Identification and Characterization of Infectious Pathogens Associated with Mass Mortalities of Pacific Oyster (Crassostrea gigas) Cultured in Northern China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
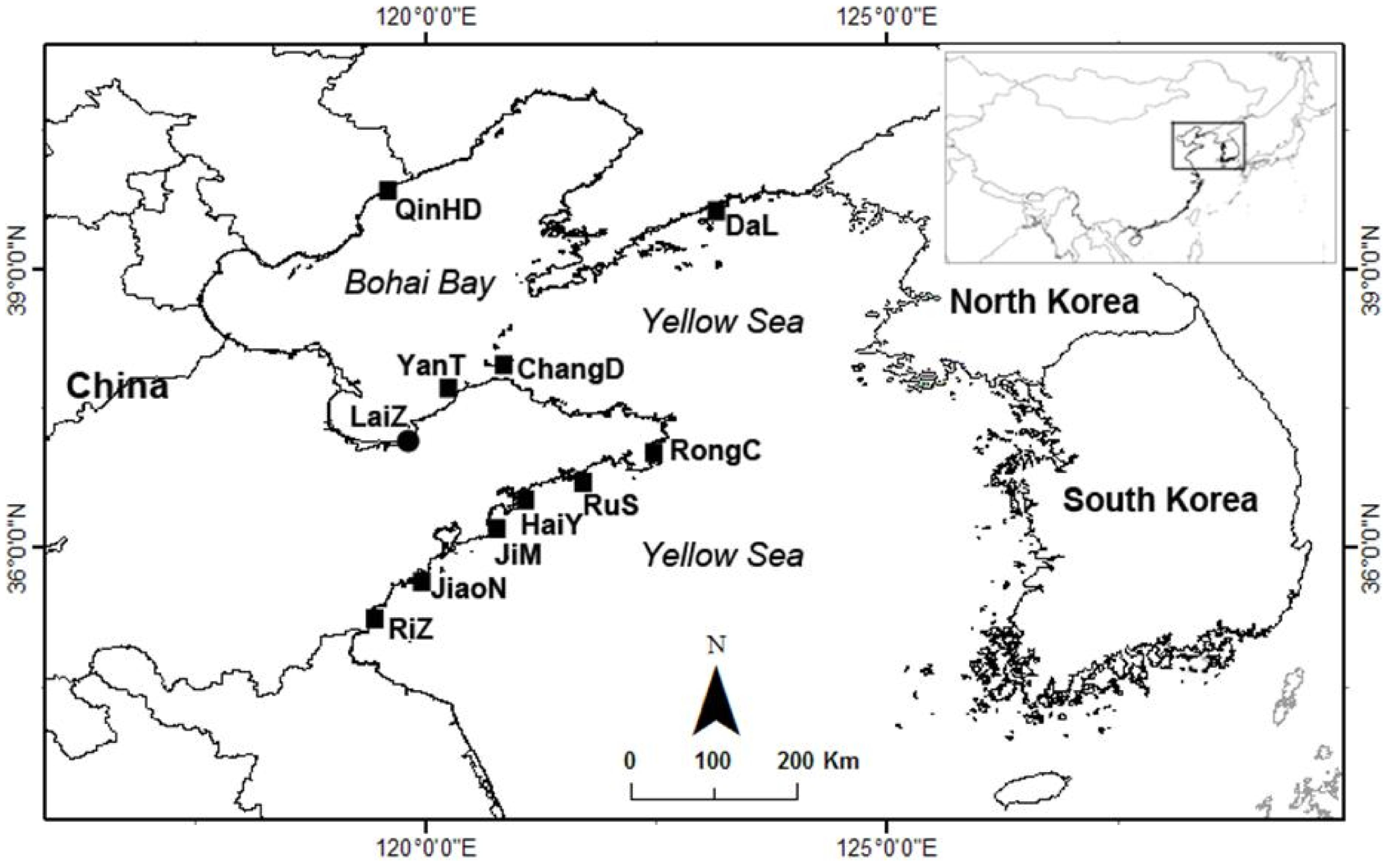
2.1. Sample Collection and Preprocessing
2.2. DNA Extraction and qPCR Screening of Known Pathogens
2.3. Bacteria Isolation and Identification
2.4. Histopathology Analysis
2.5. Morphological and Cultural Characteristic Analysis
2.6. Haemolytic Activity and Chrome Azurol S Production Analysis
2.7. Experimental Infection
3. Results
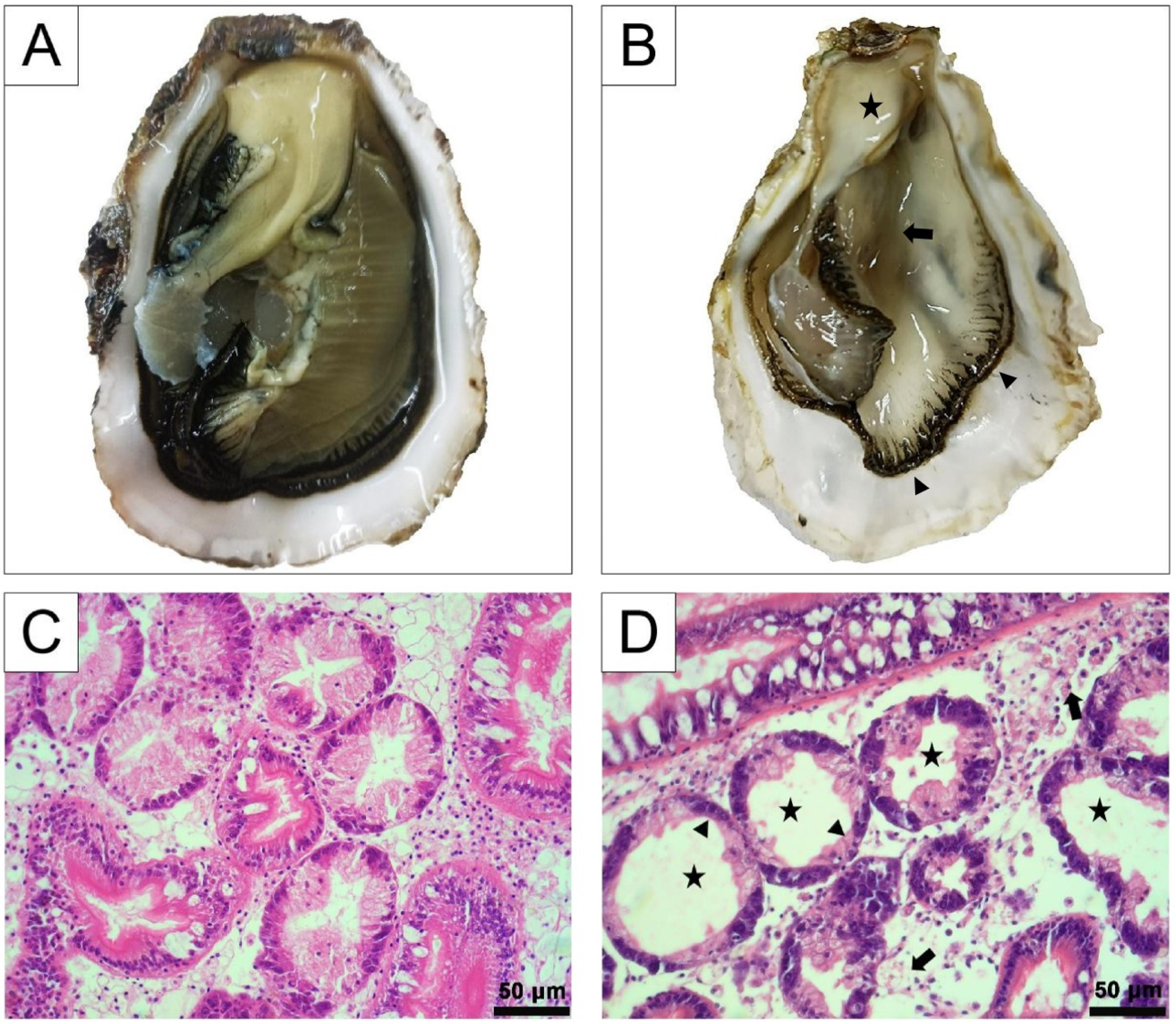
