Simple Summary
Macrobenthos is widely used as an indicator of ecological health in marine monitoring and assessment. Xiaoqing Estuary has been subjected to different degrees of freshwater injection, resulting in significant changes in salinity, which has affected macrobenthos in the area. In this paper, we determined the main environmental variables driving the macrobenthic community and established a generalized additive model (GAM) model of macrobenthos based on the Margalef diversity index (dM), following which the distribution of macrobenthos richness can be predicted. The present findings will provide useful information for future studies on the correlation between macrobenthic communities and environmental factors in salinity-stressed areas of estuaries.
Abstract
Macrobenthos is widely used as an indicator of ecological health in marine monitoring and assessment. The present study aimed to characterize the interrelationships between the distribution of the macrobenthos community and environmental factors near Xiaoqing Estuary, Laizhou Bay. Responses of species richness to environmental factors were studied using the generalized additive model (GAM) and the Margalef diversity index (dM) as indicators of species diversity instead of individual indicator species. Six factors were selected in the optimal model by stepwise regression: sediment factors (organic matter, phosphate, nitrate nitrogen, and ammonium nitrogen) and water factors (salinity, and ammonium nitrogen). The response curves generated by the GAM showed a unimodal relationship among taxa diversity, salinity in water, and sediment organic matter. dM was positively correlated with ammonium nitrogen in water and was negatively correlated with phosphate in the sediment. The model optimized by forward stepwise optimization explained 92.6% of the Margalef diversity index with a small residual (2.67). The model showed good performance, with the measured dM strongly correlated with the predicted dM (Pearson R2 = 0.845, p < 0.05). The current study examined the combined influence of multiple eco-factors on macrobenthos, and the Margalef diversity index of macrobenthos was predicted by the GAM model in a salinity-stressed estuary.
1. Introduction
Macrobenthos is widely used as an indicator of ecological health in marine monitoring and assessment due to the relatively weak ability of macrobenthos species to migrate, their long life cycles, and their differential tolerance to multiple stressors [1,2,3]. Macrobenthos communities also play a key role in the functioning of estuarine systems, which connect freshwater and oceans and are important spawning and feeding grounds for marine organisms [4]. Since estuaries are easily disturbed by anthropogenic activities, the ecological status of benthic organisms is regularly assessed in estuaries and adjacent areas [5]. There have been increasing anthropogenic pressures on coastal habitat, including coastal development and habitat degradation [6]. Consequently, there has been a decline in the biodiversity of macrobenthos due to aquatic ecosystem habitat loss and degradation [7]. In addition, the spatial and temporal distributions of the estuarine biological community may be directly or potentially affected by the changes in water and sedimentary environments [8]. Benthic communities are directly affected by a variety of physical and chemical environmental factors, including temperature, salinity, hydrodynamic status, sediment type and particle size, and nutrient content [9,10,11,12]. Therefore, there is a need to explore the habitat requirements of macrobenthos communities and their responses to changes in environmental factors.
Ecologists have attempted to explore the effects of different environmental conditions on macrobenthos communities by developing a variety of models or statistical methods suitable for understanding the community- and population-scale distributions of environmental factors [13,14]. The generalized linear model (GLM) can be used to fit regression models for univariate response data. The GLM relates a function of the mean to environmental variables through a prediction equation of a linear form. The GAM is an expansion and a nonparametric modification of GLM, which is itself a generalization of multiple linear regression (MLR) and is not only able to screen various environmental factors and fit the best model but can also intuitively evaluate the relationships between the macrobenthos community and various environmental factors in the form of a graph. It has the ability to deal with the different types of distributions that characterize ecological data [15]. Since the relationships between organisms and environmental factors are very complex, linear regression is not appropriate to study these relationships. Consequently, the nonlinear analysis methods of the GLM and GAM models have been increasingly used to investigate these relationships. The advantage of the GAM over the traditional regression method relates to its ability to integrate multiple environmental variables within a quantitative evaluation of the influence and importance of each factor [16]. The GAM has also been widely used in the study of the relationships between fishery resources and environmental factors [17,18].
Previous studies have examined disturbances to macrobenthos and changes to community structure due to the impacts of fishery activities [19]. The relationships between macrobenthic communities and ecological factors are usually nonlinear and highly complex, and it is often difficult to express these relationships using traditional mathematical equations [20]. Salinity is generally considered to be the main environmental driver of estuarine function and community dynamics. Seasonal freshwater inputs and precipitation have been shown to reduce the abundance of macrobenthos [21]. Recent applications of GAM models have mainly focused on natural river ecosystems [22]. In contrast, there has been insufficient focus on the impact of habitat factors on macrobenthic communities in estuaries, particularly in salinity-stressed areas near Xiaoqing Estuary. Xiaoqing Estuary has been subjected to different degrees of freshwater injection, resulting in significant changes in salinity, which has affected aquaculture in the area. A previous study on macrobenthos in Xiaoqing Estuary focused only on the occurrence and composition of macrobenthos [23]. To date, there has been no comprehensive analysis of the correlation between macrobenthos and environmental factors in Xiaoqing Estuary. Therefore, the present study adopted the macrobenthos community of Xiaoqing Estuary as a case study.
Laizhou Bay is in the southern Bohai Sea, China, and is the largest bay in Shandong Province. The bay is an important fishery spawning and feeding ground in the Bohai Sea. The Xiaoqing River is the second largest river flowing into Laizhou Bay after the Yellow River. This river is a large-scale artificial river with navigation, irrigation, and sewage effluent disposal functions [24]. The Xiaoqing River imports large quantities of organic matter and nutrients into Laizhou Bay [25]. Inputs of salt to this area in September and October 2021 changed due to significant rainfall and runoff. The Xiaoqing Estuary is one of the main clam production areas in Laizhou Bay. The clam species that are farmed in this area include Ruditapes philippinarum, Mactra veneriformis, Meretrix meretrix, and Cyclina sinensis, with an annual output of ~20,000 tons.
