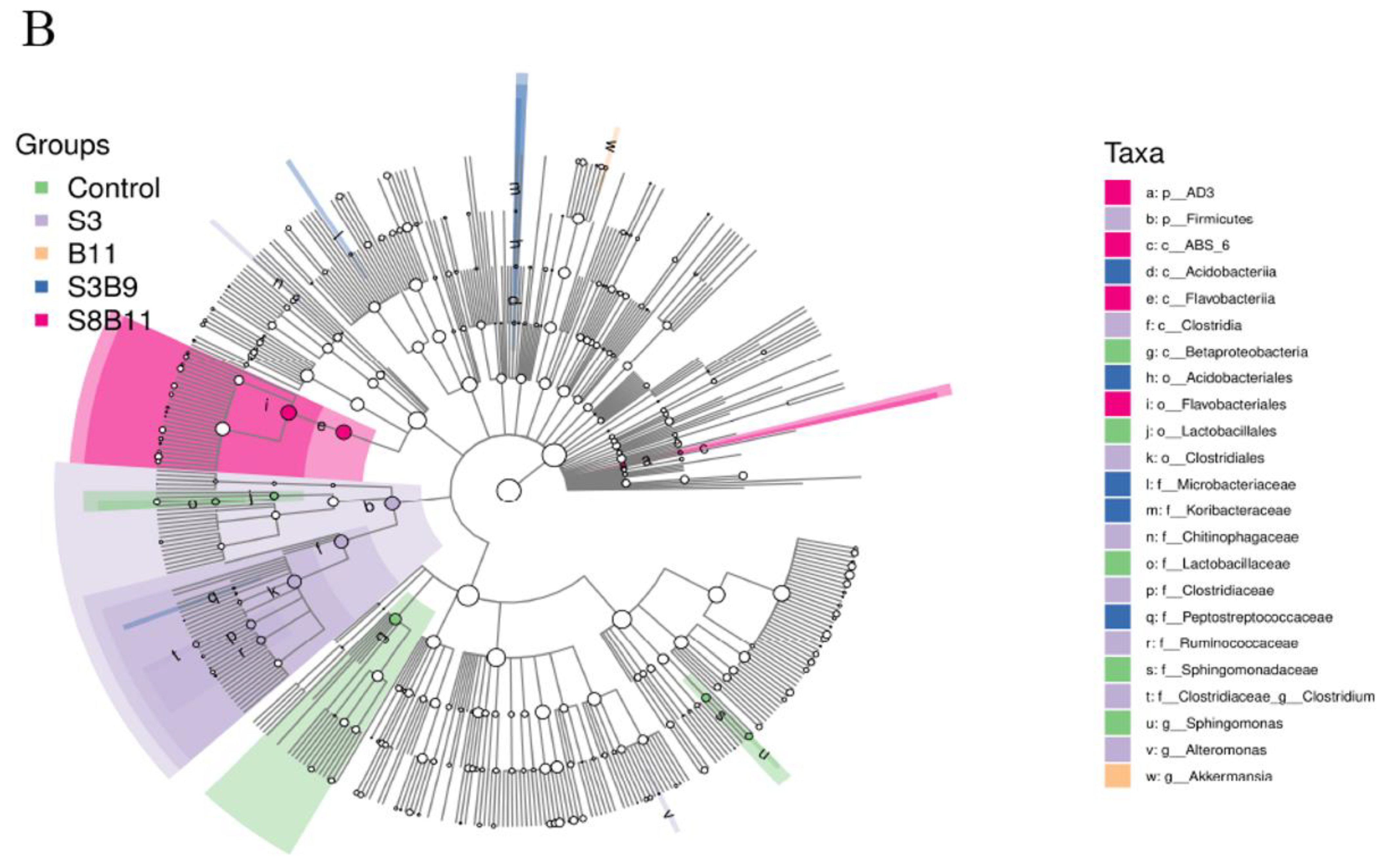
3.8. Intestinal Microbiota Changes
3.8.1. Richness and Diversity
3.8.2. Analysis of Microbial Community Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, W.Y.; Jiang, S.; Yang, Q.B.; Jiang, S.G.; Huang, J.H.; Yang, L.S.; Chen, X.; Li, Y.D.; Zhou, F.L. Effects of vitamin C on transport of Penaeus monodon. Isr. J. Aquac.-Bamidgeh 2023, 75, 1–10. [Google Scholar] [CrossRef]
- Tu, H.T.; Silvestre, F.; Wang, N.; Thome, J.P.; Phuong, N.T.; Kestemont, P. A multi-biomarker approach to assess the impact of farming systems on black tiger shrimp (Penaeus monodon). Chemosphere 2010, 81, 1204–1211. [Google Scholar] [CrossRef]
- Xie, J.J.; Chen, X.; Guo, T.Y.; Xie, S.W.; Fang, H.H.; Liu, Z.L.; Zhang, Y.M.; Tian, L.X.; Liu, Y.J.; Niu, J. Dietary values of Forsythia suspensa extract in Penaeus monodon under normal rearing and Vibrio parahaemolyticus 3HP (VP3HP) challenge conditions: Effect on growth, intestinal barrier function, immune response and immune related gene expression. Fish Shellfish Immunol. 2018, 75, 316–326. [Google Scholar] [CrossRef]
- Schleder, D.D.; Peruch, L.G.B.; Poli, M.A.; Ferreira, T.H.; Silva, C.P.; Andreatta, E.R.; Hayashi, L.; do Nascimento Vieira, F. Effect of brown seaweeds on pacific white shrimp growth performance, gut morphology, digestive enzymes activity and resistance to white spot virus. Aquaculture 2018, 495, 359–365. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Yang, Y.F.; Fei, X.G.; Song, J.M.; Hu, H.Y.; Wang, G.C.; Chung, I.K. Growth of Gracilaria lemaneiformis under different cultivation conditions and its effects on nutrient removal in Chinese coastal waters. Aquaculture 2006, 254, 248–255. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, J.; Wei, W. Application of Gracilaria lichenoides (Rhodophyta) for alleviating excess nutrients in aquaculture. J. Appl. Phycol. 2008, 20, 199–203. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweed. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Burtin, P. Nutritional value of seaweeds. J. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- Moutinho, S.; Linares, F.; Rodríguez, J.L.; Sousa, V.; Valente, L.M.P. Inclusion of 10% seaweed meal in diets for juvenile and on-growing lifestages of senegalese sole (Solea senegalensis). J. Appl. Phycol. 2018, 30, 3589–3601. [Google Scholar] [CrossRef]
- Marinho, G.; Nunes, C.; Sousa-Pinto, I.; Pereira, R.; Rema, P.; Valente, L.M.P. The IMTA-cultivated Chlorophyta Ulva spp. as a sustainable ingredient in nile tilapia (Oreochromis niloticus) diets. J. Appl. Phycol. 2013, 25, 1359–1367. [Google Scholar] [CrossRef]
- Wassef, E.A.; El-Sayed, A.F.M.; Kandeel, K.M.; Sakr, E.M. Evaluation of Pterocladia (Rhodophyta) and Ulva (Chlorophyta) meals as additives to gilthead seabream Sparus aurata diets. Egypt. J. Aquat. Res. 2005, 31, 321–332. [Google Scholar]
- Mustafa, G.; Wakamatsu, S.; Takeda, T.; Umino, T.; Nakagawa, H. Effects of algae meal as feed additive on growth, feed efficiency, andbody composition in red sea bream. Fish. Sci. 1995, 61, 25–28. [Google Scholar] [CrossRef]
- Niu, J.; Chen, X.; Lu, X.; Jiang, S.-G.; Lin, H.-Z.; Liu, Y.-J.; Huang, Z.; Wang, J.; Wang, Y.; Tian, L.-X. Effects of different levels of dietary wakame (Undaria pinnatifida) on growth, immunity and intestinal structure of juvenile Penaeus monodon. Aquaculture 2015, 435, 78–85. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Araújo, M.; Batista, S.; Peixoto, M.J.; Sousa-Pinto, I.; Brotas, V.; Cunha, L.M.; Rema, P. Carotenoid deposition, flesh quality and immunological response of nile tilapia fed increasing levels of IMTA-cultivated Ulva spp. J. Appl. Phycol. 2016, 28, 691–701. [Google Scholar] [CrossRef]