3.1. Pathological Characteristics of Diseased Pacific Oysters
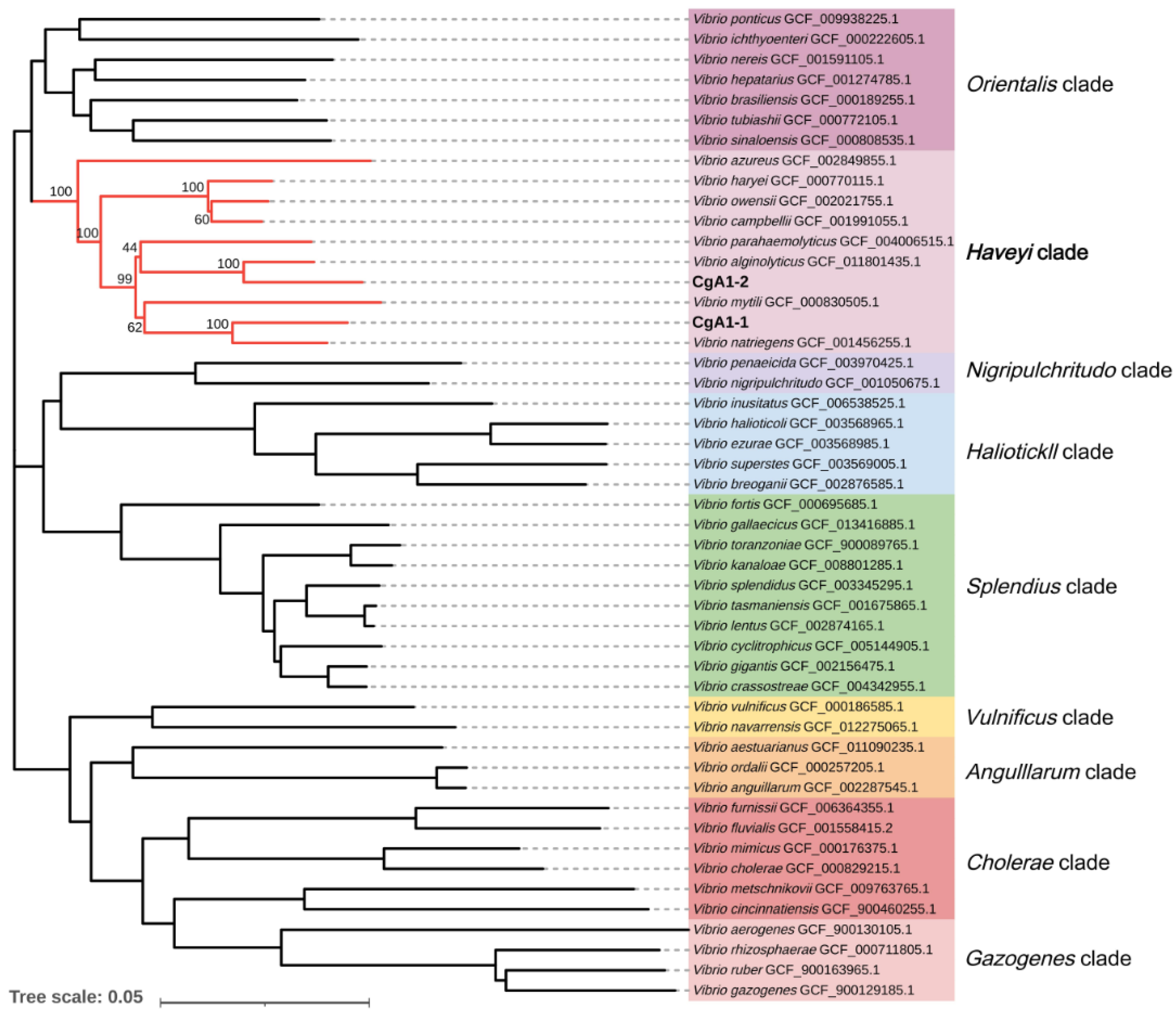
3.2. Isolation and Identification of Bacteria from Diseased Pacific Oysters
3.3. Phenotypic Characterization of CgA1-1 and CgA1-2
3.4. Haemolytic Activity and Siderophore Production Analysis
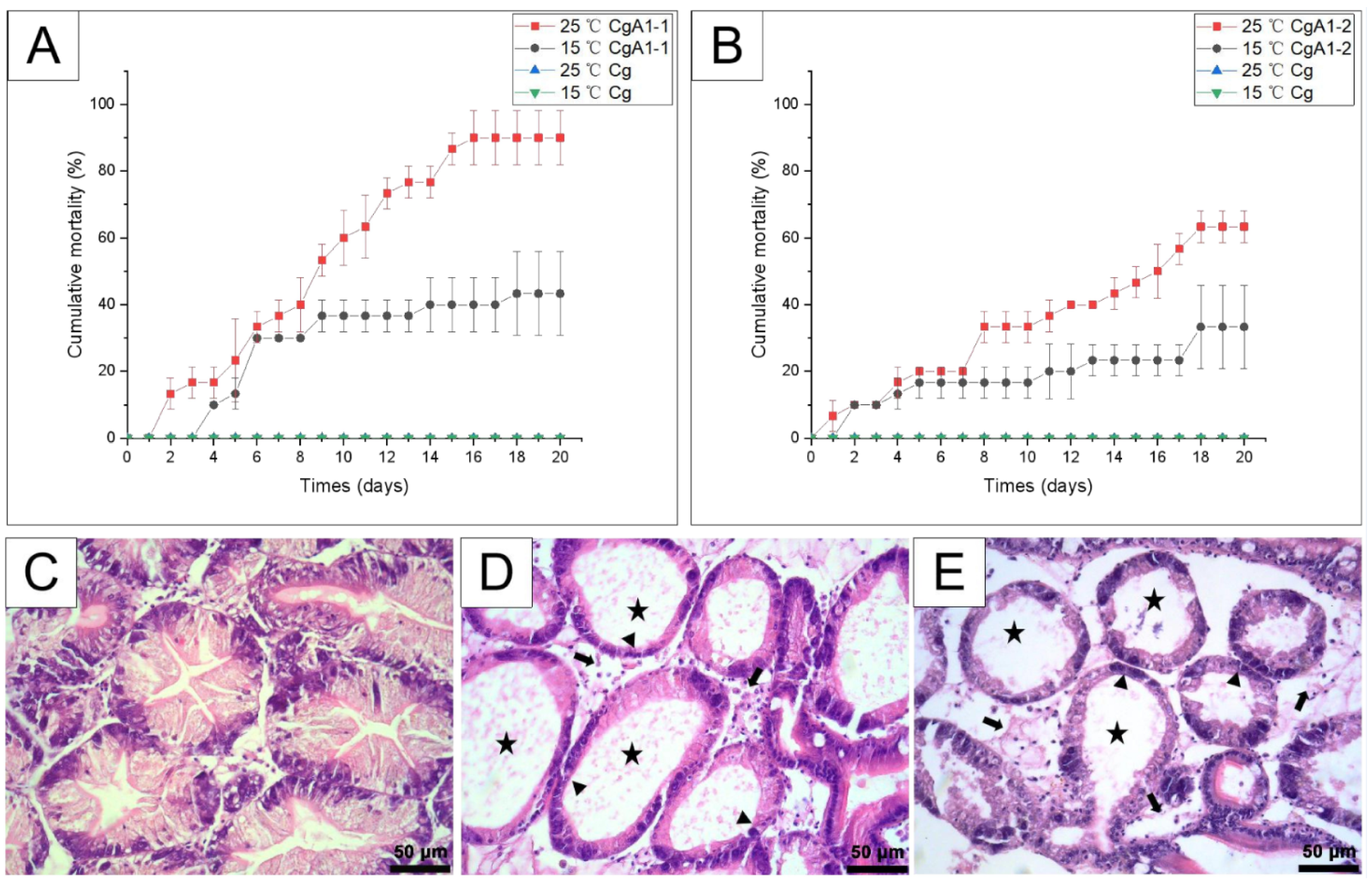
3.5. Temperature Effects on the Pathogenicity of CgA1-1 and CgA1-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayne, B.L. Developments in aquaculture and fisheries science. In Biology of Oysters; Academic Press: London, UK, 2017; p. 844. [Google Scholar]
- Guo, X. Use and exchange of genetic resources in molluscan aquaculture. Rev. Aquac. 2009, 1, 251–259. [Google Scholar] [CrossRef]
- Zhang, G.; Li, L.; Que, H. An evolution of oyster mariculture industry in china: New knowledge, variety and product. Oceanol. Limnol. Sin. 2020, 51, 740–749. [Google Scholar] [CrossRef]
- Botta, R.; Asche, F.; Borsum, J.S.; Camp, E.V. A review of global oyster aquaculture production and consumption. Mar. Policy 2020, 117, 103952. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics. Global Aquaculture Production 1950–2020 (FishstatJ). Available online: https://www.fao.org/fishery/statistics-query/en/aquaculture/aquaculture_quantity (accessed on 16 September 2022).
