Aliens on the Road: Surveying Wildlife Roadkill to Assess the Risk of Biological Invasion
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
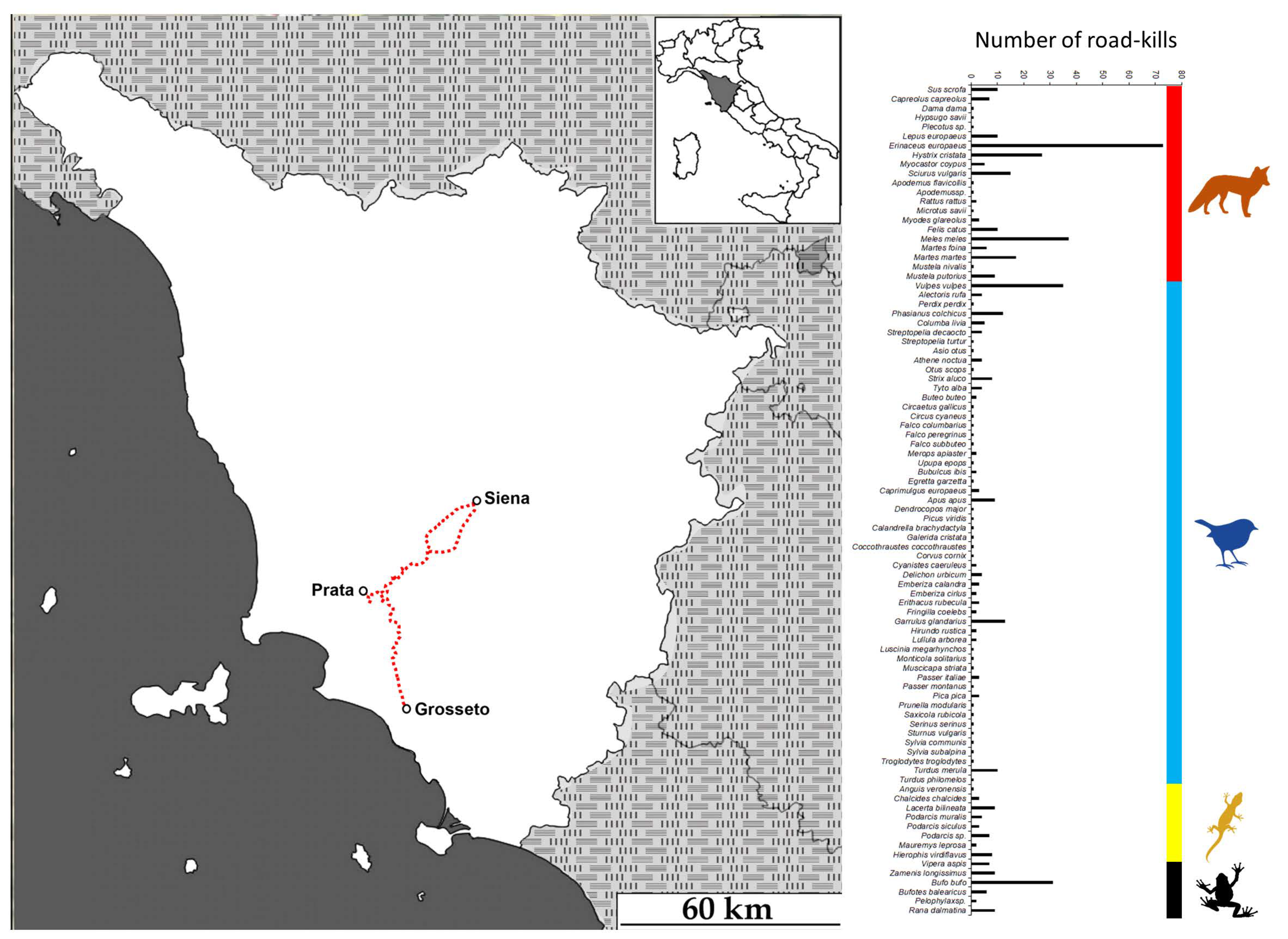
2.1. Study Area (Fieldwork Survey)
2.2. Data Collection and Analyses (Fieldwork Survey and Literature Review)
3. Results
3.1. Fieldwork Survey
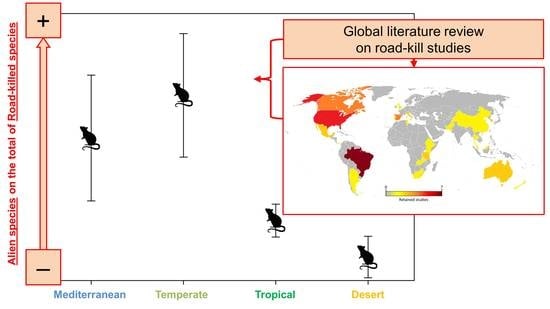
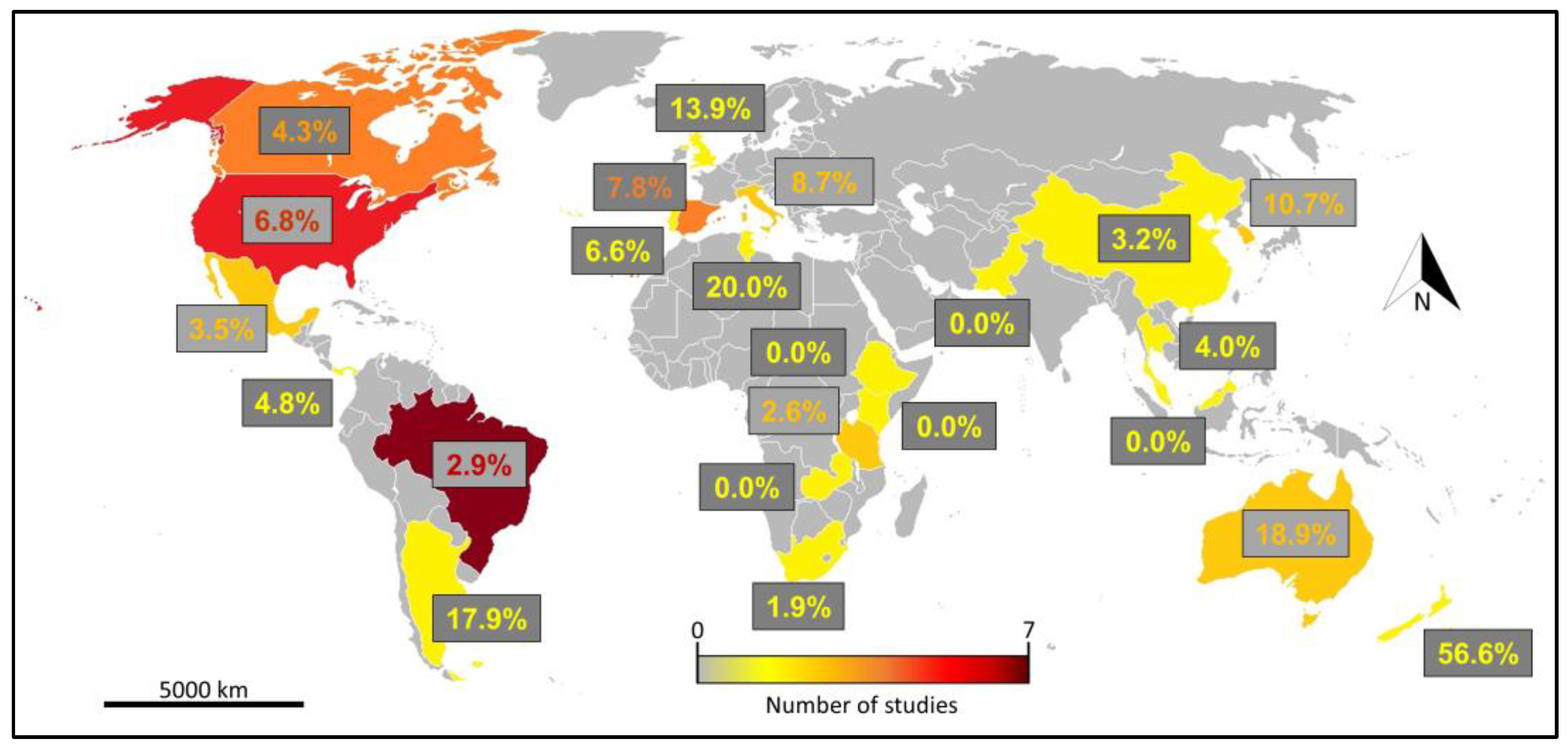
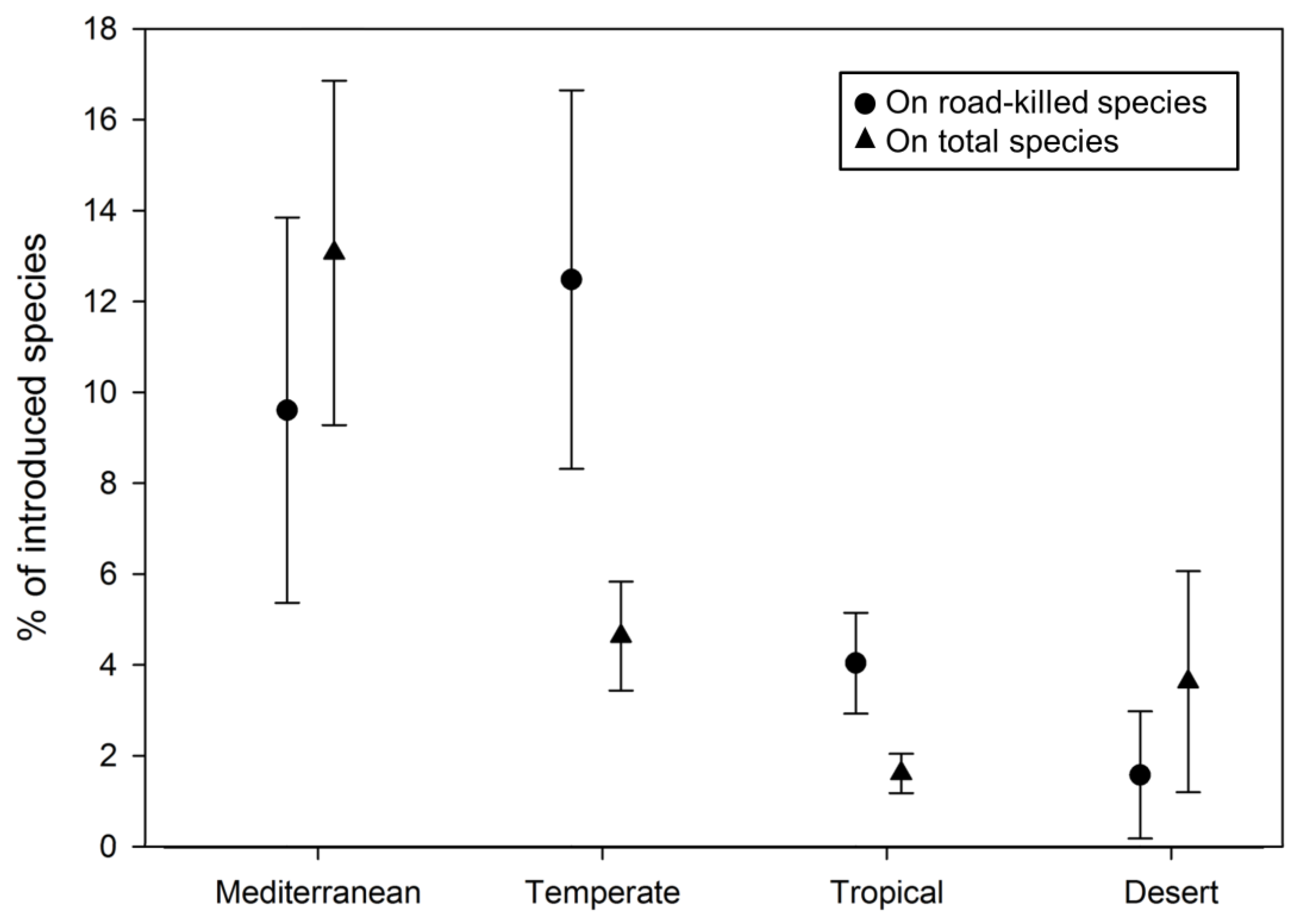
3.2. Literature Review and Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibisch, P.L.; Hoffmann, M.T.; Kreft, S.; Pe’er, G.; Kati, V.; Biber-Freudenberger, L.; Dellasala, D.A.; Vale, M.M.; Hobson, P.R.; Selva, N. A global map of roadless areas and their conservation status. Science 2016, 354, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Ascensão, F.; Fahrig, L.; Clevenger, A.P.; Corlett, R.T.; Jaeger, J.A.; Laurance, W.F.; Pereira, H.M. Environmental challenges for the Belt and Road Initiative. Nat. Sustain. 2018, 1, 206–209. [Google Scholar] [CrossRef]
- Meijer, J.R.; Huijbregts, M.A.; Schotten, K.C.; Schipper, A.M. Global patterns of current and future road infrastructure. Environ. Res. Lett. 2018, 13, 064006. [Google Scholar] [CrossRef]
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Forman, R.T.; Sperling, D.; Bissonette, J.A.; Clevenger, A.P.; Cutshall, C.D.; Dale, V.H.; Fahrig, L.; France, R.; Goldman, C.R.; Heanue, K.; et al. Road Ecology: Science and Solutions; Island Press: Washington, DC, USA, 2003; p. 481. [Google Scholar]
- Van der Ree, R.; Smith, D.J.; Grilo, C. Handbook of Road Ecology; John Wiley & Sons: Oxford, UK, 2015; p. 522. [Google Scholar]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef]
- Laurance, W.F.; Clements, G.R.; Sloan, S.; O’Connell, C.S.; Mueller, N.D.; Goosem, M.; Venter, O.; Edwards, D.P.; Phalan, B.; Balmford, A.; et al. A global strategy for road building. Nature 2014, 513, 229–232. [Google Scholar] [CrossRef]
- Leonard, R.J.; Hochuli, D.F. Exhausting all avenues: Why impacts of air pollution should be part of road ecology. Front. Ecol. Environ. 2017, 15, 443–449. [Google Scholar] [CrossRef]
- Christen, D.C.; Matlack, G.R. The habitat and conduit functions of roads in the spread of three invasive plant species. Biol. Invasions 2009, 11, 453–465. [Google Scholar] [CrossRef]
- Benedetti, Y.; Morelli, F. Spatial mismatch analysis among hotspots of alien plant species, road and railway networks in Germany and Austria. PLoS ONE 2017, 12, e0183691. [Google Scholar] [CrossRef] [PubMed]
- McDougall, K.L.; Lembrechts, J.; Rew, L.J.; Haider, S.; Cavieres, L.A.; Kueffer, C.; Milbau, A.; Naylor, B.J.; Nuñez, M.A.; Pauchard, A.; et al. Running off the road: Roadside non-native plants invading mountain vegetation. Biol. Invasions 2018, 20, 3461–3473. [Google Scholar] [CrossRef]
- Brown, G.P.; Phillips, B.L.; Webb, J.K.; Shine, R. Toad on the road: Use of roads as dispersal corridors by cane toads (Bufo marinus) at an invasion front in tropical Australia. Biol. Conserv. 2006, 133, 88–94. [Google Scholar] [CrossRef]
- D’Amico, M.; Rouco, C.; Russell, J.C.; Román, J.; Revilla, E. Invaders on the road: Synanthopic bird foraging along highways. Oecol. Aust. 2013, 17, 86–95. [Google Scholar] [CrossRef]
- Ascensão, F.; Latombe, G.; Anadón, J.D.; Abellán, P.; Cardador, L.; Carrete, M.; Tella, J.L.; Capinha, C. Drivers of compositional dissimilarity for native and alien birds: The relative roles of human activity and environmental suitability. Biol. Invasions 2020, 22, 1447–1460. [Google Scholar] [CrossRef]
