Efficacy of an Immunotherapy Combining Immunogenic Chimeric Protein Plus Adjuvant and Amphotericin B against Murine Visceral Leishmaniasis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Parasites
2.2. Production of ChimT and Soluble Leishmania Antigenic (SLA) Extract
2.3. Infection and Immunotherapeutic Schedules
2.4. Organ Toxicity
2.5. Antibody Production
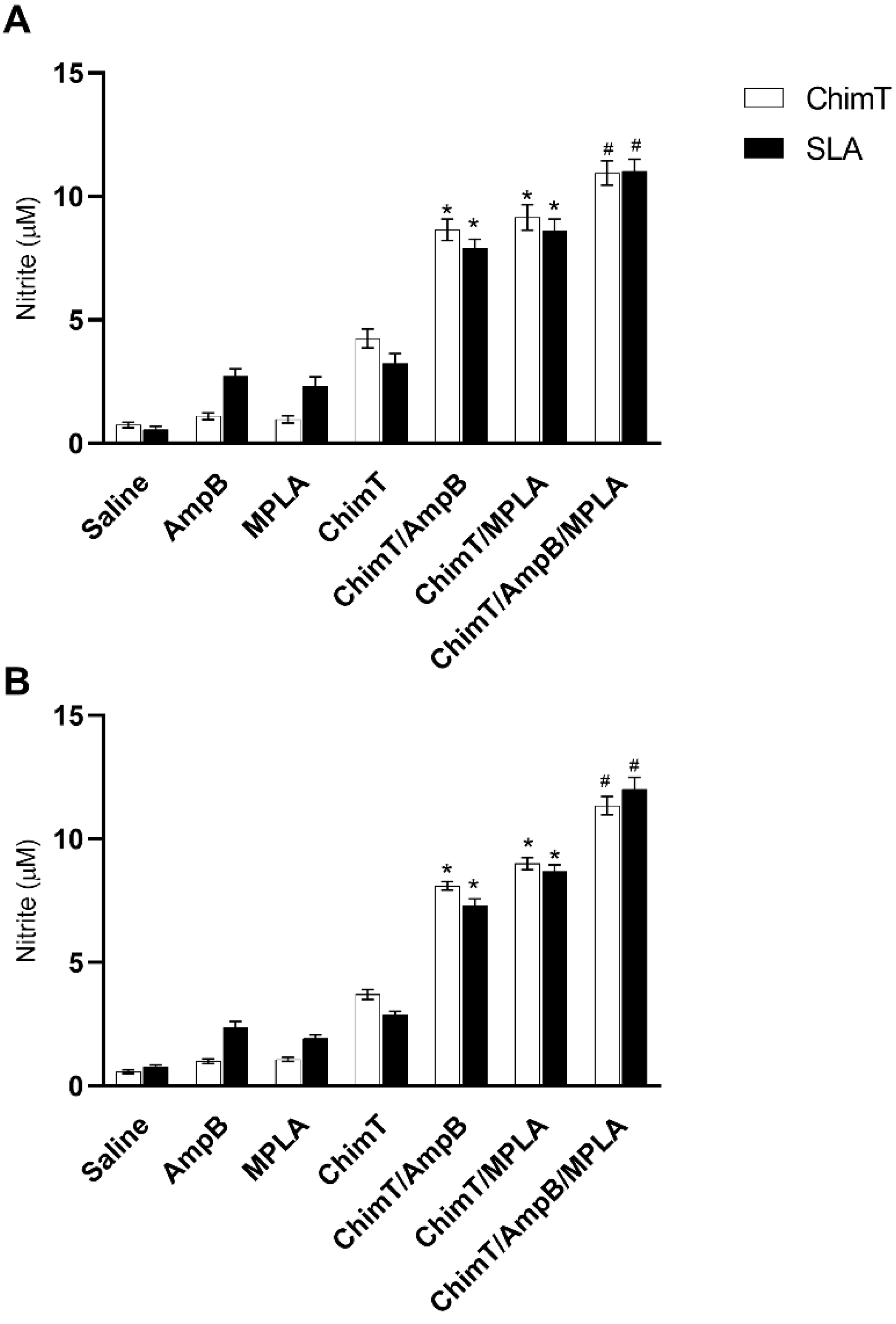
2.6. Cellular Response and Nitrite Production