- China Mollusk Research System. Developemnt report of oyster industry in China. China Fish 2021, 6, 20–31. [Google Scholar]
- Jiang, R.; Mu, Y. Analysis of agglomeration characteristics of Chinese oyster breeding industry. Chin. Fish. Econ. 2021, 39, 55–61. [Google Scholar]
- Carnegie, R.B.; Arzul, I.; Bushek, D. Managing marine mollusc diseases in the context of regional and international commerce: Policy issues and emerging concerns. Philos. Trans. R. Soc. B 2016, 371, 20150215. [Google Scholar] [CrossRef]
- Ventilla, R.F. Recent developments in the Japanese oyster culture industry. Adv. Mar. Biol. 1984, 21, 1–57. [Google Scholar] [CrossRef]
- Samain, J. Review and perspectives of physiological mechanisms underlying genetically-based resistance of the Pacific oyster Crassostrea gigas to summer mortality. Aquat. Living Resour. 2011, 24, 227–236. [Google Scholar] [CrossRef]
- Cowan, M. Exploring the Mechanisms of Pacific Oyster Summer Mortality in Baynes Sound Aquaculture. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 2020. [Google Scholar]
- Lang, R.P.; Langdon, C.J.; Taris, N.G.; Camara, M.D. Use of laboratory assays to predict subsequent growth and survival of Pacific oyster (Crassostrea gigas) families planted in coastal waters. Aquaculture 2010, 306, 68–79. [Google Scholar] [CrossRef]
- Soletchnik, P.; Ropert, M.; Mazurié, J.; Fleury, P.G.; Le Coz, F. Relationships between oyster mortality patterns and environmental data from monitoring databases along the coasts of France. Aquaculture 2007, 271, 384–400. [Google Scholar] [CrossRef]
- Yang, B.; Zhai, S.; Li, X.; Tian, J.; Li, Q.; Shan, H.; Liu, S. Identification of Vibrio alginolyticus as a causative pathogen associated with mass summer mortality of the Pacific Oyster (Crassostrea gigas) in China. Aquaculture 2021, 535, 736363. [Google Scholar] [CrossRef]
- Cotter, E.; Malham, S.K.; O’Keeffe, S.; Lynch, S.A.; Latchford, J.W.; King, J.W.; Beaumont, A.R.; Culloty, S.C. Summer mortality of the Pacific oyster, Crassostrea gigas, in the Irish Sea: The influence of growth, biochemistry and gametogenesis. Aquaculture 2010, 303, 8–21. [Google Scholar] [CrossRef]
- Malham, S.K.; Cotter, E.; O’Keeffe, S.; Lynch, S.; Culloty, S.C.; King, J.W.; Latchford, J.W.; Beaumont, A.R. Summer mortality of the Pacific oyster, Crassostrea gigas, in the Irish Sea: The influence of temperature and nutrients on health and survival. Aquaculture 2009, 287, 128–138. [Google Scholar] [CrossRef]
- Petton, B.; Boudry, P.; Alunno-Bruscia, M.; Pernet, F. Factors influencing disease-induced mortality of Pacific oysters Crassostrea gigas. Aquac. Environ. Interact. 2015, 6, 205–222. [Google Scholar] [CrossRef]
- Arzul, I.; Corbeil, S.; Morga, B.; Renault, T. Viruses infecting marine molluscs. J. Invertebr. Pathol. 2017, 147, 118–135. [Google Scholar] [CrossRef]
- Pernet, F.; Lupo, C.; Bacher, C.; Whittington, R.J. Infectious diseases in oyster aquaculture require a new integrated approach. Philos. Trans. R. Soc. B 2016, 371, 20150213. [Google Scholar] [CrossRef]
- Carnegie, R.B. Effects in mollusc culture. In Marine Parasitology; Rohde, K., Ed.; CSIRO PUBLISHING: Collingwood, Australia, 2005; Chapter 10; pp. 391–398. ISBN 0 643 09025 8. [Google Scholar]
- Hine, P.M.; Wesney, B.; Hay, B.E. Herpesviruses associated with mortalities among hatchery-reared larval Pacific oysters Crassostrea gigas. Dis. Aquat. Org. 1992, 12, 135–142. [Google Scholar] [CrossRef]
- Renault, T.; Cochennec, N.; Le Deuff, R.; Chollet, B. Herpes-like virus infecting Japanese oyster (Crassostrea gigas) spat. Bull. Eur. Assoc. Fish. Pathol. 1994, 14, 64–66. [Google Scholar]
- Da Silva, P.M.; Renault, T.; Fuentes, J.; Villalba, A. Herpesvirus infection in European flat oysters Ostrea edulis obtained from brood stocks of various geographic origins and grown in Galicia (NW Spain). Dis. Aquat. Org. 2008, 78, 181–188. [Google Scholar] [CrossRef]
- Friedman, C.S.; Estes, R.M.; Stokes, N.A.; Burge, C.A.; Hargove, J.S.; Barber, B.J.; Elston, R.A.; Burreson, E.M.; Reece, K.S. Herpes virus in juvenile Pacific oysters Crassostrea gigas from Tomales Bay, California, coincides with summer mortality episodes. Dis. Aquat. Org. 2005, 63, 33–41. [Google Scholar] [CrossRef]
- Vasquez-Yeomans, R.; Caceres-Martinez, J.; Huerta, A.F. Herpes-like virus associated with eroded gills of the Pacific oyster Crassostrea gigas in Mexico. J. Shellfish Res. 2004, 23, 417–420. [Google Scholar]
- Segarra, A.; Pepin, J.F.; Arzul, I.; Morga, B.; Faury, N.; Renault, T. Detection and description of a particular Ostreid herpesvirus 1 genotype associated with massive mortality outbreaks of Pacific oysters, Crassostrea gigas, in France in 2008. Virus Res. 2010, 153, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Barbosa Solomieu, V.; Renault, T.; Travers, M. Mass mortality in bivalves and the intricate case of the Pacific oyster, Crassostrea gigas. J. Invertebr. Pathol. 2015, 131, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Garnier, M.; Labreuche, Y.; Garcia, C.; Robert, M.; Nicolas, J.L. Evidence for the involvement of pathogenic bacteria in summer mortalities of the Pacific oyster Crassostrea gigas. Microb. Ecol. 2007, 53, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Gay, M.; Berthe, F.C.; Le Roux, F. Screening of Vibrio isolates to develop an experimental infection model in the Pacific oyster Crassostrea gigas. Dis. Aquat. Org. 2004, 59, 49–56. [Google Scholar] [CrossRef]
- Sugumar, G.; Nakai, T.; Hirata, Y.; Matsubara, D.; Muroga, K. Vibrio splendidus biovar II as the causative agent of bacillary necrosis of Japanese oyster Crassostrea gigas larvae. Dis. Aquat. Org. 1998, 33, 111–118. [Google Scholar] [CrossRef]
- de Lorgeril, J.; Lucasson, A.; Petton, B.; Toulza, E.; Montagnani, C.; Clerissi, C.; Vidal-Dupiol, J.; Chaparro, C.; Galinier, R.; Escoubas, J.M.; et al. Immune-suppression by OsHV-1 viral infection causes fatal bacteraemia in Pacific oysters. Nat. Commun. 2018, 9, 4215. [Google Scholar] [CrossRef]
- Pathirana, E.; Fuhrmann, M.; Whittington, R.; Hick, P. Influence of environment on the pathogenesis of Ostreid herpesvirus-1 (OsHV-1) infections in Pacific oysters (Crassostrea gigas) through differential microbiome responses. Heliyon 2019, 5, e2101. [Google Scholar] [CrossRef]
- Howard, D.W.; Lewis, E.J.; Keller, B.J.; Smith, C.S. Histological Techniques for Marine Bivalve Mollusks and Crustaceans, 2nd ed.; NOAA, National Ocean Service, National Centers for Coastal Ocean Service, Cooperative Oxford Laboratory: Oxford, MD, USA, 2004; Chapter 2; pp. 7–9.
