Mesenchymal Stem Cell-Derived Exosomes Loaded with Selenium or Nano Selenium as a Novel Therapeutic Paradigm for Streptozotocin-Induced Type 1 Diabetes in Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
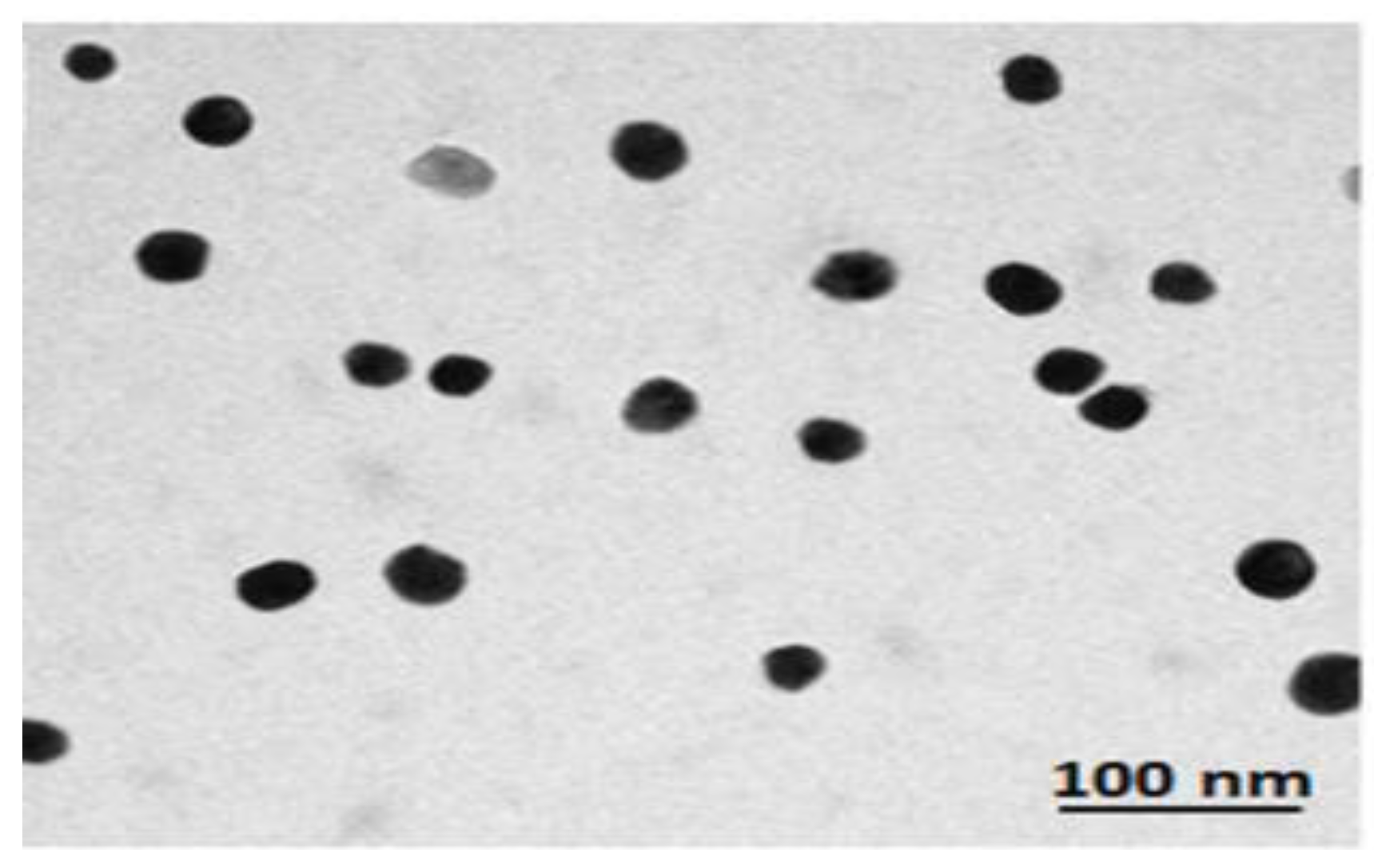
2.2. AD-MSCs-Exs Preparation and Characterization
2.3. Nano-Selenium (NSe) Preparation and Characterization
2.4. Loading of EXs with Either Se or NSe
2.5. Induction of Diabetes
2.6. Experimental Design
- A.
- Control groups
- 1.
- Normal group: Rats received an IP single dose of citrate buffer (2 mL/kg, pH 4.6).
- 2.
- Ex group: Rats received an IV single dose of 0.5 mL unloaded EXs.
- 3.
- Ex+Se group: Rats received an IV single dose of 0.5 mL EXs loaded with Se.
- 4.
- Ex+NSe group: Rats received an IV single dose of 0.5 mL EXs loaded with NSe.
- B.
