Expression and Immune Characterization of Major Histocompatibility Complex in Paralichthys olivaceus after Antigen Stimulation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Leukocytes, mRNA, and HEK293 Cells
2.3. Antibodies
2.4. Cloning of mhcIIα and mhcIIβ
2.5. Sequence Analysis
2.6. Production of Anti-Flounder MhcIIα and MhcIIβ Abs
2.7. Expression of mhcIIα and mhcIIβ in Flounder
2.8. Eukaryotic Transfection
2.9. Western Blot
2.10. Immunofluorescence Staining (IF)
2.11. Stimulation of Leukocytes by Antigens
2.12. Flow Cytometry (FCM)
2.13. Statistical Analysis
3. Results
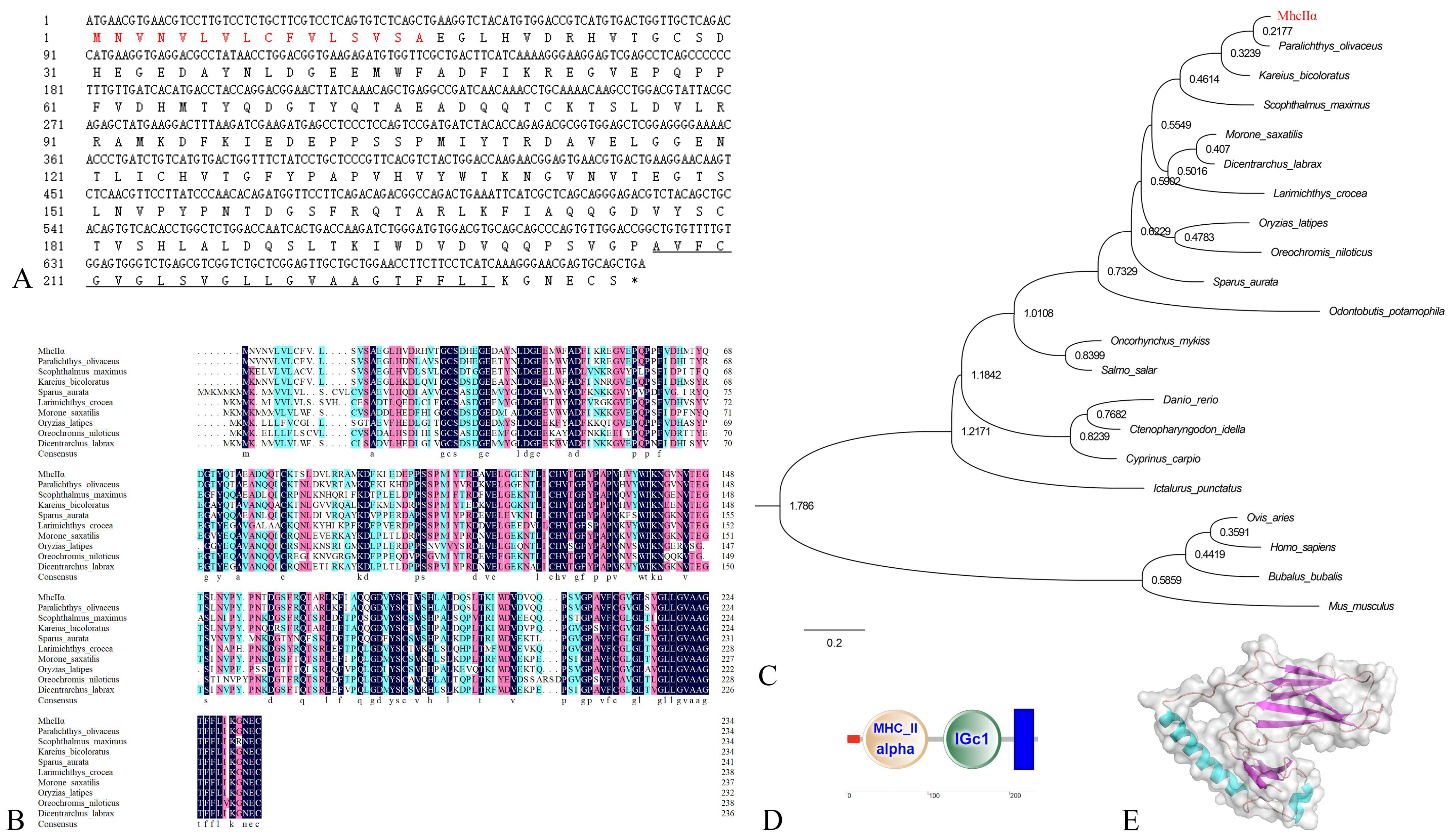
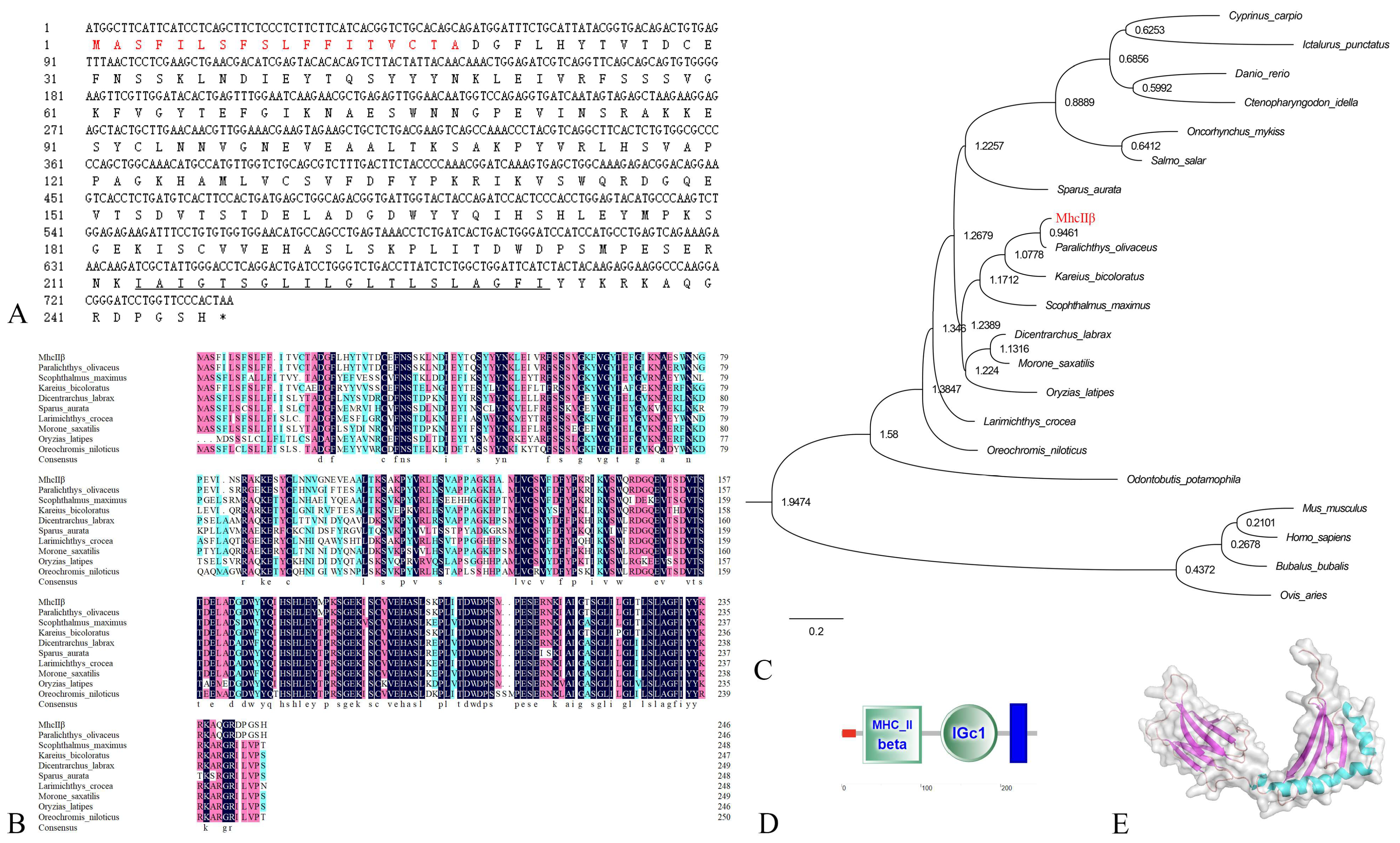
3.1. Characterizations of fMhcIIα and fMhcIIβ
3.2. Tissue Distribution of mhcIIα and mhcIIβ
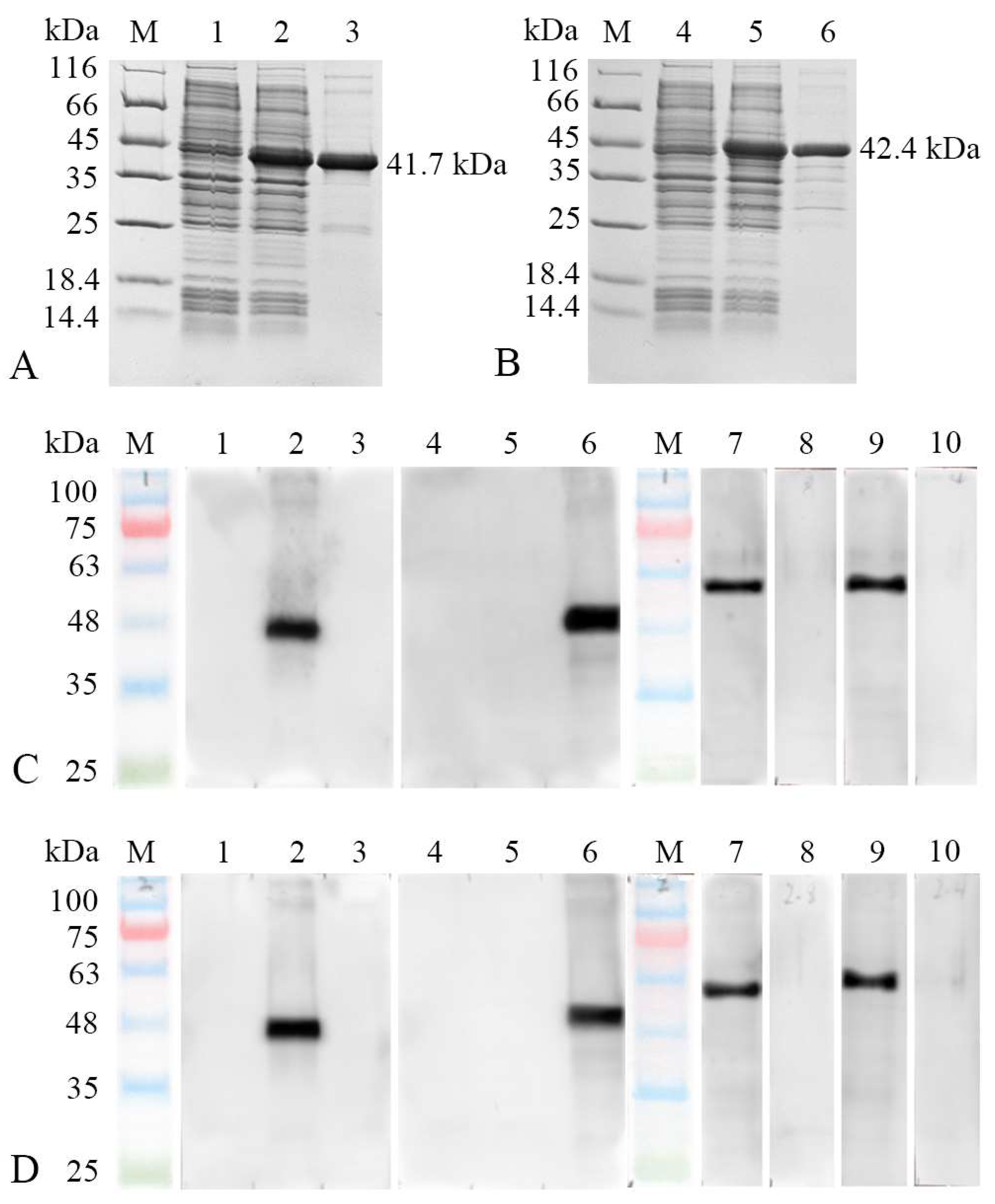
3.3. Abs Specificity of MhcIIα and MhcIIβ
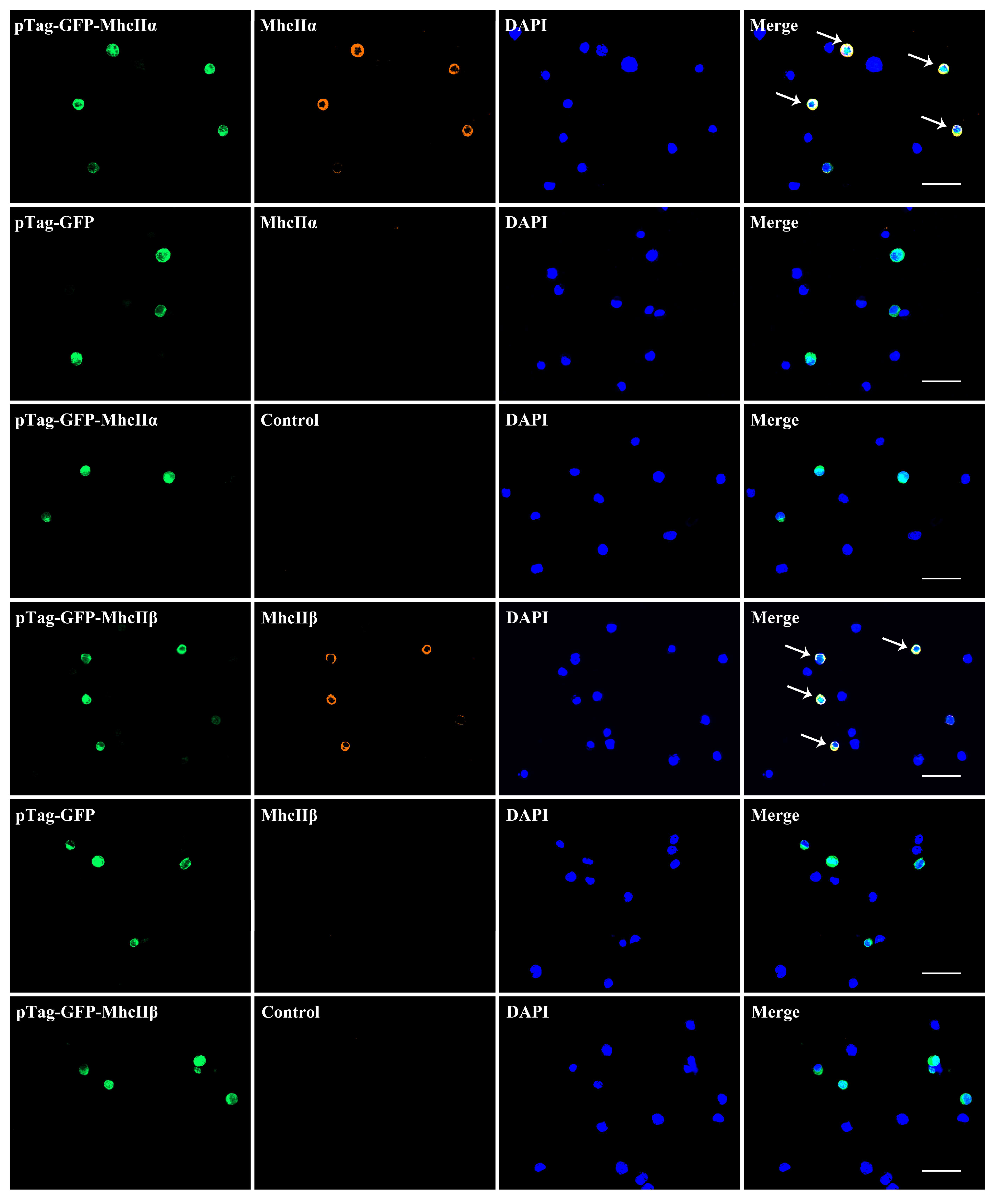
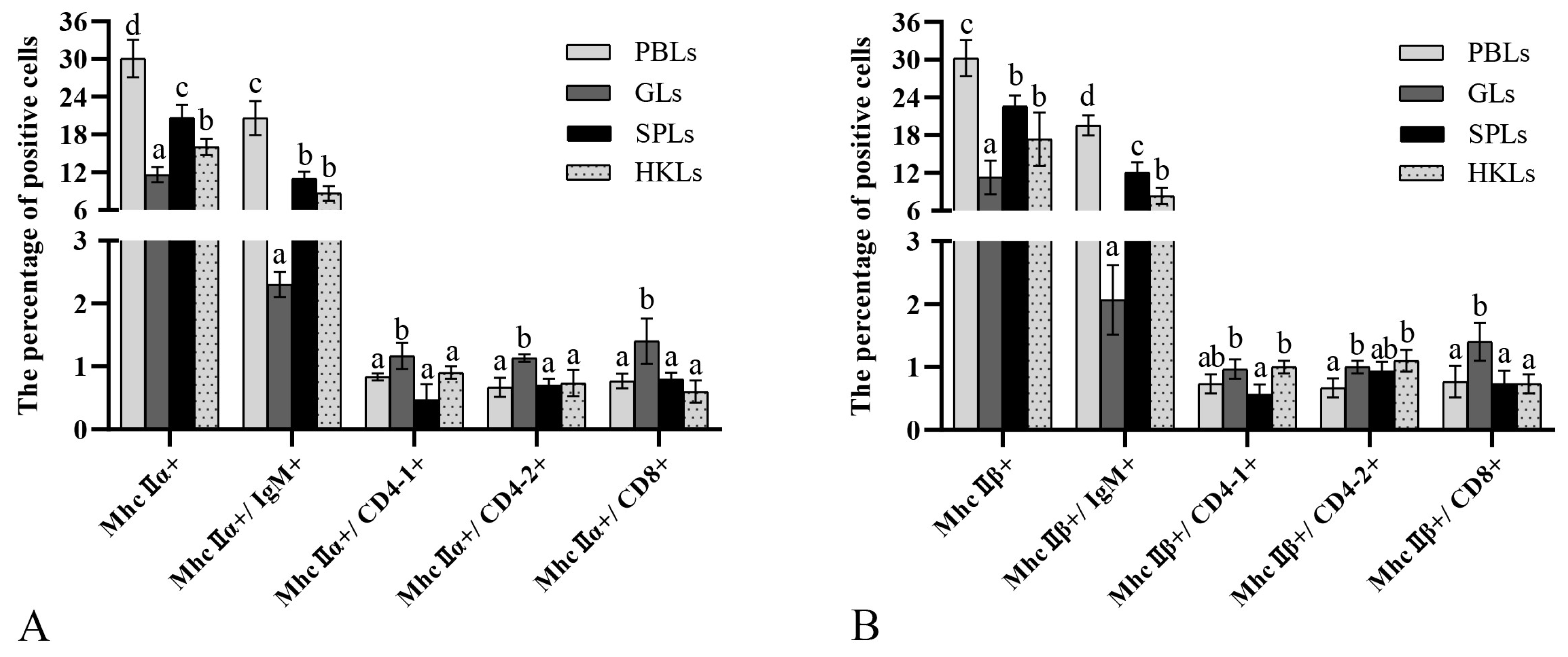
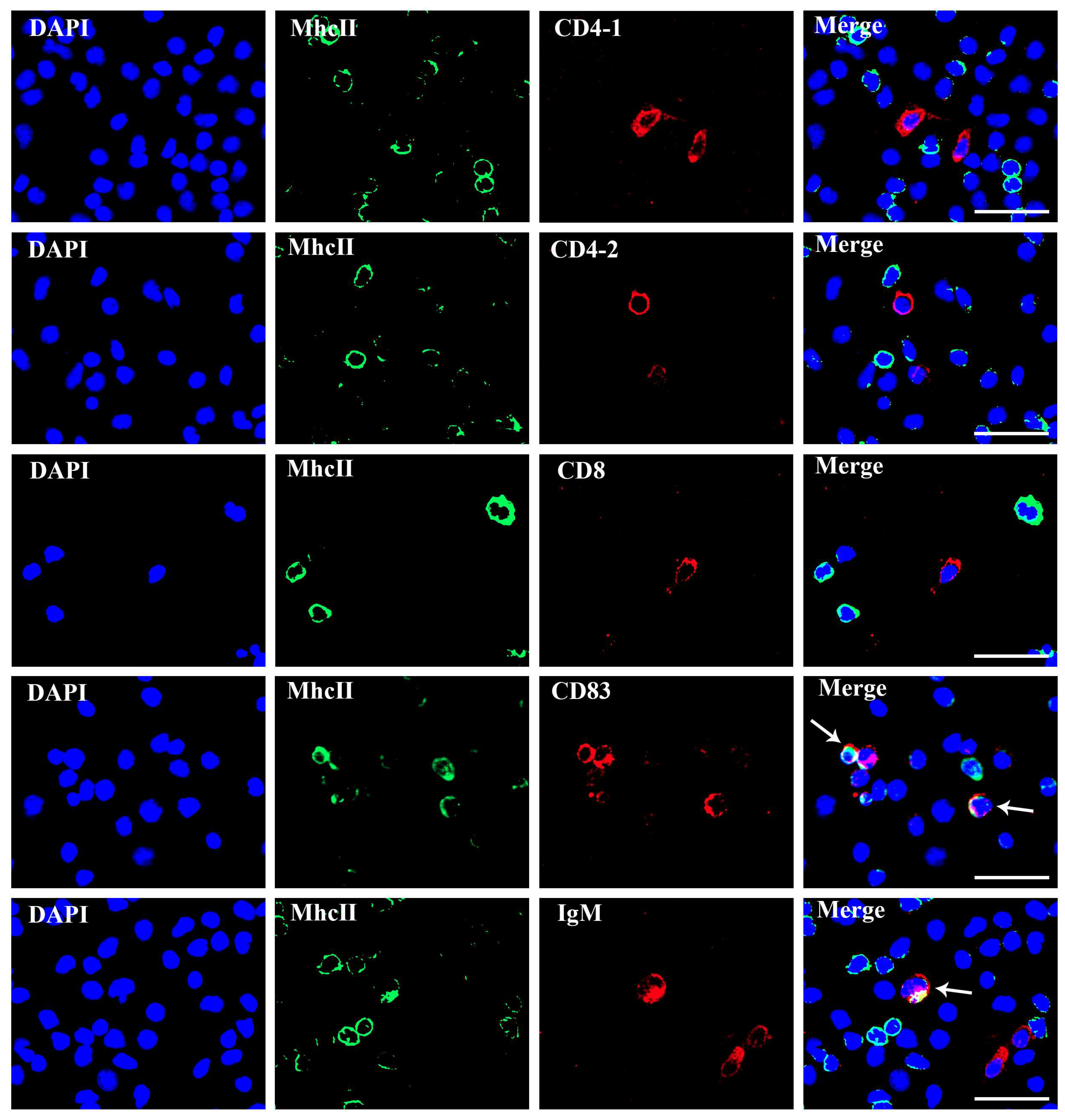
3.4. The Localization of MhcIIα and MhcIIβ in T and B Lymphocyte
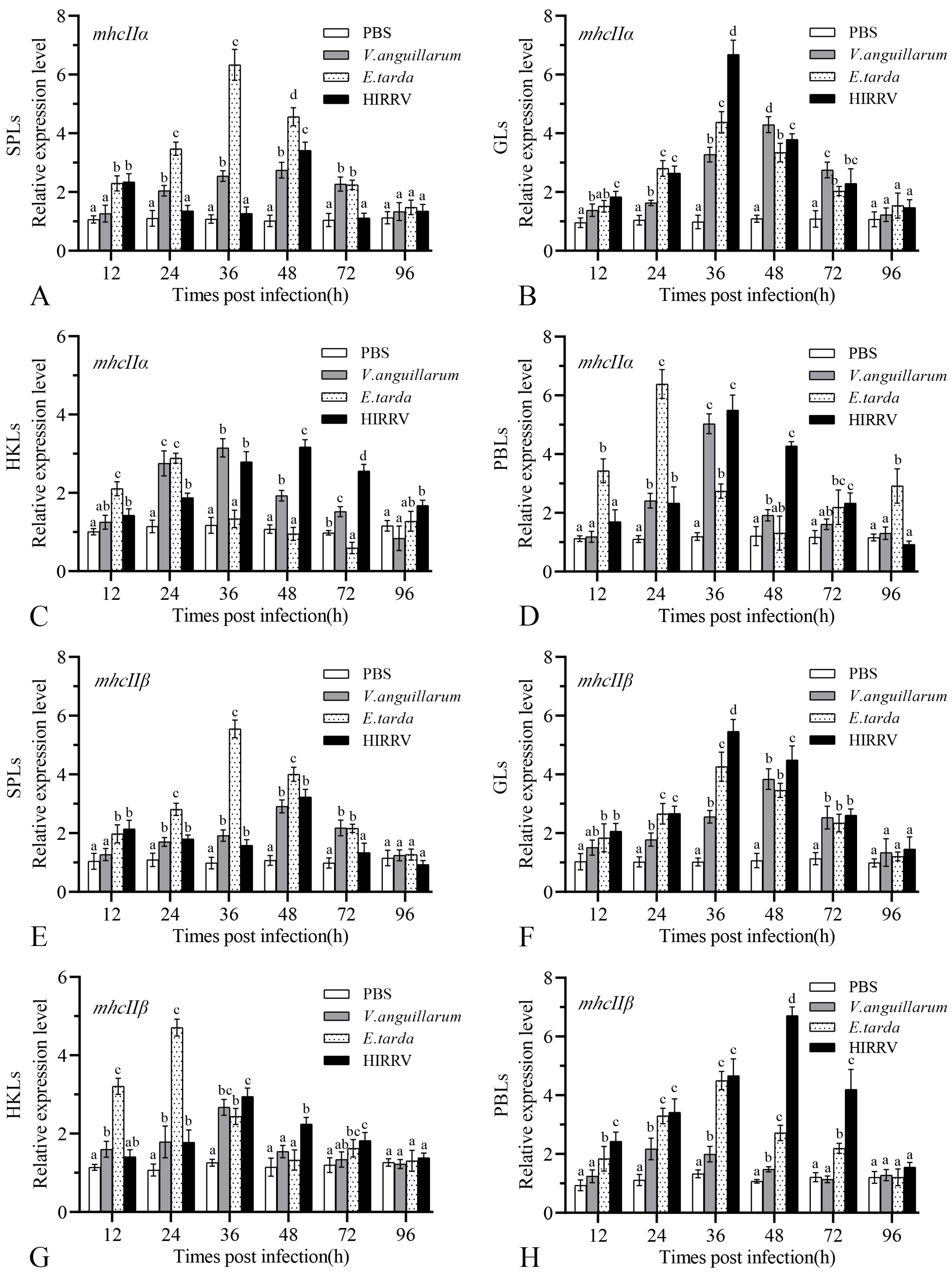
3.5. Gene Expression of mhcIIα and mhcIIβ after Infection
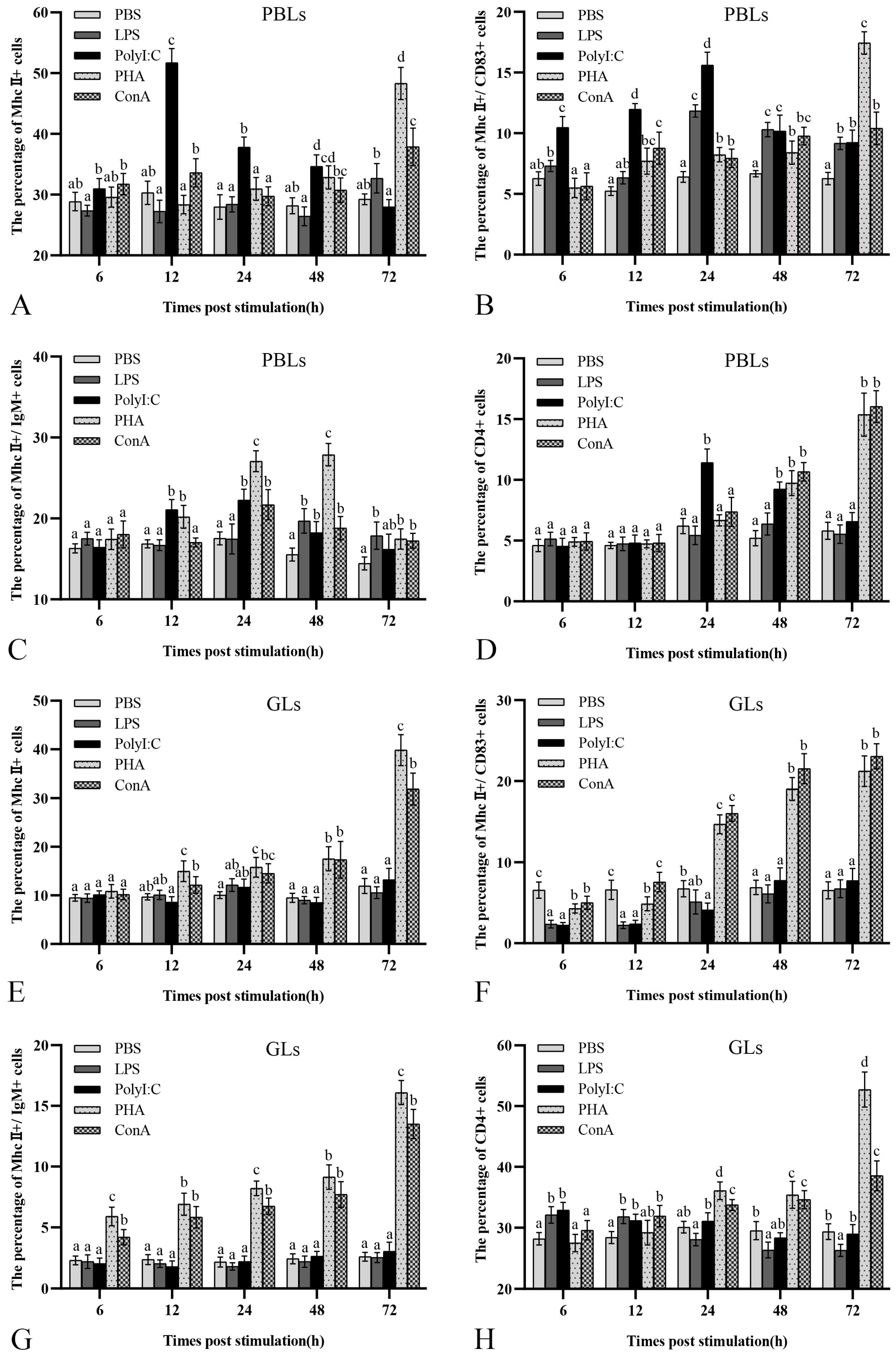
3.6. Variations in MhcII+, MhcII+/IgM+, MhcII+/CD83+, and CD4+ Leukocytes after Antigen Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a Systems Understanding of MHC Class I and MHC Class II Antigen Presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Drozina, G.; Kohoutek, J.; Jabrane-Ferrat, N.; Peterlin, B.M. Expression of MHC II Genes. Curr. Top. Microbiol. Immunol. 2005, 290, 147–170. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Dijkstra, J.M. Major Histocompatibility Complex (MHC) Genes and Disease Resistance in Fish. Cells 2019, 8, 378. [Google Scholar] [CrossRef]
