Proteomic and Phosphoproteomic Analysis Reveals Differential Immune Response to Hirame Novirhabdovirus (HIRRV) Infection in the Flounder (Paralichthys olivaceus) under Different Temperature
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Virus
2.2. Experimental Infection and Sampling
2.3. Protein Extraction and iTRAQ Labeling
2.4. HPLC Fractionation
2.5. Affinity Enrichment
2.6. LC-MS/MS Analysis and Data Analysis
2.7. Quantitative Real-Time Polymerase Chain Reaction
2.8. Statistical Analysis
3. Results
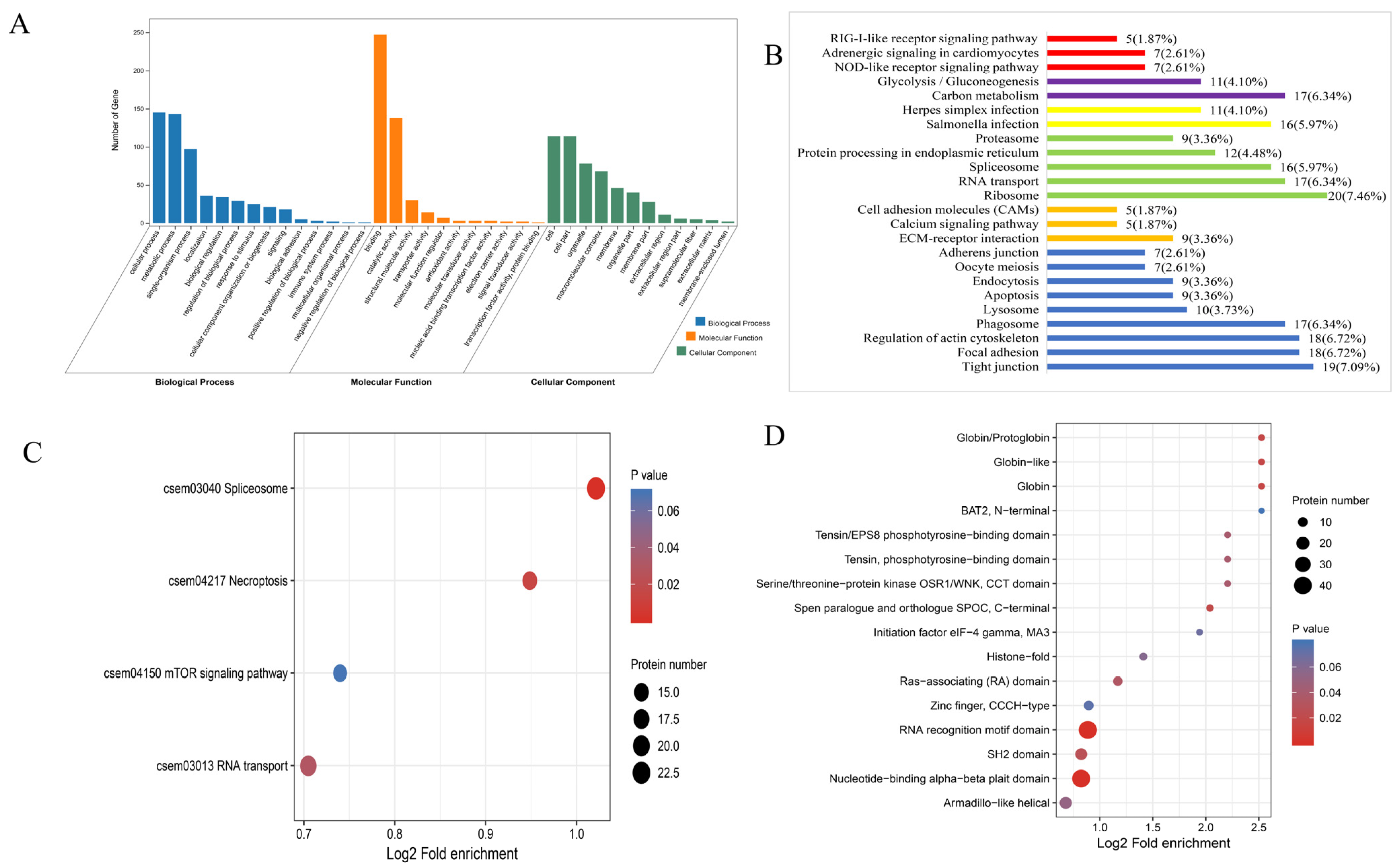
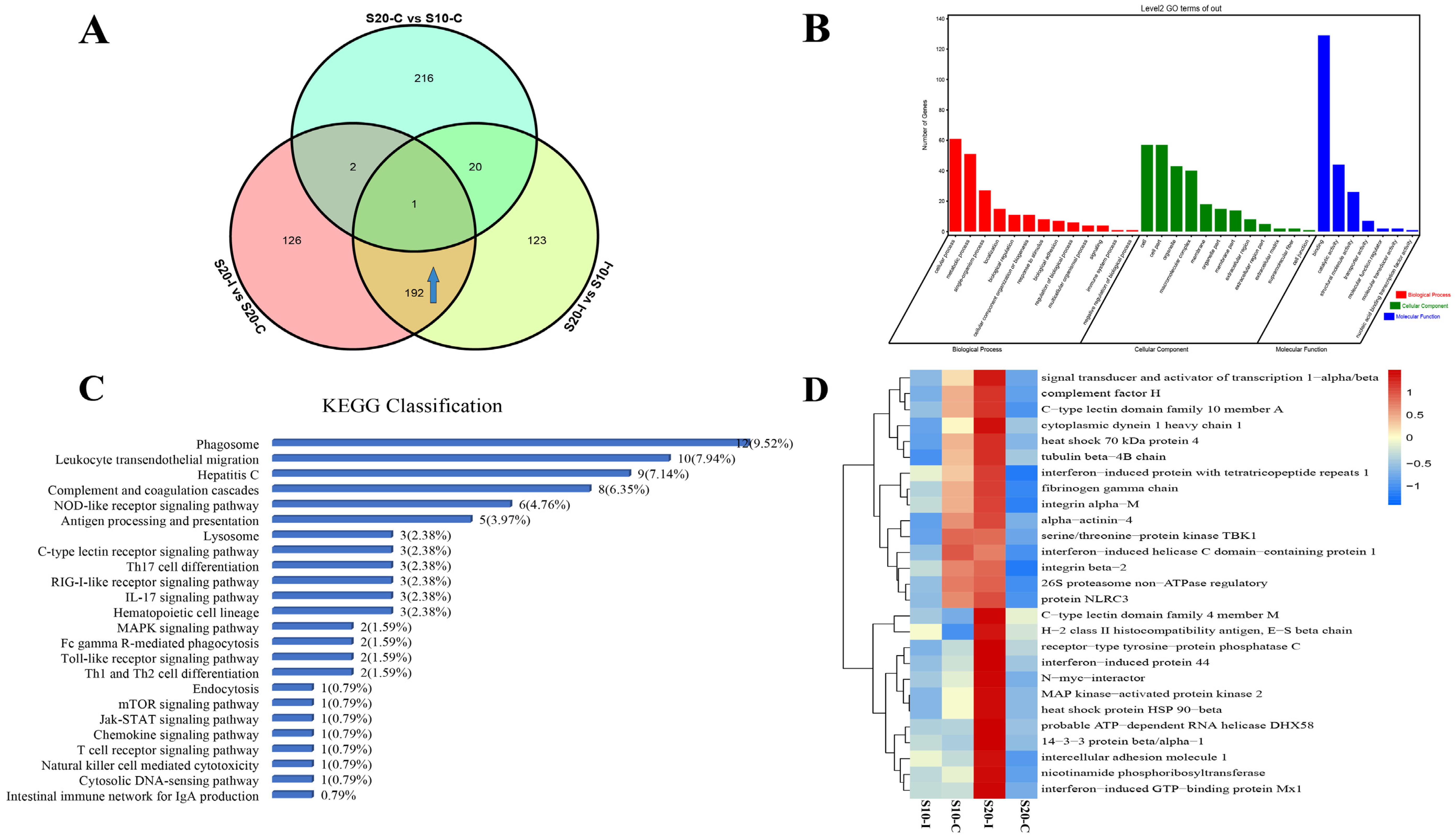
3.1. Analysis of DEPs and DEPPs under 10 °C
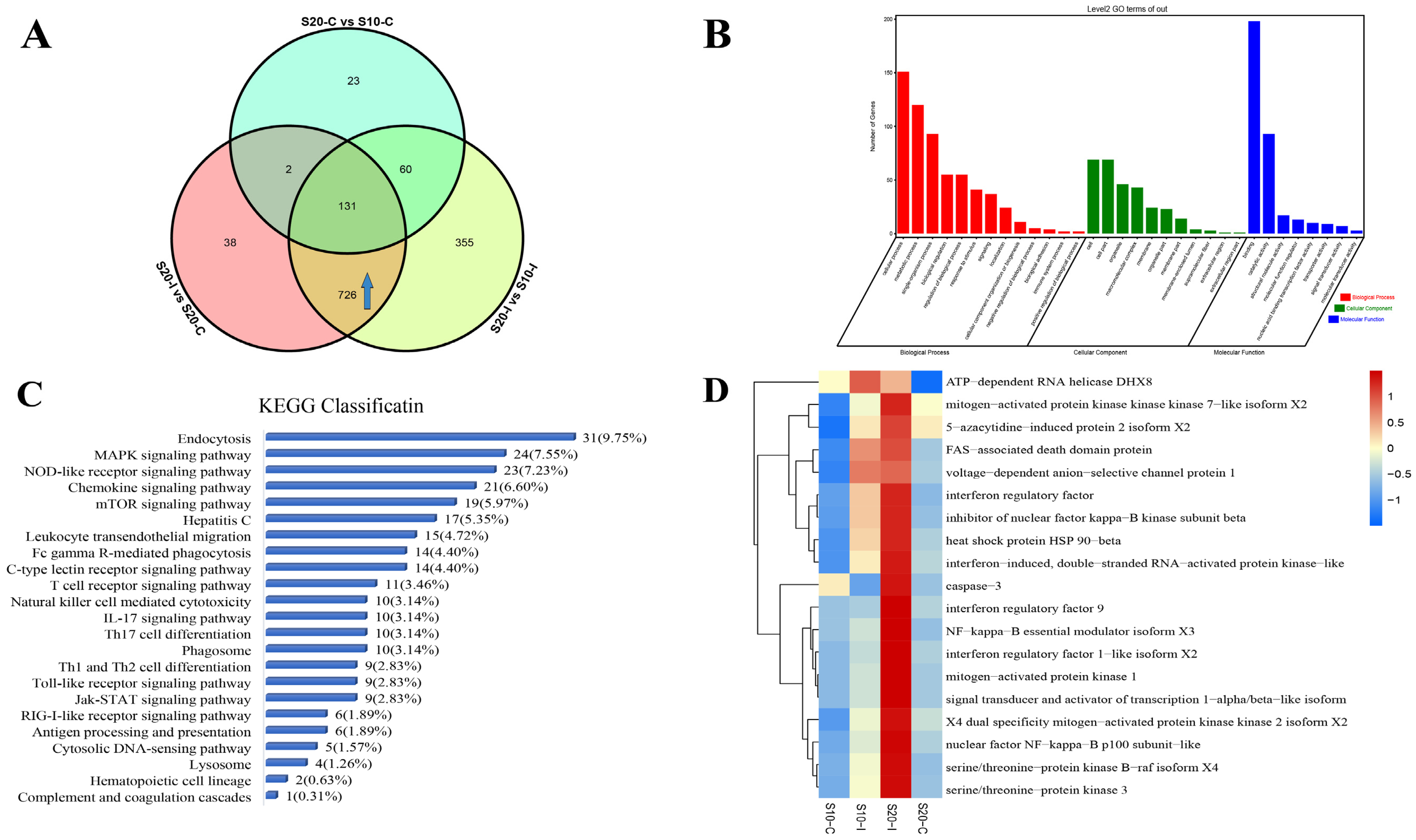
3.2. Analysis of DEPs and DEPPs under 20 °C
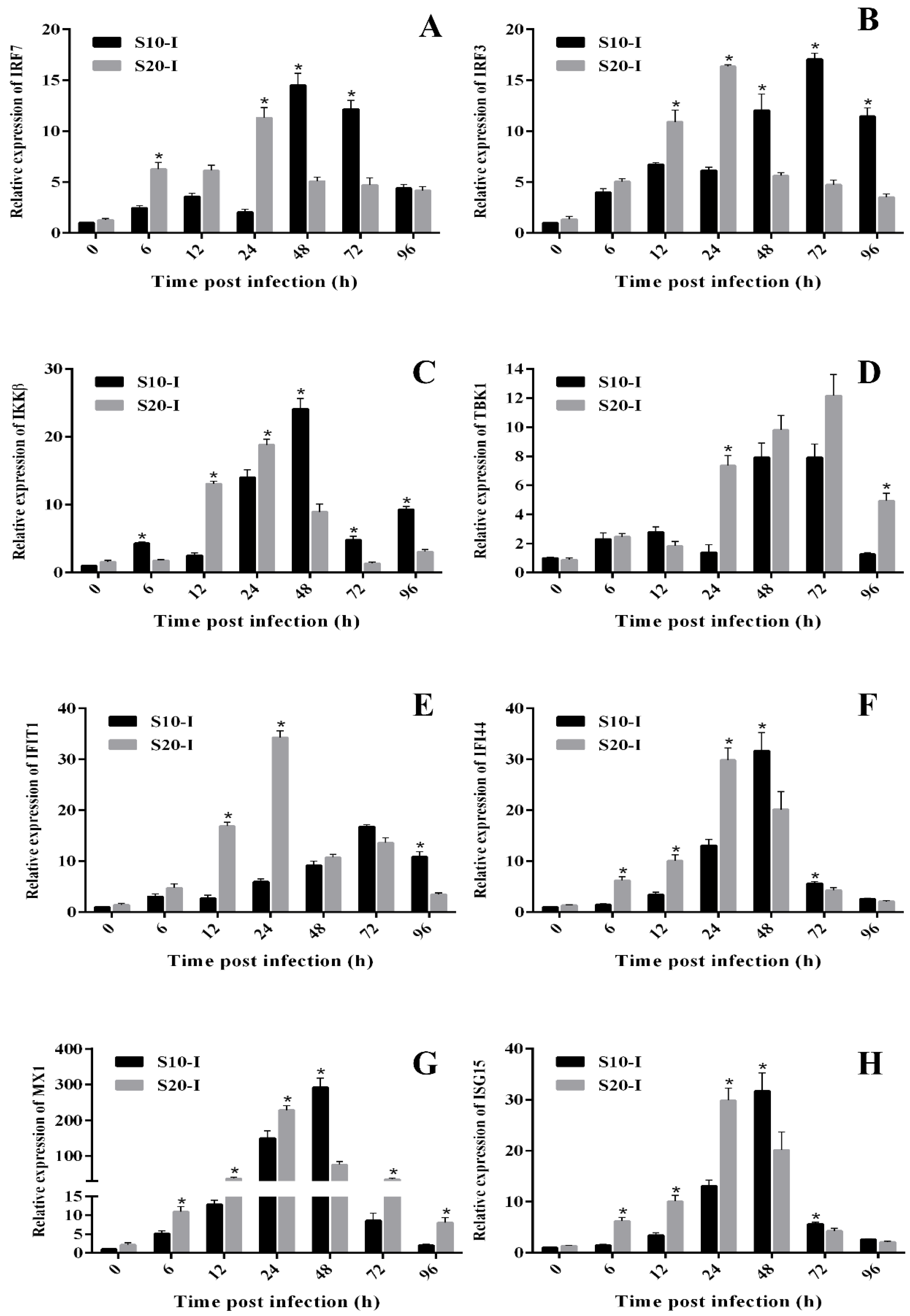
3.3. Temperature Affects the Anti-HIRRV Response of Flounder
3.4. Temporal Expressions of Genes Involved in Interferon Antiviral Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kimura, T.; Yoshimizu, M.; Gorie, S. A New Rhabdovirus Isolated in Japan from Cultured Hirame (Japanese Flounder) Paralichthys olivaceus and Ayu Plecoglossus altivelis. Dis. Aquat. Organ. 1985, 1, 209–217. [Google Scholar] [CrossRef]
- Sano, T.; Fukuda, H. Principal Microbial Diseases of Mariculture in Japan. Aquaculture 1987, 67, 59–69. [Google Scholar] [CrossRef]
- Kim, W.S.; Oh, M.-J. Hirame Rhabdovirus (HIRRV) as the Cause of a Natural Disease Outbreak in Cultured Black Seabream (Acanthopagrus schlegeli) in Korea. Arch. Virol. 2015, 160, 3063–3066. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.G.; Do, J.W.; Jung, S.H.; Han, H.J. Outbreak of hirame rhabdovirus infection in cultured spotted sea bass Lateolabrax maculatus on the western coast of Korea. J. Fish Dis. 2016, 39, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, Y.; Liu, H.; Gao, L.; Shi, X.; He, J.; Wang, Z. The isolation and characterization of a rhabdovirus from stone flounder, Kareius bicoloratus. Chin. J. Vet. Sci. 2009, 29, 277–282. [Google Scholar]
- Borzym, E.; Matras, M.; Maj-Paluch, J.; Baud, M.; De Boisséson, C.; Talbi, C.; Olesen, N.J.; Bigarré, L. First Isolation of Hirame Rhabdovirus from Freshwater Fish in Europe. J. Fish Dis. 2014, 37, 423–430. [Google Scholar] [CrossRef]
- Oseko, N.; Yoshimizu, M.; Kimura, T. Pathogenicity of Rhabdovirus Olivaceus (Hirame Rhabdovirus; HRV) for Salmonid Fish; Hokkaido University Press: New York, NY, USA, 1992; pp. 80–87. [Google Scholar]