- Lazo, J.P.; Dinis, M.T.; Holt, G.J.; Faulk, C.; Arnold, C.R. Co-Feeding microparticulate diets with algae: Toward eliminating the need of zooplankton at first feeding in larval red drum (Sciaenops ocellatus). Aquaculture 2000, 188, 339–351. [Google Scholar] [CrossRef]
- Sotoudeh, E.; Jafari, M. Effects of dietary supplementation with Red Seaweed, Gracilaria pygmaea, on growth, carcass composition and hematology of juvenile rainbow trout, Oncorhynchus Mykiss. Aquacult. Int. 2017, 25, 1857–1867. [Google Scholar] [CrossRef]
- Passos, R.; Correia, A.P.; Ferreira, I.; Pires, P.; Pires, D.; Gomes, E.; Do Carmo, B.; Santos, P.; Simões, M.; Afonso, C.; et al. Effect on health status and pathogen resistance of gilthead seabream (Sparus aurata) fed with diets supplemented with Gracilaria gracilis. Aquaculture 2021, 531, 735888. [Google Scholar] [CrossRef]
- Ragaza, J.A.; Koshio, S.; Mamauag, R.E.; Ishikawa, M.; Yokoyama, S.; Villamor, S.S. Dietary supplemental effects of red seaweed Eucheuma denticulatum on growth performance, carcass composition and blood chemistry of juvenile flounder, Paralichthys olivaceus. Aquac. Res. 2015, 46, 647–657. [Google Scholar] [CrossRef]
- Adel, M.; Omidi, A.H.; Dawood, M.A.O.; Karimi, B.; Shekarabi, S.P.H. Dietary Gracilaria persica mediated the growth performance, fillet colouration, and immune response of persian sturgeon (Acipenser persicus). Aquaculture 2021, 530, 735950. [Google Scholar] [CrossRef]
- Negm, S.S.; Ismael, N.E.M.; Ahmed, A.I.; Asely, A.M.E.; Naiel, M.A.E. The efficiency of dietary Sargassum aquifolium on the performance, innate immune responses, antioxidant activity, and intestinal microbiota of nile tilapia (Oreochromis niloticus) raised at high stocking density. J. Appl. Phycol. 2021, 33, 4067–4082. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, C.; Zhu, D.; Li, S.; Wen, X. Effects of dietary Sargassum horneri on resisting hypoxia stress, which changes blood biochemistry, antioxidant status, and Hepatic HSP mRNA expressions of juvenile Black sea bream Acanthopagrus Schlegelii. J. Appl. Phycol. 2020, 32, 3457–3466. [Google Scholar] [CrossRef]
- Bakky, M.A.H.; Tran, N.T.; Zhang, Y.; Hu, H.; Lin, H.; Zhang, M.; Liang, H.; Zhang, Y.; Li, S. Effects of dietary supplementation of Gracilaria lemaneiformis-derived sulfated polysaccharides on the growth, antioxidant capacity, and innate immunity of rabbitfish (Siganus canaliculatus). Fish Shellfish Immunol. 2023, 139, 108933. [Google Scholar] [CrossRef]
- Heidarieh, M.; Mirvaghefi, A.R.; Akbari, M.; Farahmand, H.; Sheikhzadeh, N.; Shahbazfar, A.A.; Behgar, M. Effect of dietary ergosan on growth performance, digestive enzymes, intestinal histology, hematological parameters and body composition of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2012, 38, 1169–1174. [Google Scholar] [CrossRef]
- Silva, D.M.; Valente, L.M.P.; Sousa-Pinto, I.; Pereira, R.; Pires, M.A.; Seixas, F.; Rema, P. Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. J. Appl. Phycol. 2015, 27, 1671–1680. [Google Scholar] [CrossRef]