The aim of the present study was to evaluate the correlation between environmental factors and macrobenthic communities in the salinity-stressed area near Xiaoqing Estuary. The specific objectives of the present study were to (1) characterize the composition of the macrobenthic community in the salinity-stressed area; (2) determine the main environmental variables driving the macrobenthic community; and (3) establish a GAM model of macrobenthos based on the dM, following which the distribution of macrobenthos richness can be predicted. The present study can act as a reference for future studies on the correlation between macrobenthic communities and environmental factors in salinity-stressed areas of estuaries.
2. Materials and Methods
2.1. Study Area
The present study conducted four surveys (in March, May, August, and October 2021) to describe the distribution of the macrobenthos community and analyze the responses between macrobenthos and habitat factors in southwest Laizhou Bay. The depth ranges from 1 m to 3.5 m in the study area. The present study established 12 sampling stations (S1–S12) in the bivalve aquaculture area between longitude 37.20° and 37.35° and latitude 119.05° and 119.15° (Figure 1) (no sediment was collected for S5 and S12 in March and S8 and S9 in October due to adverse weather conditions).
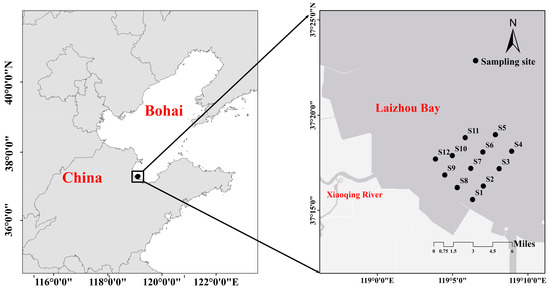
Figure 1.
Locations of the sampling sites of the present study in Xiaoqing Estuary.
2.2. Sampling and Data Analysis Method
Three macrobenthos subsamples were taken at each sampling station using a Van Veen grab of 0.1 m2. Subsamples were pooled to represent each site. Sampled sediments were washed through a metal sieve with a 0.5 mm mesh. The filtered-out specimens were then transferred to sample bottles filled with 5% formalin. The samples were identified, classified, counted, and wet-weighted (accurate to 0.0001 g) in the laboratory, and finally converted into abundance (ind./m2) and biomass (g/m2) according to the sampling area. All samples were collected, treated, and stored according to the “Specifications for oceanographic survey—Part 6: Marine biological survey (GB/T 12763.6–2007)”.
Apart from macrobenthos-related measures, the present study also conducted four surveys, during which samples were taken in triplicate from each sampling station for the measurement of seventeen parameters. The water quality parameters and physical features measured included water depth (H), water temperature (T), pH, dissolved oxygen (DO), salinity (Sal), chlorophyll-a (Chl-a), particulate organic matter (POM), phosphate (PO4-P), ammonium nitrogen (NH4-N), nitrate nitrogen (NO3-N), and nitrite nitrogen (NO2-N). The variables associated with the sediment measured in the present study included the quantity of sediment organic matter (SOM), median particle diameter (D50), sediment phosphate (PO4-Psoil), sediment ammonium nitrogen (NH4-Nsoil), sediment nitrate nitrogen (NO3-Nsoil), and sediment nitrite nitrogen (NO2-Nsoil). Water samples were collected on the surface with 1 L prelabeled plastic containers at each station. Some parameters (H, T, pH, DO, and Sal) were measured in the field using a multiparameter water quality analyzer (Smartroll Mp, Fort Collins, CO, USA). Sediments were sampled using a Van Veen grab of 0.1 m2 at all sampling stations. Each sediment sample was fully mixed and placed into sealed plastic bags. Water and sediment samples were collected and returned to the laboratory for the measurement of all other factors using methods corresponding to national standards [26]. The median particle diameter (D50) was examined by a laser particle sizer (Mastersizer 3000, Malvern City, UK). Nutrient concentrations in water and sediment were determined by an automatic nutrient fluid analyzer (Auto Analyzer Three, Bran Luebbe, Hamburg, Germany) based on “The specifications for oceanographic survey—Part 4: Seawater analysis (GB 17378.4–2007)”. Precipitation data were accessed from the ERA5 dataset (https://www.ecmwf.int/en/forecasts/datasets/reanalysis-datasets/era5) on 7 April 2022, which was generated by the fifth-generation ECMWF atmospheric reanalysis of the global climate covering the period from January 1950 to present.
Species richness is an integrative descriptor of the community. The Margalef diversity index reflects the species richness of the community, which performs well in distinguishing differences between communities. The present study used the Margalef diversity index (dM) to describe the diversity of benthic macroinvertebrates at each sampling station for each survey. Species-level taxa were used for calculating the index. dM was calculated as [27]:
In Equation (1), S is taxa richness, i.e., the number of taxa within a sampling area, and N is the total number of individuals.
The dominant species of macrobenthos were described by the dominance index; the dominance index (Y) was calculated as [28]:
In Equation (2), ni is the total abundance of the i-th species at all stations, N is the total abundance of individuals at all stations, and fi is the frequency of occurrence of the i-th species at all stations. Species i is defined as dominant when Y > 0.02.
A GAM was developed to quantify relationships between the Margalef diversity index (dM) and key ecological factors [26]. The general form of the GAM is:
In Equation (3), f(.) is the connection function, μ(N) is the expected value of the response variable Y, β0 is the intercept, and Yi(.) is the smoothing function for the ith explanatory variable xi.
We used 17 eco-factors, including H, T, pH, Sal, DO, POM, NH4-N, NO3-N, NO2-N, PO4-P, SOM, D50, NH4-Nsoil, NO3-Nsoil, NO2-Nsoil, PO4-Psoil, and NO3-Nsoil, as explanatory variables within the construction of the GAM. The model was constructed using the data sampled in March, May, and August. First, the taxa diversity index (dM) was examined to assess the significance of the effects of the single environmental factors on dM (a significance level of 0.01 was specified). Factors having a significant effect on dM were retained in the model. A forward selection procedure that sequentially added variables was used. Stepwise regression was used to assess the accuracy of the model according to the Akaike Information Criterion (AIC). The AIC value of the single-factor prediction function was detected, following which other environmental factors were progressively added to the single-factor prediction function until no further decrees in the AIC could be obtained.