- Radwan, J.; Babik, W.; Kaufman, J.; Lenz, T.L.; Winternitz, J. Advances in the Evolutionary Understanding of MHC Polymorphism. Trends Genet. 2020, 36, 298–311. [Google Scholar] [CrossRef]
- Grimholt, U. MHC and Evolution in Teleosts. Biology 2016, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Xu, S.; Yang, M.; Pang, S.; Yang, G. Molecular Cloning, Expression Pattern, and 3D Structural Analysis of the MHC Class IIB Gene in the Chinese Longsnout Catfish (Leiocassis Longirostris). Vet. Immunol. Immunopathol. 2011, 141, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Su, Y.-Q.; Wang, J.; Zhuang, Z.-M.; Tang, Q.-S. Molecular Cloning and Expression Analysis of Major Histocompatibility Complex Class IIB Gene of the Whitespotted Bambooshark (Chiloscyllium Plagiosum). Fish. Physiol. Biochem. 2013, 39, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, W.-X.; Meng, L.; Hong, D.-W. Molecular Cloning, Genomic Structure, Polymorphism and Expression Analysis of Major Histocompatibility Complex Class IIA Gene of Swamp Eel Monopterus Albus. Biology 2014, 69, 236–246. [Google Scholar] [CrossRef]
- Jiang, J.; Li, C.; Zhang, Q.; Wang, X. Cloning and Characterization of Major Histocompatibility Complex Class II Genes in the Stone Flounder Kareius Bicoloratus (Pleuronectidae). Genet. Mol. Res. 2013, 12, 5820–5832. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, Y.; Dong, S.; Yin, Y.; Wang, Q.; Yu, W.; Luo, Y.; Xu, J.; Zhang, L.; Yu, Y.; et al. Major Histocompatibility Complex Class IIA and IIB Genes of Loach (Misgurnus Anguillicaudatus): Molecular Cloning and Expression Analysis in Response to Bacterial and Parasitic Challenge. Aquaculture 2019, 500, 359–369. [Google Scholar] [CrossRef]
- Yu, S.; Ao, J.; Chen, X. Molecular Characterization and Expression Analysis of MHC Class II Alpha and Beta Genes in Large Yellow Croaker (Pseudosciaena Crocea). Mol. Biol. Rep. 2010, 37, 1295–1307. [Google Scholar] [CrossRef]
