Stimulation of Osteogenic Activity of Autologous Teeth Hard Tissues as Bone Augmentation Material
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of HTT Samples
2.2. Scanning Electron Microscopy and Energy-Dispersive Spectroscopy
2.3. Raman Spectroscopy
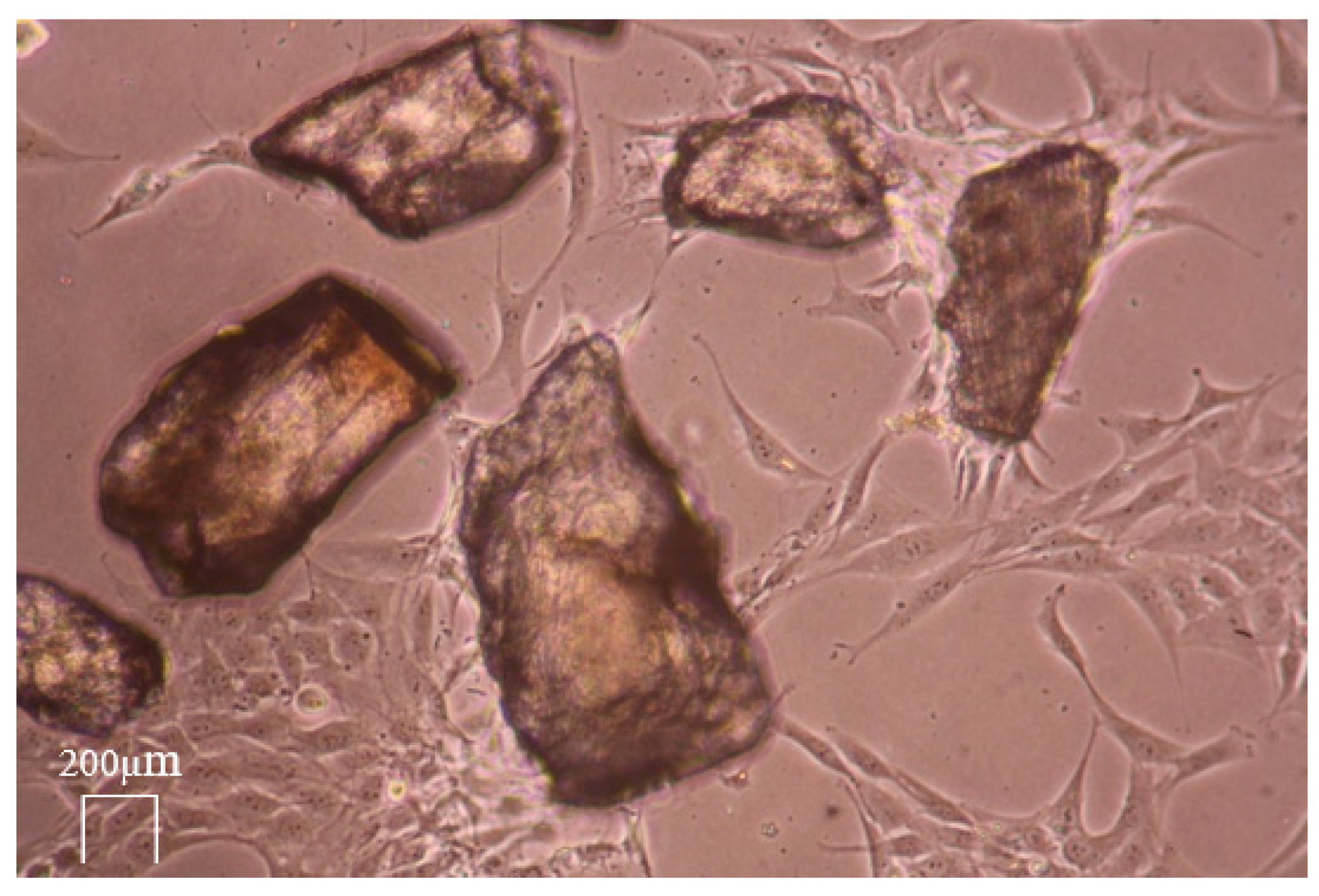
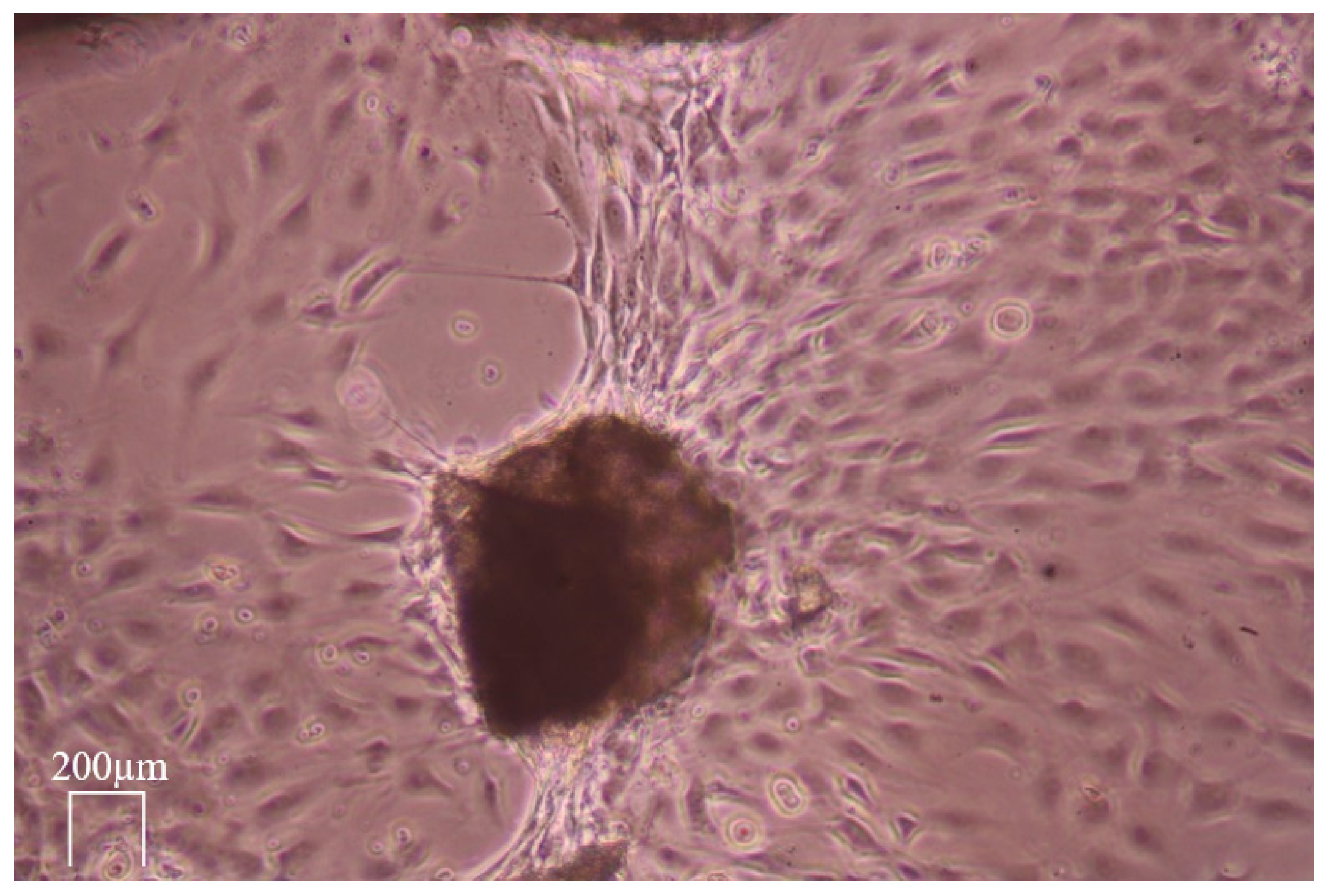
2.4. Live-Cell Dynamic Imaging
2.5. Statistical Analysis
3. Results
3.1. SEM Observations
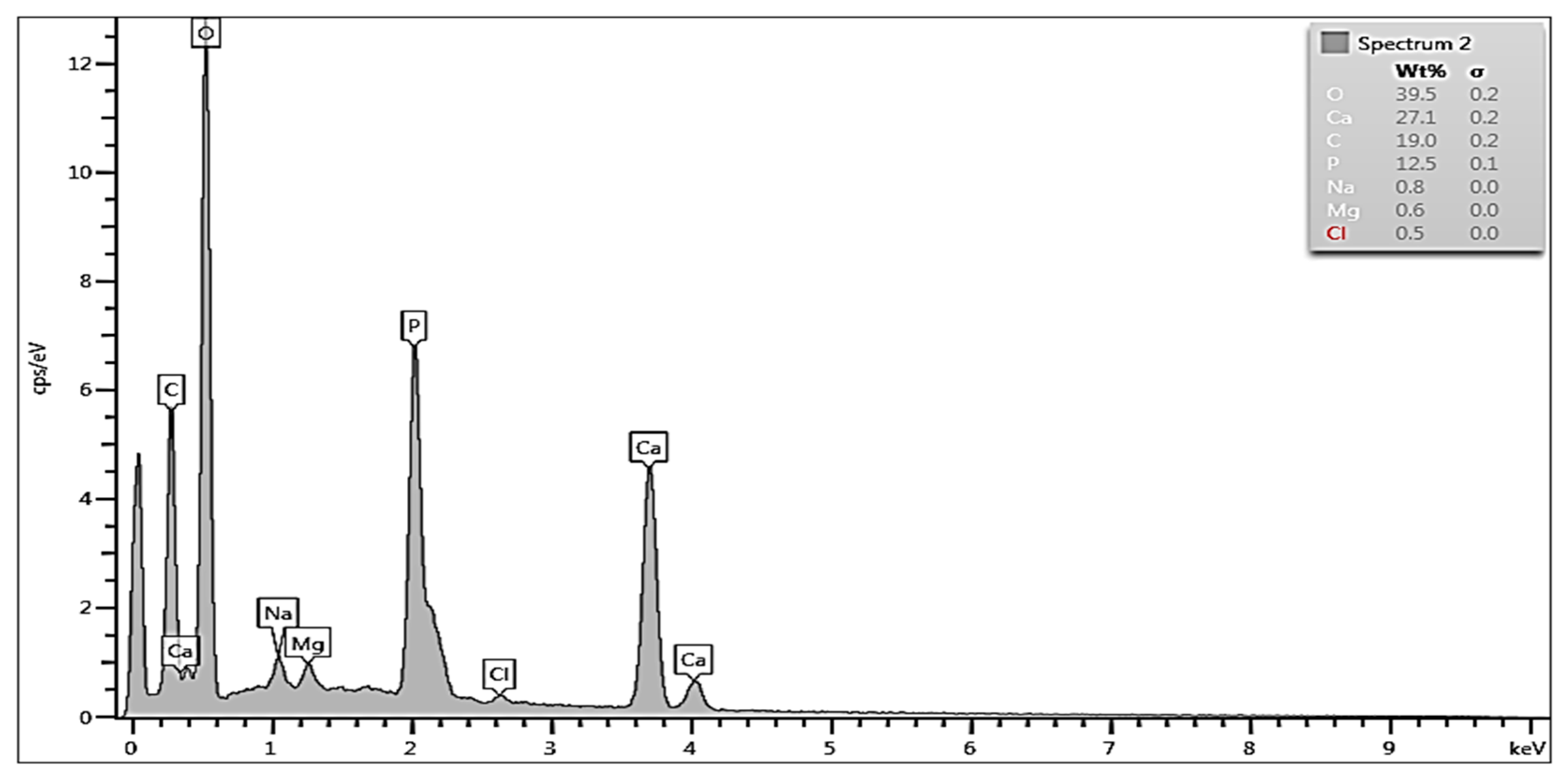
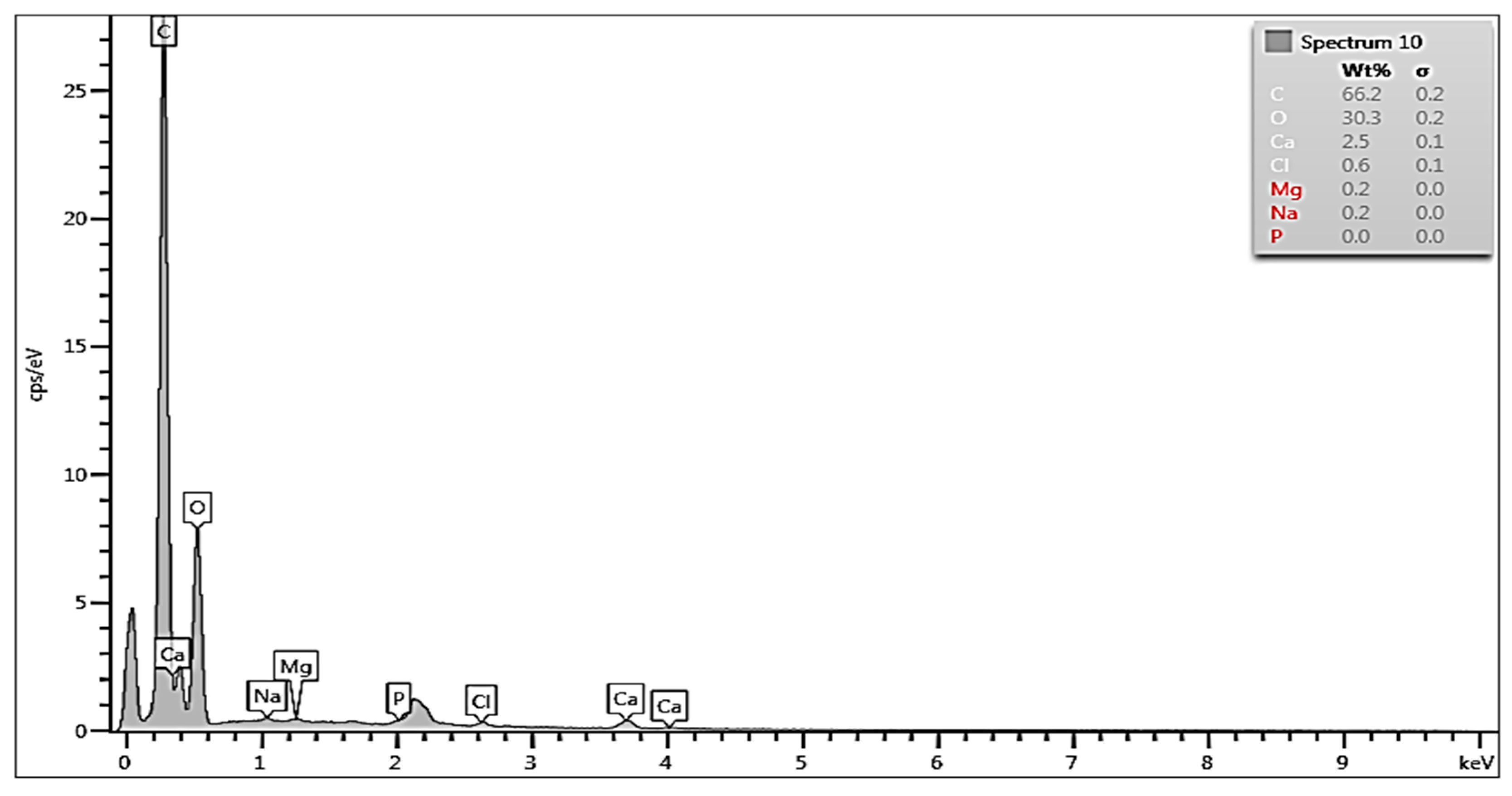
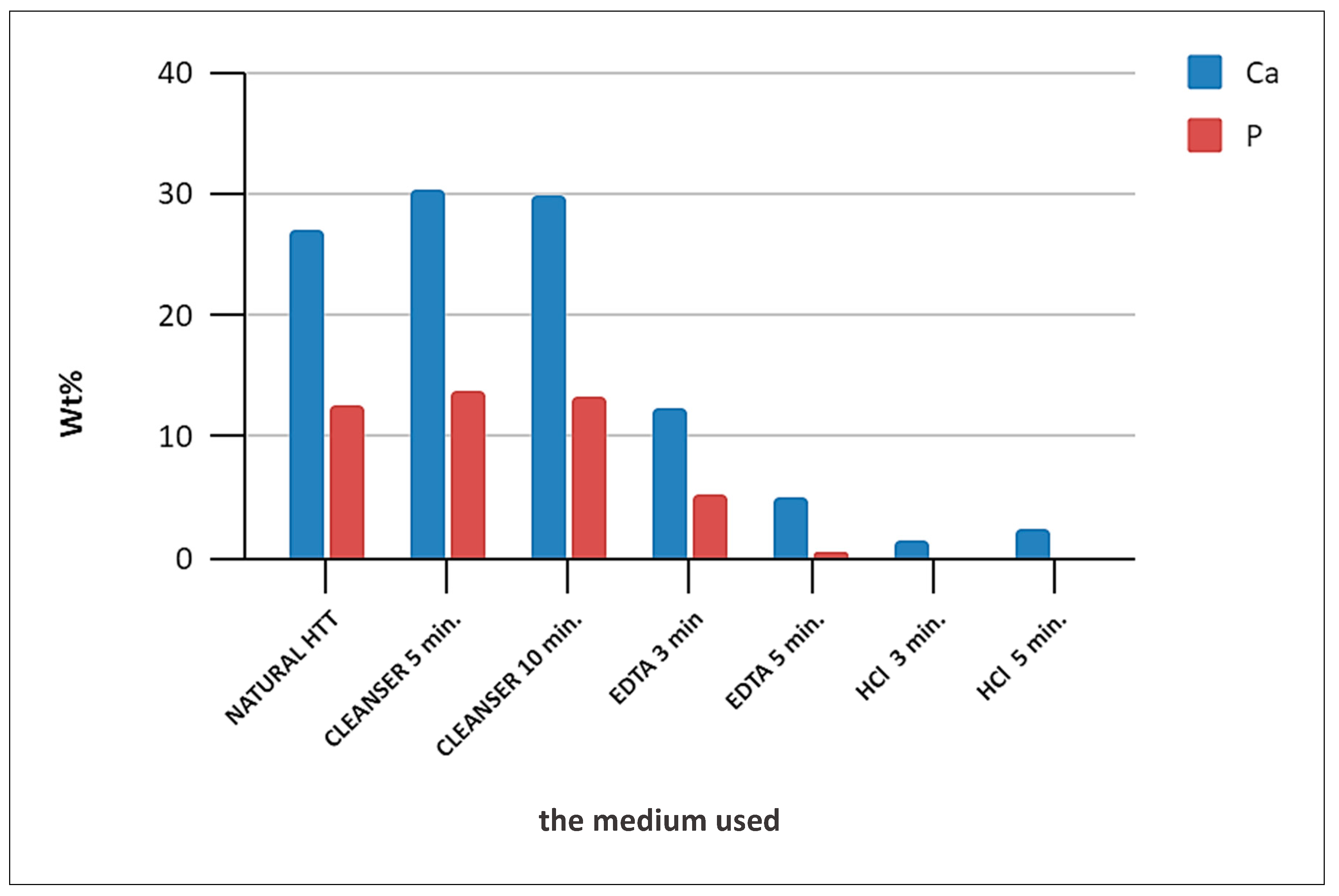
3.2. EDS Analysis
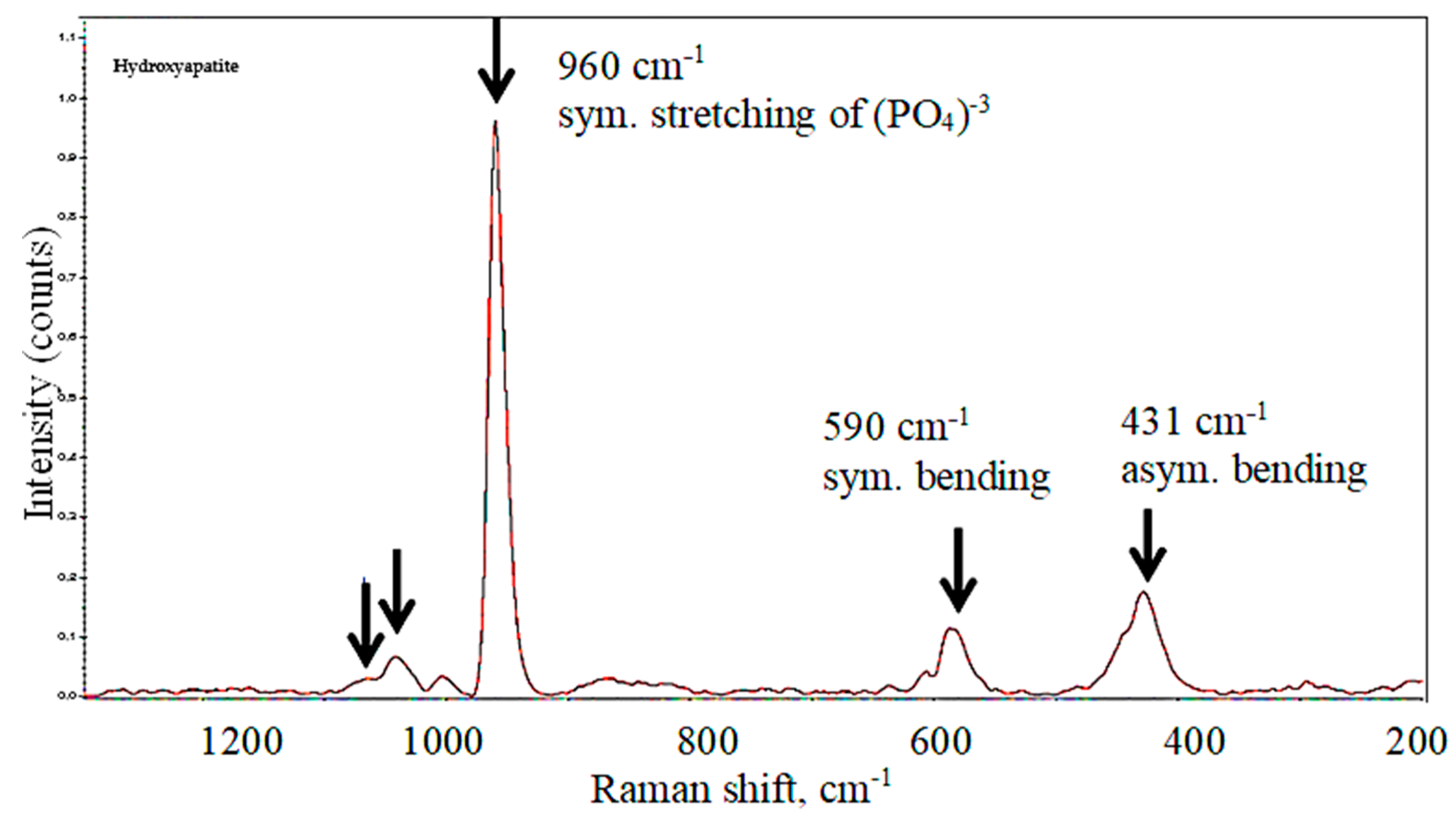
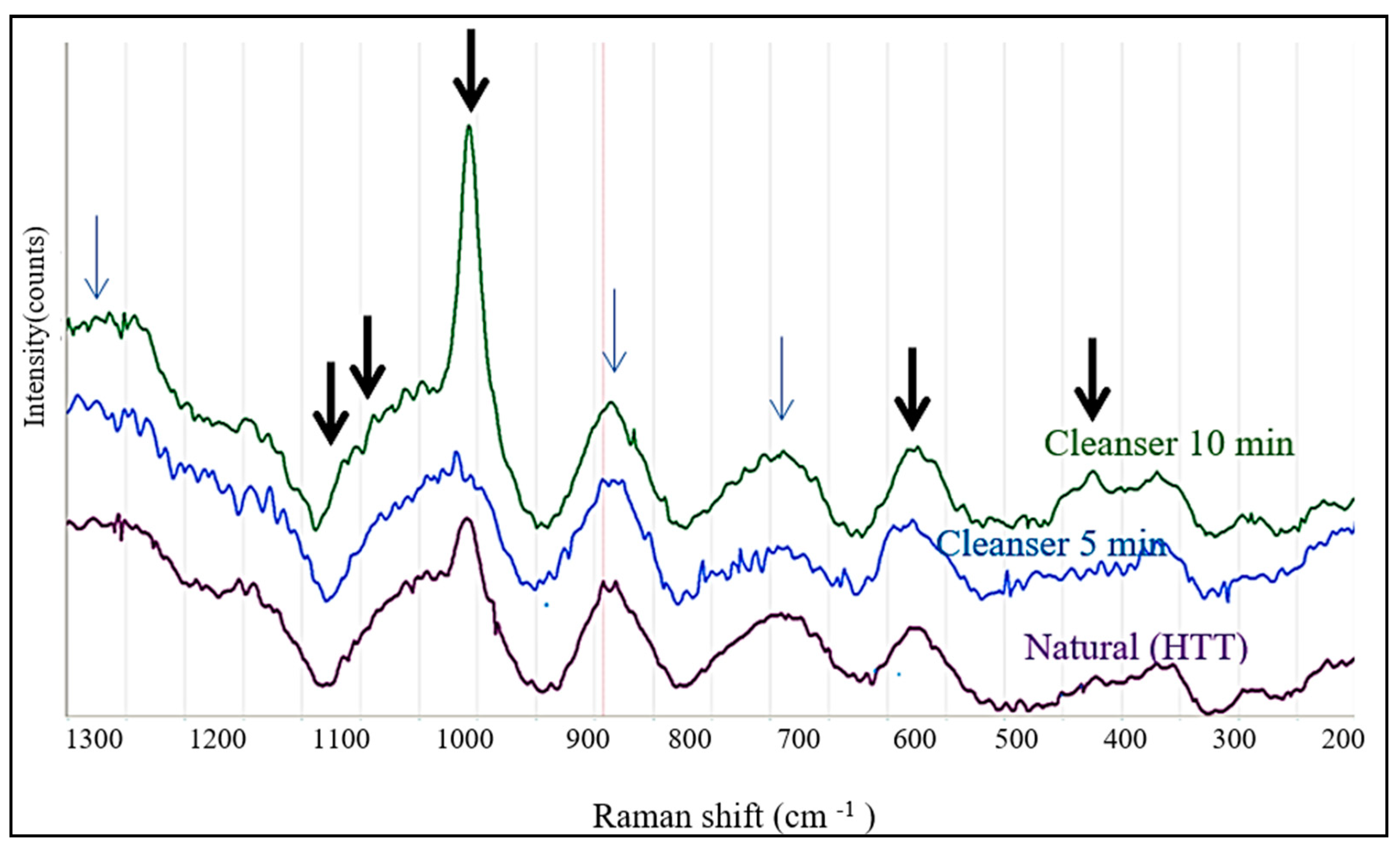
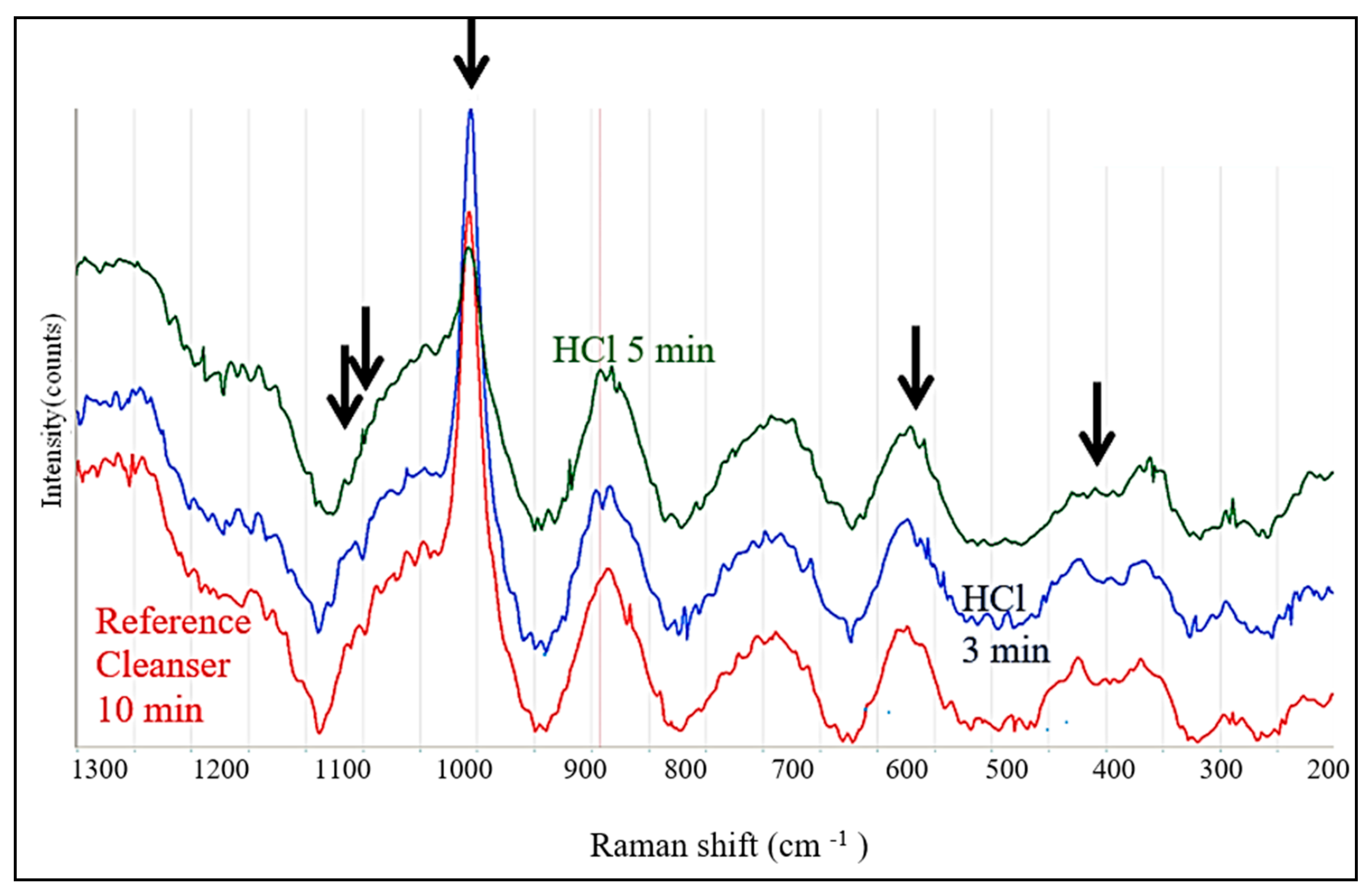
3.3. Raman Spectroscopy
3.4. Live-Cell Dynamic Imaging
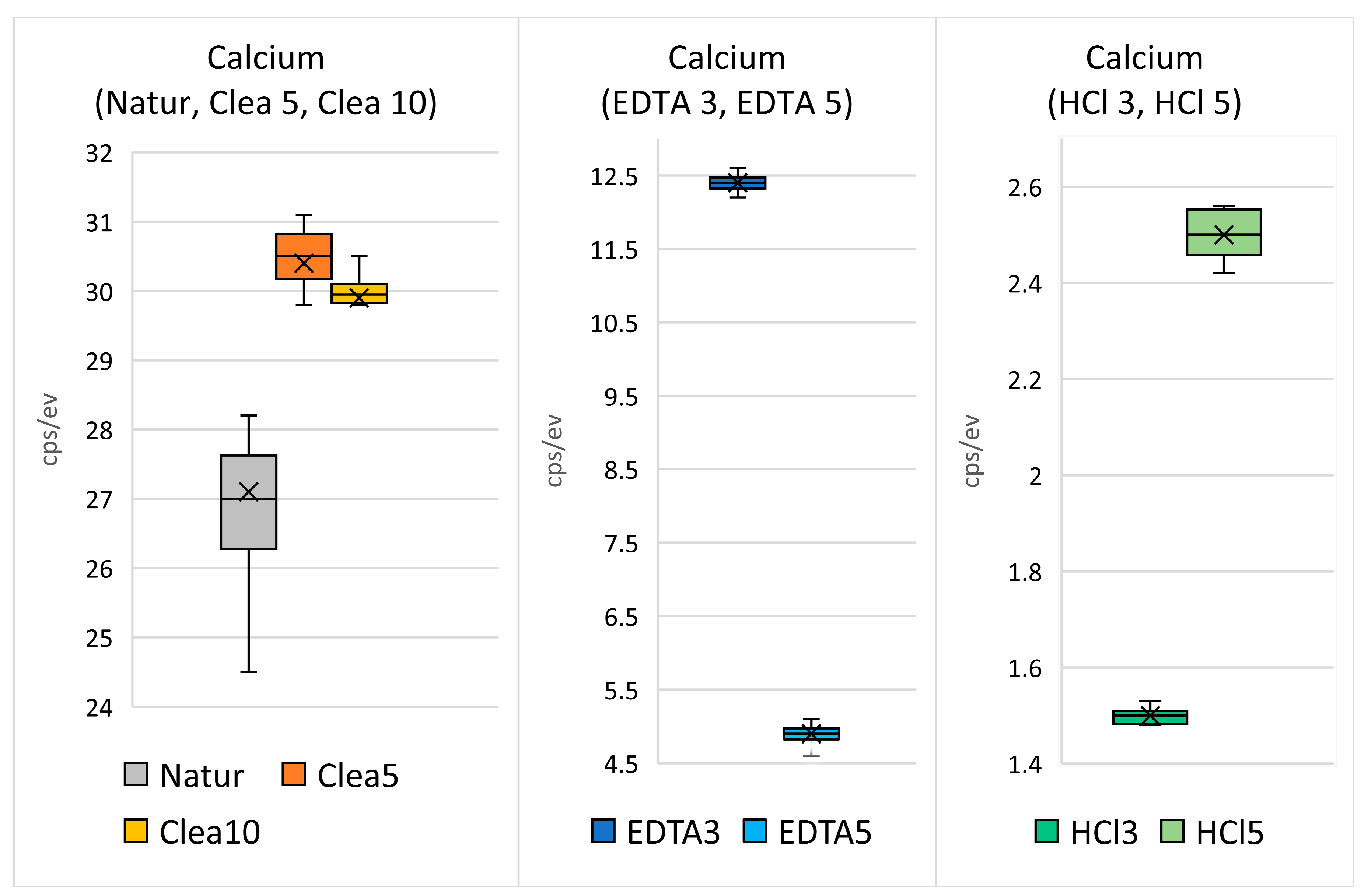
3.5. Statistical Analysis
4. Discussion
5. Conclusions
- Leaching of Ca and P in the EDTA and HCl solutions in the given concentration from the surface of HTT grains is proportional to the exposure time;
- Leaching treatment exposes organic components in the autologous dentin matrix by means of the inorganic hydroxyapatite part of the dentin;
- The increase in organic components increases the osteogenic activity of autologous dentin;
- The most effective HTT stimulation seems to be the application of Cleanser for 10 min followed by exposure to 0.6 N HCl for 5 min while applying a wash in PBS after each step of the preparation protocol.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Terminology | |
| HTT | autologous hard teeth tissues |
| DDM | dentin demineralized matrix |
| ADDM | autologous dentin demineralized matrix |
| NCPS | non-collagenous proteins |
| DSP | dentin sialoprotein |
| DMP1 | dentin matrix protein |
| DSP | bone sialoproteins |