- Araújo, M.; Rema, P.; Sousa-Pinto, I.; Cunha, L.M.; Peixoto, M.J.; Pires, M.A.; Seixas, F.; Brotas, V.; Beltrán, C.; Valente, L.M.P. Dietary inclusion of IMTA-cultivated Gracilaria vermiculophylla in rainbow trout (Oncorhynchus mykiss) diets: Effects on growth, intestinal morphology, tissue pigmentation, and immunological response. J. Appl. Phycol. 2016, 28, 679–689. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Chen, W.-D.; Liu, Y.-J.; Niu, J.; Chen, M.; Tian, L.-X. Effect of different dietary levels of Gracilaria lemaneiformis dry power on growth performance, hematological parameters and intestinal structure of juvenile pacific White shrimp (Litopenaeus vannamei). Aquaculture 2016, 450, 356–362. [Google Scholar] [CrossRef]
- Muttharasi, C.; Gayathri, V.; Muralisankar, T.; Mohan, K.; Uthayakumar, V.; Radhakrishnan, S.; Kumar, P.; Palanisamy, M. Growth Performance, Digestive enzymes and antioxidants activities in the shrimp Litopenaeus vannamei fed with Amphiroa fragilissima crude polysaccharides encapsulated Artemia nauplii. Aquaculture 2021, 545, 737263. [Google Scholar] [CrossRef]
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics: Table 1. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, S.; Meena, D.K.; Sarkar, U.K. Application of probiotics in shrimp aquaculture: Importance, mechanisms of action, and methods of administration. Rev. Fish Sci. Aquac. 2016, 24, 342–368. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, J.; Chen, X.; Zhang, S.; Xie, M.; Chen, C.; Wu, Z. Screening of intestinal probiotics and the effects of feeding probiotics on the digestive enzyme activity, immune, intestinal flora and WSSV resistance of Procambarus clarkii. Aquaculture 2021, 540, 736748. [Google Scholar] [CrossRef]
- Wang, R.; Guo, Z.; Tang, Y.; Kuang, J.; Duan, Y.; Lin, H.; Jiang, S.; Shu, H.; Huang, J. Effects on development and microbial community of shrimp Litopenaeus vannamei larvae with probiotics treatment. AMB Expr. 2020, 10, 109. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Y.; Zhang, D.; Wang, Q.; Wang, G.; Elsadek, M.; Yao, Q.; Chen, Y.; Guo, Z. The effect of adding Bacillus amyloliquefaciens LSG2-8 in diets on the growth, immune function, antioxidant capacity, and disease resistance of Rhynchocypris lagowskii. Fish Shellfish Immunol. 2022, 125, 258–265. [Google Scholar] [CrossRef]
- Truong Thy, H.T.; Tri, N.N.; Quy, O.M.; Fotedar, R.; Kannika, K.; Unajak, S.; Areechon, N. Effects of the dietary supplementation of mixed probiotic spores of Bacillus amyloliquefaciens 54A, and Bacillus pumilus 47B on growth, innate immunity and stress responses of striped catfish (Pangasianodon hypophthalmus). Fish Shellfish Immunol. 2017, 60, 391–399. [Google Scholar] [CrossRef]
- Tseng, D.-Y.; Ho, P.-L.; Huang, S.-Y.; Cheng, S.-C.; Shiu, Y.-L.; Chiu, C.-S.; Liu, C.-H. Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish Shellfish Immunol. 2009, 26, 339–344. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Saputra, F.; Chen, Y.-C.; Hu, S.-Y. Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 86, 410–419. [Google Scholar] [CrossRef]
- Xu, P.W. Optimum Dietary Lipid Requirement and Short-Chain Fatty Acids Nutritional Function for Juvenile Golden Pompano (Trachinotus ovatus). Ph.D. Thesis, Shanghai Ocean University, Shanghai, China, 2022. [Google Scholar]
- Baur, F.J.; Ensminger, L.G. The Association of Official Analytical Chemists (AOAC). J. Am. Oil Chem. Soc. 1977, 54, 171–172. [Google Scholar] [CrossRef]