- Pang, J.; Gao, F.; Lu, M.; Ye, X.; Zhu, H.; Ke, X. Major Histocompatibility Complex Class IIA and IIB Genes of Nile Tilapia Oreochromis Niloticus: Genomic Structure, Molecular Polymorphism and Expression Patterns. Fish. Shellfish. Immunol. 2013, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, L.; Han, J.; Su, H.; Yang, Q.; He, C. Major Histocompatibility Complex Class IIA and IIB Genes of the Spotted Halibut Verasper Variegatus: Genomic Structure, Molecular Polymorphism, and Expression Analysis. Fish. Physiol. Biochem. 2011, 37, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Sun, Y.; Shi, G.; Cheng, Y.; Wang, R. Characterization of the Major Histocompatibility Complex Class II Genes in Miiuy Croaker. PLoS ONE 2011, 6, e23823. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, H.; Liu, L.; You, X.; Wu, M.; Liao, Z. MHC Class II Alpha, Beta and MHC Class II-Associated Invariant Chains from Chinese Sturgeon (Acipenser Sinensis) and Their Response to Immune Stimulation. Fish. Shellfish. Immunol. 2017, 70, 1–12. [Google Scholar] [CrossRef]
- Lu, X.; Li, W.; Guo, J.; Jia, P.; Zhang, W.; Yi, M.; Jia, K. N Protein of Viral Hemorrhagic Septicemia Virus Suppresses STAT1-Mediated MHC Class II Transcription to Impair Antigen Presentation in Sea Perch, Lateolabrax Japonicus. J. Immunol. 2022, 208, 1076–1084. [Google Scholar] [CrossRef]
- Chen, S.-L.; Zhang, Y.-X.; Xu, M.-Y.; Ji, X.-S.; Yu, G.-C.; Dong, C.-F. Molecular Polymorphism and Expression Analysis of MHC Class II B Gene from Red Sea Bream (Chrysophrys Major). Dev. Comp. Immunol. 2006, 30, 407–418. [Google Scholar] [CrossRef]
- Jørgensen, S.M.; Hetland, D.L.; Press, C.M.; Grimholt, U.; Gjøen, T. Effect of Early Infectious Salmon Anaemia Virus (ISAV) Infection on Expression of MHC Pathway Genes and Type I and II Interferon in Atlantic Salmon (Salmo Salar L.) Tissues. Fish. Shellfish. Immunol. 2007, 23, 576–588. [Google Scholar] [CrossRef]