- Diabetic groups
- 5.
- Diabetic (D) untreated group: Diabetic rats received an IP single dose of STZ (60 mg/kg).
- 6.
- Ex-treated group: Diabetic rats received an IV single dose of 0.5 mL unloaded EXs.
- 7.
- Diabetic Ex+Se-treated group: Diabetic rats received an IV single dose of 0.5 mL EXs loaded with Se.
- 8.
- Diabetic Ex+NSe-treated group: Diabetic rats received an IV single dose of 0.5 mL EXs loaded with NSe.
2.7. Sample Collection
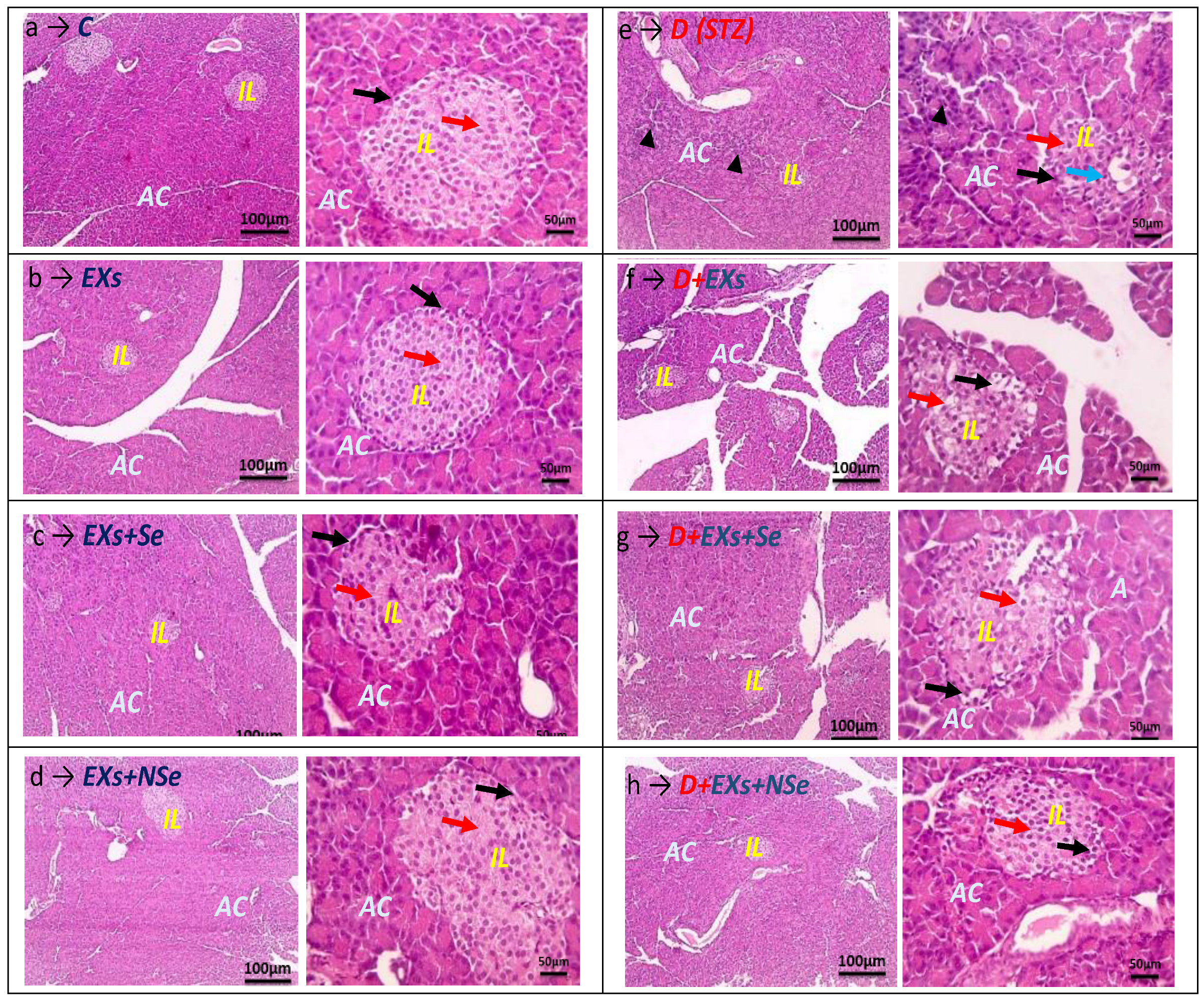
2.8. Histochemical Examination
2.9. Biochemical Assays
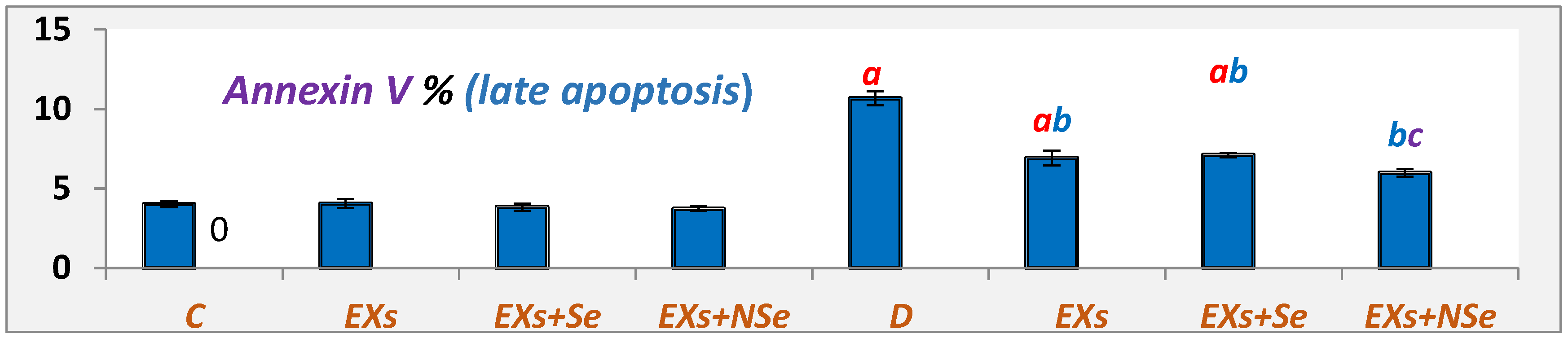
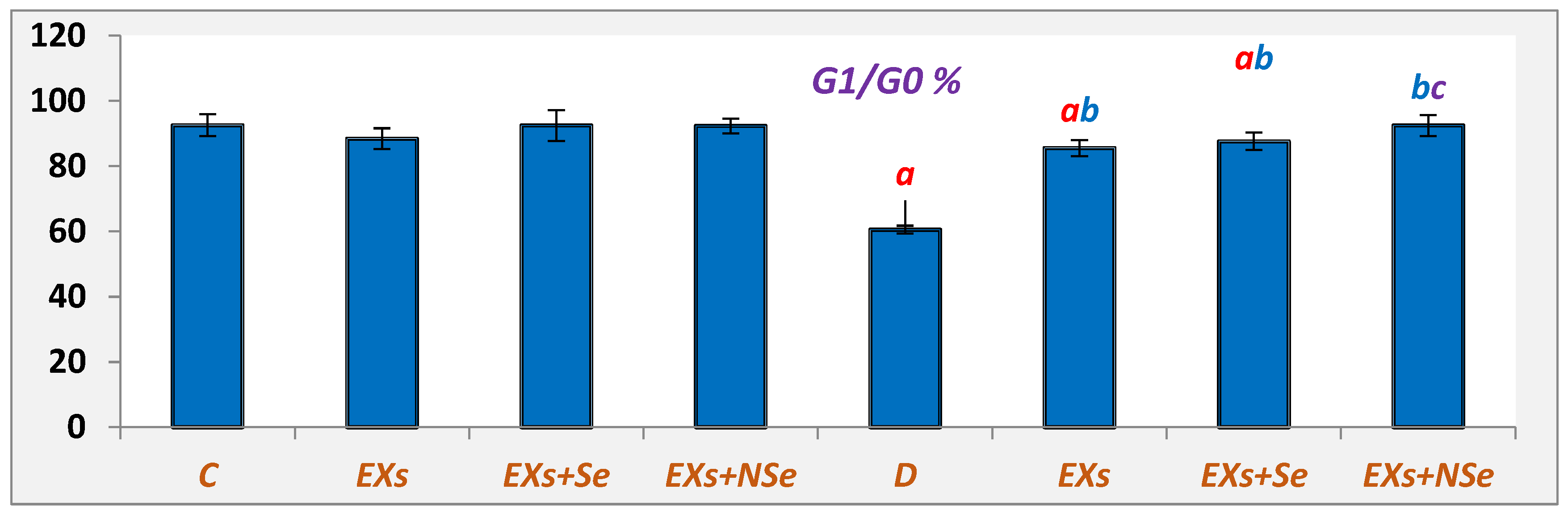
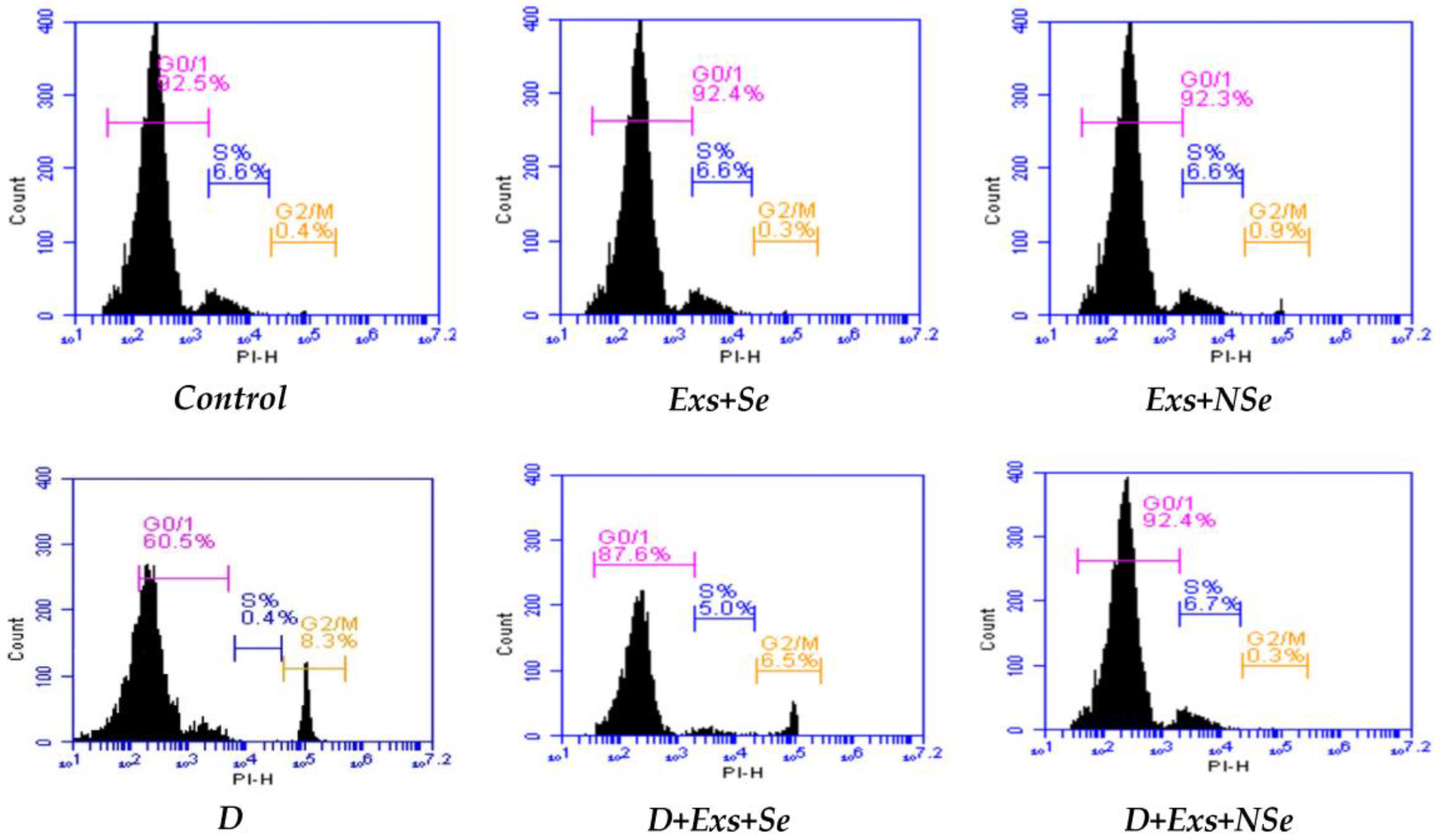
2.10. Flow Cytometric Analysis
2.11. Statistical Analysis
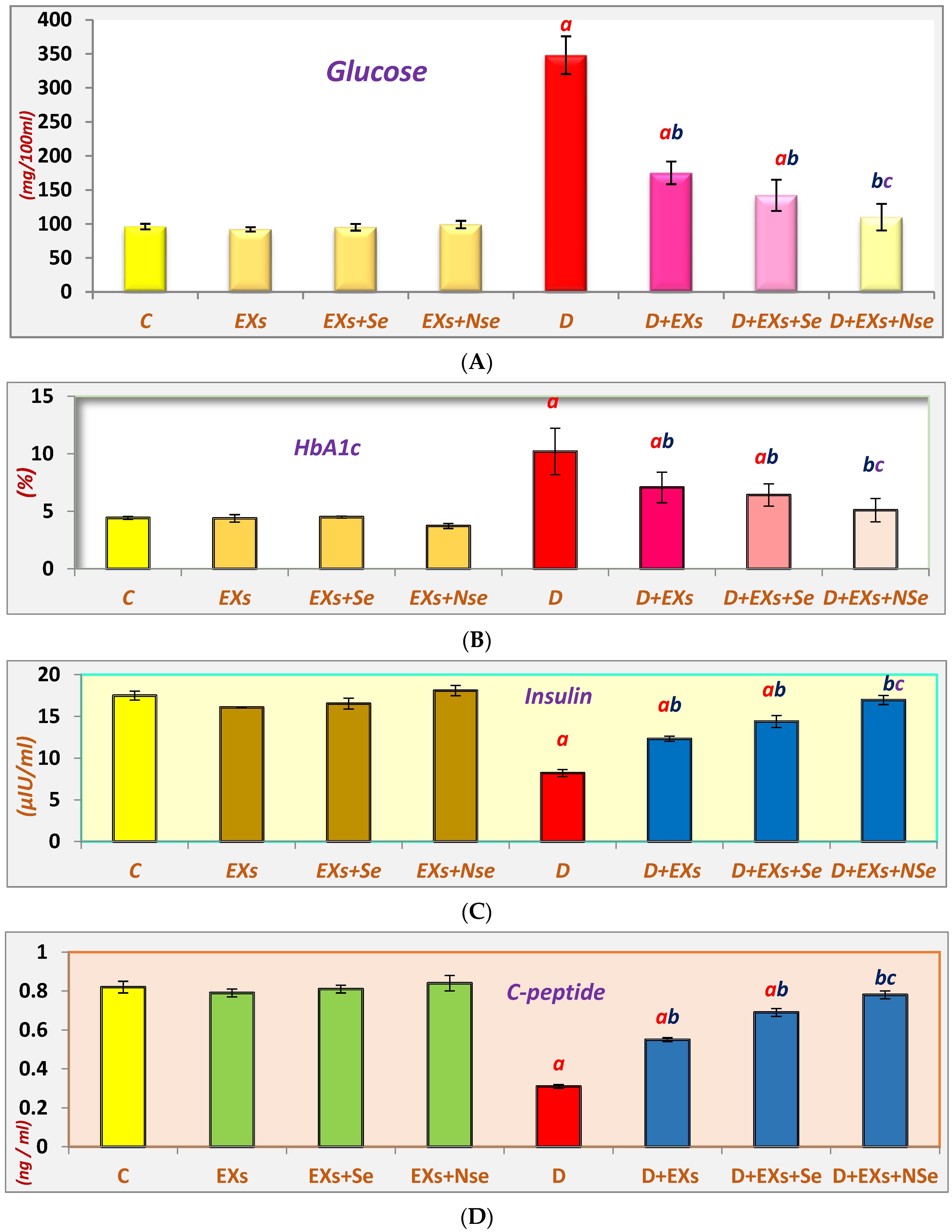
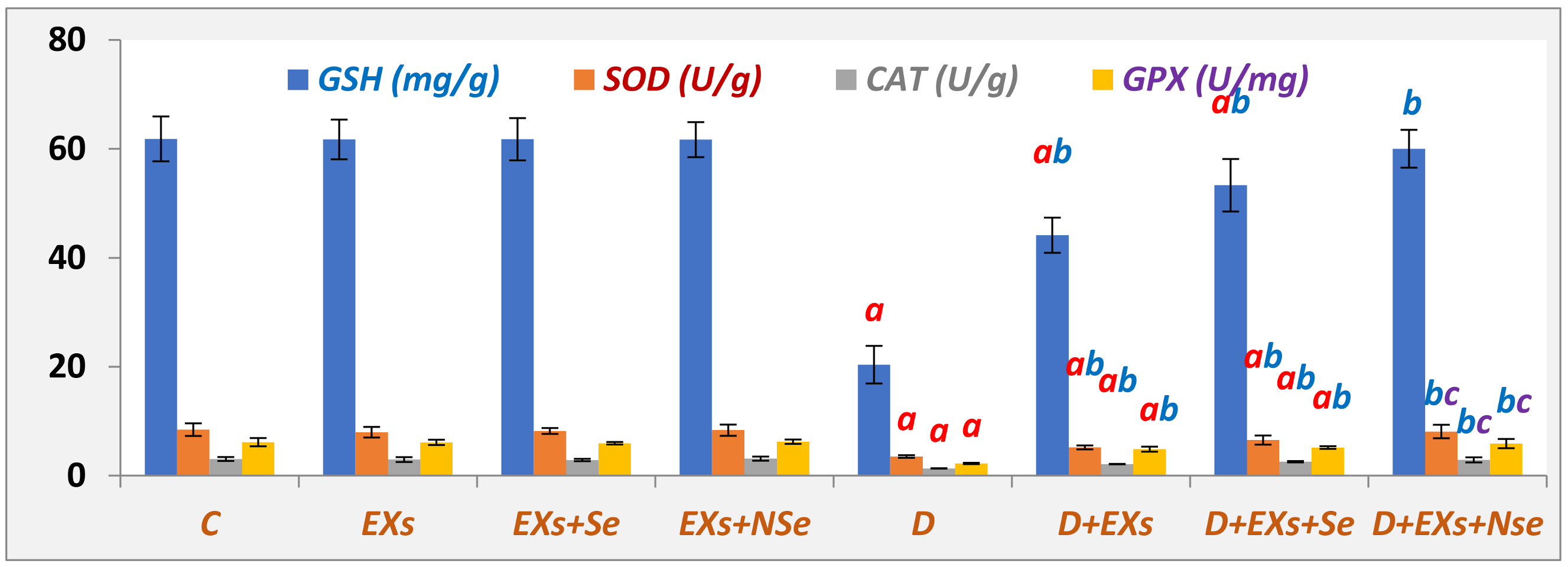
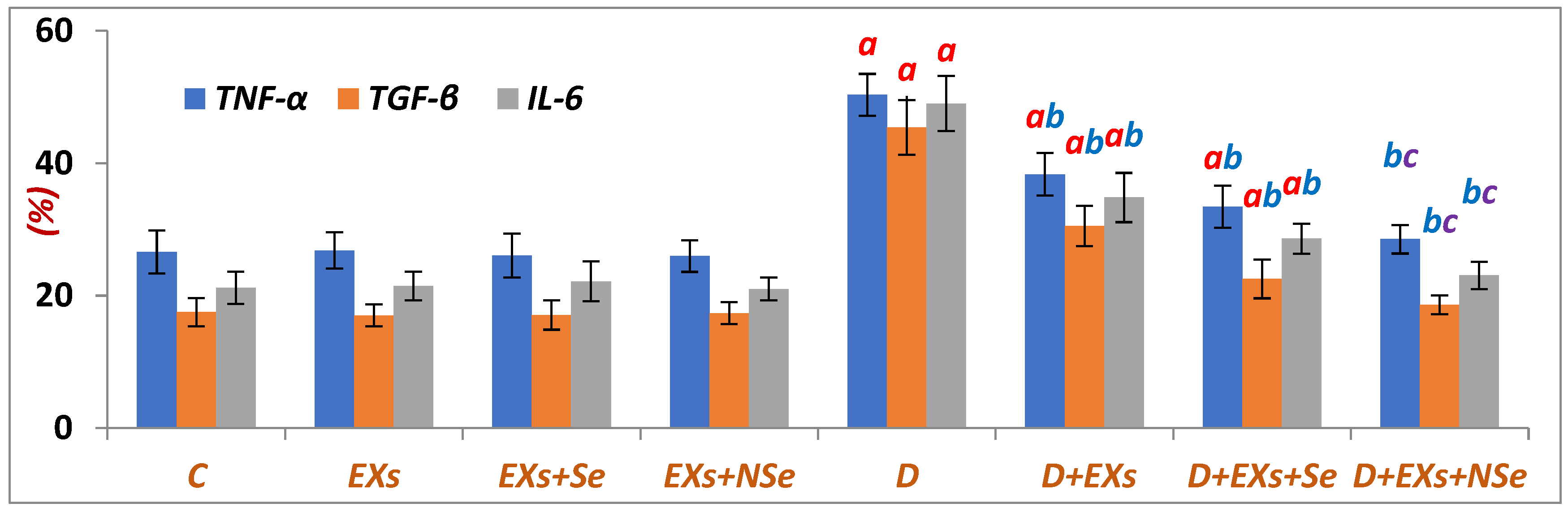
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yang, M.; Chen, J.; Chen, L. The roles of mesenchymal stem cell-derived exosomes in diabetes mellitus and its related complications. Front. Endocrinol. 2022, 13, 1027686. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wu, J.; Gao, J. Exosomes for diabetes syndrome: Ongoing applications and perspective. Biomater. Sci. 2022, 10, 2154. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tao, Q.; Wu, X.; Zhang, L.; Liu, Q.; Wang, L. The Utility of Exosomes in Diagnosis and Therapy of Diabetes Mellitus and Associated Complications. Front. Endocrinol. 2021, 12, 756581. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Volarevic, V. Therapeutic Use of Mesenchymal Stem Cell-Derived Exosomes: From Basic Science to Clinics. Pharmaceutics 2020, 12, 474. [Google Scholar] [CrossRef]
