Simple Summary
Traumatic brain injury is a leading cause of death and disability worldwide, according to the Centers for Disease Control and Prevention. It results in various long-term consequences, including brain atrophy, nerve damage, and significant economic costs. Traumatic brain injury is most common among working-age adults, highlighting the urgent need for improved diagnostic and treatment methods. Animal models serve as a valuable tool for studying the mechanisms of traumatic brain injury and developing new treatments. These models allow researchers to control injury parameters and analyze post-traumatic effects, advancing our understanding of the condition.
Abstract
According to the Centers for Disease Control and Prevention (CDC), the national public health agency of the United States, traumatic brain injury is among the leading causes of mortality and disability worldwide. The consequences of TBI include diffuse brain atrophy, local post-traumatic atrophy, arachnoiditis, pachymeningitis, meningocerebral cicatrices, cranial nerve lesions, and cranial defects. In 2019, the economic cost of injuries in the USA alone was USD 4.2 trillion, which included USD 327 billion for medical care, USD 69 billion for work loss, and USD 3.8 trillion for the value of statistical life and quality of life losses. More than half of this cost (USD 2.4 trillion) was among working-age adults (25–64 years old). Currently, the development of new diagnostic approaches and the improvement of treatment techniques require further experimental studies focused on modeling TBI of varying severity.
1. Introduction
In modern society, injury is one of the topical problems, both medical and social, related to its abundance among young able-bodied people, high mortality rate, severity of disability, and significant economic costs for the treatment and rehabilitation of patients [1,2]. Traumatic brain injury (TBI) is a leading traumatic injury that is characterized by a mortality rate of 35–38% and a disability rate of about 50% [3,4]. Globally, annual TBI statistics amount to 1.5 million deaths and 2.4 million disabilities. In the United States, the annual incidence rate of TBI is approximately 1.6 million, including 51,000 deaths and 124,000 long-term disabilities [1]. According to the Centers for Disease Control and Prevention (CDC), the national public health agency of the United States, traumatic brain injury is among the leading causes of mortality and disability worldwide [5,6]. The causes of TBI include social, demographic, geographic, climatic, and other factors. For example, the leading causes are road traffic accidents in the United States, motor scooter injuries in Taiwan, and falls from height in Scotland [7,8]. Population-based epidemiological studies conducted in various countries at the end of the 20th century played a significant role in the investigation of TBI causes and prevalence. The rate of TBI per 1000 people varies significantly: 7.3% in China, 5.3% in the USA, 9% in Russia, and 1.1% in Scotland. Injuries in males are 2- to 3-fold higher than those in females, with this trend being similar in all age groups, except infants and the elderly. TBI is most common in males between 20 and 39 years of age. Mild TBI predominates in clinical presentations (80–90%) [9].
TBI can have a variety of sequelae that include diffuse brain atrophy, local post-traumatic atrophy, arachnoiditis, pachymeningitis, meningocerebral cicatrices, cranial nerve lesions, and cranial defects [10].
The development of new approaches to the diagnosis and treatment of TBI patients has used primarily animal models that have a number of advantages: a limited range of injury mechanisms (animal models enable control of impact conditions, which provides a relatively uniform injury mechanism), genetic and demographic homogeneity (animal models usually have a genetically homogeneous population, which reduces variability in results due to genetic differences), and controllable injury parameters (injury severity can be predetermined, which enables studies using models that replicate different levels of TBI severity). Animal research is considered a key element in the advancement of biomedical science. Although its use is controversial and raises ethical challenges, the contribution of animal models in medicine is essential for understanding the physiopathology and novel treatment alternatives for several animal and human diseases [11]. The described advantages of biological test systems make them a valuable tool for studying TBI mechanisms and consequences and for evaluating new treatments [12,13].
2. Materials and Methods
2.1. Search Criteria
To conduct this review, we carried out a comprehensive literature search in the PubMed database to identify relevant publications.
Inclusion Criteria: We applied several inclusion criteria to select studies. Only full-text articles in English that described TBI models in animals, particularly mice, were considered.
Exclusion Criteria: The exclusion criteria included articles published in languages other than English, general reviews, articles with insufficient data, articles without full-text access, and studies involving other types of injuries without a detailed investigation of TBI.
All identified articles were analyzed and screened. The evaluated articles were published between May 2014 and May 2024. A total of 4948 articles were identified using the search criteria. Subsequently, 3967 articles were excluded due to a lack of relevant data (such as full-text access, publication date within the last 10 years, use of species other than mice, classical articles, clinical studies, clinical trials, meta-analyses, reviews, or systematic reviews). The remaining 133 articles were further assessed for eligibility and selected for inclusion in the study. A flowchart of the search strategy is shown in Figure 1.
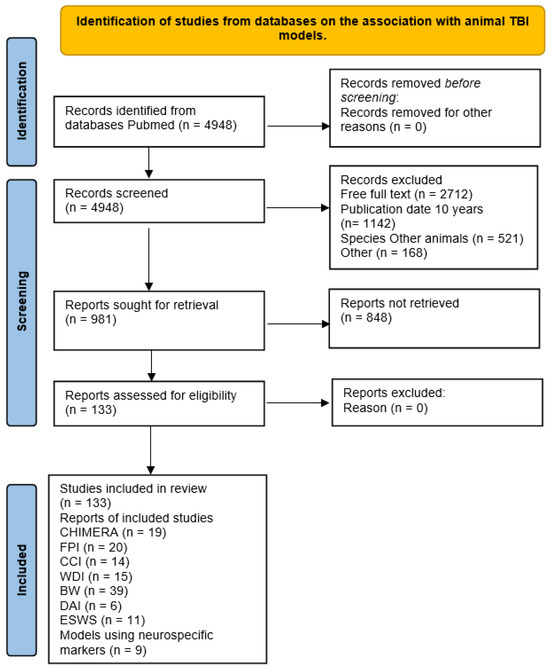
Figure 1.
PRISMA flow diagram for the review.
2.2. Inclusion/Exclusion Criteria
Abstracts and titles were selected exclusively from peer-reviewed primary research reports specific to animal models of traumatic brain injury (TBI) (FPI, CCI, WDI, DAI, BW, CHIMERA, and ESW models). All other articles were excluded.
2.3. Retrieval of Information from Full-Text Articles
To systematically retrieve data from the selected articles, we developed a structured data collection form that was implemented using the Google Forms platform. This form collected general study identifiers, such as the title, first and last authors, date of publication, and name and description of the TBI model used.
Specific information related to TBI induction techniques included identification of the injury device, anesthesia use, surgery indicator, device type, head fixation method, animal stabilization method, geometric characteristics of the impact tip (size, shape, and material), impact location and surface, weight drop parameters (weight, height), tube composition, and additional damping materials.
3. Results
The sample of 133 articles was divided into three main categories of injury models. The largest group was the blast wave impact model (n = 39), followed by the fluid percussion injury (FPI) model (n = 20), and then all “other” models (n = 74). The sample included the following models: the FPI model (n = 20), the controlled cortical impact (CCI) model (n = 14), the weight drop injury (WDI) model (n = 15), the diffuse axonal injury (DAI) model (n = 6), the blast wave (BW) model (n = 39), the CHIMERA model (n = 19), the explosive shock wave (ESW) model (n = 11), and models that use neurospecific markers indicating blood–brain barrier disruption (n = 9).
3.1. TBI Pathogenesis
The mechanism of (TBI) is a complex process that results from a combination of primary and secondary impact mechanisms and leads to temporary or permanent impairment of neurological function [14]. Primary injury is directly related to direct physical impact to the brain [15,16,17]. Secondary changes can occur within minutes or days after the primary impact and include molecular, chemical, and inflammatory responses associated with the progression of brain injury. These responses involve neuronal depolarization followed by the release of excitatory neurotransmitters, such as glutamate and aspartate, which induce an intracellular calcium surge. An elevated intracellular calcium level activates various enzymes, such as caspases and calpain, and free radicals, which leads to cell destruction through apoptosis. This process of neuronal degradation is accompanied by an inflammatory response that increases neuronal damage and disruption of the blood–brain barrier (BBB), leading to cerebral edema [13,17,18,19,20,21,22]. The secondary damage phase is followed by a recovery period that includes anatomical, molecular, and functional reorganization [23,24,25,26,27,28,29].
The Monro–Kellie doctrine has been a well-established principle of intracranial hemodynamics for over 200 years. Its fundamental concept is simple: the combined volume of brain tissue, blood, and cerebrospinal fluid (CSF) within the skull remains constant [30]. The intracranial space is filled with the brain (83%), cerebrospinal fluid (11%), and blood (6%). The relationship between these components provides a homeostatic intracranial environment. However, an increase in the volume of any of the intracranial space components activates a cascade of compensatory mechanisms. An increase in the volume of intracranial space contents often results from massive blood influx and impact, causing cytotoxic and vasogenic edema as well as venous stasis. The brain is incompressible, so edematous brain tissue initially leads to the redistribution of some cerebrospinal fluid into the spinal canal. Over time, blood, especially that of venous origin, is also removed from the brain due to compensatory mechanisms. In the absence of timely intervention, even the maximum compensatory mechanisms fail, which leads to pathological brain compression and death.
3.2. Basic Requirements for an Experimental Model
Currently, experimental TBI models require the induction of injuries similar to those seen in humans. Regardless of the physiological, behavioral, or anatomical parameters used to assess the response to injury, it is important that the results are reproducible, quantifiable, and generated under controlled injury severity. No one model can accurately replicate all the features of mechanisms of human TBI. However, this review discusses several preclinical models for a proper description of the underlying pathology.