- D’Amico, M.; Ascensão, F.; Fabrizio, M.; Barrientos, R.; Gortázar, C. Twenty years of Road Ecology: A Topical Collection looking forward for new perspectives. Eur. J. Wildl. Res. 2018, 64, 26. [Google Scholar] [CrossRef]
- Collinson, W.; Davies-Mostert, H.; Roxburgh, L.; van der Ree, R. Status of road ecology research in Africa: Do we understand the impacts of roads, and how to successfully mitigate them? Front. Ecol. Evol. 2019, 7, 479. [Google Scholar] [CrossRef]
- Barrientos, R.; Ascensão, F.; D’Amico, M.; Grilo, C.; Pereira, H.M. The lost road: Do transportation networks imperil wildlife population persistence? Perspect. Ecol. Conserv. 2021, 19, 411–416. [Google Scholar] [CrossRef]
- Grilo, C.; Borda-de-Água, L.; Beja, P.; Goolsby, E.; Soanes, K.; le Roux, A.; Koroleva, E.; Ferreira, F.Z.; Gagné, S.A.; Wang, Y.; et al. Conservation threats from roadkill in the global road network. Glob. Ecol. Biogeogr. 2021, 30, 2200–2210. [Google Scholar] [CrossRef]
- Ascensão, F.; Desbiez, A.L. Assessing the impact of roadkill on the persistence of wildlife populations: A case study on the giant anteater. Perspect. Ecol. Conserv. 2022, 20, 272–278. [Google Scholar] [CrossRef]
- Ascensão, F.; Kindel, A.; Teixeira, F.Z.; Barrientos, R.; D’Amico, M.; Borda-de-Água, L.; Pereira, H.M. Beware that the lack of wildlife mortality records can mask a serious impact of linear infrastructures. Glob. Ecol. Conserv. 2019, 19, e00661. [Google Scholar] [CrossRef]
- Hels, T.; Buchwald, E. The effect of road kills on amphibian populations. Biol. Conserv. 2001, 99, 331–340. [Google Scholar] [CrossRef]
- Spencer, R.J.; Van Dyke, J.U.; Thompson, M.B. Critically evaluating best management practices for preventing freshwater turtle extinctions. Conserv. Biol. 2017, 31, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Effects of global household proliferation on ecosystem services. In Landscape Ecology for Sustainable Environment and Culture; Fu, B., Jones, K., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2013; p. 364. [Google Scholar]
- Gilbert, S.L.; Sivy, K.J.; Pozzanghera, C.B.; DuBour, A.; Overduijn, K.; Smith, M.M.; Zhou, J.; Little, J.M.; Prugh, L.R. Socioeconomic benefits of large carnivore recolonization through reduced wildlife-vehicle collisions. Conserv. Lett. 2017, 10, 431–439. [Google Scholar] [CrossRef]
- Martin, A.E.; Graham, S.L.; Henry, M.; Pervin, E.; Fahrig, L. Flying insect abundance declines with increasing road traffic. Insect Conserv. Divers. 2018, 11, 608–613. [Google Scholar] [CrossRef]
- Conover, M.R.; Pitt, W.C.; Kessler, K.K.; DuBow, T.J.; Sanborn, W.A. Review of human injuries, illnesses, and economic losses caused by wildlife in the United States. Wildl. Soc. Bull. 1995, 23, 407–414. [Google Scholar]
- Ascensão, F.; Barrientos, R.; D’Amico, M. Wildlife collisions put a dent in road safety. Science 2021, 374, 1208. [Google Scholar] [CrossRef] [PubMed]
- Llagostera, P.; Comas, C.; López, N. Modeling road traffic safety based on point patterns of wildlife-vehicle collisions. Sci. Total Environ. 2022, 846, 157237. [Google Scholar] [CrossRef] [PubMed]
- Calenge, C.; Chadoeuf, J.; Giraud, C.; Huet, S.; Julliard, R.; Monestiez, P.; Piffady, J.; Pinaud, D.; Ruette, S. The spatial distribution of Mustelidae in France. PLoS ONE 2015, 10, e0121689. [Google Scholar] [CrossRef] [PubMed]
- Dyczkowski, J. Golden nightjar in Western Sahara, Morocco, in May 2015. Dutch Bird. 2016, 38, 80–86. [Google Scholar]
- Lapini, L.; Dreon, A.L.; Caldana, M.; Luca, M.; Villa, M. Distribuzione, espansione e problemi di conservazione di Canis aureus in Italia (Carnivora: Canidae). Quad. Mus. Civ. Stor. Nat. Ferrara 2018, 6, 89–96. [Google Scholar]