- Ming, J.; Ye, J.; Zhang, Y.; Yang, X.; Shao, X.; Qiang, J.; Xu, P. Dietary optimal reduced glutathione improves innate immunity, oxidative stress resistance and detoxification function of grass carp (Ctenopharyngodon idella) against Microcystin-LR. Aquaculture 2019, 498, 594–605. [Google Scholar] [CrossRef]
- Xie, S.; Wei, D.; Liu, Y.; Tian, L.; Niu, J. Dietary fish oil levels modulated lipid metabolism, immune response, intestinal health and salinity stress resistance of juvenile Penaeus monodon fed a low fish-meal diet. Anim. Feed Sci. Technol. 2022, 289, 115321. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sun, M.M.; Huang, J.H.; Yang, Q.B.; Zhou, F.L.; Wen, W.G.; Chen, X.; Jiang, S.G. Comparison on characteristics of growth and resistance to ammonia among 13 families of Penaeus monodon. J. Shanghai Ocean. Univ. 2011, 20, 510–516. [Google Scholar]
- Anil, K.S.; Balakrishanan, G.; Kanji, J.L.; Jitesh, S.B.; Kumaran, R. Comparison of Penaeus monodon (Crustacea, Penaeidae) growth between commercial feed vs. commercial shrimp feed supplemented with Kappaphycus alvarezii (Rhodophyta, Solieriaceae) seaweed sap. AACL Bioflux. 2011, 4, 292–300. [Google Scholar]
- Omont, A.; Quiroz-Guzman, E.; Tovar-Ramirez, D.; Peña-Rodríguez, A. Effect of diets supplemented with different seaweed extracts on growth performance and digestive enzyme activities of juvenile white shrimp Litopenaeus vannamei. J. Appl. Phycol. 2019, 31, 1433–1442. [Google Scholar] [CrossRef]
- Traifalgar, R.F.; Serrano, A.E.; CoRRE, V.; Kira, H.; TuNG, H.T.; Michael, F.R.; Koshio, S. Evaluation of dietary fucoidan supplementation effects on growth performance and vibriosis resistance of Penaeus monodon postlarvae. Aquacult. Sci. 2009, 57, 167–174. [Google Scholar]
- Guo, J.; Fu, Y.; Wu, Z.; Yu, X.; Guo, Y.; Liu, J.; Zhang, W.; Mai, K. Effects of dietary carbohydrate levels on growth performance, body composition, glucose/lipid metabolism and insulin signaling pathway in abalone Haliotis discus hannai. Aquaculture 2022, 557, 738284. [Google Scholar] [CrossRef]
- Bai, T. Study on Early Lipid Characteristics and Key Lipid Metabolism Enzymes of Schizothorax grahami. Master’s Thesis, Guizhou University, Guiyang, China, 2021. [Google Scholar]
- Lu, L.; Luo, X.G.; Ji, C.; Liu, B.; Yu, S.X. Effect of manganese supplementation and source on carcass traits, meat quality, and lipid oxidation in broilers. J. Anim. Sci. 2007, 85, 812–822. [Google Scholar] [CrossRef]
- Zhao, L.L.; Wang, D.X.; Liao, Z.B.; Bi, Q.Z.; Ma, Q.; Wei, Y.L.; Liang, M.Q.; Qiao, X.T.; Cheng, Z.Y.; Xu, H.G. Tissue distribution and nutritional regulation of fatty acid transport protein Scopthalmus maximus and Takifugu rubripes. Chin. J. Anim. Nutr. 2022, 34, 6620–6633. [Google Scholar]
- Li, H.B.; Liu, F.; Fu, J.H.; Feng, L.; Dai, C.G.; Hu, Y. Cloning and spatio-temporal expression of lipophorin receptors genes related to reproduction in the oriental armyworm Mythimna separata. J. Plant Prot. 2022, 49, 741–748. [Google Scholar]
- Knights, K.M. Long-chain-fatty-acid coA ligases: The key to fatty acid activation, formation of xenobiotic acyl-coA thioesters and lipophilic xenobiotic conjugates. Curr. Med. Chem. 2003, 3, 235–244. [Google Scholar] [CrossRef]
- Al-Fataftah, A.-R.; Abdelqader, A. Effects of dietary Bacillus subtilis on heat-stressed broilers performance, intestinal morphology and microflora composition. Anim. Feed Sci. Technol. 2014, 198, 279–285. [Google Scholar] [CrossRef]