| DPP | dentin phosphoprotein |
| TGF-β1 | transforming growth factor |
| IGF | insulin growth factor |
| AGF | angiogenic growth factor |
| BMP | bone morphogenetic protein |
| GF | growth factors |
| EDTA | ethylenediaminetetraacetic acid |
| HCL | the hydrochloric acid |
| NAOH | sodium hydroxide |
| PBS | phosphate-buffered saline |
| CO2 | carbon dioxide |
| SEM | Scanning Electron Microscopy |
| EDS | Energy Dispersive Spectroscopy |
References
- Carini, F.; Longoni, S.; Amosso, E.; Paleari, J.; Carini, S.; Porcaro, G. Bone augmentation with TiMesh. autologous bone versus autologous bone and bone substitutes. A systematic review. Ann. Stomatol. 2014, 5, 27–36. [Google Scholar]
- Um, I.-W.; Ku, J.-K.; Kim, Y.-M.; Yun, P.-Y.; Chang, N.-H.; Kim, Y.-K.; Choi, Y. Allogeneic Demineralized Dentin Matrix Graft for guided Bone Regeneration in Dental Implants. Appl. Sci. 2020, 10, 4661. [Google Scholar] [CrossRef]
- Pang, K.M.; Um, I.W.; Kim, Y.K.; Woo, J.M.; Kim, S.M.; Lee, J.H. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone. Clin. Oral Implant. Res. 2017, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, S.-G.; Oh, J.-S.; Jin, S.-C.; Son, J.-S.; Kim, S.-Y.; Lim, S.-Y. Analysis of the inorganic component of autogenous tooth bone graft material. J. Nanosci. Nanotechnol. 2011, 11, 7442–7445. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Mauad de Abreu, F.A.; Fernandes, M.L.D.M.F.; Alves, J.B. Demineralized Human Dentin Matrix as an Osteoinductor in the Dental Socket: An Experimental Study in Wistar Rats. Int. J. Oral Maxillofac. Implant. 2020, 35, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Kang, W.; Li, J.; Yu, L.; Ge, S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J. Nanobiotechnol. 