- Sawayama, E.; Tanizawa, S.; Nakayama, K.; Ito, R.; Akase, Y.; Kitamura, S.-I. Major Histocompatibility IIβ Diversity and Peptide-Binding Groove Properties Associated with Red Sea Bream Iridovirus Resistance. Aquaculture 2022, 552, 738038. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, X.; Xiang, Y.; Wang, L.; Sun, Y.; Zhou, Y. Genetic Polymorphism of MHC Class IIα Alleles and Its Association with Resistance/Susceptibility to Viral Nervous Necrosis Virus (VNNV) in Golden Pompano (Trachinotus Ovatus). Aquaculture 2019, 501, 144–152. [Google Scholar] [CrossRef]
- Kurata, O.; Miyashita, S.; Toda, S.; Wada, S.; Sakai, T. Immunohistochemistry for Detection of MHC Class II-Expressing Cells in Japanese Flounder Paralichthys Olivaceus. Fish. Pathol. 2021, 55, 151–161. [Google Scholar] [CrossRef]
- Iliev, D.B.; Thim, H.; Lagos, L.; Olsen, R.; Jørgensen, J.B. Homing of Antigen-Presenting Cells in Head Kidney and Spleen—Salmon Head Kidney Hosts Diverse APC Types. Front. Immunol. 2013, 4, 137. [Google Scholar] [CrossRef] [PubMed]
- Piertney, S.B.; Oliver, M.K. The Evolutionary Ecology of the Major Histocompatibility Complex. Heredity 2006, 96, 7–21. [Google Scholar] [CrossRef]
- Dong, F.; Song, X.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Immunological Characteristics of Dendritic Cells Marker CD83 in Flounder (Paralichthys Olivaceus). Fish. Shellfish. Immunol. Rep. 2021, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Tian, H.-F.; Tang, X.-Q.; Sheng, X.-Z.; Zhan, W.-B. Kinetics of T Lymphocyte Subsets and B Lymphocytes in Response to Immunostimulants in Flounder (Paralichthys Olivaceus): Implications for CD4+ T Lymphocyte Differentiation. Sci. Rep. 2020, 10, 13827. [Google Scholar] [CrossRef]
