Exposure to Gold Induces Autoantibodies against Nuclear Antigens in A.TL Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Gold (Aurothiomalate) Treatment
2.3. Blood and Tissue Sampling
2.4. Analysis of Antinuclear Antibodies (ANA) by Indirect Immunofluorescence
2.5. Serum Anti-dsDNA Antibodies Assessed by Indirect Immunofluorescence
2.6. Serum Anti-Chromatin Antibodies Assessed by Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Serum Anti-RNP Antibodies Assessed by Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Serum Anti-Histone Antibodies Assessed by Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Analysis of Cell Surface Markers of Splenocytes by Flow Cytometry
2.10. Glomerular Immunocomplex Deposits
2.11. Statistics and Software
3. Results
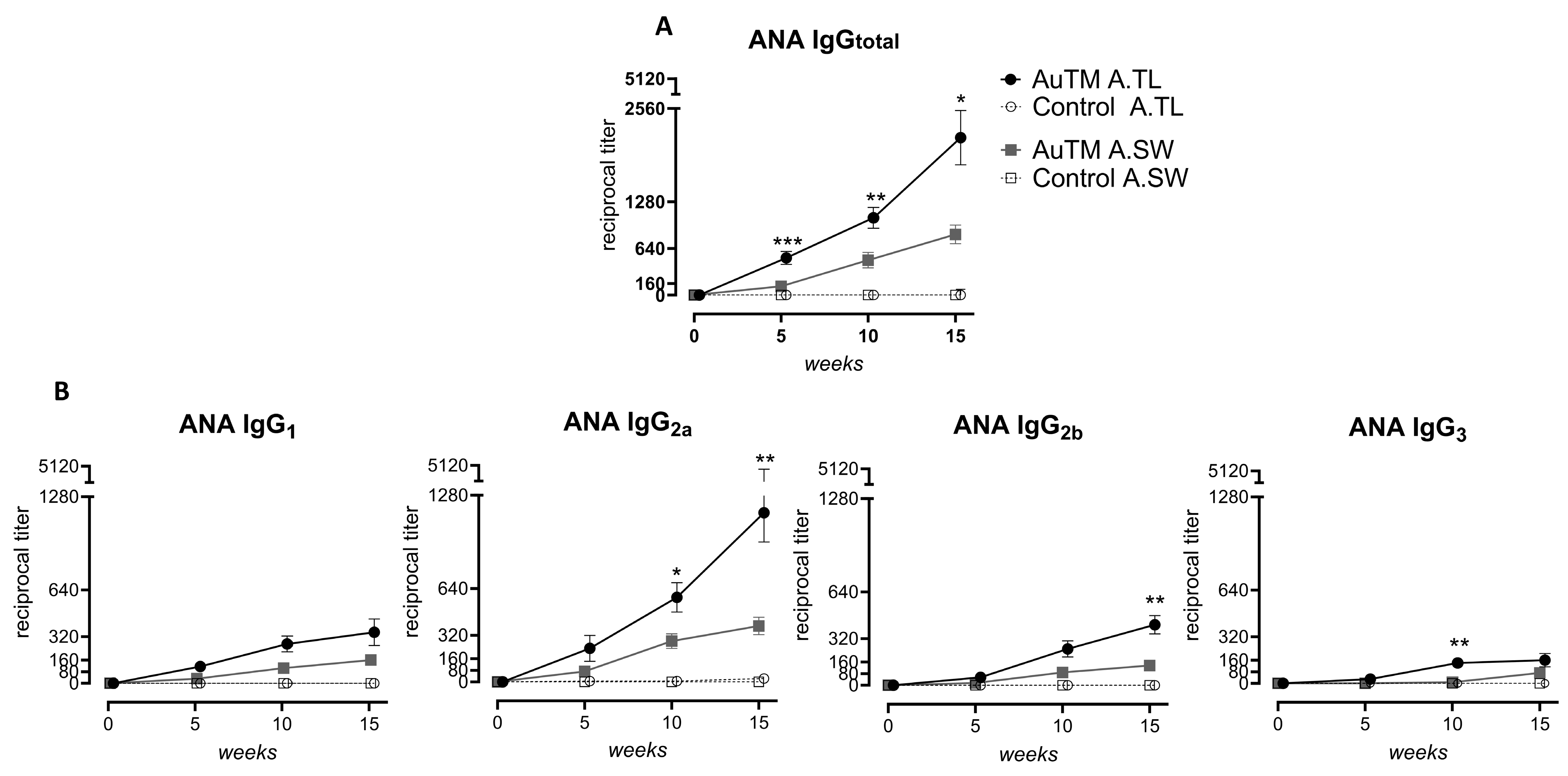
3.1. Serum Antinuclear Antibodies (ANA/ANoA) Pattern, Titer, and IC Deposits
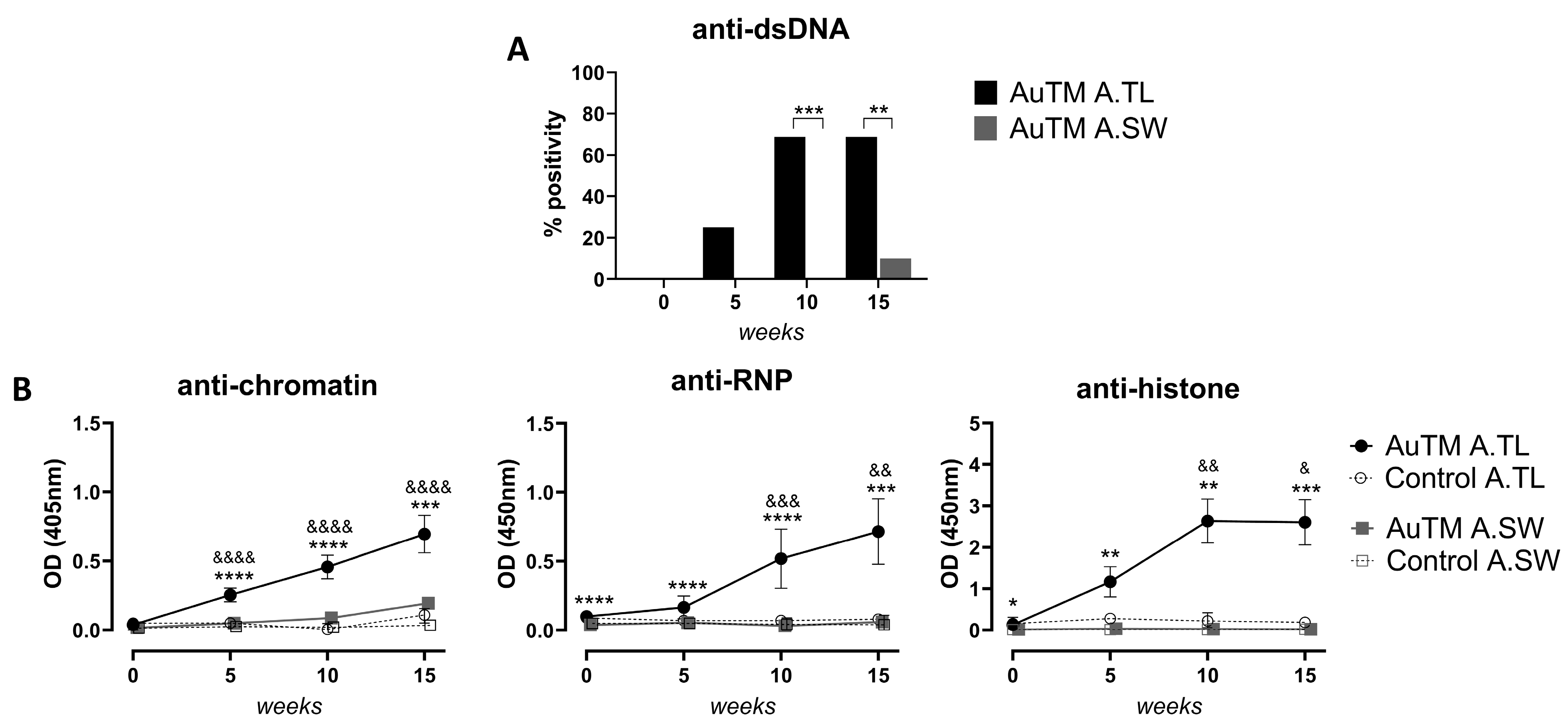
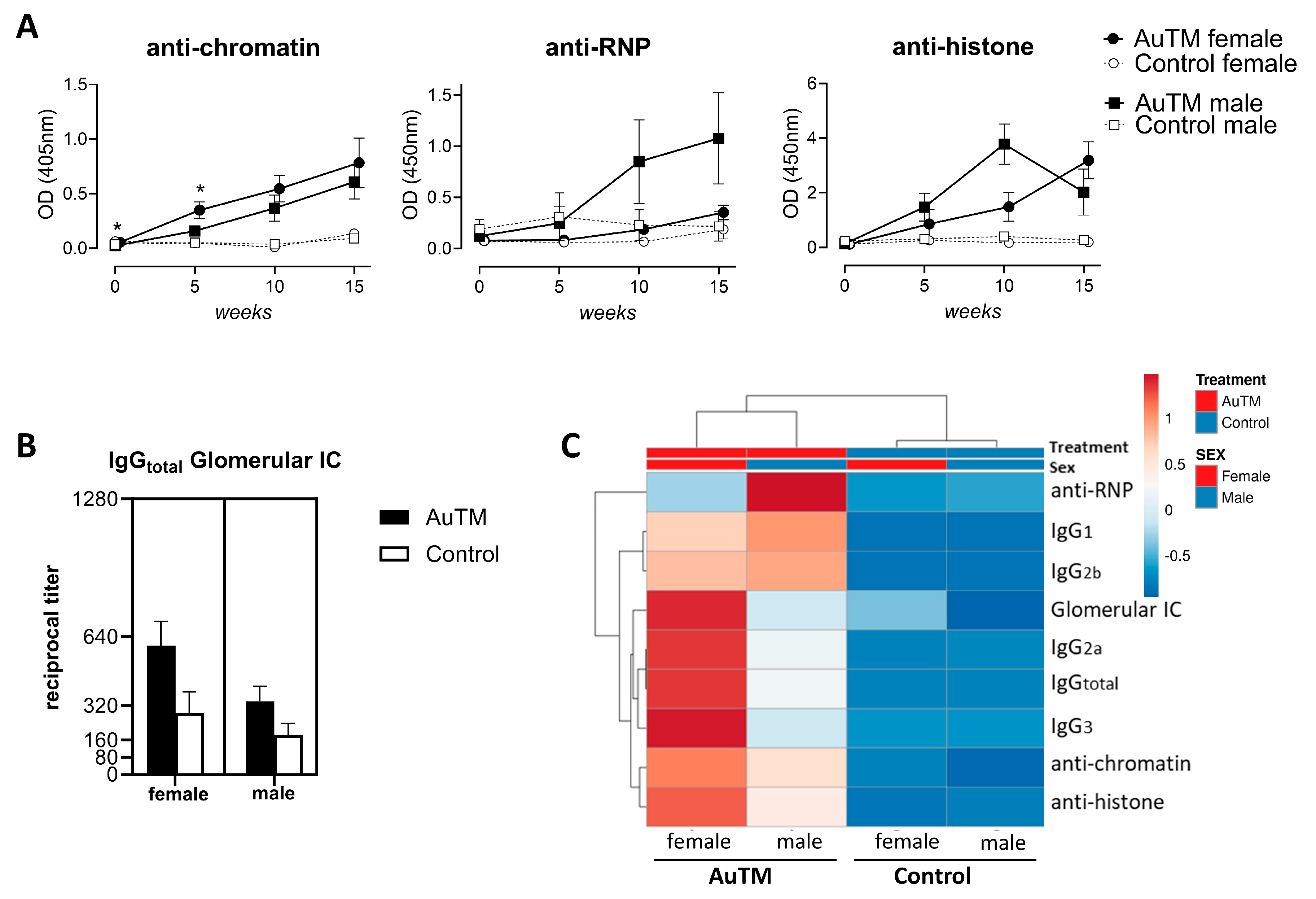
3.2. Serum Autoantibodies against dsDNA, Chromatin, Histones, and RNP
3.3. T- and B Cell Markers in Spleen Following AuTM Treatment
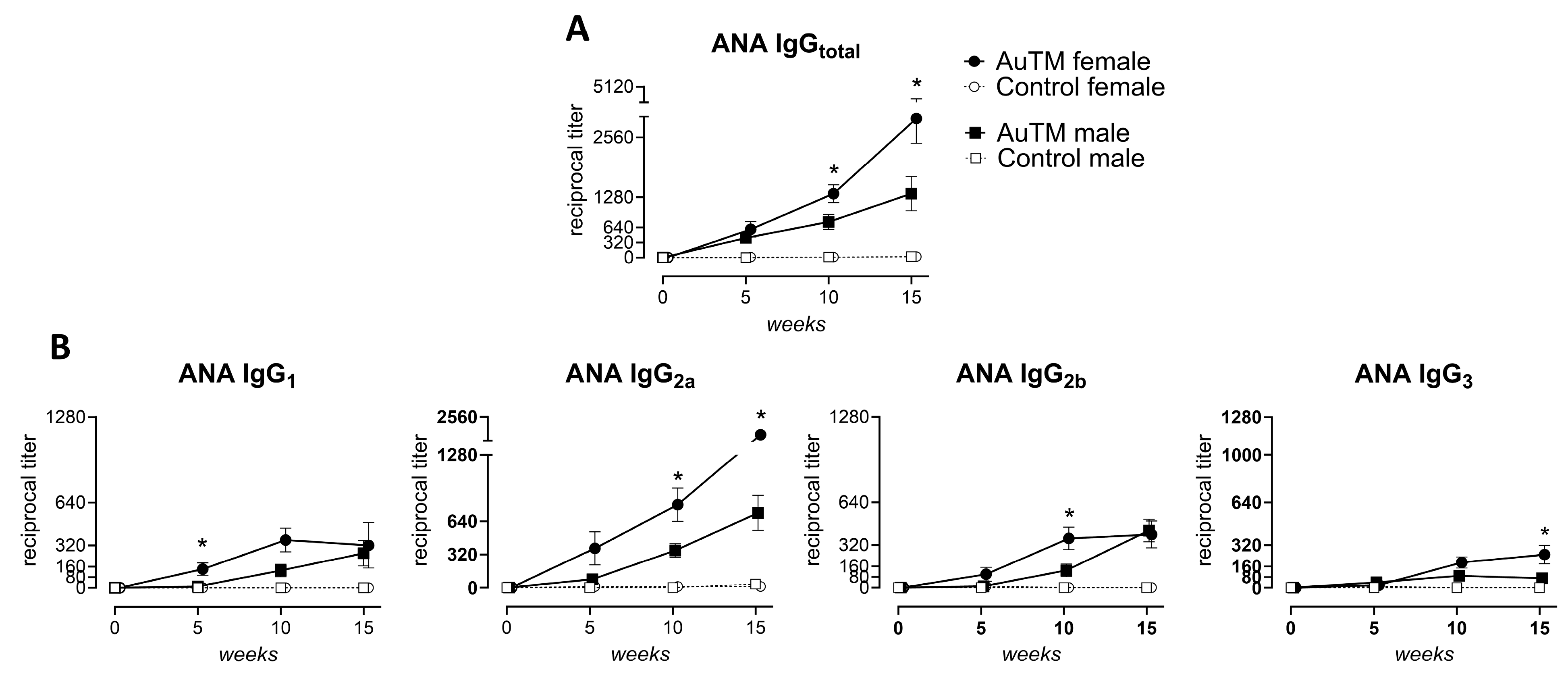
3.4. Differences in the Immune Response of A.TL Mice by Gender
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenblum, M.D.; Remedios, K.A.; Abbas, A.K. Mechanisms of human autoimmunity. J. Clin. Investig. 2015, 125, 2228–2233. [Google Scholar] [CrossRef] [PubMed]
- Cuthrell, K.M.; Tzenios, N.; Umber, J. Burden of Autoimmune Disorders; A review. Asian J. Immunol. 2022, 6, 1–13. [Google Scholar]
- Miller, F.W. The increasing prevalence of autoimmunity and autoimmune diseases: An urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr. Opin. Immunol. 2023, 80, 102266. [Google Scholar] [CrossRef]
- Xiao, Z.X.; Miller, J.S.; Zheng, S.G. An updated advance of autoantibodies in autoimmune diseases. Autoimmun. Rev. 2021, 20, 102743. [Google Scholar] [CrossRef]
- Radaelli, E.; Santagostino, S.F.; Sellers, R.S.; Brayton, C.F. Immune Relevant and Immune Deficient Mice: Options and Opportunities in Translational Research. ILAR J. 2018, 59, 211–246. [Google Scholar] [CrossRef]
- Rubin, R.L. Drug-induced lupus. Expert. Opin. Drug Saf. 2015, 14, 361–378. [Google Scholar] [CrossRef]
- Freitas, E.; de Oliveira, M.S.; Monticielo, O.A. Pristane-induced lupus: Considerations on this experimental model. Clin. Rheumatol. 2017, 36, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Nyland, J.F.; Silva, I.A.; Ventura, A.M.; de Souza, J.M.; Silbergeld, E.K. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: A cross-sectional study. Environ. Res. 2010, 110, 345–354. [Google Scholar] [CrossRef]