2021, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhang, R.; Liu, S.; Yang, Y.; Dong, W.; Wang, M.; Mi, H.; Liu, M.; Sun, J.; Zhang, X.; et al. Biomimetic, biodegradable and osteoinductive treated dentin matrix/α-calcium sulphate hemihydrate composite material for bone tissue engineering. Regen. Biomater. 2023, 10, rbad061. [Google Scholar] [CrossRef]
- Reis-Filho, C.R.; Silva, E.R.; Martins, A.B.; Pessoa, F.F.; Gomes, P.V.; de Araújo, M.S.; Miziara, M.N.; Alves, J.B. Demineralised human dentine matrix stimulates the expression of VEGF and accelerates the bone repair in tooth sockets of rats. Arch. Oral Biol. 2012, 57, 469–476. [Google Scholar] [CrossRef]
- Niwa, T.; Yamakoshi, Y.; Yamazaki, H.; Karakida, T.; Chiba, R.; Hu, J.C.-C.; Nagano, T.; Yamamoto, R.; Simmer, J.P.; Margolis, H.C.; et al. The dynamics of TGF-β in dental pulp, odontoblasts and dentin. Sci. Rep. 2018, 8, 4450. [Google Scholar] [CrossRef]
- Ferreira, C.L.; de Abreu, F.A.M.; Silva, G.A.B.; Silveira, F.F.; Barreto, L.B.A.; Paulino, T.d.P.; Miziara, M.N.; Alves, J.B. TGF-β1 and BMP-4 carried by liposomes enhance the healing process in alveolar bone. Arch. Oral Biol. 2013, 58, 646–656. [Google Scholar] [CrossRef]
- Li, R.; Guo, W.; Yang, B.; Guo, L.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011, 32, 4525–4538. [Google Scholar] [CrossRef]
- Moraes, G.F.; Caetano, R.O.; Prochnow, F.H.O.; Pupo, Y.M.; Schussel, J.L.; Schwartz-Filho, H.O. Demineralized human dentin matrix for alveolar ridge preservation using a volumetric and histologic analyses in rats. Braz. Dent. J. 2022, 33, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, F.S.; Tatari, S.; Samadi, R.; Moharamzadeh, K. Different methods of dentin processing for application in bone tissue engineering: A systematic review. J. Biomed. Mater. Res. Part A 2016, 104, 2616–2627. [Google Scholar] [CrossRef]