- Russo, L.F.; Barrientos, R.; Fabrizio, M.; Di Febbraro, M.; Loy, A. Prioritizing road-kill mitigation areas: A spatially explicit national-scale model for an elusive carnivore. Divers. Distrib. 2020, 26, 1093–1103. [Google Scholar] [CrossRef]
- Schwartz, A.L.; Shilling, F.M.; Perkins, S.E. The value of monitoring wildlife roadkill. Eur. J. Wildl. Res. 2020, 66, 18. [Google Scholar] [CrossRef]
- Vercayie, D.; Herremans, M. Citizen science and smartphones take roadkill monitoring to the next level. Nat. Conserv. 2015, 11, 29–40. [Google Scholar] [CrossRef]
- Waetjen, D.P.; Shilling, F.M. Large extent volunteer roadkill and wildlife observation systems as sources of reliable data. Front. Ecol. Evol. 2017, 5, 89. [Google Scholar] [CrossRef]
- Englefield, B.; Starling, M.; Wilson, B.; Roder, C.; McGreevy, P. The Australian roadkill reporting project—Applying integrated professional research and citizen science to monitor and mitigate roadkill in Australia. Animals 2020, 10, 1112. [Google Scholar] [CrossRef]
- Bik, H.M.; Goldstein, M.C. An introduction to social media for scientists. PLoS Biol. 2013, 11, e1001535. [Google Scholar] [CrossRef]
- Van Strien, A.J.; Van Swaay, C.A.; Termaat, T. Opportunistic citizen science data of animal species produce reliable estimates of distribution trends if analysed with occupancy models. J. Appl. Ecol. 2013, 50, 1450–1458. [Google Scholar] [CrossRef]
- Mori, E.; Di Bari, P.; Coraglia, M. Interference between roe deer and Northern chamois in the Italian Alps: Are Facebook groups effective data sources? Ethol. Ecol. Evol. 2018, 30, 277–284. [Google Scholar] [CrossRef]
- Chyn, K.; Lin, T.E.; Chen, Y.K.; Chen, C.Y.; Fitzgerald, L.A. The magnitude of roadkill in Taiwan: Patterns and consequences revealed by citizen science. Biol. Conserv. 2019, 237, 317–326. [Google Scholar] [CrossRef]
- Heigl, F.; Teufelbauer, N.; Resch, S.; Schweiger, S.; Stückler, S.; Dörler, D. A dataset of road-killed vertebrates collected via citizen science from 2014–2020. Sci. Data 2022, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Panzeri, M.; Mori, E.; Mazza, G.; Menchetti, M. Records of introduced stripe-necked terrapins (Mauremys species) in Italy. Acta Herpetol. 2014, 9, 97–100. [Google Scholar]
- Caley, P.; Ramsey, D.S.; Barry, S.C. Inferring the distribution and demography of an invasive species from sighting data: The red fox incursion into Tasmania. PLoS ONE 2015, 10, e0116631. [Google Scholar] [CrossRef] [PubMed]
- Balčiauskas, L.; Stratford, J.; Balčiauskienė, L.; Kučas, A. Roadkills as a method to monitor raccoon dog populations. Animals 2021, 11, 3147. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Amerini, R.; Mazza, G.; Bertolino, S.; Battiston, R.; Sforzi, A.; Menchetti, M. Alien shades of grey: New occurrences and relevant spread of Sciurus carolinensis in Italy. Eur. J. Ecol. 2016, 2, 13–20. [Google Scholar] [CrossRef]
- Kwak, M.L. The first record of the introduced flea Spilopsyllus cuniculi (Dale, 1878) (Siphonaptera: Pulicidae) from the invasive red fox in Australia, with a review of the fleas associated with the red fox in Australia. Aust. Entomol. 2017, 44, 289–292. [Google Scholar]
- Ham, C.H.; Park, S.M.; Lee, J.E.; Park, J.; Lee, D.H.; Sung, H.C. First report of the Black-headed python (Aspidites melanocephalus Krefft, 1864) found in the wild in the Republic of Korea. BioInvasions Rec. 2022, 11, 571–577. [Google Scholar] [CrossRef]
- Boscherini, A.; Mazza, G.; Menchetti, M.; Laurenzi, A.; Mori, E. Time is running out! Rapid range expansion of the invasive northern raccoon in central Italy. Mammalia 2020, 84, 98–101. [Google Scholar] [CrossRef]