2.7. Flow Cytometry
2.8. IFN-γ mRNA Expression
2.9. Parasite Load Quantified by Limiting Dilution Technique
2.10. Splenic Parasitism Evaluated by qPCR
2.11. Treatment of Infected Macrophages and Evaluation of In Vitro Cytokine Production
2.12. Statistical Analyses
3. Results
3.1. The Association between ChimT, MPLA and AmpB Reduces the Organic Toxicity in the Treated Animals
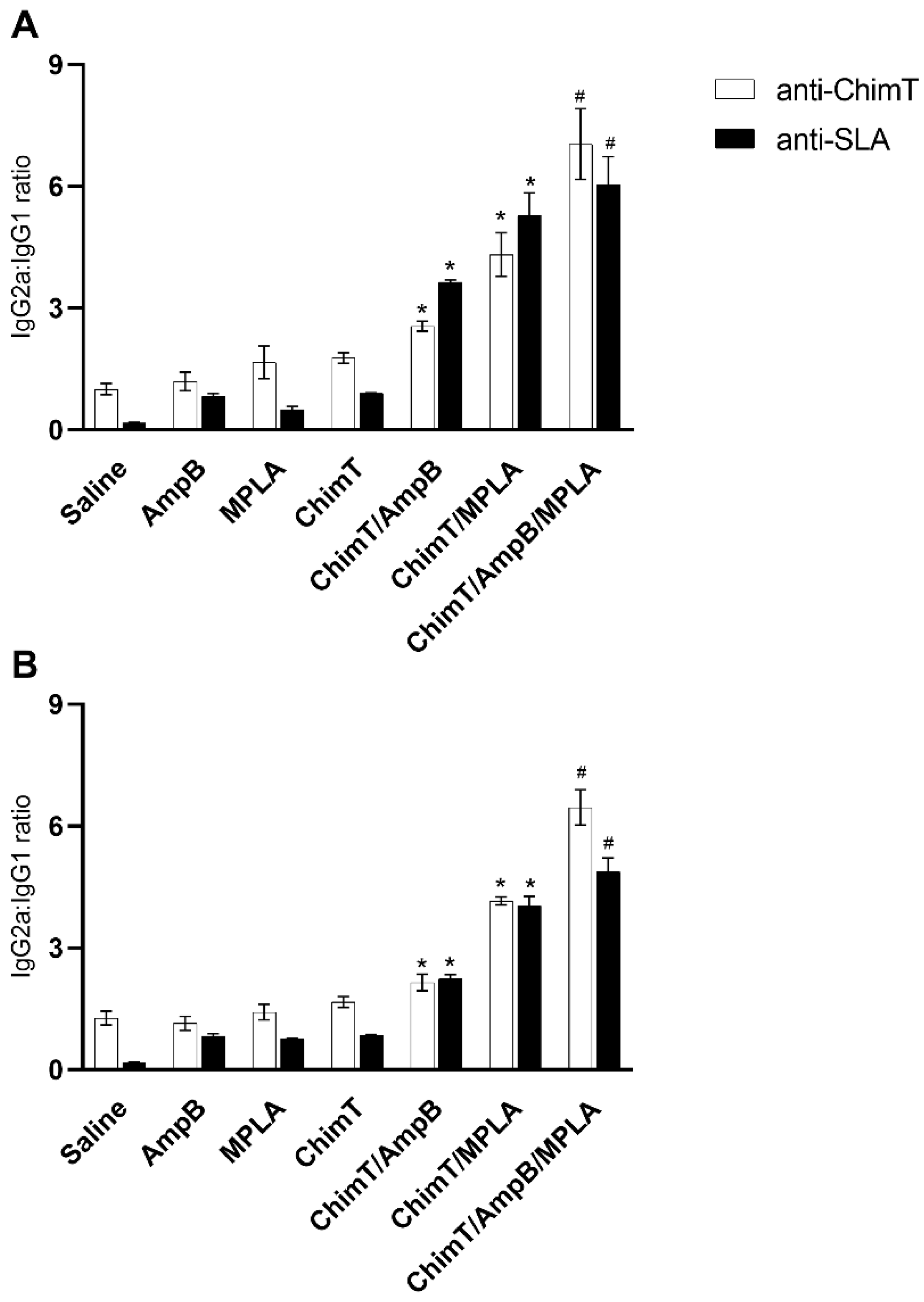