- Zhao, L.; Tang, X.; Sheng, X.; Xing, J.; Chi, H.; Zhan, W. Different Immune Responses of Flounder (Paralichthys olivaceus) towards the Full-Length and N-Terminal or C-Terminal Portion of Hirame Novirhabdovirus Glycoprotein. Fish Shellfish Immunol. 2020, 104, 279–288. [Google Scholar] [CrossRef]
- Yasuike, M.; Kondo, H.; Hirono, I.; Aoki, T. Difference in Japanese Flounder, Paralichthys olivaceus Gene Expression Profile Following Hirame Rhabdovirus (HIRRV) G and N Protein DNA Vaccination. Fish Shellfish Immunol. 2007, 23, 531–541. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. Surface Display of Hirame Novirhabdovirus (HIRRV) G Protein in Lactococcus Lactis and Its Immune Protection in Flounder (Paralichthys olivaceus). Microb. Cell Factories 2019, 18, 142. [Google Scholar] [CrossRef]
- Oseko, N.; Yoshimizu, M.; Kimura, T. Effect of water temperature on artificial infection of Rhabdovirus olivaceus (Hirame rhabdovirus: HRV) to Hirame (Japanese flounder, Paralichtys olivaceus). Fish. Pathol. 1988, 23, 125–132. [Google Scholar] [CrossRef]
- Lapatra, S.E. Factors Affecting Pathogenicity of Infectious Hematopoietic Necrosis Virus (IHNV) for Salmonid Fish. J. Aquat. Anim. Health 1998, 10, 121–131. [Google Scholar] [CrossRef]
- Goodwin, A.E.; Merry, G.E.; Noyes, A.D. Persistence of Viral RNA in Fish Infected with VHSV-IVb at 15 °C and Then Moved to Warmer Temperatures after the Onset of Disease. J. Fish Dis. 2012, 35, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Ahne, W. The Influence of Environmental Temperature and Infection Route on the Immune Response of Carp (Cyprinus carpio) to Spring Viremia of Carp Virus (SVCV). Vet. Immunol. Immunopathol. 1986, 12, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, X.; Sheng, X.; Xing, J.; Chi, H.; Zhan, W. Transcriptome Analysis Reveals Temperature-Dependent Early Immune Response in Flounder (Paralichthys olivaceus) after Hirame Novirhabdovirus (HIRRV) Infection. Fish Shellfish Immunol. 2020, 107, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, F. Responses to temperature variation: Integration of thermoregulation and metabolism in vertebrates. J. Exp. Biol. 2009, 212, 2885–2891. [Google Scholar] [CrossRef]
- Le Morvan, C.; Deschaux, P.; Troutaud, D. Effects and Mechanisms of Environmental Temperature on Carp (Cyprinus carpio) Anti-DNP Antibody Response and Non-Specific Cytotoxic Cell Activity: A Kinetic Study. Dev. Comp. Immunol. 1996, 20, 331–340. [Google Scholar] [CrossRef]
- Avunje, S.; Oh, M.-J.; Jung, S.-J. Impaired TLR2 and TLR7 Response in Olive Flounder Infected with Viral Haemorrhagic Septicaemia Virus at Host Susceptible 15 °C but High at Non-Susceptible 20 °C. Fish Shellfish Immunol. 2013, 34, 1236–1243. [Google Scholar] [CrossRef]