- Santos, S.M.; Lourenco, R.; Mira, A.; Beja, P. Relative effects of road risk, habitat suitability, and connectivity on wildlife roadkills: The case of tawny owls (Strix aluco). PLoS ONE 2013, 8, e79967. [Google Scholar] [CrossRef]
- Haigh, A.; O’Riordan, R.M.; Butler, F. Hedgehog Erinaceus europaeus mortality on Irish roads. Wildl. Biol. 2014, 20, 155–160. [Google Scholar] [CrossRef]
- Meyrom, K.; Yosef, R.; Charter, M. Are roadkills density-dependent? Case study of the barn owl (Tyto alba). Diversity 2023, 15, 412. [Google Scholar] [CrossRef]
- Lapini, L. Il cane viverrino Nyctereutes procyonoides ussuriensis Matschie, 1908 in Italia: Segnalazioni 1980–2005 (Mammalia: Carnivora: Canidae). Boll. Mus. Civ. St. Nat. Venezia 2006, 57, 235–239. [Google Scholar]
- Barrientos, R. Adult sex-ratio distortion in the native European polecat is related to the expansion of the invasive American mink. Biol. Conserv. 2015, 186, 28–34. [Google Scholar] [CrossRef]
- Chen, T.Y.; Richard, R.; Lin, T.E.; Huang, S.P. Landscape forest impacts the potential activity time of an invasive lizard and its possibilities for range expansion in Taiwan under climate warming. J. Therm. Biol. 2021, 98, 102948. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Zalewski, A.; Jędrzejewska, B.; Ansorge, H.; Bunevich, A.N. Reproduction and mortality of invasive raccoon dogs (Nyctereutes procyonoides) in the Białowieża Primeval Forest (eastern Poland). Ann. Zool. Fenn. 2009, 46, 291–301. [Google Scholar] [CrossRef]
- Boncompagni, L.; Molfini, M.; Ciampelli, P.; Fazzi, P.; Lucchesi, M.; Mori, E.; Petralia, L.; Mazza, G. No country for native crayfish: Importance of crustaceans in the diet of native and alien Northern raccoon. Ethol. Ecol. Evol. 2021, 33, 576–590. [Google Scholar] [CrossRef]
- Cuttelod, A.; García, N.; Malak, D.A.; Temple, H.J.; Katariya, V. The Mediterranean: A biodiversity hotspot under threat. In Wildlife in a Changing World—An Analysis of the 2008 IUCN Red List of Threatened Species; Viè, J.C., Hilton-Taylor, C., Stuart, S.N., Eds.; IUCN Editions: Gland, Switzerland, 2009; pp. 89–102. [Google Scholar]
- Clavero, M.; Hermoso, V.; Levin, N.; Kark, S. Biodiversity research: Geographical linkages between threats and imperilment in freshwater fish in the Mediterranean Basin. Divers. Distrib. 2010, 16, 744–754. [Google Scholar] [CrossRef]
- Cao Pinna, L.; Axmanová, I.; Chytrý, M.; Malavasi, M.; Acosta, A.T.; Giulio, S.; Attorre, F.; Bergmeier, E.; Biurrun, I.; Campos, J.A.; et al. The biogeography of alien plant invasions in the Mediterranean Basin. J. Veg. Sci. 2021, 32, e12980. [Google Scholar] [CrossRef]
- Laubier, L. Mediterranean Sea and humans: Improving a conflictual partnership. In The Mediterranean Sea; Saliot, A., Ed.; Springer: Berlin, Germany, 2005; pp. 3–27. [Google Scholar]
- De Jong, Y.; Verbeek, M.; Michelsen, V.; de Place Bjørn, P.; Los, W.; Steeman, F.; Hagedorn, G.; Wetzel, F.T.; Glöcker, F.; Kroupa, A.; et al. Fauna Europaea—All European animal species on the web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef] [PubMed]
- Martín-Forés, I. Exotic plant species in the Mediterranean biome: A reflection of cultural and historical relationships. In Mediterranean Identities—Environment, Society, Culture; Fuerst-Bjelis, B., Ed.; IntechOpen Limited: London, UK, 2017; pp. 179–201. [Google Scholar]
- Maiorano, L.; Amori, G.; Capula, M.; Falcucci, A.; Masi, M.; Montemaggiori, A.; Pottier, J.; Psomas, A.; Rondinini, C.; Russo, D.; et al. Threats from climate change to terrestrial vertebrate hotspots in Europe. PLoS ONE 2013, 8, e74989. [Google Scholar] [CrossRef] [PubMed]