Any experimental TBI model should satisfy certain criteria: conformity of age, sex, body weight, and genetic characteristics of used animals, housing conditions, and circadian rhythms to the human’s pathology as well as no differences in these parameters between the control and study groups. This definitely helps to get more precise results with high correlation regarding people’s disease. It is also important to clearly define the physical parameters, including the exact location and specific degree of injury. The central nervous system response to injury should be measurable, quantifiable, and replicable across different laboratories. The degree of injury should be consistent with the mechanical force applied to the head or brain and characterized by a specific dependence.
Modern TBI models include standardized experimental protocols and surgical techniques. An important aspect is control measurements in special sham-operated animals that undergo the same surgical procedures as animals in the experimental group, except the injury, e.g., access to the brain for injury equipment, anesthesia, maintenance of body and brain temperature, introduction of intracranial probes and cannulas, etc. [31,32]. The mechanical characteristics underlying the degree of injury are evaluated using computer measurements of the applied load that, depending on the model, may include pressure gradients of a fluid acting on the brain, the impactor velocity, and the energy of forces causing head acceleration or deceleration. This enables the tuning of injury devices and maintaining a narrow range of changes in injury severity within a single experiment.
3.3. TBI Models
The perfect modeling of all aspects of TBI within a single test system has proven impossible, so the animals used in TBI models are selected with allowance for specific features. For example, pigs provide a valuable biological TBI model because their physiology and size are similar to those of humans, which enables important physiological monitoring. In addition, the pig brain has a complex cellular structure close to that of the human brain, in contrast to that of small rodents [31,32].
Although large animals may more closely replicate the mechanical aspects of human TBI, and some models, such as primates, may better reflect the neurophysiological processes and functional impairments seen in humans, rodent models remain the most common ones in TBI research [33]. The advantages of rodent models include their relatively low cost, small size, reduced impact on regulatory systems, availability of genetically modified lines, and low variability of measurements. In addition, the relatively short lifespan of 2 to 3 years in most mouse and rat strains offers a significant advantage for aging research, a topic that will be discussed in more detail below [13].
To date, several different TBI models have been developed and used experimentally (Table 1), each of which has its own advantages and disadvantages [13,23,34,35,36,37,38,39,40,41,42,43,44].

Table 1.
Methods for inducing TBI in animal models.
3.4. Fluid Percussion Brain Injury (FPI) Model
The fluid percussion injury model can induce diffuse (central FPI) and mixed (focal and diffuse; lateral FPI) pathological changes that are frequently seen in humans after TBI [59,60,61]. This is the most commonly used and studied rodent TBI model. FPI has been successfully used in various test systems, including rabbits, cats, rats, mice, and pigs [37,39,40,44]. Injury (after scalp incision and trepanation) results from the rapid administration of a bolus of fluid to the intact dura mater, followed by the concentric spreading of the fluid into the epidural space, which causes a diffuse effect on the brain [36]. In this model, the injury can be medial (the burr hole is made in the midline) or lateral. Fluid pressure can be adjusted to simulate mild, moderate, and severe TBI [36,62,63]. This model replicates both local traumatic changes (injured cerebral cortex areas with petechial, intraparenchymal, and subarachnoid hemorrhages) and traumatic changes distant to the primary source (in the hippocampus, thalamus optica, and others), which are caused by secondary brain damages [64,65]. The fluid percussion TBI model enables assessing motor and cognitive impairments caused by injury and their changes during treatment [45,47,66].
The disadvantage of this method is that the use of this model, especially the central FPI model, is associated with highly severe injuries and mortality due to uncontrolled brainstem injury and neurogenic pulmonary edema. In terms of biomechanics, this model is most distant from human TBI, and researchers suggest that axonal damage in distant structures, such as the hippocampus and thalamus, results from secondary neurochemical reactions [13,46,48]. This model lacks standards characterizing injury parameters, such as differences in peak pressure and impact duration, which significantly affects the reproducibility and comparability of research results. In the FPI model, responses to injury can vary significantly even for the same impact parameters, which complicates data interpretation. The craniectomy procedure required in the FPI model may lead to complications, such as infection and hemorrhage [67,68]. These limitations should be considered upon interpretation of the results of research based on the FPI model and their extrapolation to clinical practice.
3.5. Controlled Cortical Injury Model
This model uses the impact of a solid impactor on the intact dura mater of the brain [50,69]. In this case, the animal’s head is usually fixed. The impactor is driven by a pneumatic [70,71,72] or electromagnetic [73] device that enables controlling the time, rate, and depth of impact to the brain [74,75]. The use of low mechanical energy enables the simulation of concussion, but this model is mainly used to study focal injuries, in particular TBI, accompanied by epidural and/or subdural hematomas [76]. Like the fluid percussion model, this model also allows studying traumatic changes in distant, injury-sensitive brain areas, such as the hippocampus, dentate gyrus, and optic tubercle, and assessing movement disorders, changes in motor coordination, and cognitive deficits [77,78,79].
This and previous methods cause severe brain injury, neuroinflammation, and behavioral impairments, including cognitive impairments. However, they have similar disadvantages, such as the rapid spontaneous recovery of brain function (within 2 weeks) and technical difficulties in modeling injuries [49,51,80].
3.6. Weight Drop Injury (WDI) Model of TBI
There are two ways to parametrically modulate the intensity of impact injury: changing the weight and initial height of the object [81]. The animal’s head is fixed, but researchers have not ensured this in most studies. After incising the scalp and exposing the surface of the skull, the injury is produced under general anesthesia. The severity of TBI is varied by adjusting the weight and height of the load [53,54,58]. In 1994, A. Marmarou proposed focal trauma that is simulated using a brass weight dropped from a predetermined height through a plexiglass tube. The weight of the object varies from 20 to 200 g, and the drop height can reach 2 m. Skull fracture is prevented by resting the rat’s head on a foam pad instead of a hard plastic disk. A metal helmet protects the exposed skull and serves as a target for the weight [57]. The short time required to prepare the animal for injury and lack of need for a surgical skull hole make this model simple and convenient [56,62].
The disadvantage is the high rate of skull fractures upon modeling severe TBI [58]. Like other rodent TBI methods, this model reveals a variety of brain injuries, ranging from mild brain injuries emulating concussions to focal contusions (where impact is applied to the skull) that are accompanied by long-term neuronal loss. Impairment of motor and cognitive functions was also observed in this model [13,46,48,55,62,66].
To overcome the risk of skull fractures that might not occur in human TBI, the model was modified [58,82]. After incising the scalp and exposing the rat’s skull, a protective “helmet”—a round steel plate of 1 cm in diameter—is firmly fixed to the bone (using dental cement). The injury is induced by dropping a weight with a blunt surface onto the “helmet” from a height, which ensures head acceleration upon minimal local impact at the point of application of the traumatic force. The animal’s head is not fixed. The “helmet” ensures the wide spread of the traumatic force across the skull surface. As in the previous method, the severity of injury is regulated by the load weight and drop height. This model is characterized mainly by diffuse brain injury, local injury of the cortex adjacent to the injured skull area, and cell death in the injury-sensitive brain area (hippocampus) [83,84].
3.7. Diffuse Axonal Injury (DAI) Model of TBI
The modeling of TBI in animals uses ether anesthesia and fixation of the limbs in the supine position [85]. The surgical level of anesthesia is assessed based on the lack of a corneal reflex on the right side of the animal’s skull. The hair on the parietal region is cut off, and the site is treated with an aseptic solution. A longitudinal skin incision is made, and the skull bones are trepanned at a distance of 2 mm from the midline using a cranial bone bur, while keeping the dura mater intact. The weight is a 114.6 g steel cylinder that is dropped from a height of 20 cm along a guide polyethylene tube, providing maximum impact to the trepanation window area in the right parietal region of the brain, with an impact force of 0.224 N. After injury, the animal’s wound is sutured with surgical thread (0.2 mm); the sutures are treated with an antiseptic, and a gentamicin solution is administered intramuscularly [75,86,87,88].
Modeling TBI in animals using ether anesthesia promotes the development of persistent neurological deficit, with the animal remaining alive; spontaneous recovery of brain function after 4–6 weeks; and control and evaluation of the therapy efficacy, in particular cell therapy, to restore brain function after TBI. A technical result using ether anesthesia is achieved by the following: influencing the cortical motor areas in the fronto-parieto-temporal region and preventing rupture of the dura mater and deep damage to brain tissue (2 mm), provided that the impact energy does not exceed 0.224 N. It should be taken into account that injuries caused by an impact energy of more than 0.224 N can lead to the death of the animal or result in severe focal lesions that lead to neuronal destruction, glial scar formation, and persistent neurological deficit. Conversely, injuries caused by an impact energy of less than 0.224 N almost do not induce severe motor neurological deficits and can sometimes cause mild hemiparesis/monoparesis that resolves within 5 to 7 days [89].
3.8. Blast Wave (BW) Model of TBI
Blast-related traumatic brain injuries are one of the most common injuries sustained by soldiers and veterans serving on military installations, which, in turn, has necessitated the development of a mechanistically relevant TBI model [90,91,92,93]. Over the years, various injury techniques have been developed, e.g., shock tubes [94,95,96], open space explosions [23,38,97], and blast tubes to simulate blast-induced injuries [42,98] in humans. Of these, the most widely popular laboratory models [23,38,42,99] are those where blast waves (shock wave plus gust of wind) are generated through the detonation of an explosive charge [13,49,51,52,80]. Currently, there are no standardized parameters for shock tubes (e.g., type of gas or explosive, tube design), test system type and location, protection of the body or head mobility, and peak overpressure or its duration. These factors can significantly affect the nature of injury, emphasizing the need for a critical approach to studies that use gaseous media (e.g., air, nitrogen, helium). Differences in the implementation of blast research regulations partly explain the variability in threshold values and pathological changes reported by laboratories from different countries. Given the recent development of various models and the lack of clinical and neuropathological descriptions of blast-induced TBI, these models are perhaps the most diverse experimental TBI models.