The present study regarded the model with the smallest AIC value to be the optimal model. The significance of the prediction model was evaluated based on the results of the F-test. The generalized additive model was implemented by the “mgcv” package in R software [16].
The similarities between sites were calculated by means of the Bray–Curtis coefficient [29] and hierarchical clustering (CLUSTER), and non-metric multidimensional scaling (NMDS) analyses were used to reveal macrofaunal assemblage groups in the sampling sites in different sites, and they were applied using PRIMER 6.0 [30]. Analysis of similarity (ANOSIM) was widely used in ecology to determine whether there was a significant difference between intergroup and intra-group distance [31], and an ANOSIM test was calculated in R using the Vegan package. The data were analyzed and plotted using Excel 2019 and R 3.6.3 statistical analysis software (Lucent Technologies, State of New Jersey, USA), and the results were presented as mean ± standard deviation. The environmental factors, biomass, and abundance of macrobenthos were analyzed using a one-way analysis of variance (ANOVA) (p < 0.05). Following the identification of significant differences, Duncan’s multiple comparison test was conducted to assess differences between the groups.
3. Results
3.1. Ecological Factors
There were significant differences in environmental variables in the water column among the four months, in addition to H, pH, and POM (Table 1). There were significant differences in environmental variables in the sediment over the four months. The results of the four surveys showed no significant differences in salinity at all sites in March, May, and August (p > 0.05). However, Sal over these months was significantly higher than in October (p < 0.05) (Table 1 and Figure 2). The contents of DIN, DINsoil, PO4-P, and PO4-Psoil in October were significantly higher than in the other months. The average daily precipitation in September significantly exceeded the value in other months (p < 0.05) (Figure 3).

Table 1.
Values for each of the measured ecological factors in March, May, August, and October 2021, respectively.
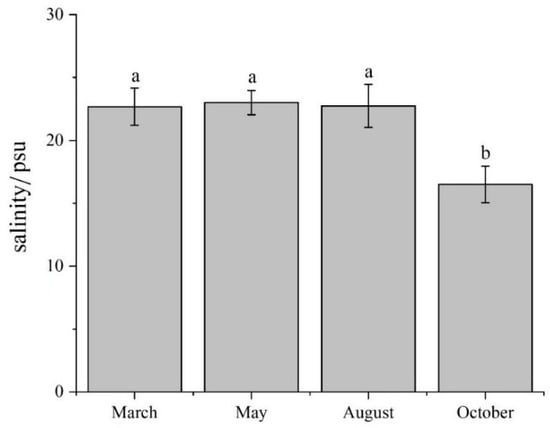
Figure 2.
The average value of salinity in March, May, August, and October 2021, respectively (mean ± standard deviation). We used lowercase letters to indicate the significant difference between cruises. Data labeled without the same letter (s) were significantly (p < 0.05) different from each other.
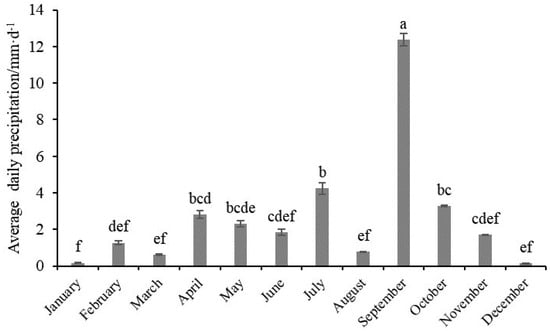
Figure 3.
Average daily precipitation for each month in 2021 (mean ± standard deviation). The superscript letter to value indicates a significant difference at the different months (p < 0.05). We used lowercase letters to indicate the significant difference between different months. Data labeled without the same letter (s) were significantly (p < 0.05) different from each other.
3.2. Composition of the Macrobenthos Fauna
The macrobenthos taxa were classified to species level for further analysis. The four surveys obtained eighty-four macrobenthos species, including sixty-one families falling into nine phyla, nine classes, and thirty-one orders. Among these, there were 30 species of Polychaeta, including twelve orders, twenty-one families, and twenty-five genera, accounting for 35.7% of the total taxonomic unit. This was followed by twenty-nine species of Mollusca, including nine orders, twenty-one families, and twenty-four genera, accounting for 34.5% of the total taxonomic unit. There were twenty species of crustaceans, including six orders, twelve families, and fifteen genera, accounting for 23.8% of the total taxonomic unit, and five other species. There were four orders, five families, and five genera, accounting for 6% of the total taxonomic unit (Figure 4). The highest number of species was in August, whereas only polychaetes, crustaceans, and mollusks were found in October (Figure 5). In March, 32 taxa were collected, with Mactra chinensis and Notomastus latericeus Sars being the dominant taxa. In May, 33 taxa were collected, with the dominant taxa being Mactra chinensis, Cultellus attenuates, Nephtys polybranchia, and Heterocuma sarst. In August, 43 taxa were collected, with Mactra veneriformis, Ruditapes philippinarum, and Musculus senhousei being the dominant taxa. In October, 28 taxa were collected, with the dominant taxa being Mactra veneriformis, Ruditapes philippinarum, Musculus senhousei, and Decorifera matusimana (Figure 5 and Table 2). Overall, there were significant differences in the composition of dominant macrobenthic species between the four voyages.
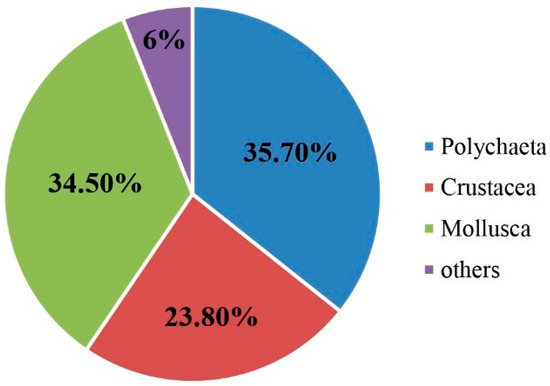
Figure 4.