- Hori, T.S.; Gamperl, A.K.; Booman, M.; Nash, G.W.; Rise, M.L. A Moderate Increase in Ambient Temperature Modulates the Atlantic Cod (Gadus morhua) Spleen Transcriptome Response to Intraperitoneal Viral Mimic Injection. BMC Genom. 2012, 13, 431. [Google Scholar] [CrossRef]
- Tang, X.; Ma, X.; Cao, J.; Sheng, X.; Xing, J.; Chi, H.; Zhan, W. The Influence of Temperature on the Antiviral Response of MIgM+ B Lymphocytes Against Hirame Novirhabdovirus in Flounder (Paralichthys olivaceus). Front. Immunol. 2022, 13, 802638. [Google Scholar] [CrossRef]
- Ewen, K.; Baker, M.; Wilhelm, D.; Aitken, R.J.; Koopman, P. Global Survey of Protein Expression during Gonadal Sex Determination in Mice. Mol. Cell Proteom. MCP 2009, 8, 2624–2641. [Google Scholar] [CrossRef]
- Ma, M.; Guo, X.; Wang, F.; Zhao, C.; Liu, Z.; Shi, Z.; Wang, Y.; Zhang, P.; Zhang, K.; Wang, N.; et al. Protein Expression Profile of the Mouse Metaphase-II Oocyte. J. Proteome Res. 2008, 7, 4821–4830. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, P.; Strating, J.R.P.M.; Defourny, K.A.Y.; Cesonyte, I.; Bottino, A.M.S.; Post, H.; Viktorova, E.G.; Ho, V.Q.T.; Langereis, M.A.; Belov, G.A.; et al. Dynamic Remodelling of the Human Host Cell Proteome and Phosphoproteome upon Enterovirus Infection. Nat. Commun. 2020, 11, 4332. [Google Scholar] [CrossRef] [PubMed]
- Bouhaddou, M.; Memon, D.; Meyer, B.; White, K.M.; Rezelj, V.V.; Correa Marrero, M.; Polacco, B.J.; Melnyk, J.E.; Ulferts, S.; Kaake, R.M.; et al. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell 2020, 182, 685–712. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, L.; Wang, X.; He, S.; Shi, W.; Chen, S. Temporal Proteomic and Phosphoproteomic Analysis of EV-A71-Infected Human Cells. J. Proteome Res. 2022, 21, 2367–2384. [Google Scholar] [CrossRef]
- Lemeer, S.; Heck, A.J. The Phosphoproteomics Data Explosion. Curr. Opin. Chem. Biol. 2009, 13, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ram, M.; Kumar, R.; Prasad, R.; Roy, B.K.; Singh, K.K. Phosphorylation: Implications in Cancer. Protein J. 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Söderholm, S.; Kainov, D.E.; Öhman, T.; Denisova, O.V.; Schepens, B.; Kulesskiy, E.; Imanishi, S.Y.; Corthals, G.; Hintsanen, P.; Aittokallio, T.; et al. Phosphoproteomics to Characterize Host Response During Influenza A Virus Infection of Human Macrophages. Mol. Cell Proteom. 2016, 15, 3203–3219. [Google Scholar] [CrossRef]