- Martenot, C.; Oden, E.; Travaillé, E.; Malas, J.P.; Houssin, M. Comparison of two real-time PCR methods for detection of ostreid herpesvirus 1 in the Pacific oyster Crassostrea gigas. J. Virol. Methods 2010, 170, 86–89. [Google Scholar] [CrossRef]
- Audemard, C.; Reece, K.S.; Burreson, E.M. Real-Time PCR for Detection and Quantification of the Protistan Parasite Perkinsus marinus in Environmental Waters. Appl. Environ. Microb. 2004, 70, 6611–6618. [Google Scholar] [CrossRef]
- Canier, L.; Dubreuil, C.; Noyer, M.; Serpin, D.; Chollet, B.; Garcia, C.; Arzul, I. A new multiplex real-time PCR assay to improve the diagnosis of shellfish regulated parasites of the genus Marteilia and Bonamia. Prev. Vet. Med. 2020, 183, 105126. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 1992, 89, 5685–5689. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.C.; Thompson, F.L.; Vicente, A.C.P.; Swings, J. Phylogenetic analysis of vibrios and related species by means of atpA gene sequences. Int. J. Syst. Evol. Microbiol. 2007, 57, 2480–2484. [Google Scholar] [CrossRef] [PubMed]
- Sawabe, T.; Kita-Tsukamoto, K.; Thompson, F.L. Inferring the Evolutionary History of Vibrios by Means of Multilocus Sequence Analysis. J. Bacteriol. 2007, 189, 7932–7936. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Macián, M.C.; Arahal, D.R.; Garay, E.; Pujalte, M.J. Multilocus sequence analysis of the central clade of the genus Vibrio by using the 16S rRNA, recA, pyrH, rpoD, gyrB, rctB and toxR genes. Int. J. Syst. Evol. Microbiol. 2010, 60, 154–165. [Google Scholar] [CrossRef]
- Thompson, F.L.; Gevers, D.; Thompson, C.C.; Dawyndt, P.; Naser, S.; Hoste, B.; Munn, C.B.; Swings, J. Phylogeny and Molecular Identification of Vibrios on the Basis of Multilocus Sequence Analysis. Appl. Environ. Microb. 2005, 71, 5107–5115. [Google Scholar] [CrossRef]
- Le Roux, F.; Gay, M.; Lambert, C.; Nicolas, J.L.; Gouy, M.; Berthe, F. Phylogenetic study and identification of Vibrio splendidus-related strains based on gyrB gene sequences. Dis. Aquat. Org. 2004, 58, 143–150. [Google Scholar] [CrossRef]
- Pérez-Cataluña, A.; Lucena, T.; Tarazona, E.; Arahal, D.R.; Macián, M.C.; Pujalte, M.J. An MLSA approach for the taxonomic update of the Splendidus clade, a lineage containing several fish and shellfish pathogenic Vibrio spp. Syst. Appl. Microbiol. 2016, 39, 361–369. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Takemoto, Y.; Matsubara, T. Haematological study of bacteria affected oysters. Hiroshima-Ken Suisan Shikenjo Hokoku (Hiroshima Prefect. Fish. Exp. Stn.) 1960, 22, 1–7. [Google Scholar]
- Elston, R.A. Infectious diseases of the Pacific oyster, Crassostrea gigas. Annu. Rev. Fish Dis. 1993, 3, 259–276. [Google Scholar] [CrossRef]
- Moore, J.D.; Juhasz, C.I.; Robbins, T.T. A histopathology survey of California oysters. Calif. Fish Game Rep. 2011, 97, 68–83. [Google Scholar]
- Samain, J.; Degremont, L.; Soletchnik, P.; Haure, J.; Bédier, E.; Ropert, M.; Moal, J.; Huvet, A.; Bacca, H.; Van Wormhoudt, A. Genetically based resistance to summer mortality in the Pacific oyster (Crassostrea gigas) and its relationship with physiological, immunological characteristics and infection processes. Aquaculture 2007, 268, 227–243. [Google Scholar] [CrossRef]
- Saulnier, D.; De Decker, S.; Haffner, P.; Cobret, L.; Robert, M.; Garcia, C. A large-scale epidemiological study to identify bacteria pathogenic to Pacific oyster Crassostrea gigas and correlation between virulence and metalloprotease-like activity. Microb. Ecol. 2010, 59, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Travers, M.; Miller, K.B.; Roque, A.; Friedman, C.S. Bacterial diseases in marine bivalves. J. Invertebr. Pathol. 2015, 131, 11–31. [Google Scholar] [CrossRef]
- Renault, T.; Qin, J.G. Pacific cupped oyster, Crassostrea gigas, mortality outbreaks and infectious diseases. Oysters Physiol. Ecol. Distrib. Mortal. 2012, 203, 225. [Google Scholar]
- Green, T.J.; Siboni, N.; King, W.L.; Labbate, M.; Seymour, J.R.; Raftos, D. Simulated marine heat wave alters abundance and structure of Vibrio populations associated with the Pacific Oyster resulting in a mass mortality event. Microb. Ecol. 2019, 77, 736–747. [Google Scholar] [CrossRef]
- Wendling, C.C.; Wegner, K.M. Relative contribution of reproductive investment, thermal stress and Vibrio infection to summer mortality phenomena in Pacific oysters. Aquaculture 2013, 412–413, 88–96. [Google Scholar] [CrossRef]
- Wang, H.; Yang, B.; Li, X.; Li, Q.; Liu, S. Screening of bacterial pathogens associated with mass summer mortality of the Pacific oyster, Crassostrea gigas, in China. Aquac. Rep. 2021, 20, 100672. [Google Scholar] [CrossRef]