Community structure of macrobenthos over four months in 2021.
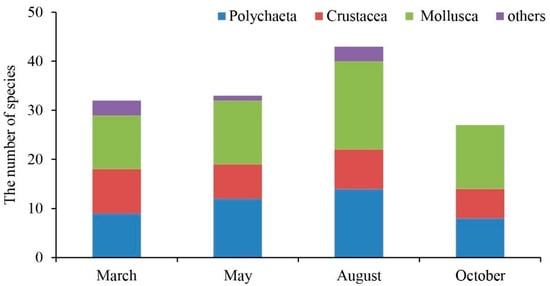
Figure 5.
The number of macrobenthos species in March, May, August, and October 2021, respectively.

Table 2.
Dominant species of macrobenthos in March, May, August, and October 2021, respectively.
There were no significant differences in the biomass of macrobenthos between the different months (Kruskal–Wallis test, p = 0.478, Figure 6A). October and August showed the highest and lowest biomass, respectively (mean ± SD of 248.34 ± 80.39 g/m2 and 99.63 ± 26.98 g/m2, respectively). There were significant differences in the densities of macrobenthos among the different months (Kruskal–Wallis test, p = 0.027). October showed the lowest density at 560.30 ± 58.27 ind./m2 (Figure 6B).
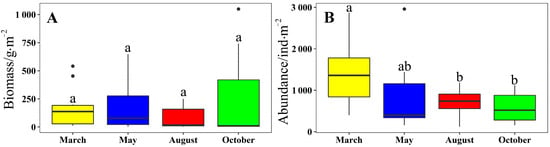
Figure 6.
(A,B) Biomass and abundance of macrobenthos in March, May, August, and October 2021, respectively (mean ± standard deviation). We used lowercase letters to indicate the significant difference between cruises. Data labeled without the same letter (s) were significantly (p < 0.05) different from each other.
The proportion of crustaceans in macrobenthos in October significantly exceeded those in other months (Figure 7A), which could be attributed to larger hermit crab (Pagurus sp.) samples from station S5. Mollusks contributed higher proportions of total macrobenthos biomass in March, May, August, and October at 89.60%, 99.10%, 62.35%, and 53.07%, respectively (Figure 7A). Mollusks showed higher contributions to macrobenthos density in March, May, August, and October at 77.71%, 49.80%, 74.42%, and 81.35%, respectively. In contrast to the other months, only polychaetes, mollusks, and crustaceans were observed in October (Figure 7B).
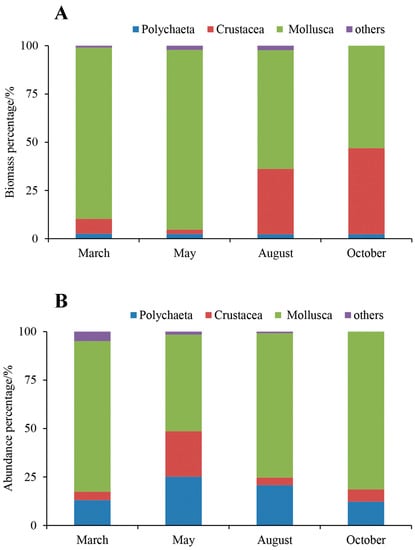
Figure 7.
(A,B) Biomass and abundance percentage of macrobenthos in March, May, August, and October 2021, respectively.
3.3. The Community Structure of Macrobenthos
The Bray–Curtis coefficient indicated low similarities between macrobenthos communities at each station between the four voyages of 20%. The results of the cluster analysis were consistent with those of the non-metric multidimensional scaling (NMDS) analysis in different months (Figure 8). All stations could be divided into two communities in March at a similarity level of 20%, and four communities in May, August, and October, respectively, at a similarity level of 20%. NMDS indicated the stress coefficients in March, May, August, and October to be 0.05, 0.13, 0.17, and 0.05, respectively. The results of ANOSIM showed significant differences between the different cluster groups in four surveys (March, R = 0.9259, p < 0.01; May, R = 0.7685, p < 0.01; August, R = 0.5789, p < 0.01; October, R = 0.8707, p < 0.01).
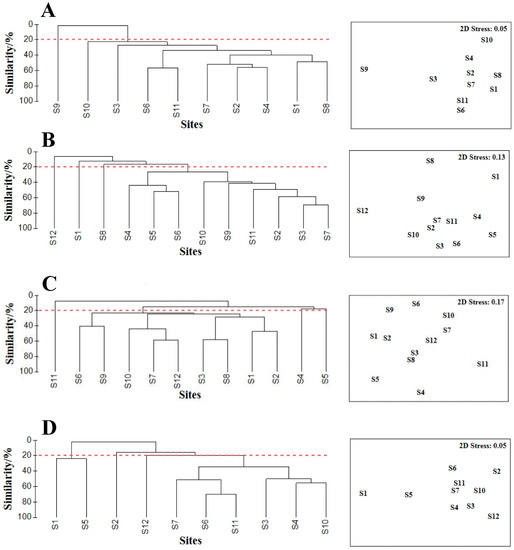
Figure 8.
(A–D) The results of cluster analysis and non-metric multidimensional scaling (NMDS) for macrobenthos in March, May, August, and October 2021, respectively. Notes: the dashed line represents the level of similarity in dividing the community structure.