- He, Q.; Wang, L.; Zhao, R.; Yan, F.; Sha, S.; Cui, C.; Song, J.; Hu, H.; Guo, X.; Yang, M.; et al. Retraction Note: Mesenchymal stem cell-derived exosomes exert ameliorative effects in type 2 diabetes by improving hepatic glucose and lipid metabolism via enhancing autophagy. Stem Cell Res. Ther. 2022, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Kaviani, M.; Sarvestani, F.S.; Ghahremani, M.H.; Aghdaei, M.H.; Al-Abdullah, I.H.; Azarpira, N. Exosomes derived from human mesenchymal stem cells preserve mouse islet survival and insulin secretion function. EXCLI J. 2020, 19, 1064–1080. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.-C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef]
- Nakano, M.; Kubota, K.; Kobayashi, E.; Chikenji, T.S.; Saito, Y.; Konari, N.; Fujimiya, M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in the hippocampus. Sci. Rep. 2020, 10, 10772. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63–70. [Google Scholar] [CrossRef]
- Lv, Q.; Deng, J.; Chen, Y.; Wang, Y.; Liu, B.; Liu, J. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol. Pharm. 2020, 17, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Lai, J. Synthesis, bioactive properties, and biomedical applications of intrinsically therapeutic nanoparticles for disease treatment. Chem. Eng. J. 2022, 435, 134970. [Google Scholar] [CrossRef]