When blast tubes are used in an animal TBI model, head immobilization with a metallic holder is necessary to minimize the inertial forces acting on the skull [41]. The shock tube model, which is based on the compression–expansion mechanism of chambers separated by a membrane, can generate high-speed shock waves with controlled peak pressure due to the rupture of a membrane of a certain thickness [100]. The closed-head shock tube model replicates the brain injury mechanism through rapid angular acceleration, which determines the magnitude of the mechanical load based on the spatial location of the animal in the device [98,101,102,103]. This model is used in experiments on various animal species (rodents, pigs, primates), leading to focal and diffuse neuronal injury as well as edema and ischemia typical of TBI [42,97].
Shock wave tubes can vary significantly in size, from centimeters to tens of meters in length. A membrane, e.g., a mylar membrane, separates the driver and driven section of the tube. Compressed air or other gas fills the driver section to a pressure sufficient to rupture the membrane. This produces a shock wave that propagates through the driven section. Experimental samples (animals or materials) are located inside or outside the driven section at a different distance to manage the environment conditions and severity of damage (Table 2).

Table 2.
Methods for modeling blast wave impact on animals.
Disadvantages of the shock tube include the differences in the brain size, geometry, and white/gray matter ratio, which complicates the comparison of animal models with humans. The mass effect induced by injury differs between large human brains and smaller rodent brains, which requires scaling of injuries to get a similar effect. The shock tube method used for modeling head injuries is not standardized, which leads to variability in results and may cause additional injuries, such as hypoxia and blood vessel damage [66,104,105,106].
The pathophysiological features of brain injury induced by low-intensity blast exposure, given the abundance and nature of TBI in combat settings, require an urgent need to study its underlying pathological mechanisms. In 2018, H. Song et al. proposed a model of low-intensity blast (LIB) brain injury that replicates the mechanical impact to the body with parameters of 46.6 kPa and a maximum impulse of 8.7 psi (or 60 kPa/ms), which is significantly different from a high-intensity blast (>100 kPa) [114,115]. Despite the absence of lethal or gross macroscopic damages, this model leads to measurable impairments in neurobehavioral functions, decreased mitochondrial fission–fusion activity, bioenergetic deficiency, increased oxidative stress, reduced mitophagy, increased compensatory respiration, and axonal myelin sheath degradation [108]. Importantly, damage to myelinated axons is most pronounced in the subacute period (7 days after injury), in contrast to the chronic period (30 days after injury). A single LIB exposure causes an increase in total tau, phosphorylated tau, and amyloid-β [116].
The disadvantage of the blast injury method is associated with a deep understanding of blast physics. A model that is aimed at replicating the multifaceted effects of blast injury needs to accurately define the parameters typical of combat settings. The lack of precise specification reduces practical value and leads to the risk of misleading conclusions. The widespread use of disparate experimental conditions in different models complicates the comparison and generalization of the research results [108,109,110,117].
Although each injury model has its own characteristics, none of the models can entirely replicate the full range of pathologies related to human TBI. Of the three common experimental animal TBI models, the fluid percussion, controlled cortical injury, and weight drop/acceleration impact ones are the most important. The fluid percussion device produces injury by a brief pulse of fluid pressure to the dura mater through a craniectomy. Unlike the fluid percussion model, controlled cortical injury causes mechanical damage to the dura mater through a directed forceful blow by using pneumatic pressure. The weight drop/weight impact model involves dropping a rod of a specific mass onto a closed skull. The TBI models listed above require the use of anesthetics at the time of injury for ethical reasons. Several anesthetics, including isofluorane and ketamine, are known to have neuroprotective effects, improving functional and histological outcomes in TBI models when present at the time of injury [69,118,119]. This limits clinical application because patients have no anesthetic agents at the time of injury.
3.9. Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA) Model
TBI associated with physical activity and traffic accidents is usually caused by the sudden acceleration of the head, which causes abnormal changes in cerebral tissue that are different from the effects of direct localized impact to the head [120,121]. This type of injury affects key white matter regions whose damage is thought to result from the shear forces typical of rotational acceleration. These injuries induce inflammatory processes that can persist long after the initial traumatic event.
Given the abundance of TBI, a novel model has been developed to rapidly and non-invasively induce injury by closed-head impact using engineered rotational acceleration (CHIMERA). Recent studies using the CHIMERA model have revealed not only the underlying pathological post-traumatic changes but also concomitant subtle changes in the accumulation of misfolded proteins, receptor expression, and neuronal death [120,122,123]. CHIMERA differs from existing neurotrauma simulation systems in that it uses a completely non-surgical procedure to precisely impact a closed skull with defined dynamic characteristics of traumatic impact, which enables kinematic analysis of unrestricted head movements [124,125].
Researchers have achieved significant progress in applications of the model by focusing on what is needed for its testing. However, most CHIMERA studies have used only adult male mice [126,127]. The development of this model requires further work with female animals of different age groups as well as research aimed at generating and standardizing additional models for methodological studies of clinical relevance.
Experimental rodent TBI models have limited validity because they do not fully account for the biomechanical and physiological features of human injury. In particular, rodent brains are largely lissencephalic (no gyri), while human brains are gyrencephalic (with gyri). These differences in anatomy may significantly influence the dynamic characteristics of intracranial brain movement in TBI, leading to varying degrees of deformity and injury [32].
In non-military settings, a significant number of head injuries are reported in motor vehicle accidents, which do not involve a direct impact to the head but result from the rotational forces of the collision. These traumas lead to diffuse axonal injury to the brain [128,129]. Currently, there are no adequate rodent models to replicate this type of injury. The studies on pigs were the only models to come close to imitating the diffuse axonal brain injury associated with rotational forces [109,130,131,132,133].
The use of large animals for TBI research has several limitations. First, large animals require significant financial expenses on both acquisition and maintenance, as well as specialized housing. Second, the long lifespan of large animals implies increased requirements for care both before and after injury. However, the use of large animals in TBI research has unique advantages. Large animal models more accurately replicate the cortical injury mechanisms typical of clinical settings and provide the opportunity to comprehensively investigate neurorecovery after TBI [134,135].
3.10. Extracorporeal Shock Wave (ESW) Model
The integration of an advanced non-invasive approach, which involves an extracorporeal shock wave (ESW) and microbubbles, enables the induction of local and temporary TBI in discrete areas [136]. Additionally, ESW can induce thermal injury during shock wave treatment, but the conversion depth at higher frequencies is limited [137]. Shock waves initiate cavitation [138,139,140], which is considered to be the main mechanism involved in the local TBI during modeling [141,142], and the imitation of this mechanism does not require craniectomy [143]. Suitable shock wave devices are commercially available, which eliminates the need for developing complex ESW devices for modeling TBI [144]. To minimize the impact of the human factor, LabVIEW 2022is used, ensuring a coordinate accuracy of 0.1 mm (2.5 mm ± 0.1 mm) at various distances from the scalp. The structural arrangement of four positioning lasers on the outer perimeter of the device optimizes the positioning mechanism and improves accuracy [143,144].
A concave ESW probe is combined with gel buffer to ensure the localization of the shock wave focus at 5 mm from the proximal surface of the gel buffer. The probe combined with the gel buffer is positioned on the dorsal parietal surface of the rat’s head, providing the focus at 5 mm below the parietal surface and 3 mm caudal, lateral, or medial to the bregma suture of the rat’s skull (Table 3). Acoustic gel is applied to the interface between the probe and the gel buffer, and between the bottom of the gel pad and the rat’s scalp [93,144,145].

Table 3.
Main parameters of shock wave intensity levels for TBI modeling.
The ESW model is a non-interventional preclinical platform for modeling focal TBI, which is characterized by high reproducibility, rapidity, easy to use, and replication of injuries histologically identified as traumatic contusion and intracranial hemorrhage. Parameters and focusing adjustments help vary the intensity and location of injury, providing highly predictable results.
3.11. Blood Biomarkers in Animal TBI Models
Animal models provide the most uniform and reproducible method for studying TBI because experimental TBI models are free of confounding factors, such as age, medications, comorbidities, and polytrauma. Thus, changes in serum or plasma levels of biochemical markers can only be explained by injury if appropriate sham controls are used [66,146].
There are five neuroglial proteins used as biomarkers of tissue damage in the nervous system: neurofilament heavy polypeptide (NF-H), the glial fibrillary acidic protein (GFAP), ubiquitin carboxy-terminal hydrolase L1 (UCHL1), neuron-specific enolase (NSE), the myelin basic protein (MBP), the tau protein, and the S100β protein. These markers are elevated in the acute phase of severe head injury and are informative for prognosis [147,148,149,150,151].
In a porcine TBI model, changes in serum neurofilament heavy chain (NF-H) protein concentrations predicted injury severity and outcome. The study examined the time profiles of four serum protein biomarkers: S100β, NSE, the myelin basic protein (MBP), and NF-H over two weeks after injury. However, only a relative NF-H concentration correlated with the outcome. Compared with animals with favorable and unfavorable outcomes, serum NF-H concentrations peaked 6 h after injury, returned to baseline levels after three days, and remained at this level until the end of the two-week observation period [152].
Recent studies have attempted to extrapolate the correlation between plasma NF-H activity in an experimental TBI model, focusing on the hyperactive axonal form of NF-H (pNF-H). However, the obtained data did not reveal significant differences in the NF-H concentration in the adult population outside the acute injury period [153].
Experimental models of blast-induced TBI in rats and mice have demonstrated a correlation between the dynamics of serum biomarker concentrations and changes in metabolism, cell adhesion, extracellular matrix, proliferation, neuronal and glial damage, axonal degeneration, and inflammation [97]. Several specific biomarkers demonstrated complex and dynamically changing temporal patterns within a 30-day period after injury. These studies, along with others, have highlighted the importance of longitudinal biofluid profiling for monitoring the dynamics of biofluid biomarker concentrations following a single traumatic exposure. Therefore, single biomarker measurements may be misleading. Without serial sampling, key pathobiological processes, indicators of disease progression, and optimal therapeutic intervals may be missed [147,148,149,150].