3.4. Responses of Community Diversity to Ecological Factors
Twelve environmental factors that had significant effects on taxa diversity (dM) were identified (H, T, pH, Sal, DO, POM, SOM, NH4-N, PO4-Psoil, NH4-Nsoil, NO3-Nsoil, and D50). Since the correlation coefficients between DO and NH4-N and between T and NH4-Nsoil exceeded 0.5, these variables were not simultaneously added to the model to avoid collinearity, and higher correlations between dM and NH4-N, NH4-Nsoil, and Sal were retained. The remaining nine environmental indicators were used to establish the GAM, and the model structure was optimized by forward stepwise regression (Table 3). The addition of the variables SOM, PO4-Psoil, NO3-Nsoil, NH4-Nsoil, Sal, and NH4-N significantly increased model performance (p < 0.05). Variables H, T, and pH were removed since their inclusion did not improve model performance. Model 9 represents the final form of the model: dM ~ s (SOM) + s (PO4-Psoil) + s (NO3-Nsoil) + s (NH4-Nsoil) + s (Sal) + s (NH4-N). The model explained 92.6% of the variance (adjusted coefficient of determination R2 = 0.845). Pearson’s correlation coefficient between the calculated dM and the measured dM was highly correlated at 0.9635 (p < 0.05, Figure 9).

Table 3.
Variance analysis table of the forward stepwise regression process.
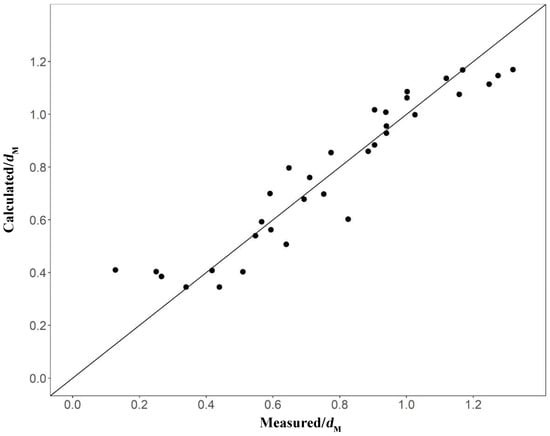
Figure 9.
Calculated Margalef diversity index (dM) versus dM based on Model 9.
The response curve of dM to environmental factors shows a unimodal relationship between species diversity, SOM, and Sal. In addition, there was a linear relationship between species diversity and NH4-N and PO4-Psoil, and dM was positively correlated with NH4-N and negatively correlated with PO4-Psoil (Figure 10).
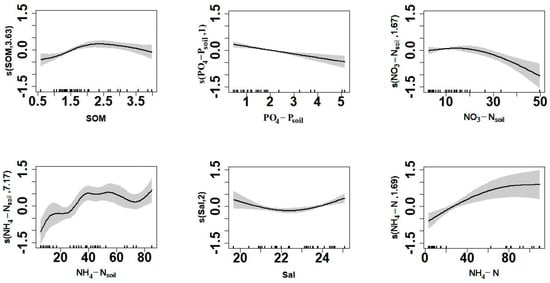
Figure 10.
Response curves of the Margalef diversity index (dM) to ecological factors in the generalized additive model (GAM) analysis.
3.5. Validation of the Model
Sampling data collected in October 2021 were used to validate Model 9. As shown in Table 4, measured dM values were strongly correlated with the dM predicted by Model 9 (Pearson R2 = 0.845, p < 0.05), with a small mean squared error (MSE). This result confirmed the good performance of the model and its ability to effectively simulate the distribution of benthic fauna diversity in the bivalve farming and salinity-stressed area in the Xiaoqing estuary, Laizhou Bay (Figure 11).

Table 4.
Statistical summary of the performance of the optimal model (Model 9) in October 2021.
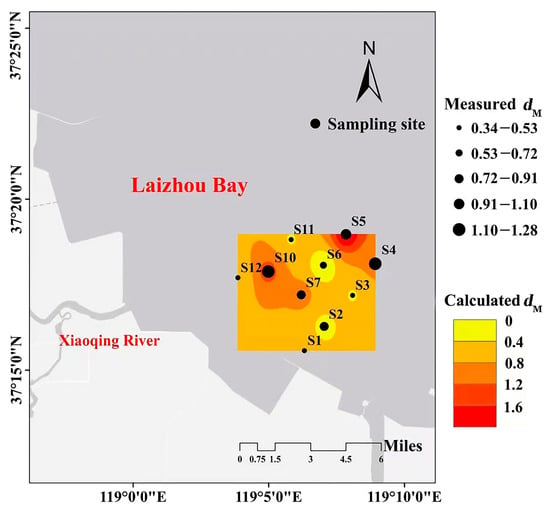
Figure 11.
The spatial distributions of the measured and predicted Margalef diversity index (dM) in October 2021.
4. Discussion
The salinity of the study area in October was significantly lower than the three other investigated months (p < 0.05). This result could possibly be attributed to heavy precipitation. The hydrological data indicated that the precipitation dilution of salinity increased significantly in September. Previous studies have shown that heavy rainfall can result in a significant short-term increase in river runoff, which not only reduces the salinity of surface water in Laizhou Bay but also facilitates the transportation of large quantities of nutrients of terrestrial origin into the Bay. This input of nutrients, in turn, leads to an increase in nutrient concentrations (dissolved inorganic nitrogen and phosphate) in the sea area [32,33]. Consistent with the above point, the present study determined that the contents of dissolved inorganic nitrogen and phosphate in surface and interstitial water were significantly higher in October than in other months (Table 1).
The survey identified 84 species of macrobenthos. The order of the major taxonomic groups in terms of species numbers was polychaeta > mollusks > crustaceans. This result is consistent with a previous study [34]. The results of the present study indicated that species number and abundance in October were lower than in other months, which was possibly related to lower salinity. Some previous studies have shown that salinity is a vital environmental factor affecting the distribution of macrobenthic species in estuarine areas. Salinity concentration shows an inverse relationship to macrobenthic species abundance [23,35]. The structure of the macrobenthic community may be influenced by unstable environmental factors, particularly the influence of river runoff and rainfall changes on the marine environment. In addition, studies in other sea areas have shown that the complex marine environment results in the formation of different habitat niches, which are inhabited by different benthic community structures, resulting in low similarities between the benthic communities of each station [36,37]. Mollusks had larger contributions to biomass over the four months due to their relatively larger body sizes.