- Minetti, E.; Palermo, A.; Malcangi, G.; Inchingolo, A.D.; Mancini, A.; Dipalma, G.; Inchingolo, F.; Patano, A.; Inchingolo, A.M. Dentin, Dentin Graft, and Bone Graft: Microscopic and Spectroscopic Analysis. J. Funct. Biomater. 2023, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Solyom, E.; Szalai, E.; Czumbel, M.L.; Szabo, B.; Váncsa, S.; Mikulas, K.; Radoczy-Drajko, Z.; Varga, G.; Hegyi, P.; Molnar, B.; et al. The use of autogenous tooth bone graft is an efficient method of alveolar ridge preservation-meta-analysis and systematic review. BMC Oral Health 2023, 23, 226. [Google Scholar] [CrossRef]
- Elfana, A.; El-Kholy, S.; Saleh, H.A.; Fawzy El-Sayed, K. Alveolar ridge preservation using autogenous whole-tooth versus demineralized dentin grafts: A randomized controlled clinical trial. Clin. Oral Implant. Res. 2021, 32, 539–548. [Google Scholar] [CrossRef]
- Kadkhodazadeh, M.; Ghasemianpour, M.; Soltanian, N.; Sultanian, G.R.; Ahmadpour, S.; Amid, R. Effects of fresh mineralized dentin and cementum on socket healing: A preliminary study in dogs. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 119–123. [Google Scholar] [CrossRef]
- Lee, H.-J.; Hong, J.-S.; Kim, Y.-K.; Um, I.-W.; Lee, J.-I. Osteogenic potential of demineralized dentin matrix as bone graft material. J. Hard Tissue Biol. 2017, 26, 223–230. [Google Scholar] [CrossRef]
- Jin, S.-Y.; Kim, S.-G.; Oh, J.-S.; You, J.-S.; Lim, S.-C.; Jeong, M.-A.; Kim, J.-S. Histomorphometric Analysis of Contaminated Autogenous Tooth Graft Materials after Various Sterilization. Implant. Dent. 2016, 25, 83–89. [Google Scholar] [CrossRef]