- Fang, H. Epidemiological Investigation and Virulence Gene Detection of Shellfish Vibrio in Shandong Province. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2019. [Google Scholar]
- Wei, Y. Study on Molecular Response to Heat Stress and Vibrio alginolytic Infection in the Immature and Post-Spawning Populations of Pacific Oyster (Crassostrea gigas). Master’s Thesis, Guangxi University, Nanning, China, 2020. [Google Scholar]
- Kahla-Nakbi, A.B.; Chaieb, K.; Besbes, A.; Zmantar, T.; Bakhrouf, A. Virulence and enterobacterial repetitive intergenic consensus PCR of Vibrio alginolyticus strains isolated from Tunisian cultured gilthead sea bream and sea bass outbreaks. Vet. Microbiol. 2006, 117, 321–327. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, W.; Hsu, J.; Chen, J. Vibrio alginolyticus infection in the white shrimp Litopenaeus vannamei confirmed by polymerase chain reaction and 16S rDNA sequencing. Dis. Aquat. Org. 2004, 61, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Gómez-León, J.; Villamil, L.; Lemos, M.L.; Novoa, B.; Figureueras, A. Isolation of Vibrio alginolyticus and Vibrio splendidus from aquacultured carpet shell clam (Ruditapes decussatus) larvae associated with mass mortalities. Appl. Environ. Microb. 2005, 71, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Burge, C.A.; Mark Eakin, C.; Friedman, C.S.; Froelich, B.; Hershberger, P.K.; Hofmann, E.E.; Petes, L.E.; Prager, K.C.; Weil, E.; Willis, B.L. Climate change influences on marine infectious diseases: Implications for management and society. Annu. Rev. Mar. Sci. 2014, 6, 249–277. [Google Scholar] [CrossRef] [PubMed]
- Oberbeckmann, S.; Fuchs, B.M.; Meiners, M.; Wichels, A.; Wiltshire, K.H.; Gerdts, G. Seasonal dynamics and modeling of a Vibrio community in coastal waters of the North Sea. Microb. Ecol. 2012, 63, 543–551. [Google Scholar] [CrossRef]
- Darshanee Ruwandeepika, H.A.; Sanjeewa Prasad Jayaweera, T.; Paban Bhowmick, P.; Karunasagar, I.; Bossier, P.; Defoirdt, T. Pathogenesis, virulence factors and virulence regulation of vibrios belonging to the Harveyi clade. Rev. Aquac. 2012, 4, 59–74. [Google Scholar] [CrossRef]
- Gundogan, N.; Yakar, U.A. Siderophore production, serum resistance, hemolytic activity and extended-spectrum β-lactamase-producing Klebsiella species isolated from milk and milk products. J. Food Saf. 2007, 27, 251–264. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, X.; Wang, C.; Bai, C.; Li, C.; Li, C.; Xin, L. Isolation and Characterization of Vibrio kanaloae as a Major Pathogen Associated with Mass Mortalities of Ark Clam, Scapharca broughtonii, in Cold Season. Microorganisms 2021, 9, 2161. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.A.; González, C.J.; Otero, A.; García-López, M. Hemolytic activity and siderophore production in different Aeromonas species isolated from fish. Appl. Environ. Microb. 1999, 65, 5612–5614. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, F.; Wegner, K.M.; Baker-Austin, C.; Vezzulli, L.; Osorio, C.R.; Amaro, C.; Ritchie, J.M.; Defoirdt, T.; Destoumieux-Garzón, D.; Blokesch, M. The emergence of Vibrio pathogens in Europe: Ecology, evolution, and pathogenesis (Paris, 11–12th March 2015). Front. Microbiol. 2015, 6, 830. [Google Scholar] [CrossRef] [PubMed]
- Paul-Pont, I.; Dhand, N.K.; Whittington, R.J. Influence of husbandry practices on OsHV-1 associated mortality of Pacific oysters Crassostrea gigas. Aquaculture 2013, 412, 202–214. [Google Scholar] [CrossRef]
- Azéma, P.; Lamy, J.; Boudry, P.; Renault, T.; Travers, M.; Dégremont, L. Genetic parameters of resistance to Vibrio aestuarianus, and OsHV-1 infections in the Pacific oyster, Crassostrea gigas, at three different life stages. Genet. Sel. Evol. 2017, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, F.; Wegner, K.M.; Polz, M.F. Oysters and vibrios as a model for disease dynamics in wild animals. Trends Microbiol. 2016, 24, 568–580. [Google Scholar] [CrossRef]
- Pernet, F.; Tamayo, D.; Fuhrmann, M.; Petton, B. Deciphering the effect of food availability, growth and host condition on disease susceptibility in a marine invertebrate. J. Exp. Biol. 2019, 222, b210534. [Google Scholar] [CrossRef]
- Nicolas, J.L.; Comps, M.; Cochennec, N. Herpes-like virus infecting Pacific oyster larvae. Bull. Eur. Ass. Fish Pathol. 1992, 12, 11. [Google Scholar]
- Abbadi, M.; Zamperin, G.; Gastaldelli, M.; Pascoli, F.; Rosani, U.; Milani, A.; Schivo, A.; Rossetti, E.; Turolla, E.; Gennari, L. Identification of a newly described OsHV-1 µvar from the North Adriatic Sea (Italy). J. Gen. Virol. 2018, 99, 693. [Google Scholar] [CrossRef]