- Germolec, D.; Kono, D.H.; Pfau, J.C.; Pollard, K.M. Animal models used to examine the role of the environment in the development of autoimmune disease: Findings from an NIEHS Expert Panel Workshop. J. Autoimmun. 2012, 39, 285–293. [Google Scholar] [CrossRef]
- Pollard, K.M.; Cauvi, D.M.; Toomey, C.B.; Hultman, P.; Kono, D.H. Mercury-induced inflammation and autoimmunity. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129299. [Google Scholar] [CrossRef]
- Hansson, M.; Abedi-Valugerdi, M. Xenobiotic metal-induced autoimmunity: Mercury and silver differentially induce antinucleolar autoantibody production in susceptible H-2s, H-2q and H-2f mice. Clin. Exp. Immunol. 2003, 131, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Johansson, U.; Hansson-Georgiadis, H.; Hultman, P. Murine silver-induced autoimmunity: Silver shares induction of antinucleolar antibodies with mercury, but causes less activation of the immune system. Int. Arch. Allergy Immunol. 1997, 113, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Hultman, P.; Enestrom, S.; Turley, S.J.; Pollard, K.M. Selective induction of anti-fibrillarin autoantibodies by silver nitrate in mice. Clin. Exp. Immunol. 1994, 96, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Havarinasab, S.; Johansson, U.; Pollard, K.M.; Hultman, P. Gold causes genetically determined autoimmune and immunostimulatory responses in mice. Clin. Exp. Immunol. 2007, 150, 179–188. [Google Scholar] [CrossRef]
- Havarinasab, S.; Pollard, K.M.; Hultman, P. Gold- and silver-induced murine autoimmunity—Requirement for cytokines and CD28 in murine heavy metal-induced autoimmunity. Clin. Exp. Immunol. 2009, 155, 567–576. [Google Scholar] [CrossRef]
- Mahler, M.; Kim, G.; Roup, F.; Bentow, C.; Fabien, N.; Goncalves, D.; Palterer, B.; Fritzler, M.J.; Villalta, D. Evaluation of a novel particle-based multi-analyte technology for the detection of anti-fibrillarin antibodies. Immunol. Res. 2021, 69, 239–248. [Google Scholar] [CrossRef]
- Pollard, K.M.; Hultman, P. Fibrillarin autoantibodies. In Autoantibodies; Elsevier: Amsterdam, The Netherlands, 2014; pp. 319–325. [Google Scholar]
- Arefieva, A.; Krasilshchikova, M.; Zatsepina, O. Immune complex deposits as a characteristic feature of mercury-induced SLE-like autoimmune process in inbred and outbred mice. In Autoimmune Diseases—Contributing Factors, Specific Cases of Autoimmune Diseases, and Stem Cell and other Therapies; InTech: Rijeka, Croatia, 2012; pp. 119–150. [Google Scholar]
- Hultman, P.; Bell, L.J.; Enestrom, S.; Pollard, K.M. Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic, and intra-H-2 recombinant strains. Clin. Immunol. Immunopathol. 1992, 65, 98–109. [Google Scholar] [CrossRef]