- Shi, F.; Zi, Y.; Lu, Z.; Li, F.; Yang, M.; Zhan, F.; Li, Y.; Li, J.; Zhao, L.; Lin, L.; et al. Bacillus subtilis H2 modulates immune response, fat metabolism and bacterial flora in the gut of grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2020, 106, 8–20. [Google Scholar] [CrossRef]
- Zheng, J.; Duan, Y.; Yu, J.; Li, F.; Guo, Q.; Li, T.; Yin, Y. Effects of Long-term protein restriction on meat quality and muscle metabolites of shaziling pigs. Animals 2022, 12, 2007. [Google Scholar] [CrossRef]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free. Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Wang, P.; Geng, J.; Gao, J.; Zhao, H.; Li, J.; Shi, Y.; Yang, B.; Xiao, C.; Linghu, Y.; Sun, X.; et al. Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 2019, 10, 755. [Google Scholar] [CrossRef]
- Abbas, H.H.H.; Authman, M.M.N. Effects of accumulated selenium on some physiological parameters and oxidative stress indicators in tilapia fish (Oreochromis spp.). Am. -Eurasian J. Agric. Environ. Sci. 2009, 5, 219–222. [Google Scholar]
- Chen, M.; Chen, X.-Q.; Tian, L.-X.; Liu, Y.-J.; Niu, J. Enhanced intestinal health, immune responses and ammonia resistance in Pacific white shrimp (Litopenaeus vannamei) fed dietary hydrolyzed yeast (Rhodotorula mucilaginosa) and Bacillus licheniformis. Aquac. Rep. 2020, 17, 100385. [Google Scholar] [CrossRef]
- Mohammadi, G.; Hafezieh, M.; Karimi, A.A.; Azra, M.N.; Van Doan, H.; Tapingkae, W.; Abdelrahman, H.A.; Dawood, M.A.O. The synergistic effects of plant polysaccharide and Pediococcus acidilactici as a synbiotic additive on growth, antioxidant status, immune response, and resistance of nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Fish Shellfish Immunol. 2022, 120, 304–313. [Google Scholar] [CrossRef]
- Liu, P.-C.; Lin, P.-W.; Huang, C.-L.; Hsu, C.-H.; Chen, J.-C. Long-term administration of diets containing Gracilaria tenuistipitata extract induce the expression of immune-related genes and increase the immune response and resistance against Vibrio harveyi in white shrimp Litopenaeus vannamei. Gene Rep. 2019, 15, 100378. [Google Scholar] [CrossRef]
- Liu, W.-C.; Zhou, S.-H.; Balasubramanian, B.; Zeng, F.-Y.; Sun, C.-B.; Pang, H.-Y. Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol. 2020, 104, 202–212. [Google Scholar] [CrossRef]
- Arjeh, E.; Akhavan, H.-R.; Barzegar, M.; Carbonell-Barrachina, Á.A. Bio-active compounds and functional properties of pistachio hull: A review. Trends Food Sci. 2020, 97, 55–64. [Google Scholar] [CrossRef]
- Akbary, P.; Ajdari, A.; Ajang, B. Growth, survival, nutritional value and phytochemical, and antioxidant state of Litopenaeus vannamei shrimp fed with premix extract of brown Sargassum ilicifolium, Nizimuddinia zanardini, Cystoseira indica, and Padina australis macroalgae. Aquac. Int. 2023, 31, 681–701. [Google Scholar] [CrossRef]
- Mohammadi, G.; Adorian, T.J.; Rafiee, G. Beneficial effects of Bacillus subtilis on water quality, growth, immune responses, endotoxemia and protection against lipopolysaccharide-induced damages in oreochromis niloticus under biofloc technology system. Aquac. Nutr. 2020, 26, 1476–1492. [Google Scholar] [CrossRef]
- Tong, H.D.; Wang, J.M.; Song, Y. The role of Keap1-Nrf2-ARE in organismal defence against oxidative stress injury. Carcinog.,Teratog. Mutagen. 2013, 25, 71–75. [Google Scholar]