4. Future Prospects for TBI Modeling
4.1. Modeling TBI in Aged Laboratory Animals
Historically, a disproportionate share of TBI-related injuries occurs in older adults. The hospitalization and death rates of people over 75 years of age exceed those of any other age group. However, despite the high prevalence of TBI in older adults, preclinical studies are predominantly conducted in relatively young animals. Obviously, age is a modifier of the outcome after TBI, and the development of aged animal models to study TBI is a promising area for further research [154,155].
In their article “Models of Traumatic Brain Injury in Aged Animals: A Clinical Perspective” (2019), Iboaya et al. provide the following relevant conclusions and results of a review of studies in laboratory mice and rats [156]:
- The mean lifespan of rats and mice in studies ranged from 24 to 30 months. Thus, extrapolating human age as a fraction of the total lifespan, a 20-month-old mouse or rat is equivalent to a 50–60-year-old human. The use of animals in the “old” age will allow covering the heterogeneity of the geriatric population, which is a specialized branch of gerontology devoted to the examination, prevention, and treatment of diseases of the elderly population.
- Modeling TBI should replicate the special mechanical properties typical of injuries in older adults. This should include studies that emulate concussion and mild to moderate TBI, which are the most common forms of clinical injury.
- Modeling TBI in combination with comorbidities frequently seen in older adults (e.g., hypertension, diabetes, cardiovascular disease). These comorbidities may be present in inbred and transgenic animals or induced in the laboratory settings (e.g., obesity, inactivity). Modeling of multiple comorbidities should also be considered.
- Modeling TBI in combination with medications commonly prescribed to older adults (e.g., preinjury antiplatelet therapy).
- Development of more accurate age-specific measures of functional outcomes in aged animals.
- Response to TBI should be assessed from acute to chronic post-TBI period.
- Use of biomarkers as outcome indicators in aged animals: measurements to confirm cerebrovascular reactivity and brain metabolism (magnetic resonance spectroscopy).
- Inclusion of both sexes in aged animal studies of TBI (rather than predominant use of males).
- Larger animal models of TBI should also be considered, including animals with gyrencephalic brains, such as sheep, pigs, and primates.
4.2. The Problem of Modeling TBI in Laboratory Animals
Modeling diffuse TBI in animals is limited by the induction of pathophysiological processes seen in humans due to the differences in size and anatomy between species. To extrapolate the model results to diffuse axonal brain injury in humans, injury parameters should be scaled depending on the animal size to replicate the mechanical impact characteristic of human TBI. The fact is that the larger mass of the human brain sustains more severe deformation and damage during acceleration than the smaller animal brain under the same acceleration/deceleration forces. For example, a scaling factor of up to 500% and 630% should be used for the baboon (140 g) and pig (90 g) brain, respectively, to model severe acceleration-induced diffuse axonal injury of the human brain. This calls into question the validity of head rotational acceleration models in small animals, in particular rodents. In rats (brain mass < 2 g), unattainable inertial forces (~8000% acceleration) are required to induce equivalent injury. However, there are small animal models of axonal injury that demonstrate clinically and morphologically relevant changes.
Animal TBI models have limitations, in particular the use of anesthesia which complicates the interpretation of traumatic pathology. In addition, certain human clinical manifestations following TBI, such as prolonged coma and post-traumatic depression, are not typically reproduced in rodent TBI models. However, despite these limitations, rodent TBI models remain valuable tools for research and development of new TBI treatments.
5. Conclusions
TBI causes significant morbidity and mortality. Animal TBI models provide a biological substrate for studying pathophysiology and consequences. They can be used to control biomechanical parameters and perform post-traumatic analysis. Animal TBI models are also valuable for studying disease-modifying therapeutics. The lack of specific treatment for TBI highlights the need for improved models that are as similar as possible in the course of pathophysiological processes [87]. The most recent models, e.g., CHIMERA, meet this need. The combination of TBI biomarker studies and animal TBI models will provide an important basis for the development of new diagnostic and treatment methods. The generalized rodent TBI models in this systematic review indicate tests that can be used in future studies to correlate pathology and behavioral changes in experimental animals.
Author Contributions
Conceptualization, E.B. and V.P.; methodology, K.Y. and V.N.; investigation, T.F.; resources, A.I.; data curation, E.B. and T.F.; writing—original draft preparation, K.Y. and A.I.; writing—review and editing, T.B. and A.K.; supervision, V.P.; project administration, A.K. and V.P.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the Ministry of Science and Higher Education of the Russian Federation within the framework of state support for the creation and development of World-Class Research Centers ‘Digital Biodesign and Personalized Healthcare’ No. 075-15-2022-305.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Parfenov, V.A. [E.I. Gusev, A.N. Konovalova, V.I. Skvortsova “Neurology and neurosurgery”. Textbook, 4th Ed]. Zhurnal Nevrol. I Psikhiatrii Im. SS Korsakova 2015, 115, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.M.; Titus, D.J.; Wilson, N.M.; Freund, J.E.; Atkins, C.M. Early Life Stress Exacerbates Outcome after Traumatic Brain Injury. J. Neurotrauma 2021, 38, 555–565. [Google Scholar] [CrossRef]
- Cognitive Outcome 1 Year after Mild Traumatic Brain Injury: Results from the TRACK-TBI Study-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35173018/ (accessed on 25 July 2024).
- Klinicheskoe Rukovodstvo Po Cherepno-Mozgovoĭ Travme-NLM Catalog-NCBI. Available online: https://www.ncbi.nlm.nih.gov/nlmcatalog/100893725 (accessed on 23 July 2024).
- Peterson, A.B. Deaths from Fall-Related Traumatic Brain Injury—United States, 2008–2017. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 225–230. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Greig, N.H.; Tweedie, D.; Jung, Y.J.; Chiang, Y.-H.; Hoffer, B.J.; Miller, J.P.; Chang, K.-H.; Wang, J.-Y. The P53 Inactivators Pifithrin-μ and Pifithrin-α Mitigate TBI-Induced Neuronal Damage through Regulation of Oxidative Stress, Neuroinflammation, Autophagy and Mitophagy. Exp. Neurol. 2020, 324, 113135. [Google Scholar] [CrossRef]
- Covington, N.V.; Duff, M.C. Heterogeneity Is a Hallmark of Traumatic Brain Injury, Not a Limitation: A New Perspective on Study Design in Rehabilitation Research. Am. J. Speech Lang. Pathol. 2021, 30, 974–985. [Google Scholar] [CrossRef]
- Likhterman, L.B. Classification of Traumatic Brain Injury• Part II. Modern Principles of Classification of CCT. Zhurnal Sud. Meditsiny 2015, 1, 37–48. [Google Scholar]
- Silver, J.M.; McAllister, T.W.; Arciniegas, D.B. Textbook of Traumatic Brain Injury; American Psychiatric Pub: Washington, DC, USA, 2018; ISBN 978-1-61537-247-8. [Google Scholar]
- Lihterman, L.B.; Potapov, A.A.; Klevno, V.A.; Kravchuk, A.D.; Ohlopkov, V.A. Aftereffects of Head Injury. Russ. J. Forensic Med. 2016, 2, 4–20. [Google Scholar] [CrossRef]
- Domínguez-Oliva, A.; Hernández-Ávalos, I.; Martínez-Burnes, J.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Mota-Rojas, D. The Importance of Animal Models in Biomedical Research: Current Insights and Applications. Animals 2023, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.J.; Fesharaki-Zadeh, A.; Takahashi, H.; Nies, S.H.; Smith, L.M.; Luo, A.; Chyung, A.; Chiasseu, M.; Strittmatter, S.M. Fyn Kinase Inhibition Reduces Protein Aggregation, Increases Synapse Density and Improves Memory in Transgenic and Traumatic Tauopathy. Acta Neuropathol. Commun. 2020, 8, 96. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal Models of Traumatic Brain Injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.-R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transpl. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Martin, G. Traumatic Brain Injury: The First 15 Milliseconds. Brain Inj. 2016, 30, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- [PDF] Reduced Mortality Rate in Patients with Severe Traumatic Brain Injury Treated with Brain Tissue Oxygen Monitoring.|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Reduced-mortality-rate-in-patients-with-severe-with-Stiefel-Spiotta/2ad355337c2d4b55d68586537b782d06af147a33 (accessed on 25 July 2024).
- Werner, C.; Engelhard, K. Pathophysiology of Traumatic Brain Injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Iverson, G.L. Outcome from Mild Traumatic Brain Injury. Curr. Opin. Psychiatry 2005, 18, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Pocivavsek, A.; Moussa, C.E.-H.; Thompson, R.; Matsuoka, Y.; Faden, A.I.; Rebeck, G.W.; Burns, M.P. Amyloid Precursor Protein Secretases as Therapeutic Targets for Traumatic Brain Injury. Nat. Med. 2009, 15, 377–379. [Google Scholar] [CrossRef]
- Monoubiquitination and Cellular Distribution of XIAP in Neurons after Traumatic Brain Injury|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Monoubiquitination-and-Cellular-Distribution-of-in-Lotocki-Alonso/1798ec9c891caccf4d363ff276a97608dcb7bef9 (accessed on 25 July 2024).
- Yao, X.; Liu, J.; McCabe, J.T. Ubiquitin and Ubiquitin-Conjugated Protein Expression in the Rat Cerebral Cortex and Hippocampus Following Traumatic Brain Injury (TBI). Brain Res. 2007, 1182, 116–122. [Google Scholar] [CrossRef]
- Traumatic Brain Injury and Mitochondrial Dysfunction—The American Journal of the Medical Sciences. Available online: https://www.amjmedsci.org/article/S0002-9629(15)41244-3/abstract (accessed on 26 July 2024).