- Alkaissi, H.; Havarinasab, S.; Nielsen, J.B.; Soderkvist, P.; Hultman, P. Bank1 and NF-kappaB as key regulators in anti-nucleolar antibody development. PLoS ONE 2018, 13, e0199979. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Wongsasuluk, P.; Tun, A.Z.; Chotpantarat, S.; Siriwong, W. Related health risk assessment of exposure to arsenic and some heavy metals in gold mines in Banmauk Township, Myanmar. Sci. Rep. 2021, 11, 22843. [Google Scholar] [CrossRef]
- Vasconcellos, A.C.S.D.; Hallwass, G.; Bezerra, J.G.; Aciole, A.N.S.; Meneses, H.N.D.M.; Lima, M.D.O.; Jesus, I.M.D.; Hacon, S.D.S.; Basta, P.C. Health Risk Assessment of Mercury Exposure from Fish Consumption in Munduruku Indigenous Communities in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2021, 18, 7940. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Dong, S.Y. Mercury Contamination in Fish and Its Effects on the Health of Pregnant Women and Their Fetuses, and Guidance for Fish Consumption—A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 15929. [Google Scholar] [CrossRef] [PubMed]
- Skvortsova, N.N.; Akhmadullina, N.S.; Vafin, I.Y.; Obraztsova, E.A.; Hrytseniuk, Y.S.; Nikandrova, A.A.; ALukianov, D.; Gayanova, T.E.; Voronova, E.V.; Shishilov, O.N.; et al. The Synthesis and Analysis of the Cytotoxicity of Al2O3-Supported Silver Nanoparticles Prepared by the Plasma Chemical Process Initiated by Pulsed MW Radiation in the Al2O3-Ag Powder Mixtures. Int. J. Mol. Sci. 2024, 25, 5326. [Google Scholar] [CrossRef]
- Marassi, V.; Di Cristo, L.; Smith, S.G.; Ortelli, S.; Blosi, M.; Costa, A.L.; Reschiglian, P.; Volkov, Y.; Prina-Mello, A. Silver nanoparticles as a medical device in healthcare settings: A five-step approach for candidate screening of coating agents. Roy Soc. Open Sci. 2018, 5, 171113. [Google Scholar] [CrossRef]
- Massai, L.; Zoppi, C.; Cirri, D.; Pratesi, A.; Messori, L. Reactions of Medicinal Gold(III) Compounds With Proteins and Peptides Explored by Electrospray Ionization Mass Spectrometry and Complementary Biophysical Methods. Front. Chem. 2020, 8, 581648. [Google Scholar] [CrossRef]
- Balfourier, A.; Kolosnjaj-Tabi, J.; Luciani, N.; Carn, F.; Gazeau, F. Gold-based therapy: From past to present. Proc. Natl. Acad. Sci. USA 2020, 117, 22639–22648. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Griem, P.; Goebel, C.; Gonzalez, J.; Gleichmann, E. The Antirheumatic Drug Gold, a Coin With Two Faces: AU(I) and AU(III). Desired and Undesired Effects on the Immune System. Met. Based Drugs 1994, 1, 483–496. [Google Scholar] [CrossRef]