- Binderman, I.; Hallel, G.; Nardy, G.; Yaffe, A.; Sapoznikov, L.A. Novel procedure to process extracted teeth for immediate grafting of autogenous dentin. J. Interdiscip. Med. Dent. Sci. 2014, 2, 6. [Google Scholar]
- Kosasih, F.U.; Cacovich, S.; Divitini, G.; Ducati, C. Nanometric Chemical Analysis of Beam-Sensitive Materials: A Case Study of STEM-EDX on Perovskite Solar Cells. Small Methods 2021, 5, e2000835. [Google Scholar] [CrossRef] [PubMed]
- Matousek, P.; Stone, N. Prospects for the diagnosis of breast cancer by noninvasive probing of calcifications using transmission Raman spectroscopy. J. Biomed. Opt. 2007, 12, 024008. [Google Scholar] [CrossRef]
- Rehman, I.; Movasaghi, Z.; Rehman, S. Vibrational Spectroscopy for Tissue Analysis; Series in Medical Physics and Biomedical Engineering; CRC Press: Boca Raton, FL, USA, 2012; p. 271. [Google Scholar]
- Andrejovská, J.; Petruš, O.; Medveď, D.; Vojtko, M.; Riznič, M.; Kizek, P.; Dusza, J. Hardness and indentation modulus of human enamel and dentin. Surf. Interface Anal. 2022, 55, 270–278. [Google Scholar] [CrossRef]
- Meijering, E.; Dzyubachyk, O.; Smal, I. Methods for cell and particle tracking. Methods Enzymol. 2012, 504, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.J.; Allan, V.J. Light microscopy techniques for live cell imaging. Science 2003, 300, 82–86. [Google Scholar] [CrossRef]
- Yeomans, J.D.; Urist, M.R. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch. Oral Biol. 1967, 12, 999–1008. [Google Scholar] [CrossRef]
- MacBeth, N.; Trullenque-Eriksson, A.; Donos, N.; Mardas, N. Hard and soft tissue changes following alveolar ridge preservation: A systematic review. Clin. Oral Implant. Res. 2017, 28, 982–1004. [Google Scholar] [CrossRef] [PubMed]