The stress coefficient of the NMDS analysis was less than 0.2, which reflected the relationships among species within the macrobenthos community in each month. The results of the clustering and non-metric multidimensional scale analysis indicated a low similarity of macrobenthos communities in the survey area, which is consistent with the results of previous studies [19,23]. Despite the differences in the community structure of macrobenthos between the various months, the model showed a total residual deviation after optimization of 2.67 and an AIC of −20.54. Pearson’s correlation coefficient between the calculated and measured dM was high at 0.9635 (p < 0.05). This result was consistent with a previous study, which showed that the GAM performed well in predicting the dM of macrobenthos under unchanging salinity in October.
Since the comprehensive multiparameter evaluation index relies heavily on the weights of parameters, the biological index is more suitable for the assessment of ecological health under the influences of anthropogenic activities [20]. Identifying the spatiotemporal distribution of the benthic community is essential for the conservation and sustainable development of local benthic resources. The relationships between various environmental factors, such as temperature and salinity, on benthic species richness are often not linear. However, the GAM typically shows higher performance in analyzing the nonlinear relationship between dependent and multiple independent variables. Thus, the application of the GAM has great significance for the study of benthic communities. The results of the GAM analysis showed that each factor had different effects on the changes to the macrobenthic abundance index in the coastal waters of Xiaoqing River Estuary in Laizhou Bay, and the relationships among them were mostly nonlinear. The present study screened and fitted the response curves of the macrobenthic richness index to key environmental factors based on the GAM. The results of the GAM indicated that environmental variables (Sal, SOM, NH4-N, PO4-Psoil, NH4-Nsoil, and NO3-Nsoil) had the greatest influence on the benthic community in the study area. Organic matter and nutrients are often the factors limiting the survival of benthic communities [38,39]. Salinity is an important environmental factor affecting the survival, growth, and distribution of benthic communities [40]. The salinity of the benthic environment ranged between 21 and 25 psu in March, May, and August. The model results showed a minimum dM under a salinity of 22.5 psu. The rate of decline in dM was highest under a salinity of 20–22 psu, whereas there was a gradually increasing trend at a salinity of 24–25 psu. The GAM indicated that dM was positively correlated with NH4-N and negatively correlated with PO4-Psoil, which was consistent with the results of previous studies. NH4-N is an essential nutrient for the growth of aquatic plants and algae in water, and the application of nitrogen to aquatic plants in previous studies improved the productivity of macrobenthos [22,41]. Eutrophication results from increases in PO4-Psoil increase the biomass and diversity of plankton but result in changes to the community structure and a reduction in the species richness of benthic communities [42]. There were high correlations between DO and NH4-N, T, and NH4-Nsoil, with increases in T and DO. Although there were clear changes in NH4-N and NH4-Nsoil, the factors T and DO were removed to increase the degree of fit of the model. Other studies have suggested that DO is an important factor regulating the survival of benthos, with impacts on the abundance and distribution of macrobenthos [43]. Therefore, the present study considered the interactions between these environmental factors, and the above environmental factors were added to the GAM to allow comprehensive future studies.
The distribution of target species was predicted by exploring the relationship between species distribution and related variables using the species distribution model. The GAM has been widely used to explore relationships between species distribution and environmental factors in fish and submerged plants [44,45]. However, there have been few studies on the relationships between macrobenthos and environmental variables in estuaries. The present study applied the GAM in combination with the common zero-value richness index to analyze the distribution of benthic resources in the bivalve farming and salinity-stressed estuary area, Laizhou Bay. Due to the relationship between the mean of the response variable and a smoothed function of the predictor variables, which was established by a link function, a parametric function of the model was not produced as one potential drawback of the GAM. But, we can make predictions based on the model [15]. In addition, the present study did not consider the influences of spatial and temporal auto-correlation on the modeling. Future studies can improve the accuracy of the model by considering the effect of time through the addition of an autoregressive process.
It was also worth noting that the establishment of the GAM model was based on environmental data surveyed in March, May, and August 2021. Although salinity in October was significantly lower than in March, May, and August, the distribution of benthic diversity was effectively predicted by the GAM model at each site in October. The GAM model was able to predict the species richness of the macrobenthos when salinity was significantly lower in October. Therefore, the present study provides a preliminary exploration of the relationships between the macrobenthic Margalef diversity index and environmental factors. Future studies should apply different methods (such as the habitat index, linear partial differential equation with first-order variable coefficient, and quantile regression) to integrate long-term quantitative and environmental data into future habitat suitability models. These models can then be used to more comprehensively analyze the distribution and dynamics of benthic organisms. Moreover, there have been changes to some environmental factors in the study area, such as salinity and inorganic salts, due to heavy rain, which may partially explain the deviation in the model results.
5. Conclusions
Among the seventeen environmental factors investigated in the present study, sediment factors (organic matter, phosphate, nitrate nitrogen, and ammonium nitrogen) and water factors (salinity and ammonium nitrogen) were the key factors affecting the structure and diversity of the macrobenthic community in the coastal waters of Xiaoqing Estuary. The response curve of dM to environmental factors shows unimodal relationships between species diversity and sediment organic matter and between species diversity and salinity. In addition, there were linear relationships between species diversity and ammonium nitrogen and between species diversity and phosphate in interstitial water, and dM was positively and negatively correlated with ammonium nitrogen and sediment phosphate, respectively. The optimal GAM model explained 92.6% of the observed variation in the macrobenthic Margalef diversity index with a small residual (2.67). The measured dM was strongly correlated with the predicted dM (Pearson R2 = 0.845, p < 0.05). In general, the model showed good performance and could effectively simulate the distribution of benthic fauna diversity in the salinity-stressed area in Xiaoqing Estuary in Laizhou Bay. The complementary use of different indices is recommended to assess the richness of macrobenthos in China.