- Moon, S.; Oh, C.M. Selenium and Risk of Diabetes. Biomarkers in Diabetes. In Biomarkers in Disease: Methods, Discoveries, and Applications; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Fontenelle, L.C.; Feitosa, M.M.; Morais, J.B.S.; Severo, J.S.; de Freitas, T.E.C.; Beserra, J.B.; Henriques, G.S.; Marreiro, D.N. The role of selenium in insulin resistance. Braz. J. Pharm. Sci. 2018, 54, e00139. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Selenium, and redox signaling. Arch. Biochem. Biophys. 2017, 617, 48–59. [Google Scholar] [CrossRef]
- Barakat, G.M.; Moustafa, M.E.; Khalifeh, I.; Hodroj, M.H.; Bikhazi, A.; Rizk, S. Effects of exendin-4 and selenium on the expression of GLP-1R, IRS-1, and preproinsulin in the pancreas of diabetic rats. J. Physiol. Biochem. 2016, 73, 387–394. [Google Scholar] [CrossRef]
- Meng, X.-L.; Zhang, H.-L.; Feng, L.-L.; Chen, M.-L.; Liu, Y.-Y.; Yu, X.; Huan, F.-N.; Lu, J.; Wang, D.; Liu, H.-S.; et al. Selenoprotein SelK increases the secretion of insulin from MIN6 β cells. RSC. Adv. 2017, 7, 35038–35047. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Alkhudhayri, A.; Al-Shaebi, E.M.; Qasem, A.A.; Murshed, M.; Mares, M.H.; Al-Quraishy, S.; Dkhil, M.A. Antioxidant and anti-apoptotic effects of selenium nanoparticles against murine eimeriosis. An. Acad. Bras. Ciênc. 2020, 92, e20191107. [Google Scholar] [CrossRef]