- Xing, J.; Ma, J.; Tang, X.; Sheng, X.; Zhan, W. Characterizations of CD4-1, CD4-2 and CD8β T Cell Subpopulations in Peripheral Blood Leucocytes, Spleen and Head Kidney of Japanese Flounder (Paralichthys Olivaceus). Mol. Immunol. 2017, 85, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhan, W.; Xing, J.; Sheng, X. Production, Characterisation and Applicability of Monoclonal Antibodies to Immunoglobulin of Japanese Flounder (Paralichthys Olivaceus). Fish. Shellfish. Immunol. 2007, 23, 982–990. [Google Scholar] [CrossRef]
- Zhao, M.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Expression of Interleukin-2 Receptor Subunit Gamma (IL-2Rγ) and Its Binding with IL-2 Induced Activation of CD4 T Lymphocytes in Flounder (Paralichthys Olivaceus). Fish. Shellfish. Immunol. 2022, 122, 426–436. [Google Scholar] [CrossRef]
- Jones, E.Y.; Fugger, L.; Strominger, J.L.; Siebold, C. MHC Class II Proteins and Disease: A Structural Perspective. Nat. Rev. Immunol. 2006, 6, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, C.; Zhang, Q.; Wang, X. Locus Number Estimation of MHC Class II B in Stone Flounder and Japanese Flounder. Int. J. Mol. Sci. 2015, 16, 6000–6017. [Google Scholar] [CrossRef]
- Buonocore, F.; Randelli, E.; Casani, D.; Costantini, S.; Facchiano, A.; Scapigliati, G.; Stet, R.J.M. Molecular Cloning, Differential Expression and 3D Structural Analysis of the MHC Class-II β Chain from Sea Bass (Dicentrarchus Labrax L.). Fish. Shellfish. Immunol. 2007, 23, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Morales-Lange, B.; Ramírez-Cepeda, F.; Schmitt, P.; Guzmán, F.; Lagos, L.; Øverland, M.; Wong-Benito, V.; Imarai, M.; Fuentes, D.; Boltaña, S.; et al. Interferon Gamma Induces the Increase of Cell-Surface Markers (CD80/86, CD83 and MHC-II) in Splenocytes From Atlantic Salmon. Front. Immunol. 