- Mohl, B.-P.; Emmott, E.; Roy, P. Phosphoproteomic Analysis Reveals the Importance of Kinase Regulation During Orbivirus Infection. Mol. Cell Proteom. 2017, 16, 1990–2005. [Google Scholar] [CrossRef]
- Liu, P.; Du, Y.; Meng, L.; Li, X.; Yang, D.; Liu, Y. Phosphoproteomic Analyses of Kidneys of Atlantic Salmon Infected with Aeromonas salmonicida. Sci. Rep. 2019, 9, 2101. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Xiao, Z.; Yang, J.; Xue, M.; Jiang, N.; Meng, Y.; Liu, W.; Fan, Y.; Zhou, Y. Proteomic and Phosphoproteomic Analyses Reveal Gibel Carp Responses to Cyprinid Herpesvirus 2 Infection. J. Proteome Res. 2022, 21, 1961–1973. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Y.; Yan, J.; Wang, J.; Wei, J.; Xiao, J.; Zeng, Y.; Feng, H. Multi-Omics Analysis Revealed Crucial Genes and Pathways Associated with Black Carp Antiviral Innate Immunity. Fish Shellfish Immunol. 2020, 106, 724–732. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. Isolation and Identification of a New Strain of Hirame Rhabdovirus (HIRRV) from Japanese Flounder Paralichthys olivaceus in China. Virol. J. 2017, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Leifer, C.A.; Medvedev, A.E. Molecular Mechanisms of Regulation of Toll-like Receptor Signaling. J. Leukoc. Biol. 2016, 100, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like Receptor and RIG-1-like Receptor Signaling. Ann. N. Y. Acad. Sci. 2008, 1143, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.; Bazan, J.F. Pathogen Recognition: TLRs Throw Us a Curve. Immunity 2005, 23, 347–350. [Google Scholar] [CrossRef]
- Loo, Y.-M.; Gale, M. Immune Signaling by RIG-I-like Receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef]
- Motta, V.; Soares, F.; Sun, T.; Philpott, D.J. NOD-Like Receptors: Versatile Cytosolic Sentinels. Physiol. Rev. 2015, 95, 149–178. [Google Scholar] [CrossRef]
- Bowie, A.G.; Unterholzner, L. Viral Evasion and Subversion of Pattern-Recognition Receptor Signalling. Nat. Rev. Immunol. 2008, 8, 911–922. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like Receptors and Innate Immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Jacobs, S.R.; Damania, B. NLRs, Inflammasomes, and Viral Infection. J. Leukoc. Biol. 2012, 92, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Rajendran, R.; Jang, Y.-S.; Kim, J.-O.; Yoon, S.-Y.; Oh, M.-J. NLRC3 Attenuates Antiviral Immunity and Activates Inflammasome Responses in Primary Grouper Brain Cells Following Nervous Necrosis Virus Infection. Fish Shellfish Immunol. 2022, 127, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.X.; Xiong, F.; Wu, X.M.; Hu, Y.W. The Expanding and Function of NLRC3 or NLRC3-like in Teleost Fish: Recent Advances and Novel Insights. Dev. Comp. Immunol. 2021, 114, 103859. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Fujita, T. RIG-I family RNA helicases: Cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 2007, 18, 545–551. [Google Scholar] [CrossRef]