- Gippoliti, S.; Groves, C.P. Overlooked mammal diversity and conservation priorities in Italy: Impacts of taxonomic neglect on a Biodiversity Hotspot in Europe. Zootaxa 2018, 4434, 511–528. [Google Scholar] [CrossRef]
- Loy, A.; Aloise, G.; Ancillotto, L.; Angelici, F.M.; Bertolino, S.; Capizzi, D.; Castiglia, R.; Colangelo, P.; Contoli, L.; Cozzi, B.; et al. Mammals of Italy: An annotated checklist. Hystrix 2019, 30, 87–106. [Google Scholar]
- Lorenzoni, M.; Borghesan, F.; Carosi, A.; Ciuffardi, L.; De Curtis, O.; Delmastro, G.B.; Di Tizio, L.; Franzoi, P.; Maio, G.; Mojetta, A.; et al. Check-list dell’ittiofauna delle acque dolci italiane. Ital. J. Freshw. Ichthyol. 2019, 1, 239–254. [Google Scholar]
- Saliot, A. The Mediterranean Sea; Springer: Berlin, Germany, 2005; p. 408. [Google Scholar]
- Blondel, J. The ‘design’ of Mediterranean landscapes: A millennial story of humans and ecological systems during the historic period. Hum. Ecol. 2006, 34, 713–729. [Google Scholar] [CrossRef]
- Oro, D.; Pueyo, Y.; Bauzà, J.; Errea, M.P.; Arroyo, A.I. Long transient response of vegetation dynamics after four millennia of anthropogenic impacts in an island ecosystem. Glob. Chang. Biol. 2022, 28, 6318–6332. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.A.; Westerman, E.L.; Harris, L.G. Elevated seasonal temperatures eliminate thermal barriers of reproduction of a dominant invasive species: A community state change for northern communities? Divers. Distrib. 2017, 23, 1182–1192. [Google Scholar] [CrossRef]
- Hulme, P.E. Climate change and biological invasions: Evidence, expectations, and response options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Westphal, M.I.; Browne, M.; MacKinnon, K.; Noble, I. The link between international trade and the global distribution of invasive alien species. Biol. Invasions 2008, 10, 391–398. [Google Scholar] [CrossRef]
- Hulme, P.E. Biological invasions in Europe: Drivers, pressures, states, impacts and responses. In Biodiversity under Threat; Hester, R.E., Harrison, R.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2007; pp. 56–80. [Google Scholar]
- Gallardo, B. Europe’s top 10 invasive species: Relative importance of climatic, habitat and socio-economic factors. Ethol. Ecol. Evol. 2014, 26, 130–151. [Google Scholar] [CrossRef]
- Margolis, M.; Shogren, J.F.; Fischer, C. How trade politics affect invasive species control. Ecol. Econ. 2005, 52, 305–313. [Google Scholar] [CrossRef]
- Hulme, P.E. Invasion pathways at a crossroad: Policy and research challenges for managing alien species introductions. J. Appl. Ecol. 2015, 52, 1418–1424. [Google Scholar] [CrossRef]
- Cantini, M.; Menchetti, M.; Vannini, A.; Bruni, G.; Borri, B.; Mori, E. Checklist of Amphibians and Reptiles in a hilly area of Southern Tuscany (Central Italy): An update. Herpetol. Notes 2013, 6, 223–228. [Google Scholar]
- Vannini, A.; Menchetti, M.; Mori, E. L’avifauna del SIC “Poggi di Prata” (Grosseto, Italia Centrale): Analisi faunistica, quantitativa e considerazioni sulla gestione ambientale del sito. Alula 2013, 20, 101–112. [Google Scholar]
- Mori, E.; Menchetti, M.; Dondini, G.; Biosa, D.; Vergari, S. Theriofauna of Site of Community Importance Poggi di Prata (Grosseto, Central Italy): Terrestrial mammals and preliminary data on Chiroptera. Check List 2014, 10, 718–723. [Google Scholar] [CrossRef]