- Bauman, R.A.; Ling, G.; Tong, L.; Januszkiewicz, A.; Agoston, D.; Delanerolle, N.; Kim, Y.; Ritzel, D.; Bell, R.; Ecklund, J.; et al. An Introductory Characterization of a Combat-Casualty-Care Relevant Swine Model of Closed Head Injury Resulting from Exposure to Explosive Blast. J. Neurotrauma 2009, 26, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R.; Sell, S.L.; Micci, M.-A.; Crookshanks, J.M.; Parsley, M.; Uchida, T.; Prough, D.S.; DeWitt, D.S.; Hellmich, H.L. Traumatic Brain Injury-Induced Dysregulation of the Circadian Clock. PLoS ONE 2012, 7, e46204. [Google Scholar] [CrossRef]
- Freire, M.A.M.; Rocha, G.S.; Bittencourt, L.O.; Falcao, D.; Lima, R.R.; Cavalcanti, J.R.L.P. Cellular and Molecular Pathophysiology of Traumatic Brain Injury: What Have We Learned So Far? Biology 2023, 12, 1139. [Google Scholar] [CrossRef]
- Mannix, R.; Berglass, J.; Berkner, J.; Moleus, P.; Qiu, J.; Andrews, N.; Gunner, G.; Berglass, L.; Jantzie, L.L.; Robinson, S.; et al. Chronic Gliosis and Behavioral Deficits in Mice Following Repetitive Mild Traumatic Brain Injury. J. Neurosurg. 2014, 121, 1342–1350. [Google Scholar] [CrossRef]
- Tucker, L.B.; Fu, A.H.; McCabe, J.T. Performance of Male and Female C57BL/6J Mice on Motor and Cognitive Tasks Commonly Used in Pre-Clinical Traumatic Brain Injury Research. J. Neurotrauma 2016, 33, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Single Cell Molecular Alterations Reveal Target Cells and Pathways of Concussive Brain Injury-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30254269/ (accessed on 25 July 2024).
- Traumatic Brain Injury Induces Genome-Wide Transcriptomic, Methylomic, and Network Perturbations in Brain and Blood Predicting Neurological Disorders-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S2352396417300506 (accessed on 25 July 2024).
- Benson, J.C.; Madhavan, A.A.; Cutsforth-Gregory, J.K.; Johnson, D.R.; Carr, C.M. The Monro-Kellie Doctrine: A Review and Call for Revision. Am. J. Neuroradiol. 2022, 44, 2–6. [Google Scholar] [CrossRef]
- Sorby-Adams, A.J.; Vink, R.; Turner, R.J. Large Animal Models of Stroke and Traumatic Brain Injury as Translational Tools. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R165–R190. [Google Scholar] [CrossRef] [PubMed]
- Vink, R. Large Animal Models of Traumatic Brain Injury. J. Neurosci. Res. 2018, 96, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-X.; Ma, Y.-B.; Le, N.-Y.; Cao, J.; Wang, Y. Large Animal Models of Traumatic Brain Injury. Int. J. Neurosci. 2018, 128, 243–254. [Google Scholar] [CrossRef] [PubMed]
- RU2641569C1. Method for Severe Craniocerebral Injury Simulation-Google Patents. Available online: https://patents.google.com/patent/RU2641569C1/en (accessed on 25 July 2024).
- Radkov, I.V.; Laptev, V.V.; Plekhova, N.G. Technologies of Modeling the Diffuse Traumatic Brain Injury. Sovremen. Probl. Nauk. Obraz. 2018, 4, 148. [Google Scholar]
- Eakin, K.; Rowe, R.K.; Lifshitz, J. Modeling Fluid Percussion Injury: Relevance to Human Traumatic Brain Injury. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; Frontiers in Neuroengineering; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015; ISBN 978-1-4665-6598-2. [Google Scholar]
- Hayes, R.L.; Stalhammar, D.; Povlishock, J.T.; Allen, A.M.; Galinat, B.J.; Becker, D.P.; Stonnington, H.H. A New Model of Concussive Brain Injury in the Cat Produced by Extradural Fluid Volume Loading: II. Physiological and Neuropathological Observations. Brain Inj. 1987, 1, 93–112. [Google Scholar] [CrossRef]
- de Lanerolle, N.C.; Bandak, F.; Kang, D.; Li, A.Y.; Du, F.; Swauger, P.; Parks, S.; Ling, G.; Kim, J.H. Characteristics of an Explosive Blast-Induced Brain Injury in an Experimental Model. J. Neuropathol. Exp. Neurol. 2011, 70, 1046–1057. [Google Scholar] [CrossRef]
- Marmarou, A.; Shima, K. Comparative Studies of Edema Produced by Fluid Percussion Injury with Lateral and Central Modes of Injury in Cats. Adv. Neurol. 1990, 52, 233–236. [Google Scholar]
- Millen, J.E.; Glauser, F.L.; Fairman, R.P. A Comparison of Physiological Responses to Percussive Brain Trauma in Dogs and Sheep. J. Neurosurg. 1985, 62, 587–591. [Google Scholar] [CrossRef]
- Mechanisms of Blast Induced Brain Injuries, Experimental Studies in Rats—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1053811910007639 (accessed on 26 July 2024).
- Säljö, A.; Bao, F.; Haglid, K.G.; Hansson, H.A. Blast Exposure Causes Redistribution of Phosphorylated Neurofilament Subunits in Neurons of the Adult Rat Brain. J. Neurotrauma 2000, 17, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Shah, E.J.; Gurdziel, K.; Ruden, D.M. Mammalian Models of Traumatic Brain Injury and a Place for Drosophila in TBI Research. Front. Neurosci. 2019, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Thibault, L.E.; Meaney, D.F.; Anderson, B.J.; Marmarou, A. Biomechanical Aspects of a Fluid Percussion Model of Brain Injury. J. Neurotrauma 1992, 9, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.; Fujioka, W.; Lifshitz, J.; Crockett, D.; Thakker-Varia, S. Lateral Fluid Percussion: Model of Traumatic Brain Injury in Mice. J. Vis. Exp. JoVE 2011, 54, e3063. [Google Scholar] [CrossRef]
- Briones, T.L. Chapter 3 Animal Models of Traumatic Brain Injury: Is There an Optimal Model That Parallels Human Brain Injury? Annu. Rev. Nurs. Res. 2015, 33, 31–73. [Google Scholar] [CrossRef]
- Liu, Y.R.; Cardamone, L.; Hogan, R.E.; Gregoire, M.C.; Williams, J.P.; Hicks, R.J.; Binns, D.; Koe, A.; Jones, N.C.; Myers, D.E.; et al. Progressive Metabolic and Structural Cerebral Perturbations after Traumatic Brain Injury: An in Vivo Imaging Study in the Rat. J. Nucl. Med. 2010, 51, 1788–1795. [Google Scholar] [CrossRef]
- Rostami, E.; Davidsson, J.; Ng, K.C.; Lu, J.; Gyorgy, A.; Walker, J.; Wingo, D.; Plantman, S.; Bellander, B.-M.; Agoston, D.V.; et al. A Model for Mild Traumatic Brain Injury That Induces Limited Transient Memory Impairment and Increased Levels of Axon Related Serum Biomarkers. Front. Neurol. 2012, 3, 115. [Google Scholar] [CrossRef]
- Dixon, C.E.; Lyeth, B.G.; Povlishock, J.T.; Findling, R.L.; Hamm, R.J.; Marmarou, A.; Young, H.F.; Hayes, R.L. A Fluid Percussion Model of Experimental Brain Injury in the Rat. J. Neurosurg. 1987, 67, 110–119. [Google Scholar] [CrossRef]
- Dixon, C.E.; Clifton, G.L.; Lighthall, J.W.; Yaghmai, A.A.; Hayes, R.L. A Controlled Cortical Impact Model of Traumatic Brain Injury in the Rat. J. Neurosci. Methods 1991, 39, 253–262. [Google Scholar] [CrossRef]
- Friess, S.H.; Lapidus, J.B.; Brody, D.L. Decompressive Craniectomy Reduces White Matter Injury after Controlled Cortical Impact in Mice. J. Neurotrauma 2015, 32, 791–800. [Google Scholar] [CrossRef]
- Hoogenboom, W.S.; Branch, C.A.; Lipton, M.L. Animal Models of Closed-Skull, Repetitive Mild Traumatic Brain Injury. Pharmacol. Ther. 2019, 198, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Responses to Cortical Injury: II. Widespread Depression of the Activity of an Enzyme in Cortex Remote from a Focal Injury—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/6784887/ (accessed on 26 July 2024).