- Su, J.; Huang, T.; Dong, J.; Heng, J.; Zhang, R.; Peng, L. Molecular Cloning and Immune Responsive Expression of MDA5 Gene, a Pivotal Member of the RLR Gene Family from Grass Carp Ctenopharyngodon idella. Fish Shellfish Immunol. 2010, 28, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M.; Hikima, J.; Kondo, H.; Hirono, I.; Jung, T.-S.; Aoki, T. Characterization and Antiviral Function of a Cytosolic Sensor Gene, MDA5, in Japanese Flounder, Paralichthys olivaceus. Dev. Comp. Immunol. 2011, 35, 554–562. [Google Scholar] [CrossRef]
- Ohtani, M.; Hikima, J.; Kondo, H.; Hirono, I.; Jung, T.-S.; Aoki, T. Evolutional Conservation of Molecular Structure and Antiviral Function of a Viral RNA Receptor, LGP2, in Japanese Flounder, Paralichthys olivaceus. J. Immunol. 2010, 185, 7507–7517. [Google Scholar] [CrossRef]
- Der, S.D.; Zhou, A.; Williams, B.R.G.; Silverman, R.H. Identification of Genes Differentially Regulated by Interferon α, β, or γ Using Oligonucleotide Arrays. Proc. Natl. Acad. Sci. USA 1998, 95, 15623–15628. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Rice, C.M. Interferon-Stimulated Genes and Their Antiviral Effector Functions. Curr. Opin. Virol. 2011, 1, 519–525. [Google Scholar] [CrossRef]
- Busse, D.C.; Habgood-Coote, D.; Clare, S.; Brandt, C.; Bassano, I.; Kaforou, M.; Herberg, J.; Levin, M.; Eléouët, J.-F.; Kellam, P.; et al. Interferon-Induced Protein 44 and Interferon-Induced Protein 44-Like Restrict Replication of Respiratory Syncytial Virus. J. Virol. 2020, 94, e00297-20. [Google Scholar] [CrossRef]
- Geiss, G.; Jin, G.; Guo, J.; Bumgarner, R.; Katze, M.G.; Sen, G.C. A Comprehensive View of Regulation of Gene Expression by Double-Stranded RNA-Mediated Cell Signaling. J. Biol. Chem. 2001, 276, 30178–30182. [Google Scholar] [CrossRef] [PubMed]
- Fensterl, V.; Sen, G.C. The ISG56/IFIT1 Gene Family. J. Interferon Cytokine Res. 2011, 31, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Ahn, S.J.; Kwon, M.-G.; Seo, J.S.; Hwang, S.D.; Son, M.-H. Interferon-Induced Protein 56 (IFI56) Is Induced by VHSV Infection but Not by Bacterial Infection in Olive Flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2017, 66, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Sun, L. CsIFIT1, an Interferon-Induced Protein with Tetratricopeptide Repeat, Inhibits Viral Infection in Tongue Sole (Cynoglossus semilaevis). Fish Shellfish Immunol. 