- Morilla, M.J.; Ghosal, K.; Romero, E.L. More than pigments: The potential of astaxanthin and bacterioruberin-based nanomedicines. Pharmaceutics 2023, 15, 1828. [Google Scholar] [CrossRef]
- Niemcharoen, S. Effects of Microplastics on Gene Expression to Nonspecific Immune System in Pacific White Shrimp (Litopenaeus vannamei). Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 2022. [Google Scholar]
- Hoseinifar, S.H.; Yousefi, S.; Capillo, G.; Paknejad, H.; Khalili, M.; Tabarraei, A.; Van Doan, H.; Spanò, N.; Faggio, C. Mucosal immune parameters, immune and antioxidant defence related genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish Shellfish Immunol. 2018, 83, 232–237. [Google Scholar] [CrossRef]
- Ji, K.; Liang, H.; Ren, M.; Ge, X.; Mi, H.; Pan, L.; Yu, H. The immunoreaction and antioxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala) involves the PI3K/Akt/Nrf2 and NF-κB signal pathways in response to dietary methionine levels. Fish Shellfish Immunol. 2020, 105, 126–134. [Google Scholar] [CrossRef]
- Paul, S.S.; Vantharam Venkata, H.G.R.; Raju, M.V.; Rama Rao, S.V.; Nori, S.S.; Suryanarayan, S.; Kumar, V.; Perveen, Z.; Prasad, C.S. Dietary supplementation of extracts of red sea weed (Kappaphycus alvarezii) improves growth, intestinal morphology, expression of intestinal genes and immune responses in broiler chickens. J. Sci. Food Agric. 2021, 101, 997–1008. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, M.; Feng, J.; Chen, Y.; Li, M.; Chang, X. Effects of dietary Bacillus licheniformis on growth performance, intestinal morphology, intestinal microbiome, and disease resistance in common carp (Cyprinus carpio L.). Aquac. Int. 2021, 29, 1343–1358. [Google Scholar] [CrossRef]
- Hasyimi, W.; Widanarni, W.; Yuhana, M. Growth performance and intestinal microbiota diversity in pacific white shrimp Litopenaeus vannamei fed with a probiotic bacterium, honey prebiotic, and synbiotic. Curr. Microbiol. 2020, 77, 2982–2990. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Xu, K.; Zhang, X.; Sun, H.; Fan, L.; Yan, M. White spot syndrome virus (WSSV) infection impacts intestinal microbiota composition and function in Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 84, 130–137. [Google Scholar] [CrossRef]
- Liu, L. Effects of Lactobacillus Pentosus Combined with Arthrospira platensis on the Growth Performance, Digestive Ability, Immunity, Intestinal Microbiota and Disease Resistance of Litopenaeus vannamei. Master’s Thesis, Hainan University, Haikou, China, 2022. [Google Scholar]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Sha, Y.; Liu, M.; Wang, B.; Jiang, K.; Qi, C.; Wang, L. Bacterial population in intestines of Litopenaeus vannamei fed different probiotics or probiotic supernatant. J. Microbiol. Biotechnol. 2016, 26, 1736–1745. [Google Scholar] [CrossRef]
- Costello, E.K.; Gordon, J.I.; Secor, S.M.; Knight, R. Postprandial remodeling of the gut microbiota in burmese pythons. ISME J. 2010, 4, 1375–1385. [Google Scholar] [CrossRef]
- Yuan, H.; Song, W.; Tan, J.; Zheng, Y.; Wang, H.; Shi, L.; Zhang, S. The effects of dietary protein level on the growth performance, body composition, intestinal digestion and microbiota of Litopenaeus vannamei fed Chlorella Sorokiniana as the main protein source. Animals 2023, 13, 2881. [Google Scholar] [CrossRef]