- BirdLife International. Streptopelia turtur. In The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Gippoliti, S.; Amori, G. Ancient introductions of mammals in the Mediterranean Basin and their implications for conservation. Mammal Rev. 2006, 36, 37–48. [Google Scholar] [CrossRef]
- Kemp, M.E.; Mychajliw, A.M.; Wadman, J.; Goldberg, A. 7000 years of turnover: Historical contingency and human niche construction shape the Caribbean’s Anthropocene biota. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200447. [Google Scholar] [CrossRef]
- Halevi, G.; Moed, H.; Bar-Ilan, J. Suitability of Google Scholar as a source of scientific information and as a source of data for scientific evaluation—Review of the literature. J. Informetr. 2017, 11, 823–834. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E. The Global 200: A representation approach to conserving the Earth’s most biologically valuable ecoregions. Conserv. Biol. 1998, 12, 502–515. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference. A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.; Huyvaert, K. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- D’Amico, M.; Román, J.; de los Reyes, L.; Revilla, E. Vertebrate road-kill patterns in Mediterranean habitats: Who, when and where. Biol. Conserv. 2015, 191, 234–242. [Google Scholar] [CrossRef]
- Grilo, C.; Koroleva, E.; Andrášik, R.; Bíl, M.; González-Suárez, M. Roadkill risk and population vulnerability in European birds and mammals. Front. Ecol. Environ. 2020, 18, 323–328. [Google Scholar] [CrossRef]
- Medrano-Vizcaíno, P.; Grilo, C.; Silva Pinto, F.A.; Carvalho, W.D.; Melinski, R.D.; Schultz, E.D.; González-Suárez, M. Roadkill patterns in Latin American birds and mammals. Glob. Ecol. Biogeogr. 2022, 31, 1756–1783. [Google Scholar] [CrossRef]
- Eberhardt, E.; Mitchell, S.; Fahrig, L. Road kill hotspots do not effectively indicate mitigation locations when past road kill has depressed populations. J. Wildl. Manag. 2013, 77, 1353–1359. [Google Scholar] [CrossRef]
- Quiles, P.; Ascensão, F.; D’Amico, M.; Revilla, E.; Barrientos, R. Are road-kills representative of wildlife community obtained from atlas data? Hystrix 2021, 32, 89–94. [Google Scholar]
- Delgado, J.D.; Arévalo, J.R.; Fernández-Palacios, J.M. Road and topography effects on invasion: Edge effects in rat foraging patterns in two oceanic island forests (Tenerife, Canary Islands). Ecography 2001, 24, 539–546. [Google Scholar] [CrossRef]
- Hollings, T.; Jones, M.; Mooney, N.; McCallum, H. Disease-induced decline of an apex predator drives invasive dominated states and threatens biodiversity. Ecology 2016, 97, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.L.; Williams, H.F.; Chadwick, E.; Thomas, R.J.; Perkins, S.E. Roadkill scavenging behaviour in an urban environment. J. Urban Ecol. 2018, 4, juy006. [Google Scholar] [CrossRef]
- Dhiab, O.; D’Amico, M.; Selmi, S. Experimental evidence of increased carcass removal along roads by facultative scavengers. Environ. Monit. Assess. 2023, 195, 216. [Google Scholar] [CrossRef] [PubMed]
- Ascensão, F.; D’Amico, M.; Martins, R.C.; Rebelo, R.; Barbosa, A.M.; Bencatel, J.; Barrientos, R.; Abellán, P.; Tella, J.L.; Cardador, L.; et al. Distribution of alien tetrapods in the Iberian Peninsula. NeoBiota 2021, 64, 1–21. [Google Scholar] [CrossRef]
- Underwood, E.C.; Viers, J.H.; Klausmeyer, K.R.; Cox, R.L.; Shaw, M.R. Threats and biodiversity in the Mediterranean biome. Divers. Distrib. 2009, 15, 188–197. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Change Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Shi, J.; Luo, Y.Q.; Zhou, F.; He, P. The relationship between invasive alien species and main climatic zones. Biodivers. Conserv. 2010, 19, 2485–2500. [Google Scholar] [CrossRef]