- Adachi, J.D.; Bensen, W.G.; Singal, D.P.; Powers, P.J. Gold induced thrombocytopenia: Platelet associated IgG and HLA typing in three patients. J. Rheumatol. 1984, 11, 355–357. [Google Scholar] [PubMed]
- Bendix, G.; Bjelle, A. Adding low-dose cyclosporin A to parenteral gold therapy in rheumatoid arthritis: A double-blind placebo-controlled study. Br. J. Rheumatol. 1996, 35, 1142–1149. [Google Scholar] [CrossRef][Green Version]
- Fernando, M.M.; Stevens, C.R.; Walsh, E.C.; De Jager, P.L.; Goyette, P.; Plenge, R.M.; Vyse, T.J.; Rioux, J.D. Defining the role of the MHC in autoimmunity: A review and pooled analysis. PLoS Genet. 2008, 4, e1000024. [Google Scholar] [CrossRef]
- Hakala, M.; Vanassendelft, A.H.W.; Ilonen, J.; Jalava, S.; Tiilikainen, A. Association of Different Hla Antigens with Various Toxic Effects of Gold Salts in Rheumatoid-Arthritis. Ann. Rheum. Dis. 1986, 45, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, G.; Lu, H.; Li, H.; Tang, M.; Tong, A. Development of therapeutic antibodies for the treatment of diseases. Mol. Biomed. 2022, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Natural History of the Major Histocompatibility Complex; Wiley: Hoboken, NJ, USA, 1986. [Google Scholar]
- Sontheimer, R.D.; Gilliam, J.N. An immunofluorescence assay for double-stranded DNA antibodies using the Crithidia luciliae kinetoplast as a double-stranded DNA substrate. J. Lab. Clin. Med. 1978, 91, 550–558. [Google Scholar] [PubMed]
- Burlingame, R.W.; Rubin, R.L. Subnucleosome Structures as Substrates in Enzyme-Linked Immunosorbent Assays. J. Immunol. Methods 1990, 134, 187–199. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.; Andrade, L.E.C.; Carballo, O.G.; Conrad, K.; Francescantonio, P.L.C.; Fritzler, M.J.; de la Torre, I.G.; Herold, M.; Klotz, W.; de Melo Cruvinel, W.; et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: The International Consensus on ANA patterns (ICAP) perspective. Ann. Rheum. Dis. 2019, 78, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.J.G.; Balazs, T.; Egorov, I.K. Mercuric Chloride-Induced, Gold Sodium Thiomalate-Induced, and D-Penicillamine-Induced Antinuclear Antibodies in Mice. Toxicol. Appl. Pharm. 1986, 86, 159–169. [Google Scholar] [CrossRef]
- Johansson, U.; Hansson-Georgiadis, H.; Hultman, P. The genotype determines the B cell response in mercury-treated mice. Int. Arch. Allergy Immunol. 1998, 116, 295–305. [Google Scholar] [CrossRef]
- Mirtcheva, J.; Pfeiffer, C.; De Bruijn, J.A.; Jacquesmart, F.; Gleichmann, E. Immunological alterations inducible by mercury compounds. III. H-2A acts as an immune response and H-2E as an immune “suppression” locus for HgCl2-induced antinucleolar autoantibodies. Eur. J. Immunol. 1989, 19, 2257–2261. [Google Scholar] [CrossRef]