- Bai, C.; Morga, B.; Rosani, U.; Shi, J.; Li, C.; Xin, L.; Wang, C. Long-range PCR and high-throughput sequencing of Ostreid herpesvirus 1 indicate high genetic diversity and complex evolution process. Virology 2019, 526, 81–90. [Google Scholar] [CrossRef]
- Martenot, C.; Oden, E.; Travaillé, E.; Malas, J.; Houssin, M. Detection of different variants of Ostreid Herpesvirus 1 in the Pacific oyster, Crassostrea gigas between 2008 and 2010. Virus Res. 2011, 160, 25–31. [Google Scholar] [CrossRef]
- Peeler, E.J.; Reese, R.A.; Cheslett, D.L.; Geoghegan, F.; Power, A.; Thrush, M.A. Investigation of mortality in Pacific oysters associated with Ostreid herpesvirus-1 μVar in the Republic of Ireland in 2009. Prev. Vet. Med. 2012, 105, 136–143. [Google Scholar] [CrossRef]
- Song, W.; Wang, C. New Research Porgress on Massive Mortality of Cultured Scallop Chlamys farreri. Mar. Sci. Qingdao Chin. Ed. 2001, 25, 23–29. [Google Scholar]
- Wang, C.; Wang, X.; Song, X.; Huang, J.; Song, W. Purification and ultrastructure of a spherical virus in cultured scallop Chlamys farreri. Shuichan Xuebao 2002, 26, 180–183. [Google Scholar]
- Bai, C.; Xin, L.; Wang, C. Malacoherpesviruses and their associated damages to mollusk aquaculture industry. Prog. Fish. Sci. 2021, 42, 214–226. [Google Scholar] [CrossRef]
- Yao, S.; Li, L.; Guan, X.; He, Y.; Jouaux, A.; Xu, F.; Guo, X.; Zhang, G.; Zhang, L. Pooled resequencing of larvae and adults reveals genomic variations associated with Ostreid herpesvirus 1 resistance in the Pacific oyster Crassostrea gigas. Front. Immunol. 2022, 13, 928628. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Wang, C.; Xia, J.; Sun, H.; Zhang, S.; Huang, J. Emerging and endemic types of Ostreid herpesvirus 1 were detected in bivalves in China. J. Invertebr. Pathol. 2015, 124, 98–106. [Google Scholar] [CrossRef]
- Xia, J.; Bai, C.; Wang, C.; Song, X.; Huang, J. Complete genome sequence of Ostreid herpesvirus-1 associated with mortalities of Scapharca broughtonii broodstocks. Virol. J. 2015, 12, 1–9. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Park, J.J.; Yu, H.J.; Hur, Y.B.; Arzul, I.; Couraleau, Y.; Park, M.A. Ostreid herpesvirus 1 infection in farmed Pacific oyster larvae Crassostrea gigas (Thunberg) in Korea. J. Fish Dis. 2013, 36, 969–972. [Google Scholar] [CrossRef]
- Jee, B.Y.; Lee, S.J.; Cho, M.Y.; Lee, S.J.; Kim, J.W.; Choi, S.H.; Jeong, H.D.; Kim, K.H. Detection of Ostreid Herpesvirus 1 from adult Pacific oysters Crassostrea gigas cultured in Korea. Fish. Aquat. Sci. 2013, 16, 131–135. [Google Scholar] [CrossRef]
- Shimahara, Y.; Kurita, J.; Kiryu, I.; Nishioka, T.; Yuasa, K.; Kawana, M.; Kamaishi, T.; Oseko, N. Surveillance of Type 1 Ostreid Herpesvirus (OsHV-1) Variants in Japan. Gyobyō Kenkyu 2012, 47, 129–136. [Google Scholar] [CrossRef]
- Tun, K.L.; Itoh, N.; Shimizu, Y.; Yamanoi, H.; Yoshinaga, T.; Ogawa, K. Pathogenicity of the protozoan parasite Marteilioides chungmuensis in the Pacific oyster Crassostrea gigas. Int. J. Parasitol. 2008, 38, 211–217. [Google Scholar] [CrossRef]
- Enriquez-Espinoza, T.L.; Grijalva-Chon, J.M.; Castro-Longoria, R.; Ramos-Paredes, J. Perkinsus marinus in Crassostrea gigas in the Gulf of California. Dis. Aquat. Org. 2010, 89, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Wu, L. Studies on Distribution and Detection Methods of Perkinsus spp. in Mollusk along the Coast of China. Master’s Thesis, Shanghai Ocean Universtiy, Shanghai, China, 2019. [Google Scholar]
- Arzul, I.; Garcia, C.; Chollet, B.; Serpin, D.; Lupo, C.; Noyer, M.; Tourbiez, D.; Berland, C.; Dégremont, L.; Travers, M.A. First characterization of the parasite Haplosporidium costale in France and development of a real-time PCR assay for its rapid detection in the Pacific oyster, Crassostrea gigas. Transbound. Emerg. Dis. 2022, 69, e2041–e2058. [Google Scholar] [CrossRef] [PubMed]
- Bower, S.M.; Hervio, D.; Meyer, G.R. Infectivity of Mikrocytos mackini, the causative agent of Denman Island disease in Pacific oysters Crassostrea gigas, to various species of oysters. Dis. Aquat. Org. 1997, 29, 111–116. [Google Scholar] [CrossRef]
- Grijalva-Chon, J.M.; Castro-Longoria, R.; Enríquez-Espinoza, T.L.; Maeda-Martínez, A.N.; Mendoza-Cano, F. Molecular evidence of the protozoan parasite Marteilia refringens in Crassostrea gigas and Crassostrea corteziensis from the Gulf of California. Lat. Am. J. Aquat. Res. 2015, 43, 776–780. [Google Scholar] [CrossRef]
- OIE Ad Hoc Group. Report of the OIE ad Hoc Group on Susceptibility; OIE: Paris, France, 2020; pp. 1–23. [Google Scholar]