- Feeney, D.M.; Boyeson, M.G.; Linn, R.T.; Murray, H.M.; Dail, W.G. Responses to Cortical Injury: I. Methodology and Local Effects of Contusions in the Rat. Brain Res. 1981, 211, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Kallakuri, S.; Zhou, R.; Cavanaugh, J.M. Quantitative Relationship between Axonal Injury and Mechanical Response in a Rodent Head Impact Acceleration Model. J. Neurotrauma 2011, 28, 1767–1782. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, A.F.; Lucke-Wold, B.P.; Turner, R.C.; Huber, J.D.; Rosen, C.L.; Simpkins, J.W. Role of Microvascular Disruption in Brain Damage from Traumatic Brain Injury. Compr. Physiol. 2015, 5, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Aravind, A.; Pfister, B.J.; Chandra, N.; Haorah, J. Animal Models of Traumatic Brain Injury and Assessment of Injury Severity. Mol. Neurobiol. 2019, 56, 5332–5345. [Google Scholar] [CrossRef]
- Marmarou, A.; Foda, M.A.; van den Brink, W.; Campbell, J.; Kita, H.; Demetriadou, K. A New Model of Diffuse Brain Injury in Rats. Part I: Pathophysiology and Biomechanics. J. Neurosurg. 1994, 80, 291–300. [Google Scholar] [CrossRef]
- Morales, D.M.; Marklund, N.; Lebold, D.; Thompson, H.J.; Pitkanen, A.; Maxwell, W.L.; Longhi, L.; Laurer, H.; Maegele, M.; Neugebauer, E.; et al. Experimental Models of Traumatic Brain Injury: Do We Really Need to Build a Better Mousetrap? Neuroscience 2005, 136, 971–989. [Google Scholar] [CrossRef]
- Kabadi, S.V.; Hilton, G.D.; Stoica, B.A.; Zapple, D.N.; Faden, A.I. Fluid-Percussion-Induced Traumatic Brain Injury Model in Rats. Nat. Protoc. 2010, 5, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Morales-Villagrán, A.; Salazar-Sánchez, J.C.; Chiprés-Tinajero, G.A.; Medina-Ceja, L.; Ortega-Ibarra, J. A Novel Hydro-Pneumatic Fluid Percussion Device for Inducing Traumatic Brain Injury: Assessment of Sensory, Motor, Cognitive, Molecular, and Morphological Outcomes in Rodents. Front. Mol. Neurosci. 2023, 16, 1208954. [Google Scholar] [CrossRef]
- Ouyang, W.; Wu, W.; Fan, Z.; Wang, J.; Pan, H.; Yang, W. Modified Device for Fluid Percussion Injury in Rodents. J. Neurosci. Res. 2018, 96, 1412–1429. [Google Scholar] [CrossRef]
- Chitturi, J.; Li, Y.; Santhakumar, V.; Kannurpatti, S.S. Early Behavioral and Metabolomic Change after Mild to Moderate Traumatic Brain Injury in the Developing Brain. Neurochem. Int. 2018, 120, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.S.; Molina, P.E. A Lateral Fluid Percussion Injury Model for Studying Traumatic Brain Injury in Rats. Methods Mol. Biol. 2018, 1717, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.; Soderstrom, M.; Saha, B.; Sharma, P. Animal Models of Traumatic Brain Injury: A Review of Pathophysiology to Biomarkers and Treatments. Exp. Brain Res. 2021, 239, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- A Systematic Review of Closed Head Injury Models of Mild Traumatic Brain Injury in Mice and Rats-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30661454/ (accessed on 26 July 2024).
- Lifshitz, J.; Rowe, R.K.; Griffiths, D.R.; Evilsizor, M.N.; Thomas, T.C.; Adelson, P.D.; McIntosh, T.K. Clinical Relevance of Midline Fluid Percussion Brain Injury: Acute Deficits, Chronic Morbidities, and the Utility of Biomarkers. Brain Inj. 2016, 30, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Lyeth, B.G. Historical Review of the Fluid-Percussion TBI Model. Front. Neurol. 2016, 7, 217. [Google Scholar] [CrossRef]
- Dean, D.D.; Frank, J.A.; Turtzo, L.C. Controlled Cortical Impact in the Rat. Curr. Protoc. Neurosci. 2017, 81, 9.62.1–9.62.12. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Furuya, H.; Patel, P.M. Neuroprotective Effects of Anesthetic Agents. J. Anesth. 2005, 19, 150–156. [Google Scholar] [CrossRef]
- Songarj, P.; Luh, C.; Staib-Lasarzik, I.; Engelhard, K.; Moosmann, B.; Thal, S.C. The Antioxidative, Non-Psychoactive Tricyclic Phenothiazine Reduces Brain Damage after Experimental Traumatic Brain Injury in Mice. Neurosci. Lett. 2015, 584, 253–258. [Google Scholar] [CrossRef]
- Talley Watts, L.; Sprague, S.; Zheng, W.; Garling, R.J.; Jimenez, D.; Digicaylioglu, M.; Lechleiter, J. Purinergic 2Y1 Receptor Stimulation Decreases Cerebral Edema and Reactive Gliosis in a Traumatic Brain Injury Model. J. Neurotrauma 2013, 30, 55–66. [Google Scholar] [CrossRef]
- Brody, D.L.; Mac Donald, C.; Kessens, C.C.; Yuede, C.; Parsadanian, M.; Spinner, M.; Kim, E.; Schwetye, K.E.; Holtzman, D.M.; Bayly, P.V. Electromagnetic Controlled Cortical Impact Device for Precise, Graded Experimental Traumatic Brain Injury. J. Neurotrauma 2007, 24, 657–673. [Google Scholar] [CrossRef]
- Fesharaki-Zadeh, A.; Miyauchi, J.T.; Laurent-Arriot, K.S.; Tsirka, S.E.; Bergold, P.J. Increased Behavioral Deficits and Inflammation in a Mouse Model of Co-Morbid Traumatic Brain Injury and Post-Traumatic Stress Disorder. 2020. Available online: https://journals.sagepub.com/doi/10.1177/1759091420979567 (accessed on 26 July 2024).
- Osier, N.D.; Dixon, C.E. The Controlled Cortical Impact Model: Applications, Considerations for Researchers, and Future Directions. Front. Neurol. 2016, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Siebold, L.; Obenaus, A.; Goyal, R. Criteria to Define Mild, Moderate, and Severe Traumatic Brain Injury in the Mouse Controlled Cortical Impact Model. Exp. Neurol. 2018, 310, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Schwulst, S.J.; Trahanas, D.M.; Saber, R.; Perlman, H. Traumatic Brain Injury-Induced Alterations in Peripheral Immunity. J. Trauma. Acute Care Surg. 2013, 75, 780–788. [Google Scholar] [CrossRef]
- Svirsky, S.E.; Henchir, J.; Li, Y.; Carlson, S.W.; Dixon, C.E. Temporal-Specific Sex and Injury-Dependent Changes on Neurogranin-Associated Synaptic Signaling After Controlled Cortical Impact in Rats. Mol. Neurobiol. 2024, 61, 7256–7268. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, X.; Michalski, S.; Zhao, S.; Chen, J. Traumatic Brain Injury Severity Affects Neurogenesis in Adult Mouse Hippocampus. J. Neurotrauma 2016, 33, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Whalen, M.J. Weight Drop Models in Traumatic Brain Injury. Methods Mol. Biol. 2016, 1462, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Animal Models of Traumatic Brain Injury: Is There an Optimal Model to Reproduce Human Brain Injury in the Laboratory?—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20416875/ (accessed on 26 July 2024).
- Kishimoto, Y.; Shishido, H.; Sawanishi, M.; Toyota, Y.; Ueno, M.; Kubota, T.; Kirino, Y.; Tamiya, T.; Kawai, N. Data on Amyloid Precursor Protein Accumulation, Spontaneous Physical Activity, and Motor Learning after Traumatic Brain Injury in the Triple-Transgenic Mouse Model of Alzheimer׳s Disease. Data Brief. 2016, 9, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Cai, J.; Shields, L.B.E.; Liu, N.; Xu, X.-M.; Shields, C.B. Traumatic Brain Injury Using Mouse Models. Transl. Stroke Res. 2014, 5, 454–471. [Google Scholar] [CrossRef]
- Statler, K.D.; Alexander, H.; Vagni, V.; Dixon, C.E.; Clark, R.S.B.; Jenkins, L.; Kochanek, P.M. Comparison of Seven Anesthetic Agents on Outcome after Experimental Traumatic Brain Injury in Adult, Male Rats. J. Neurotrauma 2006, 23, 97–108. [Google Scholar] [CrossRef]
- Fesharaki-Zadeh, A.; Datta, D. An Overview of Preclinical Models of Traumatic Brain Injury (TBI): Relevance to Pathophysiological Mechanisms. Front. Cell Neurosci. 2024, 18, 1371213. [Google Scholar] [CrossRef]
- Osier, N.D.; Korpon, J.R.; Dixon, C.E. Controlled Cortical Impact Model. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; Frontiers in Neuroengineering; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015; ISBN 978-1-4665-6598-2. [Google Scholar]
- Smith, D.H.; Soares, H.D.; Pierce, J.S.; Perlman, K.G.; Saatman, K.E.; Meaney, D.F.; Dixon, C.E.; McIntosh, T.K. A Model of Parasagittal Controlled Cortical Impact in the Mouse: Cognitive and Histopathologic Effects. J. Neurotrauma 1995, 12, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Hicks, R.R.; Johnson, V.E.; Bergstrom, D.A.; Cummings, D.M.; Noble, L.J.; Hovda, D.; Whalen, M.; Ahlers, S.T.; LaPlaca, M.; et al. Pre-Clinical Traumatic Brain Injury Common Data Elements: Toward a Common Language Across Laboratories. J. Neurotrauma 2015, 32, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, H.; Hjelmqvist, H.; Medin, A.; Persson, J.K.; Suneson, A. Physiological Changes in Pigs Exposed to a Blast Wave from a Detonating High-Explosive Charge. Mil. Med. 2000, 165, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Guley, N.H.; Rogers, J.T.; Del Mar, N.A.; Deng, Y.; Islam, R.M.; D’Surney, L.; Ferrell, J.; Deng, B.; Hines-Beard, J.; Bu, W.; et al. A Novel Closed-Head Model of Mild Traumatic Brain Injury Using Focal Primary Overpressure Blast to the Cranium in Mice. J. Neurotrauma 2016, 33, 403–422. [Google Scholar] [CrossRef] [PubMed]
- Kallakuri, S.; Desai, A.; Feng, K.; Tummala, S.; Saif, T.; Chen, C.; Zhang, L.; Cavanaugh, J.M.; King, A.I. Neuronal Injury and Glial Changes Are Hallmarks of Open Field Blast Exposure in Swine Frontal Lobe. PLoS ONE 2017, 12, e0169239. [Google Scholar] [CrossRef]
- Nakagawa, A.; Manley, G.T.; Gean, A.D.; Ohtani, K.; Armonda, R.; Tsukamoto, A.; Yamamoto, H.; Takayama, K.; Tominaga, T. Mechanisms of Primary Blast-Induced Traumatic Brain Injury: Insights from Shock-Wave Research. J. Neurotrauma 2011, 28, 1101–1119. [Google Scholar] [CrossRef]
- Cernak, I.; Merkle, A.C.; Koliatsos, V.E.; Bilik, J.M.; Luong, Q.T.; Mahota, T.M.; Xu, L.; Slack, N.; Windle, D.; Ahmed, F.A. The Pathobiology of Blast Injuries and Blast-Induced Neurotrauma as Identified Using a New Experimental Model of Injury in Mice. Neurobiol. Dis. 2011, 41, 538–551. [Google Scholar] [CrossRef]
- Koliatsos, V.; Cernak, I.; Xu, L.; Song, Y.; Savonenko, A.; Crain, B.; Eberhart, C.; Frangakis, C.; Melnikova, T.; Kim, H.; et al. A Mouse Model of Blast Injury to Brain: Initial Pathological, Neuropathological, and Behavioral Characterization. J. Neuropathol. Exp. Neurol. 2011, 70, 399–416. [Google Scholar] [CrossRef]
- Rafaels, K.A.; ‘Dale’ Bass, C.R.; Panzer, M.B.; Salzar, R.S.; Woods, W.A.; Feldman, S.H.; Walilko, T.; Kent, R.W.; Capehart, B.P.; Foster, J.B.; et al. Brain Injury Risk from Primary Blast. J. Trauma. Acute Care Surg. 2012, 73, 895. [Google Scholar] [CrossRef]
- Garman, R.H.; Jenkins, L.W.; Switzer, R.C.; Bauman, R.A.; Tong, L.C.; Swauger, P.V.; Parks, S.A.; Ritzel, D.V.; Dixon, C.E.; Clark, R.S.B.; et al. Blast Exposure in Rats with Body Shielding Is Characterized Primarily by Diffuse Axonal Injury. J. Neurotrauma 2011, 28, 947–959. [Google Scholar] [CrossRef]
- Reneer, D.V.; Hisel, R.D.; Hoffman, J.M.; Kryscio, R.J.; Lusk, B.T.; Geddes, J.W. A Multi-Mode Shock Tube for Investigation of Blast-Induced Traumatic Brain Injury. J. Neurotrauma 2011, 28, 95–104. [Google Scholar] [CrossRef] [PubMed]
- (PDF) Blast Traumatic Brain Injury in the Rat Using a Blast Overpressure Model. Available online: https://www.researchgate.net/publication/234124806_Blast_Traumatic_Brain_Injury_in_the_Rat_Using_a_Blast_Overpressure_Model (accessed on 26 July 2024).