2014, 41, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Staeheli, P.; Pitossi, F.; Pavlovic, J. Mx Proteins: GTPases with Antiviral Activity. Trends Cell Biol. 1993, 3, 268–272. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Lu, Y.-F.; Chi, S.-C. Anti-Viral Mechanism of Barramundi Mx against Betanoda virus Involves the Inhibition of Viral RNA Synthesis through the Interference of RdRp. Fish Shellfish Immunol. 2010, 28, 467–475. [Google Scholar] [CrossRef]
- Larsen, R.; Røkenes, T.P.; Robertsen, B. Inhibition of Infectious Pancreatic Necrosis Virus Replication by Atlantic Salmon Mx1 Protein. J. Virol. 2004, 78, 7938–7944. [Google Scholar] [CrossRef] [PubMed]
- Caipang, C.M.A.; Hirono, I.; Aoki, T. In Vitro Inhibition of Fish Rhabdoviruses by Japanese Flounder, Paralichthys olivaceus Mx. Virology 2003, 317, 373–382. [Google Scholar] [CrossRef]
- Seppola, M.; Stenvik, J.; Steiro, K.; Solstad, T.; Robertsen, B.; Jensen, I. Sequence and Expression Analysis of an Interferon Stimulated Gene (ISG15) from Atlantic Cod (Gadus morhua L.). Dev. Comp. Immunol. 2007, 31, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Dios, S.; Romero, A.; Chamorro, R.; Figueras, A.; Novoa, B. Effect of the Temperature during Antiviral Immune Response Ontogeny in Teleosts. Fish Shellfish Immunol. 2010, 29, 1019–1027. [Google Scholar] [CrossRef]
- Wu, W.; Dai, C.; Duan, X.; Wang, C.; Lin, X.; Ke, J.; Wang, Y.; Zhang, X.; Liu, H. miRNAs Induced by White Spot Syndrome Virus Involve in Immunity Pathways in Shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 93, 743–751. [Google Scholar] [CrossRef]
- Tjelle, T.E.; Løvdal, T.; Berg, T. Phagosome Dynamics and Function. BioEssays 2000, 22, 255–263. [Google Scholar] [CrossRef]
- Fairn, G.D.; Grinstein, S. How Nascent Phagosomes Mature to Become Phagolysosomes. Trends Immunol. 2012, 33, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lakadamyali, M.; Rust, M.J.; Zhuang, X. Endocytosis of Influenza Viruses. Microbes Infect. 2004, 6, 929–936. [Google Scholar] [CrossRef]
- MacArthur, J.I.; Fletcher, T.C. Phagocytosis in fish. In Fish Immunology; Manning, M.J., Tatner, M.F., Eds.; Academic Press: Cambridge, MA, USA, 1985; pp. 29–46. [Google Scholar]