- Arbez, B.; Kun-Darbois, J.D.; Convert, T.; Guillaume, B.; Mercier, P.; Hubert, L.; Chappard, D. Biomaterial granules used for filling bone defects constitute 3D scaffolds: Porosity, microarchitecture and molecular composition analyzed by microCT and Raman microspectroscopy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 415–423. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Nevins, M.; Schupbach, P. New Bone Formation Using an Extracted Tooth as a Biomaterial: A Case Report with Histologic Evidence. Int. J. Periodontics Restor. Dent. 2019, 39, 157–163. [Google Scholar] [CrossRef]
- Cenicante, J.; Botelho, J.; Machado, V.; Mendes, J.J.; Mascarenhas, P.; Alcoforado, G.; Santos, A. The use of autogenous teeth for alveolar ridge preservation: A literature review. Appl. Sci. 2021, 11, 1853. [Google Scholar] [CrossRef]
- Canellas, J.; Soares, B.N.; Ritto, F.G.; Vettore, M.V.; Vidigal Júnior, G.M.; Fischer, R.G.; Medeiros, P.J.D. What grafting materials produce greater alveolar ridge preservation after tooth extraction? A systematic review and network meta-analysis. J. Cranio-Maxillofac. Surg. 2021, 49, 1064–1071. [Google Scholar] [CrossRef]
- Dwivedi, A.; Kour, M. A neoteric procedure for alveolar ridge preservation using autogenous fresh mineralized tooth graft prepared at chair side. J. Oral Biol. Craniofac. Res. 2020, 10, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Mahardawi, B.; Rochanavibhata, S.; Jiaranuchart, S.; Arunjaroensuk, S.; Mattheos, N.; Pimkhaokham, A. Autogenous tooth bone graft material prepared chairside and its clinical applications: A systematic review. Int. J. Oral Maxillofac. Surg. 2023, 52, 132–141. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.; Payne, A.G.; Ali, S.; Faggion, C.M.J.; Esposito, M. Interventions for replacing missing teeth: Alveolar ridge preservation techniques for dental implant site development. Cochrane Database Syst. Rev. 2021, 4, Cd010176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Jing, D.; Shuang, Y.; Miron, R.J. Enamel matrix derivative improves gingival fibroblast cell behavior cultured on titanium surfaces. Clin. Oral Investig. 2016, 20, 685–695. [Google Scholar] [CrossRef]
- Chappard, D.; Stancu, I.C. Porosity imaged by a vector projection algorithm correlates with fractal dimension measured on 3D models obtained by microCT. J. Microsc. 2015, 258, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chappard, D.; Terranova, L.; Mallet, R.; Mercier, P. 3D Porous Architecture of Stacks of β-TCP Granules Compared with That of Trabecular Bone: A microCT, Vector Analysis, and Compression Study. Front. Endocrinol. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Pennycook, S.J.; Li, C.; Li, M.; Tang, C.; Okunishi, E.; Varela, M.; Kim, Y.-M.; Jang, J.H. Material structure, properties, and dynamics through scanning transmission electron microscopy. J. Anal. Sci. Technol. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, L.; Wang, Q.; Wen, Z.; Liu, C.; Ding, Y. Raman Spectroscopy: A Potential Diagnostic Tool for Oral Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 775236. [Google Scholar] [CrossRef] [PubMed]
- Ralbovsky, N.M.; Zou, L.; Chen, B.; Zhang, N.R.; Hines, C.D.; Vavrek, M.; Zhong, W.; Smith, J.P.; Bu, X. Simultaneous multielement imaging of liver tissue using laser ablation inductively coupled plasma mass spectrometry. Talanta 2021, 235, 122725. [Google Scholar] [CrossRef]
- Ingendoh-Tsakmakidis, A.; Nolte, L.; Winkel, A.; Meyer, H.; Koroleva, A.; Shpichka, A.; Ripken, T.; Heisterkamp, A.; Stiesch, M. Time resolved 3D live-cell imaging on implants. PLoS ONE 2018, 13, e0205411. [Google Scholar] [CrossRef] [PubMed]