- Iacono, D.; Murphy, E.K.; Stimpson, C.D.; Leonessa, F.; Perl, D.P. Double Blast Wave Primary Effect on Synaptic, Glymphatic, Myelin, Neuronal and Neurovascular Markers. Brain Sci. 2023, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- (PDF) Blast Overpressure in Rats: Recreating a Battlefield Injury in the Laboratory. Available online: https://www.researchgate.net/publication/24364547_Blast_Overpressure_in_Rats_Recreating_a_Battlefield_Injury_in_the_Laboratory (accessed on 26 July 2024).
- Modeling the Neurobehavioral Consequences of Blast-Induced Traumatic Brain Injury Spectrum Disorder and Identifying Related Biomarkers—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26269904/ (accessed on 26 July 2024).
- Wojnarowicz, M.W.; Fisher, A.M.; Minaeva, O.; Goldstein, L.E. Considerations for Experimental Animal Models of Concussion, Traumatic Brain Injury, and Chronic Traumatic Encephalopathy-These Matters Matter. Front. Neurol. 2017, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Biomechanical Responses of a Pig Head under Blast Loading: A Computational Simulation|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Biomechanical-responses-of-a-pig-head-under-blast-Zhu-Skelton/7b575cf48e8d26b552c03cf4471932af57e1f508 (accessed on 26 July 2024).
- Albert-Weißenberger, C.; Várrallyay, C.; Raslan, F.; Kleinschnitz, C.; Sirén, A.-L. An Experimental Protocol for Mimicking Pathomechanisms of Traumatic Brain Injury in Mice. Exp. Transl. Stroke Med. 2012, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Earle, S.A.; de Moya, M.A.; Zuccarelli, J.E.; Norenberg, M.D.; Proctor, K.G. Cerebrovascular Resuscitation after Polytrauma and Fluid Restriction. J. Am. Coll. Surg. 2007, 204, 261. [Google Scholar] [CrossRef] [PubMed]
- Margulies, S.S.; Thibault, L.E.; Gennarelli, T.A. Physical Model Simulations of Brain Injury in the Primate. J. Biomech. 1990, 23, 823–836. [Google Scholar] [CrossRef]
- The Window of Risk in Repeated Head Injury—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23259734/ (accessed on 26 July 2024).
- Ultrastructural Brain Abnormalities and Associated Behavioral Changes in Mice after Low-Intensity Blast Exposure-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0166432818300718 (accessed on 26 July 2024).
- Cernak, I. Blast Injuries and Blast-Induced Neurotrauma: Overview of Pathophysiology and Experimental Knowledge Models and Findings. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; Frontiers in Neuroengineering; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015; ISBN 978-1-4665-6598-2. [Google Scholar]
- Rutter, B.; Song, H.; DePalma, R.G.; Hubler, G.; Cui, J.; Gu, Z.; Johnson, C.E. Shock Wave Physics as Related to Primary Non-Impact Blast-Induced Traumatic Brain Injury. Mil. Med. 2021, 186, 601–609. [Google Scholar] [CrossRef]
- Iacono, D.; Hatch, K.; Murphy, E.K.; Cole, R.N.; Post, J.; Leonessa, F.; Perl, D.P. Proteomic Changes in the Hippocampus after Repeated Explosive-Driven Blasts. J. Proteome Res. 2024, 23, 397–408. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Shao, Y.; Wu, Y.; He, J.; Wu, C. Mechanism of the Traumatic Brain Injury Induced by Blast Wave Using the Energy Assessment Method. Med. Eng. Phys. 2022, 101, 103767. [Google Scholar] [CrossRef]
- Liu, W.; Chai, J.K.; Qin, B.; Han, S.F.; Wang, X.T.; Jiang, S.; Bai, H.L.; Liu, L.Y.; Chang, Y.; Yue, X.T.; et al. Effects of Blast Wave-Induced Biomechanical Changes on Lung Injury in Rats. Biomed. Environ. Sci. 2020, 33, 338–349. [Google Scholar] [CrossRef]
- Song, H.; Cui, J.; Simonyi, A.; Johnson, C.E.; Hubler, G.K.; DePalma, R.G.; Gu, Z. Linking Blast Physics to Biological Outcomes in Mild Traumatic Brain Injury: Narrative Review and Preliminary Report of an Open-Field Blast Model. Behav. Brain Res. 2018, 340, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chen, M.; Chen, C.; Cui, J.; Johnson, C.E.; Cheng, J.; Wang, X.; Swerdlow, R.H.; DePalma, R.G.; Xia, W.; et al. Proteomic Analysis and Biochemical Correlates of Mitochondrial Dysfunction after Low-Intensity Primary Blast Exposure. J. Neurotrauma 2019, 36, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Konan, L.M.; Cui, J.; Johnson, C.E.; Hubler, G.K.; DePalma, R.G.; Gu, Z. Nanometer Ultrastructural Brain Damage Following Low Intensity Primary Blast Wave Exposure. Neural Regen. Res. 2018, 13, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Panzer, M.B.; Matthews, K.A.; Yu, A.W.; Morrison, B.; Meaney, D.F.; Bass, C.R. A Multiscale Approach to Blast Neurotrauma Modeling: Part I–Development of Novel Test Devices for in Vivo and in Vitro Blast Injury Models. Front. Neurol. 2012, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Dogan, G.; Karaca, O. Evaluation of Neuroprotective Effect of Sevoflurane in Acute Traumatic Brain Injury: An Experimental Study in Rats. Turk. Neurosurg. 2020, 30, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Statler, K.D.; Alexander, H.; Vagni, V.; Holubkov, R.; Dixon, C.E.; Clark, R.S.B.; Jenkins, L.; Kochanek, P.M. Isoflurane Exerts Neuroprotective Actions at or near the Time of Severe Traumatic Brain Injury. Brain Res. 2006, 1076, 216–224. [Google Scholar] [CrossRef]
- Namjoshi, D.R.; Cheng, W.H.; McInnes, K.A.; Martens, K.M.; Carr, M.; Wilkinson, A.; Fan, J.; Robert, J.; Hayat, A.; Cripton, P.A.; et al. Merging Pathology with Biomechanics Using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): A Novel, Surgery-Free Model of Traumatic Brain Injury. Mol. Neurodegener. 2014, 9, 55. [Google Scholar] [CrossRef]
- Viano, D.C.; Hamberger, A.; Bolouri, H.; Säljö, A. Concussion in Professional Football: Animal Model of Brain Injury—Part 15. Neurosurgery 2009, 64, 1162–1173, discussion 1173. [Google Scholar] [CrossRef]
- Cheng, W.H.; Martens, K.M.; Bashir, A.; Cheung, H.; Stukas, S.; Gibbs, E.; Namjoshi, D.R.; Button, E.B.; Wilkinson, A.; Barron, C.J.; et al. CHIMERA Repetitive Mild Traumatic Brain Injury Induces Chronic Behavioural and Neuropathological Phenotypes in Wild-Type and APP/PS1 Mice. Alzheimers Res. Ther. 2019, 11, 6. [Google Scholar] [CrossRef]
- Defining the Biomechanical and Biological Threshold of Murine Mild Traumatic Brain Injury Using CHIMERA (Closed Head Impact Model of Engineered Rotational Acceleration)—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28274861/ (accessed on 26 July 2024).