- Ainsworth, A.J.; Dexiang, C.; Waterstrat, P.R.; Greenway, T. Effect of Temperature on the Immune System of Channel Catfish (Ictalurus punctatus)—I. Leucocyte Distribution and Phagocyte Function in the Anterior Kidney at 10 °C. Comp. Biochem. Physiol. A Physiol. 1991, 100, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.D.; Borgman, C.A.; Cobb, B.S.; Vines, R.R.; Reynolds, A.B.; Parsons, J.T. Pp125FAK a Structurally Distinctive Protein-Tyrosine Kinase Associated with Focal Adhesions. Proc. Natl. Acad. Sci. USA 1992, 89, 5192–5196. [Google Scholar] [CrossRef] [PubMed]
- Owen, K.A.; Thomas, K.S.; Bouton, A.H. The Differential Expression of Yersinia Pseudotuberculosis Adhesins Determines the Requirement for FAK and/or Pyk2 during Bacterial Phagocytosis by Macrophages. Cell Microbiol. 2007, 9, 596–609. [Google Scholar] [CrossRef]
- Elbahesh, H.; Bergmann, S.; Russell, C.J. Focal Adhesion Kinase (FAK) Regulates Polymerase Activity of Multiple Influenza A Virus Subtypes. Virology 2016, 499, 369–374. [Google Scholar] [CrossRef]
- Parsons, J.T. Focal Adhesion Kinase: The First Ten Years. J. Cell Sci. 2003, 116, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Gayrard, C.; Bernaudin, C.; Déjardin, T.; Seiler, C.; Borghi, N. Src- and Confinement-Dependent FAK Activation Causes E-Cadherin Relaxation and β-Catenin Activity. J. Cell Biol. 2018, 217, 1063–1077. [Google Scholar] [CrossRef]
- Bozym, R.A.; Delorme-Axford, E.; Harris, K.; Morosky, S.; Ikizler, M.; Dermody, T.S.; Sarkar, S.N.; Coyne, C.B. Focal Adhesion Kinase Is a Component of Antiviral RIG-I-like Receptor Signaling. Cell Host Microbe 2012, 11, 153–166. [Google Scholar] [CrossRef]
- Upton, J.W.; Chan, F.K.-M. Staying Alive: Cell Death in Antiviral Immunity. Mol. Cell 2014, 54, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, S.; Rall, G.F. Benefits and Perils of Necroptosis in Influenza Virus Infection. J. Virol. 2020, 94, e01101-19. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, V.; Cinti, A.; Amorim, R.; Mouland, A.J. Adapting the Stress Response: Viral Subversion of the MTOR Signaling Pathway. Viruses 2016, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Franz, K.M.; Kagan, J.C. PRRs Are Watching You: Localization of Innate Sensing and Signaling Regulators. Virology 2015, 479–480, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Yamashita, M.; Zhang, Y.; Sen, G.C. The IRF-3/Bax-Mediated Apoptotic Pathway, Activated by Viral Cytoplasmic RNA and DNA, Inhibits Virus Replication. J. Virol. 2011, 85, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- White, C.L.; Chattopadhyay, S.; Sen, G.C. Phosphatidylinositol 3-Kinase Signaling Delays Sendai Virus-Induced Apoptosis by Preventing XIAP Degradation. J. Virol. 2011, 85, 5224–5227. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Marques, J.T.; Yamashita, M.; Peters, K.L.; Smith, K.; Desai, A.; Williams, B.R.G.; Sen, G.C. Viral Apoptosis Is Induced by IRF-3-Mediated Activation of Bax. EMBO J. 2010, 29, 1762–1773. [Google Scholar] [CrossRef]