- Weidema, I.R. Introduced Species in the Nordic Countries; Nord 2000 Editions: Aarhus, Denmark, 2020; p. 242. [Google Scholar]
- Zhou, Y.; Smith, S.J.; Zhao, K.; Imhoff, M.; Thomson, A.; Bond-Lamberty, B.; Asrar, G.R.; Zhang, X.; He, C.; Elvidge, C.D. A global map of urban extent from nightlights. Environ. Res. Lett. 2015, 10, 054011. [Google Scholar] [CrossRef]
- Kuemmerle, T.; Levers, C.; Erb, K.; Estel, S.; Jepsen, M.R.; Müller, D.; Plutzar, C.; Stürck, J.; Verkerk, P.J.; Reenberg, A. Hotspots of land use change in Europe. Environ. Res. Lett. 2016, 11, 064020. [Google Scholar] [CrossRef]
- Richardson, H.W.; Bae, C.H.C. Urban Sprawl in Western Europe and the United States; Routledge: London, UK, 2017; p. 344. [Google Scholar]
- Masseti, M.; Albarella, U.; De Grossi Mazzorin, J. The crested porcupine, Hystrix cristata L., 1758, in Italy. Anthropozoologia 2010, 45, 27–42. [Google Scholar] [CrossRef]
- Esattore, B.; Saggiomo, L.; Sensi, M.; Francia, V.; Cherin, M. Tell me what you eat and I’ll tell you…where you live: An updated review of the worldwide distribution and foraging ecology of the fallow deer (Dama dama). Mamm. Biol. 2022, 102, 321–338. [Google Scholar] [CrossRef]
- Moreira, F.; Allsopp, N.; Esler, K.J.; Wardell-Johnson, G.; Ancillotto, L.; Arianoutsou, M.; Clary, J.; Brotons, L.; Clavero, M.; Dimitrakopoulos, P.G.; et al. Priority questions for biodiversity conservation in the Mediterranean biome: Heterogeneous perspectives across continents and stakeholders. Conserv. Sci. Pract. 2019, 1, e118. [Google Scholar] [CrossRef]
- Newbold, T.; Oppenheimer, P.; Etard, A.; Williams, J.J. Tropical and Mediterranean biodiversity is disproportionately sensitive to land-use and climate change. Nat. Ecol. Evol. 2020, 4, 1630–1638. [Google Scholar] [CrossRef]
- Garriga, N.; Santos, X.; Montori, A.; Richter-Boix, A.; Franch, M.; Llorente, G.A. Are protected areas truly protected? The impact of road traffic on vertebrate fauna. Biodivers. Conserv. 2012, 21, 2761–2774. [Google Scholar] [CrossRef]
- Kang, W.; Minor, E.S.; Woo, D.; Lee, D.; Park, C.R. Forest mammal roadkills as related to habitat connectivity in protected areas. Biodivers. Conserv. 2016, 25, 2673–2686. [Google Scholar] [CrossRef]
- Collinson, W.J.; Marneweck, C.; Davies-Mostert, H.T. Protecting the protected: Reducing wildlife roadkill in protected areas. Anim. Conserv. 2019, 22, 396–403. [Google Scholar] [CrossRef]
- Mori, E.; Bini, A.; Viviano, A.; Bartolommei, P.; Mazza, G. Distribution of introduced American mink in the Northern Apennine area (Central Italy). Mammalia 2022, 86, 266–270. [Google Scholar] [CrossRef]
- Périquet, S.; Roxburgh, L.; Le Roux, A.; Collinson, W.J. Testing the value of citizen science for roadkill studies: A case study from South Africa. Front. Ecol. Evol. 2018, 6, 15. [Google Scholar] [CrossRef]
- Valerio, F.; Basile, M.; Balestrieri, R. The identification of wildlife-vehicle collision hotspots: Citizen science reveals spatial and temporal patterns. Ecol. Process. 2021, 10, 6. [Google Scholar] [CrossRef]
- Aristeidou, M.; Herodotou, C.; Ballard, H.L.; Young, A.N.; Miller, A.E.; Higgins, L.; Johnson, R.F. Exploring the participation of young citizen scientists in scientific research: The case of iNaturalist. PLoS ONE 2021, 16, e0245682. [Google Scholar] [CrossRef]
- Mesaglio, T.; Callaghan, C.T. An overview of the history, current contributions and future outlook of iNaturalist in Australia. Wildl. Res. 2021, 48, 289–303. [Google Scholar] [CrossRef]