2021, 12, 666356. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhujin, D.; Marana, M.H.; Dalsgaard, I.; Rzgar, J.; Heidi, M.; Asma, K.M.; Per, K.W.; Kurt, B. Immersion Vaccines against Yersinia Ruckeri Infection in Rainbow Trout: Comparative Effects of Strain Differences. J. Fish. Dis. 2021, 44, 1937–1950. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.B. MHC and Adaptive Immunity in Teleost Fishes. Immunogenetics 2017, 69, 521–528. [Google Scholar] [CrossRef]
- Mildner, A.; Jung, S. Development and Function of Dendritic Cell Subsets. Immunity 2014, 40, 642–656. [Google Scholar] [CrossRef]
- Itano, A.A.; Jenkins, M.K. Antigen Presentation to Naive CD4 T Cells in the Lymph Node. Nat. Immunol. 2003, 4, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Koppang, E.O.; Hordvik, I.; Bjerkås, I.; Torvund, J.; Aune, L.; Thevarajan, J.; Endresen, C. Production of Rabbit Antisera against Recombinant MHC Class II Beta Chain and Identification of Immunoreactive Cells in Atlantic Salmon (Salmo Salar). Fish. Shellfish. Immunol. 2003, 14, 115–132. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, F.; Shi, Z.; Huang, Z.; Sun, R.; Wang, Q.; Ouyang, G.; Ji, W. Molecular Characteristics, Polymorphism and Expression Analysis of Mhc II in Yellow Catfish (Pelteobagrus Fulvidraco) Responding to Flavobacterium Columnare Infection. Fish. Shellfish. Immunol. 2022, 125, 90–100. [Google Scholar] [CrossRef]
- Iliev, D.B.; Jørgensen, S.M.; Rode, M.; Krasnov, A.; Harneshaug, I.; Jørgensen, J.B. CpG-Induced Secretion of MHCIIβ and Exosomes from Salmon (Salmo Salar) APCs. Dev. Comp. Immunol. 2010, 34, 29–41. [Google Scholar] [CrossRef]