- Tian, Z.-K.; Zhang, Y.-J.; Feng, Z.-J.; Jiang, H.; Cheng, C.; Sun, J.-M.; Liu, C.-M. Nephroprotective effect of gastrodin against lead-induced oxidative stress and inflammation in mice by the GSH, Trx, Nrf2 antioxidant system, and the HMGB1 pathway. Toxicol. Res. 2021, 10, 249–263. [Google Scholar] [CrossRef]
- Xiong, J.B.; Wang, K.; Wu, J.F.; Qiuqian, L.L.; Yang, K.L.; Qian, Y.X.; Zhang, D. Changs in intestinal bacterial communities are closely associated with shrimp disease severity. Appl. Microbiol. Biotechnol. 2015, 99, 6911–6919. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, J.; Wang, Y.; Zhang, J. Characterization of bacterial community in intestinal and rearing water of Penaeus monodon differing growth performances in outdoor and indoor ponds. Aquac. Res. 2020, 51, 4279–4289. [Google Scholar] [CrossRef]


| Items (%) | Control | S3 | S3B9 | B9 | S8 | S8B11 | B11 |
|---|---|---|---|---|---|---|---|
| Fish meal | 31.00 | 31.00 | 31.00 | 31.00 | 31.00 | 31.00 | 31.00 |
| Soybean meal | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 |
| Peanut meal | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Wheat flour | 20.30 | 20.00 | 19.90 | 20.29 | 19.50 | 19.49 | 20.29 |
| Gracilaria lichenoides | 0.00 | 0.30 | 0.30 | 0.00 | 0.80 | 0.80 | 0.00 |
| Bacillus amyloliquefaciens | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 |
| Beer yeast | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Krill meal | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Soy lecithin | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Fish oil | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Soybean oil | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline chloride (50%) | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 |
| Monocalcium phosphate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin premix a | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral premix b | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ascorbic Phosphate ester | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Sum | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient levels c | |||||||
| Moisture | 9.66 | 9.97 | 10.17 | 9.46 | 9.86 | 10.32 | 9.40 |
| Ash | 10.35 | 11.29 | 11.53 | 11.28 | 13.18 | 13.64 | 11.27 |
| Crude protein | 40.62 | 40.73 | 43.87 | 41.78 | 40.15 | 40.44 | 39.71 |
| Crude lipid | 9.12 | 8.95 | 8.73 | 9.09 | 9.32 | 8.99 | 8.84 |
| Crude fiber | 3.94 | 3.29 | 4.30 | 4.52 | 3.21 | 3.64 | 3.73 |
| cDNA | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Size of Production | GenBank Nos. |
|---|---|---|---|---|
| mtMnSOD | TCGCCGCCAGGAGACTCTTC | GGCACAGATGACAGGTTCCAAGG | 122 | KC461130.1 |
| CAT | GTCCTTCTTCAGCAGCCTCAGTTG | CTTGGCTCGTGGTCAGGTTATCG | 155 | KR908786.1 |
| GSH-Px | CGTCCGTCCTGGCAATAACTTCG | CTGGCAGCGGCAGTCGTTC | 116 | JX912159.1 |
| Trx | CCTGAAGGTGGATGTGGACGAATG | AGTGGAATGGATTACTTGTGCTTCTCG | 168 | JF828310.1 |
| Hippo | TGAGCACAACCAAACCCACCATC | CATCGTCCGACTGTCCACTTCATC | 88 | Not acquired |
| Nrf2 | CCAACCTCCAGTAACAAGCCAAGAG | TCAACAATTCTGATGAGCACAGCAATG | 237 | MW390830.1 |
| FAS | GCGTGATAACTGGGTGTCCT | CTTTCAGGCCCTGGATGATA | 227 | XM030250746.2 |
| LR | GTGGCAGGAATGAGTGTGAGG | GCATACCCTCCAACGCACTC | 215 | NM009309.2 |
| FATP1 | GATGACACGGACATGACTACTG | CTCCTGGCTTCAGCACATTCC | 195 | NM164934.2 |
| LCL4 | CTCCAATCTGTAGTTTAACCAAGTCC | GATGACACGGACATGACTACTG | 304 | XM006583136.4 |