- Fatima, S.; Alfrayh, R.; Alrashed, M.; Alsobaie, S.; Ahmad, R.; Mahmood, A. Selenium Nanoparticles by Moderating Oxidative Stress Promote Differentiation of Mesenchymal Stem Cells to Osteoblasts. Int. J. Nanomed. 2021, 16, 331–343. [Google Scholar] [CrossRef]
- Shahidi, S.; Asl, S.S.; Gholamigeravand, B.; Afshar, S.; Hashemi-Firouzi, N.; Samzadeh-Kermani, A.; Majidi, M.; Amiri, K. Effect of co-treatment with mesenchymal stem cells and polyvinyl alcohol-coated selenium nanoparticles on rats with streptozotocin-induced Alzheimer’s disease. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Kodidela, S.; BegumShaik, F.; Chinta, V.; Mohammad, S.; Pasala, C.; Mittameedi, S.; Maddu, N.; Wudayagiri, R.; Nallanchakravarthula, V. Possible ameliorative role of green tea on chronic alcohol mediated renal toxicity of STZ-induced diabetic rats. Clin. Nutr. Exp. 2020, 34, 1–25. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Tribukait, B.; Moberger, G.; Zetterberg, A. Methodological aspects of rapid-flow cytoflurometry for DNA analysis of human urinary bladder cells. Pulse Cytophotom. 2013, 1, 50–60. [Google Scholar]
- Wang, L.; Shi, S.; Bai, R.; Wang, Y.; Guo, Z.; Li, D. Biological properties of bone marrow stem cells and adipose-derived stem cells derived from T2DM rats: A comparative study. Cell Biosci. 2020, 10, 102. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, H.; Yin, S.; Ji, C.; Zhang, X.; Zhang, B.; Wu, P.; Shi, Y.; Mao, F.; Yan, Y.; et al. Human mesenchymal stem cell-derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving beta-cell destruction. ACS Nano 2018, 12, 7613–7628. [Google Scholar] [CrossRef]