| Primer Name | Product | Primer Sequence (5′–3′) | References |
|---|---|---|---|
| OsHV-1 | [34] | ||
| BF | GTCGCATCTTTGGATTTAACAA | ||
| B4 | ACTGGGATCCGACTGACAAC | ||
| BP | FAM-TGCCCCTGTCATCTTGAGGTATAGACAATC-BHQ | ||
| Perkinsus spp. | [35] | ||
| PERK-F | TCCGTGAACCAGTAGAAATCTCAAC | ||
| PERK-R | GGAAGAAGAGCGACACTGATATGTA | ||
| PERK-P | FAM-CCCTTTGTGCAGTATGC-MGB | ||
| Marteili spp. and Bonamia spp. | [36] | ||
| Mar-18S-F | ACGATCAAAGTGAGCTCGTG | ||
| Mar-18S-R | CAGTTCCCTCACCCCTGAT | ||
| Mar18S-IN | FAM-GCATGGAATCGTGGAACGGG-BHQ | ||
| Bosp2-18S-F | CAGGATGCCCTTAGATGCTC | ||
| Bosp2-18S-R | GTACAAAGGGCAGGGACGTA | ||
| Bosp2-18S-IN | HEX-TTGACCCGGCTTGACAAGGC-BHQ | ||
| Vibrio housekeeping gene | |||
| 27F | 16S rDNA | AGAGTTTGATCCTGGCTCAG | [37] |
| 1492R | GGTTACCTTGTTACGACTT | ||
| atpA-F | ATP synthase alpha subunit | CTDAATTCHACNGAAATYAGYG | [38] |
| atpA-R | TTACCARGWYTGGGTTGC | ||
| mreB-F | Rod shaping protein B subunit | ACTTCGTGGCATGTTTTC | [39] |
| mreB-R | CCGTGCATATCGATCATTTC | ||
| pyrH-F | Uridylate kinase | ATGASNACBAAYCCWAAACC | [40] |
| pyrH-R | GTRAABGCNGMYARRTCCA | ||
| recA-F | Recombinase A | TGARAARCARTTYGGTAAAGG | [40] |
| recA-R | TCRCCNTTRTAGCTRTACC | ||
| rpoA-F | RNA polymerase alpha subunit | ATGCAGGGTTCTGTDACAG | [41] |
| rpoA-R | GHGGCCARTTTTCHARRCGC | ||
| rpoD-F | Factor σ70 RNA polymerase | ACGACTGACCCGGTACGCATGTAYATGMGNGARATGGGNACNGT | [40] |
| rpoD-R | ATAGAAATAACCAGACGTAAGTTNGCYTCNACCATYTCYTTYT | ||
| gyrB-F | Gyrase B subunit | GAAGTCATCATGACCGTTCTGCAYGCNGGNGGNAARTTYRA | [42] |
| gyrB-R | AGCAGGGTACGGATGTGCGAGCCRTCNACRTCNGCRTCNGYCAT | ||
| Sampling Date | Sampling Site | Month Age | Temp. (°C) | No. of Samples a | Shell Height (mm) | Mortality (%) | Dominant Bacteria |
|---|---|---|---|---|---|---|---|
| 2020.03 | HaiY | 10 | 6.8 | 20 (3) | 90.5 ± 8.6 | 60 | Vibrio alginolyticus |
| 2020.05 | LaiZ c | larvae | 25.3 | 5 tubes (0) | / | >80 | / |
| 2020.05 | RiZ | 12 | 18.6 | 42 (3) | 118.6 ± 9.5 | 40 | Vibrio alginolyticus |
| 2020.07 | RongC | 3 | 19.8 | 26 (0) | 35.9 ± 3.3 | 70 | / |
| 2020.08 | JiM b | 2 | 27.2 | 30 (0) | 14.6 ± 2.1 | >70 | / |
| 2020.09 | DaL | 5 | 24.6 | 20 (3) | 55.3 ± 5.1 | 80 | Vibrio natriegens |
| 2020.09 | DaL | 4 | 25.1 | 8 (2) | 53.7 ± 4.8 | 85 | Vibrio natriegens Vibrio alginolyticus |
| 2020.12 | ChangD b | 8 | 9.5 | 24 (3) | 88.9 ± 7.8 | 40–50 | Pseudoalteromonas nigrifaciens |
| 2020.12 | ChangD | 8 | 9.4 | 32 (3) | 94.3 ± 8.3 | 50 | Pseudoalteromonas elyakovii |
| 2021.04 | LaiZ | larvae | 25.2 | 3 tubes (0) | / | 80 | / |
| 2021.05 | LaiZ | larvae | 25 | 13 tubes (3) | / | 50 | Pseudoalteromonas piratica Vibrio alginolyticus |
| 2021.05 | LaiZ | larvae | 26 | 7 tubes (1) | / | 70–80 | Vibrio harveyi |
| 2021.06 | RuS | 13 | 12.3 | 12 (3) | 122.6 ± 9.6 | 50 | Vibrio aestuarianus |
| 2021.07 | YanT | 3 | 23.5 | 30 (3) | 35.4 ± 3.5 | 75 | Vibrio natriegens Vibrio alginolyticus |
| 2021.08 | DaL | 4 | 22.1 | 30 (0) | 56.8 ± 5.1 | 70 | / |
| 2021.08 | QinHD | 4 | 28.3 | 20 (3) | 52.3 ± 4.4 | 60–70 | Vibrio natriegens Vibrio alginolyticus |
| 2021.10 | JiaoN | 6 | 18.2 | 30 (5) | 64.3 ± 5.2 | 50–60 | Vibrio fortis Vibrio alginolyticus |
| 2021.10 | JiaoN b | 6 | 18.3 | 30 (5) | 56.4 ± 4.7 | 50 | Vibrio alginolyticus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Huang, B.-W.; Zheng, Y.-D.; Xin, L.-S.; Chen, W.-B.; Yu, T.; Li, C.; Wang, C.-M.; Bai, C.-M. Identification and Characterization of Infectious Pathogens Associated with Mass Mortalities of Pacific Oyster (Crassostrea gigas) Cultured in Northern China. Biology 2023, 12, 759. https://doi.org/10.3390/biology12060759
Zhang X, Huang B-W, Zheng Y-D, Xin L-S, Chen W-B, Yu T, Li C, Wang C-M, Bai C-M. Identification and Characterization of Infectious Pathogens Associated with Mass Mortalities of Pacific Oyster (Crassostrea gigas) Cultured in Northern China. Biology. 2023; 12(6):759. https://doi.org/10.3390/biology12060759
Chicago/Turabian StyleZhang, Xiang, Bo-Wen Huang, Yu-Dong Zheng, Lu-Sheng Xin, Wen-Bo Chen, Tao Yu, Chen Li, Chong-Ming Wang, and Chang-Ming Bai. 2023. "Identification and Characterization of Infectious Pathogens Associated with Mass Mortalities of Pacific Oyster (Crassostrea gigas) Cultured in Northern China" Biology 12, no. 6: 759. https://doi.org/10.3390/biology12060759
APA StyleZhang, X., Huang, B.-W., Zheng, Y.-D., Xin, L.-S., Chen, W.-B., Yu, T., Li, C., Wang, C.-M., & Bai, C.-M. (2023). Identification and Characterization of Infectious Pathogens Associated with Mass Mortalities of Pacific Oyster (Crassostrea gigas) Cultured in Northern China. Biology, 12(6), 759. https://doi.org/10.3390/biology12060759