- Wolf, S.; Mahecha, M.D.; Sabatini, F.M.; Wirth, C.; Bruelheide, H.; Kattge, J.; Martínez, A.M.; Mora, K.; Kattenborn, T. Citizen science plant observations encode global trait patterns. Nat. Ecol. Evol. 2022, 6, 1850–1859. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Mihalca, A.D.; Traub, R.J.; Lappin, M.; Baneth, G. Zoonotic parasites of sheltered and stray dogs in the era of the global economic and political crisis. Trends Parasitol. 2017, 33, 813–825. [Google Scholar] [CrossRef]
- Dong, S. Overview: Pastoralism in the World. In Building Resilience of Human-Natural Systems of Pastoralism in the Developing World; Dong, S., Kassam, K.A., Tourrand, J., Boone, R., Eds.; Springer International Publishing: Cham, Switzerland, 2016; p. 298. [Google Scholar]
- Giuntini, S.; Ancillotto, L.; Mori, E.; Viviano, A. The Alexandrine parakeet Psittacula eupatria as a naturalized breeding species: A proposal of integration to the national official checklist. Avocetta 2021, 45, 177–179. [Google Scholar]
- Di Nicola, M.; Cavigioli, L.; Luiselli, L.; Andreone, F. Anfibi & Rettili d’Italia; Belvedere, Ed.; Historia Naturae: Latina, Italy, 2021; p. 576. [Google Scholar]
- McKinney, M.L. Influence of settlement time, human population, park shape and age, visitation and roads on the number of alien plant species in protected areas in the USA. Divers. Distrib. 2002, 8, 311–318. [Google Scholar] [CrossRef]
- Cardador, L.; Carrete, M.; Gallardo, B.; Tella, J.L. Combining trade data and niche modelling improves predictions of the origin and distribution of non-native European populations of a globally invasive species. J. Biogeogr. 2016, 43, 967–978. [Google Scholar] [CrossRef]
- Mazzamuto, M.V.; Wauters, L.A.; Koprowski, J.L. Exotic pet trade as a cause of biological invasions: The case of tree squirrels of the genus Callosciurus. Biology 2021, 10, 1046. [Google Scholar] [CrossRef] [PubMed]

| Roadkill Model Selection | |||||||
| Model | K | AICc | ΔAICc | wAICc | ER | Rank | R2 |
| Latitude | 3 | 244.6 | 0.0 | 0.78 | 1.0 | 1 | 0.15 |
| Nc | 4 | 247.9 | 3.3 | 0.15 | 5.0 | 2 | 0.21 |
| Biome | 5 | 250.5 | 5.9 | 0.04 | 19.0 | 3 | 0.16 |
| Null | 2 | 251.7 | 7.1 | 0.02 | 34.7 | - | 0.00 |
| GDP | 4 | 256.2 | 11.6 | 0.01 | 324.5 | - | 0.01 |
| Full | 10 | 258.3 | 13.6 | 0.00 | 918.0 | - | 0.32 |
| All-Species Model Selection | |||||||
| Model | AICc | ΔAICc | wAICc | ER | Rank | R2 | |
| Biome | 5 | 187.9 | 0.0 | 0.99 | 1.0 | 1 | 0.19 |
| Latitude | 3 | 197.3 | 9.3 | 0.01 | 107.0 | - | 0.54 |
| Full | 10 | 199.2 | 11.2 | 0.00 | 276.7 | - | 0.57 |
| Nc | 4 | 205.5 | 17.6 | 0.00 | 6555.3 | - | 0.23 |
| Null | 2 | 210.2 | 22.3 | 0.00 | 70,829.4 | - | 0.00 |
| GDP | 4 | 214.1 | 26.1 | 0.00 | 475,931.8 | - | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viviano, A.; D’Amico, M.; Mori, E. Aliens on the Road: Surveying Wildlife Roadkill to Assess the Risk of Biological Invasion. Biology 2023, 12, 850. https://doi.org/10.3390/biology12060850
Viviano A, D’Amico M, Mori E. Aliens on the Road: Surveying Wildlife Roadkill to Assess the Risk of Biological Invasion. Biology. 2023; 12(6):850. https://doi.org/10.3390/biology12060850
Chicago/Turabian StyleViviano, Andrea, Marcello D’Amico, and Emiliano Mori. 2023. "Aliens on the Road: Surveying Wildlife Roadkill to Assess the Risk of Biological Invasion" Biology 12, no. 6: 850. https://doi.org/10.3390/biology12060850
APA StyleViviano, A., D’Amico, M., & Mori, E. (2023). Aliens on the Road: Surveying Wildlife Roadkill to Assess the Risk of Biological Invasion. Biology, 12(6), 850. https://doi.org/10.3390/biology12060850