| EF-1α | AGTATGCTCCTTTTGGACGTTTTGC | CCTTTTCTGCGGCCTTGGTAGTC | 120 | GU136229.1 |
| Items | IBW (g) | FBW (g) | WGR (%) | SGR (%) | FCR | SR (%) |
|---|---|---|---|---|---|---|
| Control | 1.06 ± 0.02 | 9.18 ± 0.48 b | 765.56 ± 56.72 b | 3.92 ± 0.12 b | 1.79 ± 0.17 | 90.00 ± 6.67 |
| S3 | 1.04 ± 0.06 | 10.49 ± 0.90 a | 908.94 ± 33.58 a | 4.20 ± 0.06 a | 1.50 ± 0.24 | 90.00 ± 0.00 |
| S3B9 | 1.05 ± 0.05 | 9.47 ± 0.62 ab | 804.95 ± 56.43 b | 4.00 ± 0.12 ab | 1.64 ± 0.14 | 94.44 ± 1.93 |
| B9 | 1.04 ± 0.02 | 8.95 ± 0.56 b | 759.42 ± 41.23 b | 3.91 ± 0.09 b | 1.53 ± 0.30 | 88.89 ± 3.85 |
| S8 | 1.02 ± 0.03 | 8.51 ± 0.45 b | 731.19 ± 49.05 b | 3.85 ± 0.11 b | 1.80 ± 0.10 | 93.33 ± 0.00 |
| S8B11 | 1.01 ± 0.04 | 9.08 ± 1.05 b | 794.15 ± 80.75 b | 3.98 ± 0.17 b | 1.46 ± 0.12 | 93.33 ± 0.00 |
| B11 | 1.04 ± 0.04 | 8.33 ± 0.29 b | 703.55 ± 17.80 b | 3.79 ± 0.04 b | 1.63 ± 0.21 | 93.33 ± 8.82 |
| Items | Control | S3 | S3B9 | B9 | S8 | S8B11 | B11 |
|---|---|---|---|---|---|---|---|
| Moisture (%) | 76.51 ± 1.20 | 77.02 ± 0.45 | 76.15 ± 0.86 | 76.76 ± 0.17 | 76.54 ± 0.47 | 76.09 ± 0.81 | 76.00 ± 0.74 |
| Protein (% dry matter) | 70.01 ± 1.30 | 70.37 ± 0.68 | 70.45 ± 0.35 | 70.56 ± 1.27 | 71.09 ± 0.42 | 71.39 ± 0.87 | 70.01 ± 0.94 |
| Lipid (% dry matter) | 10.40 ± 0.70 ab | 10.49 ± 1.11 ab | 10.73 ± 1.86 ab | 11.08 ± 0.75 a | 9.80 ± 1.06 ab | 9.35 ± 0.92 ab | 9.27 ± 0.76 b |
| Ash (% dry matter) | 16.61 ± 0.69 ab | 16.11 ± 1.13 ab | 15.67 ± 0.48 b | 16.97 ± 0.56 a | 16.66 ± 0.72 ab | 16.11 ± 0.79 ab | 16.46 ± 0.55 ab |
| Alpha name | Control | S3 | S3B9 | B9 | S8 | S8B11 | B11 |
|---|---|---|---|---|---|---|---|
| Chao1 | 1066.90 ± 445.03 | 1054.39 ± 354.66 | 876.95 ± 243.56 | 1139.16 ± 399.85 | 1229.12 ± 352.32 | 1052.77 ± 469.62 | 1298.55 ± 404.43 |
| Simpson | 0.94 ± 0.03 | 0.91 ± 0.05 | 0.91 ± 0.04 | 0.94 ± 0.03 | 0.95 ± 0.02 | 0.92 ± 0.04 | 0.93 ± 0.01 |
| Shannon | 5.74 ± 0.97 | 5.39 ± 0.41 | 5.14 ± 0.94 | 5.78 ± 0.83 | 5.94 ± 0.46 | 5.20 ± 0.97 | 5.54 ± 0.57 |
| Coverage (%) | 99.67 ± 0.17 | 99.65 ± 0.14 | 99.73 ± 0.05 | 99.59 ± 0.15 | 99.61 ± 0.15 | 99.61 ± 0.19 | 99.54 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Wang, Y.; Huang, J.; Yan, H.; Duan, Y.; Wang, J.; Zhou, C.; Huang, Z. Effects of Dietary Gracilaria lichenoides and Bacillus amyloliquefaciens on Growth Performance, Antioxidant Capacity, and Intestinal Health of Penaeus monodon. Biology 2024, 13, 252. https://doi.org/10.3390/biology13040252
Tian J, Wang Y, Huang J, Yan H, Duan Y, Wang J, Zhou C, Huang Z. Effects of Dietary Gracilaria lichenoides and Bacillus amyloliquefaciens on Growth Performance, Antioxidant Capacity, and Intestinal Health of Penaeus monodon. Biology. 2024; 13(4):252. https://doi.org/10.3390/biology13040252
Chicago/Turabian StyleTian, Jialin, Yun Wang, Jianhua Huang, Hailiang Yan, Yafei Duan, Jun Wang, Chuangpeng Zhou, and Zhong Huang. 2024. "Effects of Dietary Gracilaria lichenoides and Bacillus amyloliquefaciens on Growth Performance, Antioxidant Capacity, and Intestinal Health of Penaeus monodon" Biology 13, no. 4: 252. https://doi.org/10.3390/biology13040252
APA StyleTian, J., Wang, Y., Huang, J., Yan, H., Duan, Y., Wang, J., Zhou, C., & Huang, Z. (2024). Effects of Dietary Gracilaria lichenoides and Bacillus amyloliquefaciens on Growth Performance, Antioxidant Capacity, and Intestinal Health of Penaeus monodon. Biology, 13(4), 252. https://doi.org/10.3390/biology13040252