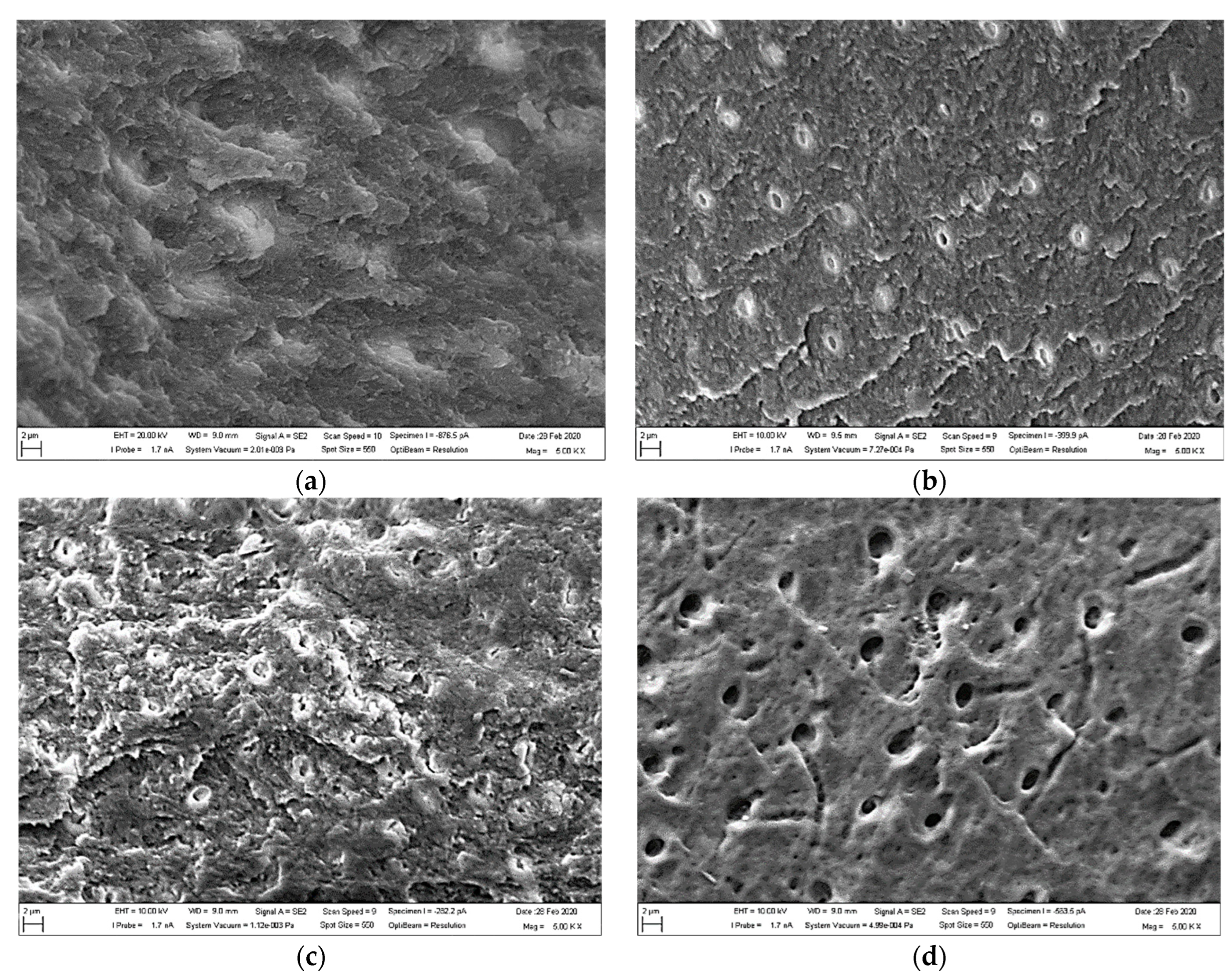
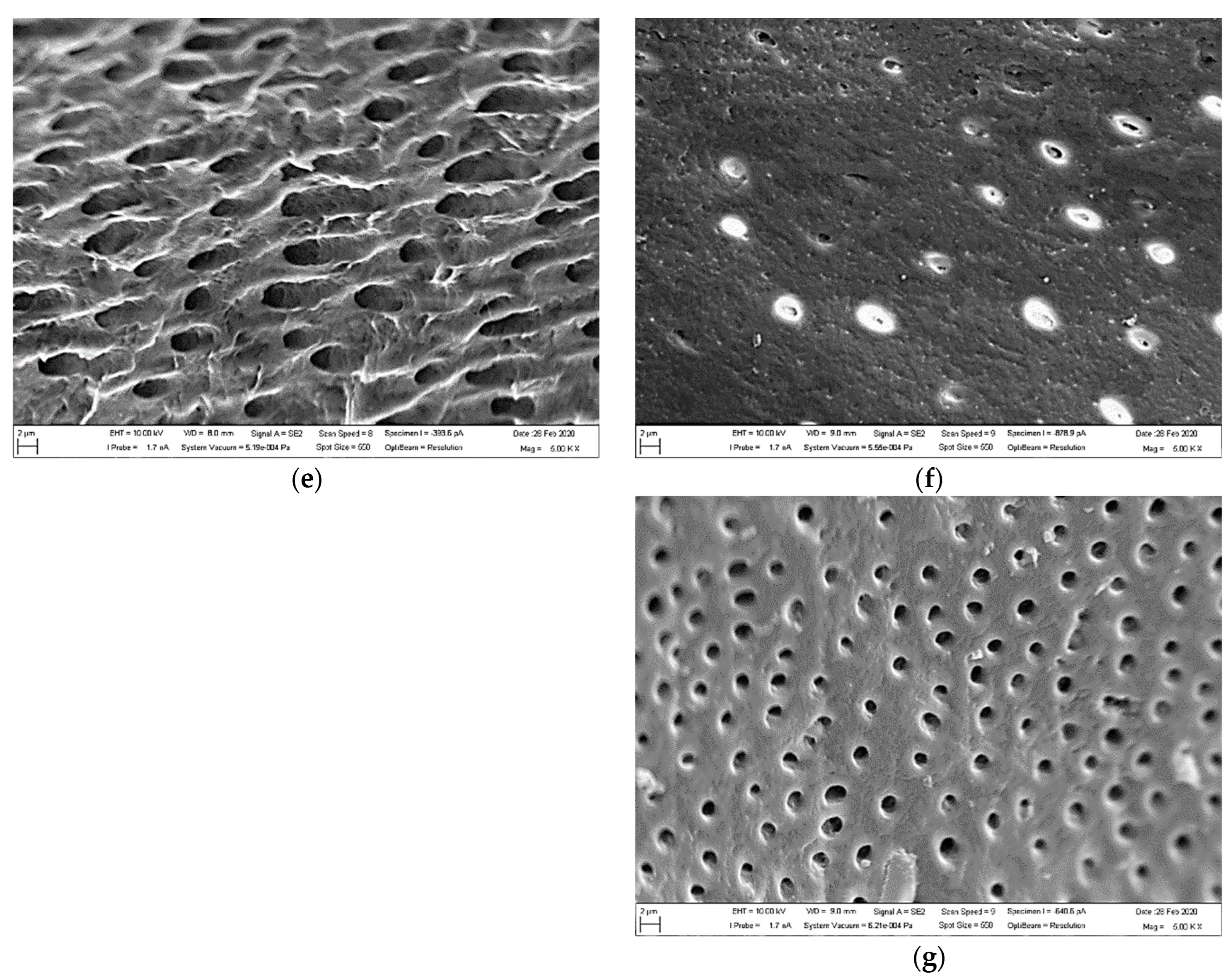




| Washing (PBS) | Cleanser | Washing (PBS) | EDTA 10% | HCl 0.6 N | Washing (PBS) | |
|---|---|---|---|---|---|---|
| Natural | 10 min | |||||
| Cleanser 5 min | 10 min | 5 min | 5 min | |||
| Cleanser 10 min | 10 min | 10 min | 5 min | |||
| EDTA 10% 3 min | 10 min | 5 min | 5 min | 3 min | 10 min | |
| EDTA 10% 5 min | 10 min | 5 min | 5 min | 5 min | 10 min | |
| HCl 0.6 N 3 min | 10 min | 5 min | 5 min | 3 min | 10 min | |
| HCl 0.6 N 5 min | 10 min | 5 min | 5 min | 5 min | 10 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kučera, J.; Lofaj, F.; Nagyová-Krchova, Z.; Šurín Hudáková, N.; Vojtko, M.; Březina, V. Stimulation of Osteogenic Activity of Autologous Teeth Hard Tissues as Bone Augmentation Material. Biology 2024, 13, 40. https://doi.org/10.3390/biology13010040
Kučera J, Lofaj F, Nagyová-Krchova Z, Šurín Hudáková N, Vojtko M, Březina V. Stimulation of Osteogenic Activity of Autologous Teeth Hard Tissues as Bone Augmentation Material. Biology. 2024; 13(1):40. https://doi.org/10.3390/biology13010040
Chicago/Turabian StyleKučera, Jan, František Lofaj, Zuzana Nagyová-Krchova, Natália Šurín Hudáková, Marek Vojtko, and Vitěslav Březina. 2024. "Stimulation of Osteogenic Activity of Autologous Teeth Hard Tissues as Bone Augmentation Material" Biology 13, no. 1: 40. https://doi.org/10.3390/biology13010040
APA StyleKučera, J., Lofaj, F., Nagyová-Krchova, Z., Šurín Hudáková, N., Vojtko, M., & Březina, V. (2024). Stimulation of Osteogenic Activity of Autologous Teeth Hard Tissues as Bone Augmentation Material. Biology, 13(1), 40. https://doi.org/10.3390/biology13010040