Author Contributions
Conceptualization, Y.M.; methodology, L.L.; software, A.L.; investigation, C.Z., W.Y., H.Z. and Z.M.; writing—original draft preparation, L.L.; writing—review and editing, Y.M.; resources and visualization, L.Z. and Z.J.; project administration S.X.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by research grants from the Marine S&T Fund of Shandong Province for the Pilot National Laboratory for Marine Science and Technology (Qingdao) (2021QNLM 050103 and 2022QNLM040003-4) and the Central Public-interest Scientific Institution Basal Research Fund, CAFS (2020TD50) and the Central Public-interest Scientific Institution Basal Research Fund, YSFRI, CAFS (20603022023007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Butkas, K.J.; Vadeboncoeur, Y.; Vander Zanden, M.J. Estimating benthic invertebrate production in lakes: A comparison of methods and scaling from individual taxa to the whole-lake level. Aquat. Sci. 2011, 73, 153–169. [Google Scholar] [CrossRef]
- Hajializadeh, P.; Safaie, M.; Naderloo, R.; Shojael, M.G.; Gammal, J.; Villnas, A.; Norkko, A. Species composition and functional traits of macrofauna in different mangrove habitats in the Persian Gulf. Front. Mar. Sci. 2020, 7, 575480. [Google Scholar] [CrossRef]
- Han, C.; Xu, Z.; Liu, X. Characteristics of macrofaunal assemblages and their relationships with environmental factors in a semi-enclosed bay. Mar. Pollut. Bull. 2021, 167, 112348. [Google Scholar] [CrossRef]
- Meng, Z.; Han, Q.; Wang, X. Distribution pattern of macrobenthic composition, diversity and secondary production in Hangzhou Bay, northern East China Sea. Reg. Stud. Mar. Sci. 2021, 47, 101956. [Google Scholar] [CrossRef]
- Aubry, A.; Elliott, M. The use of environmental integrative indicators to assess seabed disturbance in estuaries and coasts: Application to the Humber Estuary, UK. Mar. Pollut. Bull. 2006, 53, 175–185. [Google Scholar] [CrossRef]
- Lotze, H.K.; Lenihan, H.S.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.G.; Kay, M.C.; Kidwell, S.M.; Kirby, M.X.; Peterson, C.H.; Jackson, J.B. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 2006, 312, 1806–1809. [Google Scholar] [CrossRef] [PubMed]
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Dias, H.Q.; Sukumaran, S.; Neetu, S.; Ridha, H. Benthic community resilience in two differently impacted tropical estuaries: Taxonomic vs functional approaches. J. Environ. Manag. 2022, 324, 116264. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, F.; Liu, R. Community structure changes of macrobenthos in the South Yellow Sea. Chin. J. Oceanol. Limn. 2012, 30, 248–255. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, J.; Cai, K.; Xu, Z.; Wu, D.; Wang, B. Temporal and spatial distribution of macrobenthos communities and their responses to environmental factors in Lake Taihu. Acta Ecol. Sin. 2016, 36, 16–22. [Google Scholar] [CrossRef]
- Shadrin, N.; Kolesnikova, E.; Revkova, T.; Latushkin, A.; Chepyzhenko, A.; Dyakov, N.; Anufriieva, E. Macrostructure of benthos along a salinity gradient: The case of Sivash Bay (the Sea of Azov), the largest hypersaline lagoon worldwide. J. Sea. Res. 2019, 154, 101811. [Google Scholar] [CrossRef]
- Huang, X.; Xu, J.; Liu, B. Assessment of aquatic ecosystem health with Indices of Biotic Integrity (IBIs) in the Ganjiang River system, China. Water 2022, 14, 278. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z. Rationale of the multivariate statistical software PRIMER and its application in benthic community ecology. J. Ocean. Univ. China. 2003, 33, 58–64. [Google Scholar]
- Ellingsen, K.E.; Hewitt, J.E.; Thrush, S.F. Rare species, habitat diversity and functional redundancy in marine benthos. J. Sea. Res. 2007, 58, 291–301. [Google Scholar] [CrossRef]
- Ahmadi-Nedushan, B.; St-Hilaire, A.; Bérubé, M.; Robichaud, É.; Thiémonge, N.; Bobée, B. A review of statistical methods for the evaluation of aquatic habitat suitability for instream flow assessment. River. Res. Appl. 2006, 22, 503–523. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable direct fitting and smoothness selection for generalized additive models. J. R. Stat. Soc. B 2008, 70, 495–518. [Google Scholar] [CrossRef]
- Yan, L.; Li, J.; Zhang, P.; Yang, B.; Wang, T. Effects of spatiotemporal and environmental factors on the fishing ground of Sthenoteuthis oualaniensis in the South China Sea based on the Generalized Additive Model. Mar. Pollut. Bull. 2011, 40, 217–223. [Google Scholar]
- Glińska-Lewczuk, K.; Burandt, P.; Kujawa, R.; Kobus, S.; Obolewski, K.; Dunalska, J.; Grabowska, M.; Lew, S.; Chormański, J. Environmental factors structuring fish communities in Floodplain Lakes of the undisturbed system of the Biebrza River. Water 2016, 8, 146. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Wang, H.; Wang, Y.; Wang, J.; Zhang, B. Characters of a macrobenthic community off the Changjiang River estuary. Acta Zool. Sin. 2007, 53, 76–82. [Google Scholar]
- Gezie, A.; Anteneh, W.; Dejen, E.; Mereta, S.T. Effects of human-induced environmental changes on benthic macroinvertebrate assemblages of wetlands in Lake Tana Watershed, northwest Ethiopia. Environ. Monit. Assess. 2017, 189, 152. [Google Scholar] [CrossRef]