- Hultman, P.; Enestrom, S.; Pollard, K.M.; Tan, E.M. Anti-fibrillarin autoantibodies in mercury-treated mice. Clin. Exp. Immunol. 1989, 78, 470–477. [Google Scholar]
- Hultman, P.; Johansson, U.; Dagnaes-Hansen, F. Murine mercury-induced autoimmunity: The role of T-helper cells. J. Autoimmun. 1995, 8, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Hultman, P.; Ganowiak, K.; Turley, S.J.; Pollard, K.M. Genetic susceptibility to silver-induced anti-fibrillarin autoantibodies in mice. Clin. Immunol. Immunopathol. 1995, 77, 291–297. [Google Scholar] [CrossRef] [PubMed]
- LeMaoult, J.; Manavalan, J.S.; Dyall, R.; Szabo, P.; Nikolic-Zugic, J.; Weksler, M.E. Cellular basis of B cell clonal populations in old mice. J. Immunol. 1999, 162, 6384–6391. [Google Scholar] [CrossRef] [PubMed]
- Ghiggeri, G.M.; D’alessandro, M.; Bartolomeo, D.; Degl’innocenti, M.L.; Magnasco, A.; Lugani, F.; Prunotto, M.; Bruschi, M. An Update on Antibodies to Necleosome Components as Biomarkers of Sistemic Lupus Erythematosus and of Lupus Flares. Int. J. Mol. Sci. 2019, 20, 5799. [Google Scholar] [CrossRef] [PubMed]
- Kosałka-Węgiel, J.; Dziedzic, R.; Siwiec-Koźlik, A.; Spałkowska, M.; Milewski, M.; Żuk-Kuwik, J.; Zaręba, L.; Bazan-Socha, S.; Korkosz, M. Clinical and laboratory characteristics of early-onset and delayed-onset lupus nephritis patients: A single-center retrospective study. Rheumatol. Int. 2024, 44, 1283–1294. [Google Scholar] [CrossRef]
- Lundgren, M.C.; Sapkota, S.; Peterson, D.J.; Crosson, J.T. The antinuclear antibody dense fine speckled pattern and possible clinical associations: An indication of a proinflammatory microenvironment. J. Immunol. Methods 2021, 488, 112904. [Google Scholar] [CrossRef]
- Yu, H.-H.; Hsieh, P.-F.; Huang, S.-W.; Chan, T.-M.; Tai, P.-L.; Yang, S.-T.; Yu, K.-H. Discriminating between Homogeneous (AC-1) and Dense Fine Speckled (AC-2) Antinuclear Antibody Patterns: Re-Evaluation of Immunofluorescence Imaging. Biomedicines 2023, 11, 3027. [Google Scholar] [CrossRef]
- Amirhosseini, M.; Alkaissi, H.; Hultman, P.A.; Havarinasab, S. Autoantibodies in outbred Swiss Webster mice following exposure to gold and mercury. Toxicol. Appl. Pharmacol. 2021, 412, 115379. [Google Scholar] [CrossRef]
- Dixon, F.J.; Andrews, B.S.; Eisenberg, R.A.; McConahey, P.J.; Theofilopoulos, A.N.; Wilson, C.B. Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis Rheum. 1978, 21 (Suppl. S5), S64–S67. [Google Scholar] [CrossRef]