- Krieg, J.L.; Leonard, A.V.; Tuner, R.J.; Corrigan, F. Characterization of Traumatic Brain Injury in a Gyrencephalic Ferret Model Using the Novel Closed Head Injury Model of Engineered Rotational Acceleration (CHIMERA). Neurotrauma Rep. 2023, 4, 761–780. [Google Scholar] [CrossRef]
- Sauerbeck, A.D.; Fanizzi, C.; Kim, J.H.; Gangolli, M.; Bayly, P.V.; Wellington, C.L.; Brody, D.L.; Kummer, T.T. modCHIMERA: A Novel Murine Closed-Head Model of Moderate Traumatic Brain Injury. Sci. Rep. 2018, 8, 7677. [Google Scholar] [CrossRef] [PubMed]
- Increased Severity of the CHIMERA Model Induces Acute Vascular Injury, Sub-Acute Deficits in Memory Recall, and Chronic White Matter Gliosis—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0014488619302638 (accessed on 26 July 2024).
- Vonder Haar, C.; Martens, K.M.; Bashir, A.; McInnes, K.A.; Cheng, W.H.; Cheung, H.; Stukas, S.; Barron, C.; Ladner, T.; Welch, K.A.; et al. Repetitive Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA) Injury in Rats Increases Impulsivity, Decreases Dopaminergic Innervation in the Olfactory Tubercle and Generates White Matter Inflammation, Tau Phosphorylation and Degeneration. Exp. Neurol. 2019, 317, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.D.; Chen, X.-H.; Meaney, D.F.; Smith, D.H. Mild Traumatic Brain Injury and Diffuse Axonal Injury in Swine. J. Neurotrauma 2011, 28, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; McEwan, P.P.; Ameen-Ali, K.E.; Tomasevich, A.; Kennedy-Dietrich, C.; Palma, A.; Arroyo, E.J.; Dolle, J.-P.; Johnson, V.E.; Stewart, W.; et al. Concussion Leads to Widespread Axonal Sodium Channel Loss and Disruption of the Node of Ranvier. Acta Neuropathol. 2022, 144, 967–985. [Google Scholar] [CrossRef]
- Cullen, D.K.; Harris, J.P.; Browne, K.D.; Wolf, J.A.; Duda, J.E.; Meaney, D.F.; Margulies, S.S.; Smith, D.H. A Porcine Model of Traumatic Brain Injury via Head Rotational Acceleration. Methods Mol. Biol. 2016, 1462, 289–324. [Google Scholar] [CrossRef]
- Solomon, D.; Kim, B.; Scultetus, A.; Arnaud, F.; Auker, C.; Freilich, D.; McCarron, R. The Effect of rFVIIa on Pro- and Anti-Inflammatory Cytokines in Serum and Cerebrospinal Fluid in a Swine Model of Traumatic Brain Injury. Cytokine 2011, 54, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Oeur, A.; Liu, Y.; Zeineh, M.M.; Grant, G.A.; Margulies, S.S.; Camarillo, D.B. Translational Models of Mild Traumatic Brain Injury Tissue Biomechanics. Curr. Opin. Biomed. Eng. 2022, 24, 100422. [Google Scholar] [CrossRef]
- Zheng, Z.; Morykwas, M.; Campbell, D.; McGee, M.; Hollingsworth, C.; Adams, F.; Mays, J.; Tatter, S.; Argenta, L. Mechanical Tissue Resuscitation at the Site of Traumatic Brain Injuries Reduces the Volume of Injury and Hemorrhage in a Swine Model. Neurosurgery 2014, 75, 152–162. [Google Scholar] [CrossRef]
- Duhaime, A.C. Large animal models of traumatic injury to the immature brain. Dev. Neurosci. 2006, 28, 380–387. [Google Scholar] [CrossRef]
- Pareja, J.C.M.; Keeley, K.; Duhaime, A.-C.; Dodge, C.P. Modeling Pediatric Brain Trauma: Piglet Model of Controlled Cortical Impact. Methods Mol. Biol. 2016, 1462, 345–356. [Google Scholar] [CrossRef]
- Microbubble Gas Volume: A Unifying Dose Parameter in Blood-Brain Barrier Opening by Focused Ultrasound—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5196892/ (accessed on 26 July 2024).
- Ultrasound Physics and Instrumentation—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34033355/ (accessed on 26 July 2024).
- Alhelfi, A.; Sundén, B. Predictions of Temperature and Pressure Fields Due to Collapse of a Bubble in Sulfuric Acid Solution Under Ultrasound. J. Therm. Sci. Eng. Appl. 2016, 8, 041010. [Google Scholar] [CrossRef]
- Bachmann, C.E.; Gruber, G.; Konermann, W.; Arnold, A.; Gruber, G.M.; Ueberle, F.; Gerdesmeyer, L. ESWT and Ultrasound Imaging of the Musculoskeletal System; Steinkopff: Heidelberg, Germany, 2001; ISBN 978-3-7985-1252-8. [Google Scholar]
- Prieur, F.; Pialoux, V.; Mestas, J.-L.; Mury, P.; Skinner, S.; Lafon, C. Evaluation of Inertial Cavitation Activity in Tissue through Measurement of Oxidative Stress. Ultrason. Sonochem. 2015, 26, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Blast-Associated Shock Waves Result in Increased Brain Vascular Leakage and Elevated ROS Levels in a Rat Model of Traumatic Brain Injury. PLoS ONE 2015, 10, e0127971. [CrossRef]
- Wang, C.-J. Extracorporeal Shockwave Therapy in Musculoskeletal Disorders. J. Orthop. Surg. Res. 2012, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.P.-H.; Lai, D.-M.; Hsu, Y.-H.; Kung, Y.; Lan, C.; Yeh, C.-S.; Tsai, H.-H.; Lin, C.-F.; Chen, W.-S. Cavitation-Induced Traumatic Cerebral Contusion and Intracerebral Hemorrhage in the Rat Brain by Using an off-the-Shelf Clinical Shockwave Device. Sci. Rep. 2019, 9, 15614. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.; Lan, C.; Hsiao, M.-Y.; Sun, M.-K.; Hsu, Y.-H.; Huang, A.P.-H.; Liao, W.-H.; Liu, H.-L.; Inserra, C.; Chen, W.-S. Focused Shockwave Induced Blood-Brain Barrier Opening and Transfection. Sci. Rep. 2018, 8, 2218. [Google Scholar] [CrossRef]
- Takeuchi, S.; Nawashiro, H.; Sato, S.; Kawauchi, S.; Nagatani, K.; Kobayashi, H.; Otani, N.; Osada, H.; Wada, K.; Shima, K. A Better Mild Traumatic Brain Injury Model in the Rat. Acta Neurochir. Suppl. 2013, 118, 99–101. [Google Scholar] [CrossRef]
- Induction of a Transmissible Tau Pathology by Traumatic Brain Injury—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30084913/ (accessed on 26 July 2024).
- Agoston, D.V.; Shutes-David, A.; Peskind, E.R. Biofluid Biomarkers of Traumatic Brain Injury. Brain Inj. 2017, 31, 1195–1203. [Google Scholar] [CrossRef]
- Agoston, D.V.; Helmy, A. Fluid-Based Protein Biomarkers in Traumatic Brain Injury: The View from the Bedside. Int. J. Mol. Sci. 2023, 24, 16267. [Google Scholar] [CrossRef]
- Mahan, M.Y.; Thorpe, M.; Ahmadi, A.; Abdallah, T.; Casey, H.; Sturtevant, D.; Judge-Yoakam, S.; Hoover, C.; Rafter, D.; Miner, J.R.; et al. Glial Fibrillary Acidic Protein (GFAP) Outperforms S100 Calcium-Binding Protein B (S100B) and Ubiquitin C-Terminal Hydrolase L1 (UCH-L1) as Predictor for Positive Computed Tomography of the Head in Trauma Subjects. World Neurosurg. 2019, 128, e434–e444. [Google Scholar] [CrossRef]
- Svingos, A.M.; Robicsek, S.A.; Hayes, R.L.; Wang, K.K.; Robertson, C.S.; Brophy, G.M.; Papa, L.; Gabrielli, A.; Hannay, H.J.; Bauer, R.M.; et al. Predicting Clinical Outcomes 7–10 Years after Severe Traumatic Brain Injury: Exploring the Prognostic Utility of the IMPACT Lab Model and Cerebrospinal Fluid UCH-L1 and MAP-2. Neurocritical Care 2022, 37, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Trauma Diagnostic-Related Target Proteins and Their Detection Techniques—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11062145/ (accessed on 26 July 2024).
- Distribution of Five Clinically Important Neuroglial Proteins in the Human Brain—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35765081/ (accessed on 26 July 2024).
- The Use and Potential of pNF-H as a General Blood Biomarker of Axonal Loss: An Immediate Application for CNS Injury—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26269910/ (accessed on 26 July 2024).
- Increasing Rigor of Preclinical Research to Maximize Opportunities for Translation. Neurotherapeutics 2023, 20, 1433–1445. [CrossRef] [PubMed]
- Smith, D.H.; Kochanek, P.M.; Rosi, S.; Meyer, R.; Ferland-Beckham, C.; Prager, E.M.; Ahlers, S.T.; Crawford, F. Roadmap for Advancing Pre-Clinical Science in Traumatic Brain Injury. J. Neurotrauma 2021, 38, 3204–3221. [Google Scholar] [CrossRef] [PubMed]
- Iboaya, A.; Harris, J.L.; Arickx, A.N.; Nudo, R.J. Models of Traumatic Brain Injury in Aged Animals: A Clinical Perspective. Neurorehabil. Neural Repair. 2019, 33, 975–988. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).