- Morales-Lange, B.; Nombela, I.; Ortega-Villaizán, M.D.M.; Imarai, M.; Schmitt, P.; Mercado, L. Induction of Foxp3 during the Crosstalk between Antigen Presenting Like-Cells MHCII+CD83+ and Splenocytes CD4+IgM− in Rainbow Trout. Biology 2021, 10, 324. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The Ins and Outs of MHC Class II-Mediated Antigen Processing and Presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Tian, H.; Xing, J.; Tang, X.; Chi, H.; Sheng, X.; Zhan, W. Identification and Characterization of a Master Transcription Factor of Th1 Cells, T-Bet, Within Flounder (Paralichthys Olivaceus). Front. Immunol. 2021, 12, 704324. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Zhu, L.-Y.; Nie, L.; Shi, W.; Dong, W.-R.; Xiang, L.-X.; Shao, J.-Z. Characterization of Surface Phenotypic Molecules of Teleost Dendritic Cells. Dev. Comp. Immunol. 2015, 49, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Bassity, E.; Clark, T.G. Functional Identification of Dendritic Cells in the Teleost Model, Rainbow Trout (Oncorhynchus Mykiss). PLoS ONE 2012, 7, e33196. [Google Scholar] [CrossRef]
- Abós, B.; Castro, R.; González Granja, A.; Havixbeck, J.J.; Barreda, D.R.; Tafalla, C. Early Activation of Teleost B Cells in Response to Rhabdovirus Infection. J. Virol. 2015, 89, 1768–1780. [Google Scholar] [CrossRef]
- Kjøglum, S.; Larsen, S.; Bakke, H.G.; Grimholt, U. How Specific MHC Class I and Class II Combinations Affect Disease Resistance against Infectious Salmon Anaemia in Atlantic Salmon (Salmo Salar). Fish. Shellfish. Immunol. 2006, 21, 431–441. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequence(5′−3′) | Application |
|---|---|---|
| mhcIIα-F | ATGAACGTGAACGTCCTTG | Full-length cloning |
| mhcIIα-R | TCAGCTGCACTCGTTCC | |
| mhcIIβ-F | ATGGCTTCATTCATCCTCAGCTT | |
| mhcIIβ-R | TTAGTGGGAACCAGGATCCCG | |
| mhcIIαCO-F | GGCCATGGCTGATATCGGATCCGAAGGTCTACATGTGGACCGTC(BamHⅠ) | Extracellular domain cloning |
| mhcIIαCO-R | GACGGAGCTCGAATTCGGATCC CGGTCCAACACTGGGCTG(BamHⅠ) | |