- Bloom, D.D.; St Clair, E.W.; Pisetsky, D.S.; Clarke, S.H. The anti-La response of a single MRL/Mp-lpr/lpr mouse: Specificity for DNA and VH gene usage. Eur. J. Immunol. 1994, 24, 1332–1338. [Google Scholar] [CrossRef]
- Gulinello, M.; Putterman, C. The MRL/lpr Mouse Strain as a Model for Neuropsychiatric Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2011, 2011, 207504. [Google Scholar] [CrossRef] [PubMed]
- Laderach, D.; Koutouzov, S.; Bach, J.F.; Yamamoto, A.M. Concomitant early appearance of anti-ribonucleoprotein and anti-nucleosome antibodies in lupus prone mice. J. Autoimmun. 2003, 20, 161–170. [Google Scholar] [CrossRef]
- Blossom, S.; Gilbert, K.M. Antibody production in autoimmune BXSB mice. I. CD40L-expressing B cells need fewer signals for polyclonal antibody synthesis. Clin. Exp. Immunol. 1999, 118, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Brick, J.E.; Ong, S.H.; Bathon, J.M.; Walker, S.E.; O’Sullivan, F.X.; DiBartolomeo, A.G. Anti-histone antibodies in the serum of autoimmune MRL and NZB/NZW1 F1 mice. Clin. Immunol. Immunopathol. 1990, 54, 372–381. [Google Scholar] [CrossRef]
- Richard, M.L.; Gilkeson, G. Mouse models of lupus: What they tell us and what they don’t. Lupus Sci. Med. 2018, 5, e000199. [Google Scholar] [CrossRef]
- Gabriela, T.; Yessia, H.; Maria Rosa, B.; Mario, R. A Spontaneous Mouse Model of Lupus: Physiology and Therapy. In Lupus—New Advances and Challenges; Sophia, L., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 3. [Google Scholar]
- Burlingame, R.W.; Rubin, R.L.; Balderas, R.S.; Theofilopoulos, A.N. Genesis and evolution of antichromatin autoantibodies in murine lupus implicates T-dependent immunization with self antigen. J. Clin. Investig. 1993, 91, 1687–1696. [Google Scholar] [CrossRef]
- Okuma, K.; Oku, T.; Sasaki, C.; Kitagori, K.; Mimori, T.; Aramori, I.; Hirayama, Y.; Yoshifuji, H. Similarity and difference between systemic lupus erythematosus and NZB/W F1 mice by multi-omics analysis. Mod. Rheumatol. 2024, 34, 359–368. [Google Scholar] [CrossRef]
- Roubinian, J.R.; Papoian, R.; Talal, N. Androgenic Hormones Modulate Autoantibody Responses and Improve Survival in Murine Lupus. J. Clin. Investig. 1977, 59, 1066–1070. [Google Scholar] [CrossRef]
- Steinberg, A.D.; Melez, K.A.; Raveche, E.S.; Reeves, J.P.; Boegel, W.A.; Smathers, P.A.; Taurog, J.D.; Weinlein, L.; Duvic, M. Approach to the study of the role of sex hormones in autoimmunity. Arthritis Rheum. 1979, 22, 1170–1176. [Google Scholar] [CrossRef]
- Angum, F.; Khan, T.; Kaler, J.; Siddiqui, L.; Hussain, A. The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus 2020, 12, e8094. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed]