- Rahman, M.K.; Hossain, M.B.; Majumdar, P.R.; Mustafa, M.G.; Noman, M.A.; Albeshr, M.F.; Bhat, E.A.; Arai, T. Macrobenthic assemblages, distribution and functional guilds from a freshwater-dominated tropical estuary. Diversity 2022, 14, 473. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, J.; Yang, Q.; Guo, H. Zooplankton diversity and its relationships with environmental factors in the Liaohe estuary. Mari. Environ. Sci. 2020, 39, 25–30. [Google Scholar]
- Ding, J.; Li, J.; Xue, S.; Zhang, W.; Huo, E.; Ma, Z.; Yu, W.; Mao, Y. Health assessment for benthic habitats of macrobenthos in the sea area adjacent to the Xiaoqing estuary, Laizhou Bay. Acta Ecol. Sin. 2021, 41, 4806–4817. [Google Scholar]
- Wang, Z.; Wang, H.; Fan, S.; Xin, M.; Sun, X. Community structure and diversity of macrobenthos in Jiaozhou Bay. Mar. Pollut. Bull. 2021, 171, 112781. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, L.; Luo, X.; Zhang, X. Study on the water pollution and eutrophication in the Xiaoqing River estuary. Period. Ocean. Univ. China 2013, 43, 60–66. [Google Scholar]
- Yi, Y.; Sun, J.; Yang, Y.; Zhou, Y.; Tang, C.; Wang, X.; Yang, Z. Habitat suitability evaluation of a benthic macroinvertebrate community in a shallow lake. Ecol. Indic. 2018, 90, 451–459. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Li, B.; Liu, W.; Zuo, Y.; Kong, D.; Zhu, J. Relationships between characteristics of macrobenthic assemblages and environmental variables in the Heihe River Basin, China. J. Water. Supply Res. T 2021, 70, 710–730. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y. Aggregated intensity of dominate species of zooplankton in autumn in the East China Sea and Yellow Sea. J. Ecol. 1989, 8, 13–15. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Wang, L.; Fan, Y.; Yan, C.; Gao, C.; Xu, Z.; Liu, X. Assessing benthic ecological impacts of bottom aquaculture using macrofaunal assemblages. Mar. Pollut. Bull. 2017, 114, 258–268. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Fang, F.; Li, Z.; Tian, G.; Guo, J.; Zhang, C. Seasonal variation of phosphorus in Xiaojiang backwater area, Three Gorges Reservoir. Envir. Sci. 2009, 30, 3488–3493. [Google Scholar]
- Liang, S.; Li, S.; Ma, H.; Yang, Y.; Lv, H.; Xu, Z.; Duan, X. Spatial-temporal distributions and limiting factors of nutrients in Laizhou Bay based on land-sea synchronous survey. Period. Ocean Univ. China. 2022, 52, 97–110. [Google Scholar]
- Luo, X.; Zhang, S.; Yang, J.; Pan, J.; Tian, L.; Zhang, L. Macrobenthic community in the Xiaoqing River estuary in Laizhou Bay, China. J. Ocean. Univ. China. 2013, 12, 366–372. [Google Scholar] [CrossRef]
- Ysebaert, T.; Herman, P.M.J.; Meire, P.; Craeymeersch, J.; Verbeek, H.; Heip, C.H.R. Large-scale spatial patterns in estuaries: Estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuar. Coast. Shelf Sci. 2003, 57, 335–355. [Google Scholar] [CrossRef]
- Cai, W.; Meng, W.; Liu, L.; Zhu, Y.; Zhou, J. Macrozoobenthos community structure of the Bohai Bay in spring time. Acta Sci. Cir. 2013, 33, 1458–1466. [Google Scholar]
- Jia, H.; Cao, L.; Chai, X. The changes of macrobenthic community structure and cause analysis in the Yangtze Estuary during summer from 2016 to 2019. Mar. Environ. Sci. 2022, 41, 180–186. [Google Scholar]
- Levin, L.A.; Gage, J.D. Relationships between oxygen, organic matter and the diversity of bathyal macrofauna. Deep-Sea. Res. PT. II 1998, 45, 129–163. [Google Scholar] [CrossRef]
- Lv, W.; Liu, Z.; Yang, Y.; Huang, Y.; Fan, B.; Jiang, Q.; Zhao, Y. Loss and self-restoration of macrobenthic diversity in reclamation habitats of estuarine islands in Yangtze Estuary, China. Mar. Pollut. Bull. 2016, 103, 128–136. [Google Scholar] [CrossRef]
- Shou, L.; Zeng, J.; Liao, Y.; Xu, T.; Gao, A.; Chen, Z.; Chen, Q.; Yang, J. Temporal and spatial variability of benthic macrofauna communities in the Yangtze River estuary and adjacent area. Aquat. Ecosyst. Health 2013, 16, 31–39. [Google Scholar] [CrossRef]
- Miserendino, M.L.; Brand, C.; Di Prinzio, C.Y. Assessing urban impacts on water quality, benthic communities and fish in streams of the Andes Mountains, Patagonia (Argentina). Water Air Soil. Poll. 2008, 194, 91–110. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, L.; Li, J.; Huang, X. Long-term changes of nutrients and biocenoses indicating the anthropogenic influences on ecosystem in Jiaozhou Bay and Daya Bay, China. Mar. Pollut. Bull. 2021, 168, 112406. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Sun, K.; Yang, J.; Song, W.; Cui, W. A comparison of the applicability of the Shannon-Wiener index, AMBI and M-AMBI indices for assessing benthic habitat health in the Huanghe (Yellow River) Estuary and adjacent areas. Acta Oceanol. Sin. 2016, 35, 50–58. [Google Scholar] [CrossRef]
- Hua, C.; Zhu, Q.; Shi, Y.; Liu, Y. Comparative analysis of CPUE standardization of Chinese Pacific saury (Cololabis saira) fishery based on GLM and GAM. Acta Oceanol. Sin. 2019, 38, 100–110. [Google Scholar] [CrossRef]
- Yang, Y.; Yi, Y.; Wang, W.; Zhou, Y.; Yang, Z. Generalized additive models for biomass simulation of submerged macrophytes in a shallow lake. Sci. Total. Environ. 2020, 711, 135108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).