| mhcIIβCO-F | GGCCATGGCTGATATCGGATCC GATGGATTTCTGCATTATACGG(BamHⅠ) | |
| mhcIIβCO-R | GACGGAGCTCGAATTCGGATCCCTTGTTTCTTTCTGACTCAGGC(BamHⅠ) | |
| q mhcIIα-F | GACGGTGAAGAGATGTGGTT | qPCR |
| q mhcIIα-R | ATCGGACTGGAGGGAGGC | |
| q mhcIIβ-F | AAGTCTGGAGAGAAGATTTCCTGTG | |
| q mhcIIβ-R | GATGAATCCAGCCAGAGATAAGGT | |
| qβ-actin-F | GAGGGAAATCGTGCGTGACAT | |
| qβ-actin-R | ATTGCCGATGGTGATGACCTG | |
| mhcIIα-GFP-F | ACTCAGATCTCGAGCTCAAGCTTATGAACGTGAACGTCCTTG(Hind Ⅲ) | Eukaryotic transfection |
| mhcIIα-GFP-R | GTCGACTGCAGAATTCGAAGCTTGCTGCACTCGTTCCCTT(Hind Ⅲ) | |
| mhcIIβ-GFP-F | ACTCAGATCTCGAGCTCAAGCTTATGGCTTCATTCATCCTCAGC(Hind Ⅲ) | |
| mhcIIβ-GFP-R | GTCGACTGCAGAATTCGAAGCTTGTGGGAACCAGGATCCCGT(Hind Ⅲ) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, J.; An, Z.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Expression and Immune Characterization of Major Histocompatibility Complex in Paralichthys olivaceus after Antigen Stimulation. Biology 2023, 12, 1464. https://doi.org/10.3390/biology12121464
Xing J, An Z, Tang X, Sheng X, Chi H, Zhan W. Expression and Immune Characterization of Major Histocompatibility Complex in Paralichthys olivaceus after Antigen Stimulation. Biology. 2023; 12(12):1464. https://doi.org/10.3390/biology12121464
Chicago/Turabian StyleXing, Jing, Zhaoxia An, Xiaoqian Tang, Xiuzhen Sheng, Heng Chi, and Wenbin Zhan. 2023. "Expression and Immune Characterization of Major Histocompatibility Complex in Paralichthys olivaceus after Antigen Stimulation" Biology 12, no. 12: 1464. https://doi.org/10.3390/biology12121464
APA StyleXing, J., An, Z., Tang, X., Sheng, X., Chi, H., & Zhan, W. (2023). Expression and Immune Characterization of Major Histocompatibility Complex in Paralichthys olivaceus after Antigen Stimulation. Biology, 12(12), 1464. https://doi.org/10.3390/biology12121464