- Didier, K.; Bolko, L.; Giusti, D.; Toquet, S.; Robbins, A.; Antonicelli, F.; Servettaz, A. Autoantibodies Associated with Connective Tissue Diseases: What Meaning for Clinicians? Front. Immunol. 2018, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Benito-Garcia, E.; Schur, P.H.; Lahita, R.; American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: Anti-Sm and anti-RNP antibody tests. Arthritis Rheum. 2004, 51, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Habets, W.J.; Hoet, M.H.; De Jong, B.A.; Van der Kemp, A.; Van Venrooij, W.J. Mapping of B cell epitopes on small nuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally-induced mouse monoclonal antibodies. J. Immunol. 1989, 143, 2560–2566. [Google Scholar] [CrossRef]
- Orme, M.E.; Voreck, A.; Aksouh, R.; Ramsey-Goldman, R.; Schreurs, M.W.J. Systematic review of anti-dsDNA testing for systemic lupus erythematosus: A meta-analysis of the diagnostic test specificity of an anti-dsDNA fluorescence enzyme immunoassay. Autoimmun. Rev. 2021, 20, 102943. [Google Scholar] [CrossRef]
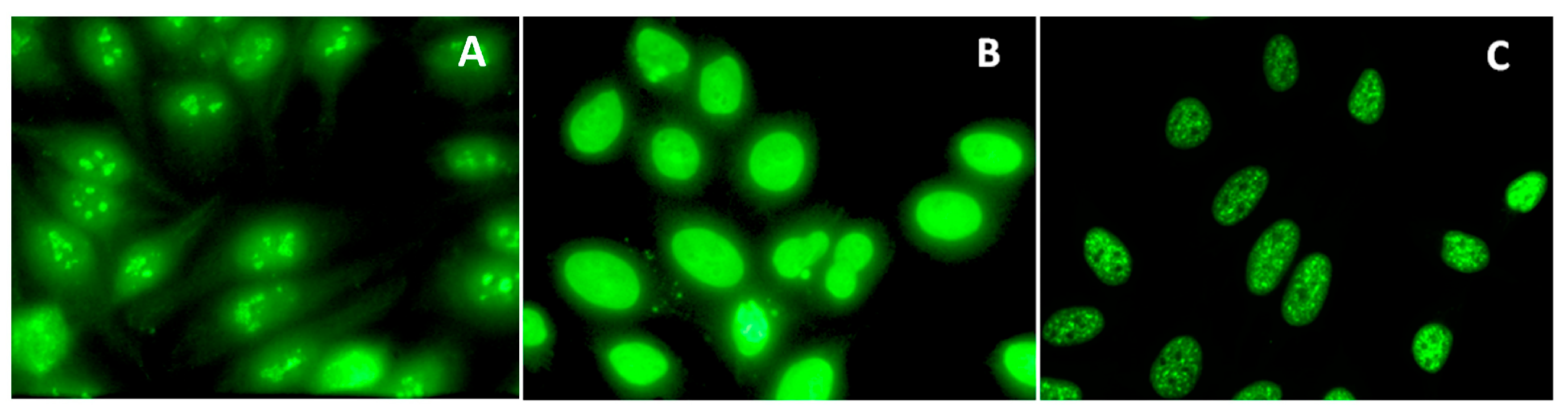
| Strain | H-2 Haplotype | H-2 Loci | Gender | AuTM | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Ab | Aa | Eβ | Ea | S | D | L | n | n | |||
| A.TL | t1 | s | k | k | k | k | k | d | d | Male | 8 | 7 |
| Female | 8 | 7 | ||||||||||
| A.SW | s | s | s | s | (s | s) a | s | s | s | Male | 5 | 5 |
| Female | 5 | 5 | ||||||||||
| Strain | Treatment | Treatment Time (Weeks) | Fraction of IgGtotal (Pos./Total No) | IgG Pattern (No) | * ANA Titer IgGtotal | Fraction of Subclasses (Pos./Total No) | Fraction of dsDNA (Pos./Total No) | * IgGtotal Titer Glomeruli | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | ||||||||
| A.TL | H2O (n = 14) | 0 | 0 | - | 0 | ND | ND | ND | ND | 0 | ND |
| 5 | 1/14 | FSp (1) | 80 ± 0 | 0 | 1/14 | 0 | 0 | 0 | ND | ||
| 10 | 2/14 | FSp (2) | 80 ± 0 | 1/14 | 2/14 | 0 | 0 | 0 | ND | ||
| 15 | 4/14 | FSp (4) | 80 ± 0 | 0 | 4/14 | 0 | 0 | 0 | 234 ± 56 | ||
| AuTM (n = 16) | 0 | 0 | - | 0 | ND | ND | ND | ND | 0/16 | ND | |
| 5 | 15/16 d | H (9), FSp (6) | 544 ± 88 d | 6/16 a | 8/16 b | 4/16 | 6/16 a | 4/16 | ND | ||
| 10 | 16/16 d | H (13), FSp (3) | 1060 ± 142 d | 12/16 d | 15/16 d | 13/16 d | 11/16 d | 11/16 d | ND | ||
| 15 | 16/16 d | H (12), FSp (4) | 2160 ± 372 d | 8/16 b | 16/16 d | 14/16 d | 10/16 c | 11/16 d | 470 ±72 b | ||
| A.SW | H2O (n = 9) | 0 | 0 | - | 0 | ND | ND | ND | ND | 0 | ND |
| 5 | 0 | - | 0 | ND | ND | ND | ND | 0 | ND | ||
| 10 | 0 | - | 0 | ND | ND | ND | ND | 0 | ND | ||
| 15 | 0 | - | 0 | ND | ND | ND | ND | 0 | 80 ± 37 | ||
| AuTM (n = 10) | 0 | 0 | - | 0 | ND | ND | ND | ND | 0 | ND | |