| Primers | Sequences(5′-3′) | Length(bp) | Accession No. |
|---|---|---|---|
| TBK1-F | GCAGAGCACCACTAACTACCT | 215 | XM_020110891 |
| TBK1-R | CAGCGAAGAGCTTCACAAT | ||
| MX1-F | ACCTGCCTGGAATCACC | 244 | XM_020086975 |
| MX1-R | GACTCCTCTGCTCCTTTG | ||
| IFI44-F | AGTGTTTAACGGACGAG | 144 | XM_020098597 |
| IFI44-R | CCCAGACCCATAGCA | ||
| IFIT1-F | AGCCACCGAACAGGA | 204 | KY399812 |
| IFIT1-R | GCCAAAGCAAGAGCC | ||
| IRF3-F | AACTCAAGCCAAACTGACCCG | 220 | GU017417 |
| IRF3-R | GAAGTCCATAATTCCTCGCACAA | ||
| IRF7-F | ATGGGCAGTGGCAAGTGGT | 184 | GU017419 |
| IRF7-R | GTTTTCTTGTCTGTGCTCGGTGT | ||
| IKKβ-F | GTCTTGGAGCCTAACG | 139 | XM_020099249 |
| IKKβ-R | GGGTGCGAGGAGTAA | ||
| ISG15-F | CTCCCATCCAGGTCTTCC | 199 | AB519717 |
| ISG15-R | GTGCTCTGTGCCTCAACG’ | ||
| 18S RNA-F | GGTCTGTGATGCCCTTAGATGTC | 107 | EF126037 |
| 18S RNA-F | AGTGGGGTTCAGCGGGTTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Zhang, Y.; Xing, J.; Sheng, X.; Chi, H.; Zhan, W. Proteomic and Phosphoproteomic Analysis Reveals Differential Immune Response to Hirame Novirhabdovirus (HIRRV) Infection in the Flounder (Paralichthys olivaceus) under Different Temperature. Biology 2023, 12, 1145. https://doi.org/10.3390/biology12081145
Tang X, Zhang Y, Xing J, Sheng X, Chi H, Zhan W. Proteomic and Phosphoproteomic Analysis Reveals Differential Immune Response to Hirame Novirhabdovirus (HIRRV) Infection in the Flounder (Paralichthys olivaceus) under Different Temperature. Biology. 2023; 12(8):1145. https://doi.org/10.3390/biology12081145
Chicago/Turabian StyleTang, Xiaoqian, Yingfeng Zhang, Jing Xing, Xiuzhen Sheng, Heng Chi, and Wenbin Zhan. 2023. "Proteomic and Phosphoproteomic Analysis Reveals Differential Immune Response to Hirame Novirhabdovirus (HIRRV) Infection in the Flounder (Paralichthys olivaceus) under Different Temperature" Biology 12, no. 8: 1145. https://doi.org/10.3390/biology12081145
APA StyleTang, X., Zhang, Y., Xing, J., Sheng, X., Chi, H., & Zhan, W. (2023). Proteomic and Phosphoproteomic Analysis Reveals Differential Immune Response to Hirame Novirhabdovirus (HIRRV) Infection in the Flounder (Paralichthys olivaceus) under Different Temperature. Biology, 12(8), 1145. https://doi.org/10.3390/biology12081145