- Yap, S.K.; Tan, K.L.; Abd Rahaman, N.Y.; Saulol Hamid, N.F.; Ooi, D.J.; Tor, Y.S.; Looi, Q.H.D.; Tan, L.K.S.; How, C.W.; Foo, J.B. Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles ameliorated insulin resistance in type 2 diabetes mellitus rats. Pharmaceutics 2022, 14, 649. [Google Scholar] [CrossRef]
- Sharma, R.; Kumari, M.; Mishra, S.; Chaudhary, D.K.; Kumar, A.; Avni, B.; Tiwari, S. Exosomes Secreted by Umbilical Cord Blood-Derived Mesenchymal Stem Cell Attenuate Diabetes in Mice. J. Diabetes Res. 2021, 2021, 9534574. [Google Scholar] [CrossRef]
- Xue, C.; Shen, Y.; Li, X.; Li, B.; Zhao, S.; Gu, J.; Chen, Y.; Ma, B.; Wei, J.; Han, Q.; et al. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis through the PKA Signaling Pathway. Stem Cells Dev. 2018, 27, 456–465. [Google Scholar] [CrossRef]
- Mahdipour, E.; Salmasi, Z.; Sabeti, N. The potential of stem cell-derived exosomes to regenerate β islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J. Cell Physiol. 2019, 234, 20310–20321. [Google Scholar] [CrossRef]
- Sabry, D.; Marzouk, S.; Zakaria, R.; Ibrahim, H.A.; Samir, M. The effect of exosomes derived from mesenchymal stem cells in the treatment of induced type 1 diabetes mellitus in rats. Biotechnol. Lett. 2020, 42, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ke, Q.F.; Tao, S.C.; Guo, S.C.; Rui, B.Y.; Guo, Y.P. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J. Mater. Chem. B 2016, 4, 6830–6841. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Karalis, D.T. The Beneficiary Role of Selenium in Type II Diabetes: A Longitudinal Study. Cureus 2019, 11, e6443. [Google Scholar] [CrossRef]
- Barakat, G.M.; Moustafa, M.E.; Bikhazi, A.B. Effects of selenium and exendin-4 on glucagon-like peptide-1 receptor, IRS-1, and Raf-1 in the liver of diabetic rats. Biochem. Genet. 2012, 50, 922–935. [Google Scholar] [CrossRef]
- Iizuka, Y.; Ueda, Y.; Yagi, Y.; Sakurai, E. Significant improvement of insulin resistance of GK rats by treatment with sodium selenate. Biol. Trace Elem. Res. 2010, 138, 265–271. [Google Scholar] [CrossRef]
- Harmon, J.S.; Bogdani, M.; Parazzoli, S.D.; Mak, S.S.M.; Oseid, E.A.; Berghmans, M.; LeBoeuf, R.C.; Robertson, R.P. Beta-cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology 2009, 150, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.G.; Vatamaniuk, M.Z. Two tales of antioxidant enzymes on β cells and diabetes. Antioxid. Redox Signal. 2011, 14, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Hotze, A.-L.; Speckmann, B.; Pinto, A.; Sies, H.; Schott, M.; Ehlers, M.; Scherbaum, W.A.; Schinner, S. Localization and regulation of pancreatic selenoprotein P. J. Mol. Endocrinol. 2013, 50, 31–42. [Google Scholar] [CrossRef]