| 5 | 6/10 a | cp (6) | 200 ± 40 c | 3/10 | 5/10 a | 2/10 | 0 | 0 | ND | ||
| 10 | 10/10 d | cp (10) | 480 ± 160 d | 7/10 b | 10/10 d | 8/10 c | 1/10 | 0 | ND | ||
| 15 | 10/10 d | cp (10) | 832 ± 128 d | 9/10 c | 10/10 d | 8/10 c | 8/10 c | 1/10 | 160 ± 86 | ||
| Strain | Treatment | No. of Splenocytes | CD19+ | CD3+ | CD4+, CD25+ | CD3+, CD90.2+ | CD3+, CD49b+, c-IFN-γ+ |
|---|---|---|---|---|---|---|---|
| A.TL | H2O (n = 4) | 12.08 ± 2.18 | 5.18 ± 0.92 | 2.55 ± 0.44 | 0.11 ± 0.02 | 2.05 ± 0.34 | 0.01 ± 0.00 |
| AuTM (n = 5) | 14.52 ± 2.10 | 9.75 ± 0.52 a | 2.9 ± 0.42 | 0.10 ± 0.2 | 1.99 ± 0.18 | 0.03 ± 0.02 | |
| A.SW | H2O (n = 5) | 10.64 ± 0.47 | 4.47 ± 0.26 | 4.92 ± 0.12 | 0.97 ± 0.05 | 0.74 ± 0.12 | 0.15 ± 0.1 |
| AuTM (n = 5) | 17.52 ± 0.74 b | 8.14 ± 0.48 b | 7.24 ± 0.83 a | 0.51 ± 0.12 | 1.42 ± 0.19 a | 0.56 ± 0.4 b |
| A.TL Gender | Treatment Time (Weeks) | Fraction of IgGtotal (Pos./Total No) | IgG Pattern (No) | * ANA Titer IgGtotal | Fraction of Subclasses (Pos./Total No) | Fraction of dsDNA (Pos./Total No) | * IgGtotal Titer Glomeruli | |||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | |||||||
| Male (n = 8) | 0 | 0/8 | - | 0 | ND | ND | ND | ND | 0/8 | ND |
| 5 | 8/8 c | H (6), FSp (2) | 420 ± 67 c | 1/8 | 3/8 | 1/8 | 4/8 | 1/8 | ND | |
| 10 | 8/8 c | H (6), FSp (2) | 760 ± 160 c | 5/8 a | 8/8 c | 5/8 a | 4/8 | 5/8 a | ND | |
| 15 | 8/8 c | H (7), FSp (1) | 1360 ± 366 c | 5/8 a | 8/8 c | 8/8 c | 3/8 | 5/8 a | 340 ± 70 | |
| Female (n = 8) | 0 | 0/8 | - | 0 | ND | ND | ND | ND | 0/8 | ND |
| 5 | 7/8 b | H (3), FSp (4) | 685 ± 162 c | 5/8 a | 5/8 a | 3/8 | 2/8 | 3/8 | ND | |
| 10 | 8/8 c | H (7), FSp (1) | 1360 ± 188 c | 7/8 b | 7/8 b | 8/8 c | 7/8 b | 6/8 b | ND | |
| 15 | 8/8 c | H (5), FSp (3) | 2960 ± 526 c | 3/8 | 8/8 c | 6/8 b | 7/8 b | 6/8 b | 600 ± 112 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puente-Marin, S.; Havarinasab, S. Exposure to Gold Induces Autoantibodies against Nuclear Antigens in A.TL Mice. Biology 2024, 13, 812. https://doi.org/10.3390/biology13100812
Puente-Marin S, Havarinasab S. Exposure to Gold Induces Autoantibodies against Nuclear Antigens in A.TL Mice. Biology. 2024; 13(10):812. https://doi.org/10.3390/biology13100812
Chicago/Turabian StylePuente-Marin, Sara, and Said Havarinasab. 2024. "Exposure to Gold Induces Autoantibodies against Nuclear Antigens in A.TL Mice" Biology 13, no. 10: 812. https://doi.org/10.3390/biology13100812
APA StylePuente-Marin, S., & Havarinasab, S. (2024). Exposure to Gold Induces Autoantibodies against Nuclear Antigens in A.TL Mice. Biology, 13(10), 812. https://doi.org/10.3390/biology13100812