- Labunsky, V.M.; Lee, B.C.; Handy, D.E.; Loscalzo, J.; Hatfield, D.L.; Gladyshev, V.N. Both maximal expression of selenoproteins and selenoprotein deficiency can promote the development of type 2 diabetes-like phenotype in mice. Antioxid. Redox Signal. 2011, 14, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraishy, S.; Dkhil, M.A.; Moneim, A.E.A. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int. J. Nanomed. 2015, 10, 6741–6756. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, D.Y.; Hussein, R.H.; El-Kholy, W.M. Mesenchymal Stem Cell-Derived Exosomes Loaded with Selenium or Nano Selenium as a Novel Therapeutic Paradigm for Streptozotocin-Induced Type 1 Diabetes in Rats. Biology 2024, 13, 253. https://doi.org/10.3390/biology13040253
Khalil DY, Hussein RH, El-Kholy WM. Mesenchymal Stem Cell-Derived Exosomes Loaded with Selenium or Nano Selenium as a Novel Therapeutic Paradigm for Streptozotocin-Induced Type 1 Diabetes in Rats. Biology. 2024; 13(4):253. https://doi.org/10.3390/biology13040253
Chicago/Turabian StyleKhalil, Dlovan Y., Ridah H. Hussein, and Wafaa M. El-Kholy. 2024. "Mesenchymal Stem Cell-Derived Exosomes Loaded with Selenium or Nano Selenium as a Novel Therapeutic Paradigm for Streptozotocin-Induced Type 1 Diabetes in Rats" Biology 13, no. 4: 253. https://doi.org/10.3390/biology13040253
APA StyleKhalil, D. Y., Hussein, R. H., & El-Kholy, W. M. (2024). Mesenchymal Stem Cell-Derived Exosomes Loaded with Selenium or Nano Selenium as a Novel Therapeutic Paradigm for Streptozotocin-Induced Type 1 Diabetes in Rats. Biology, 13(4), 253. https://doi.org/10.3390/biology13040253