3.2. Higher Production of Anti-Parasite IgG2a Antibody in Mice Treated with ChimT/MPLA/AmpB
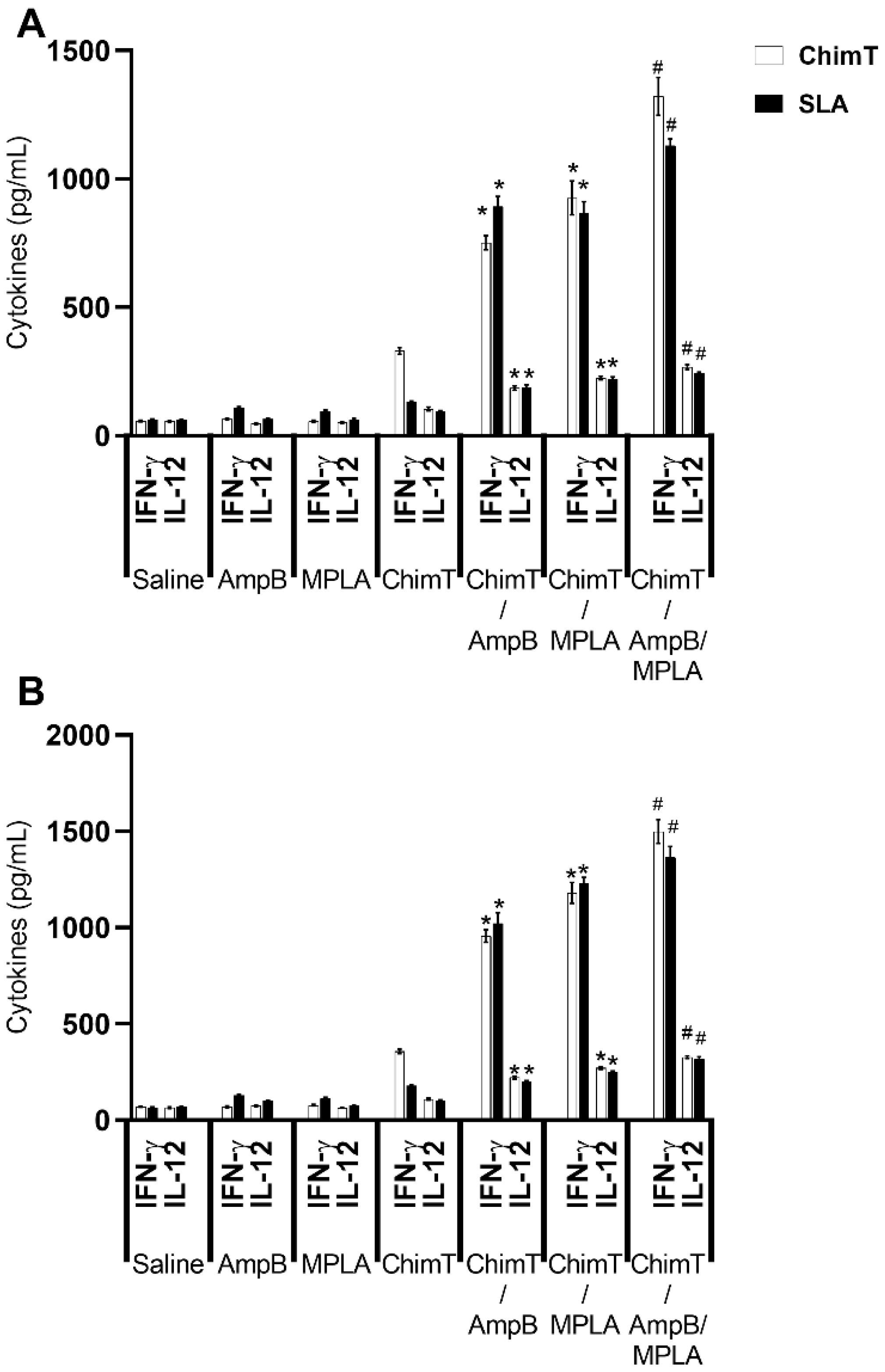
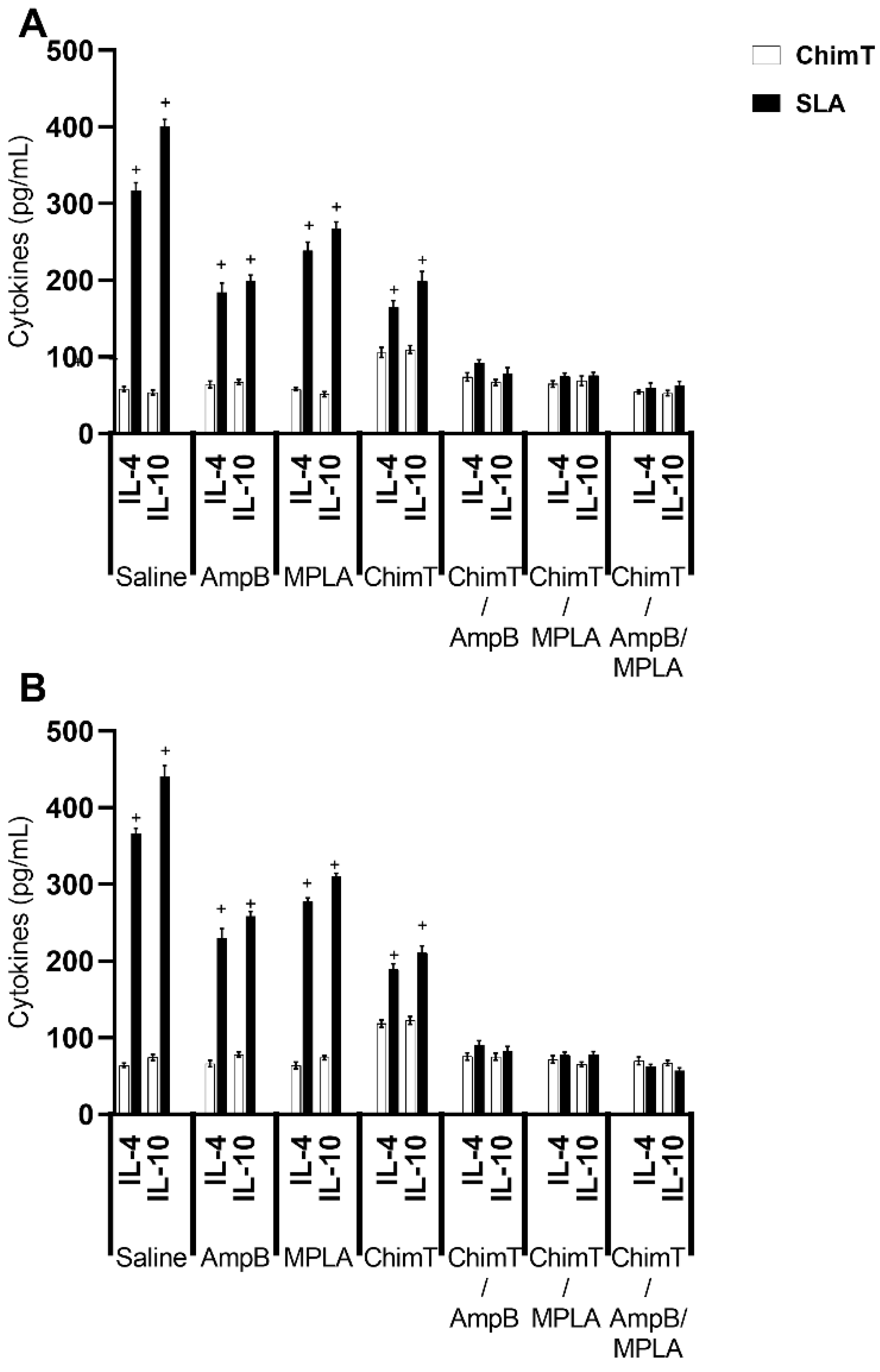
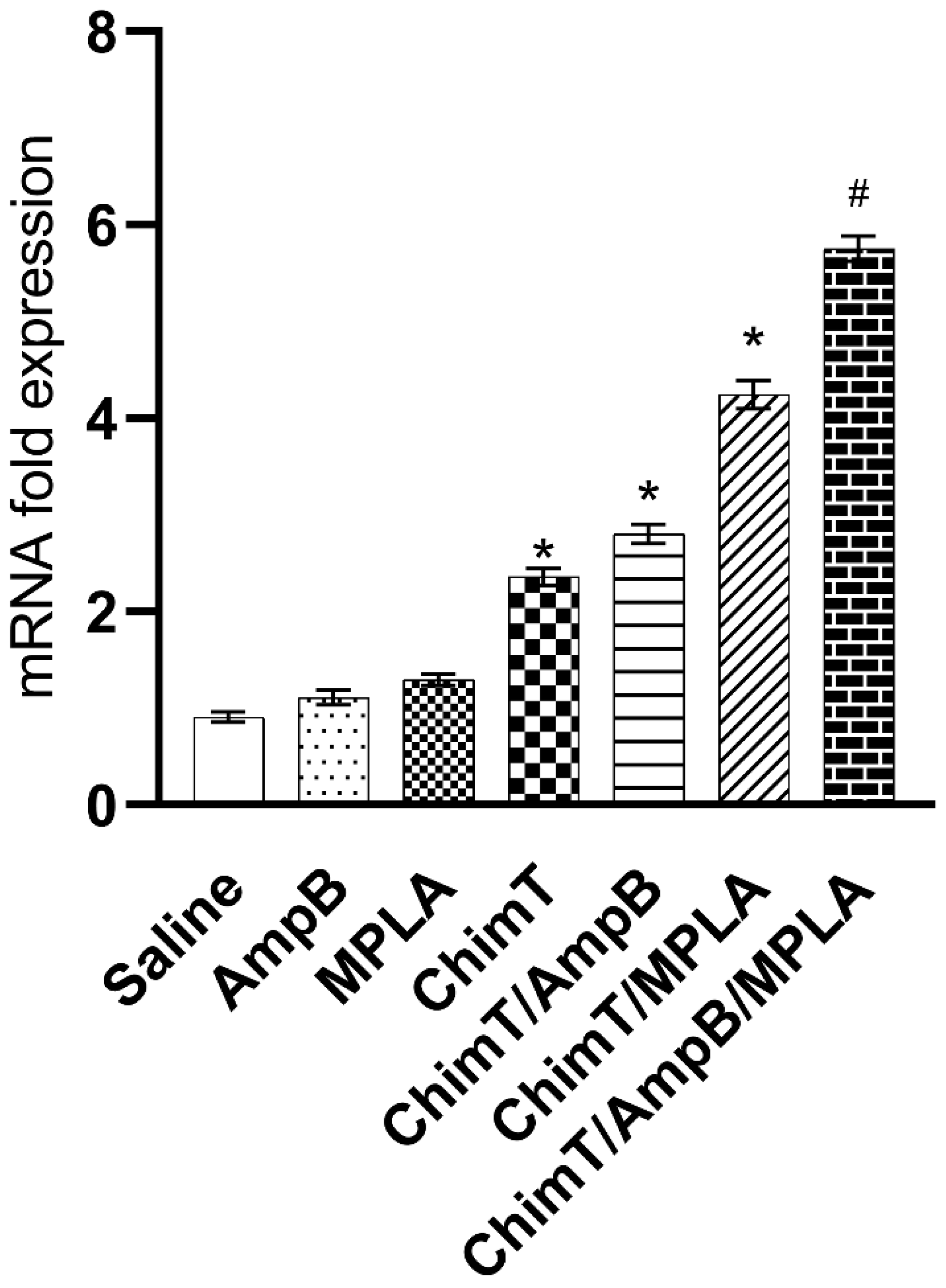
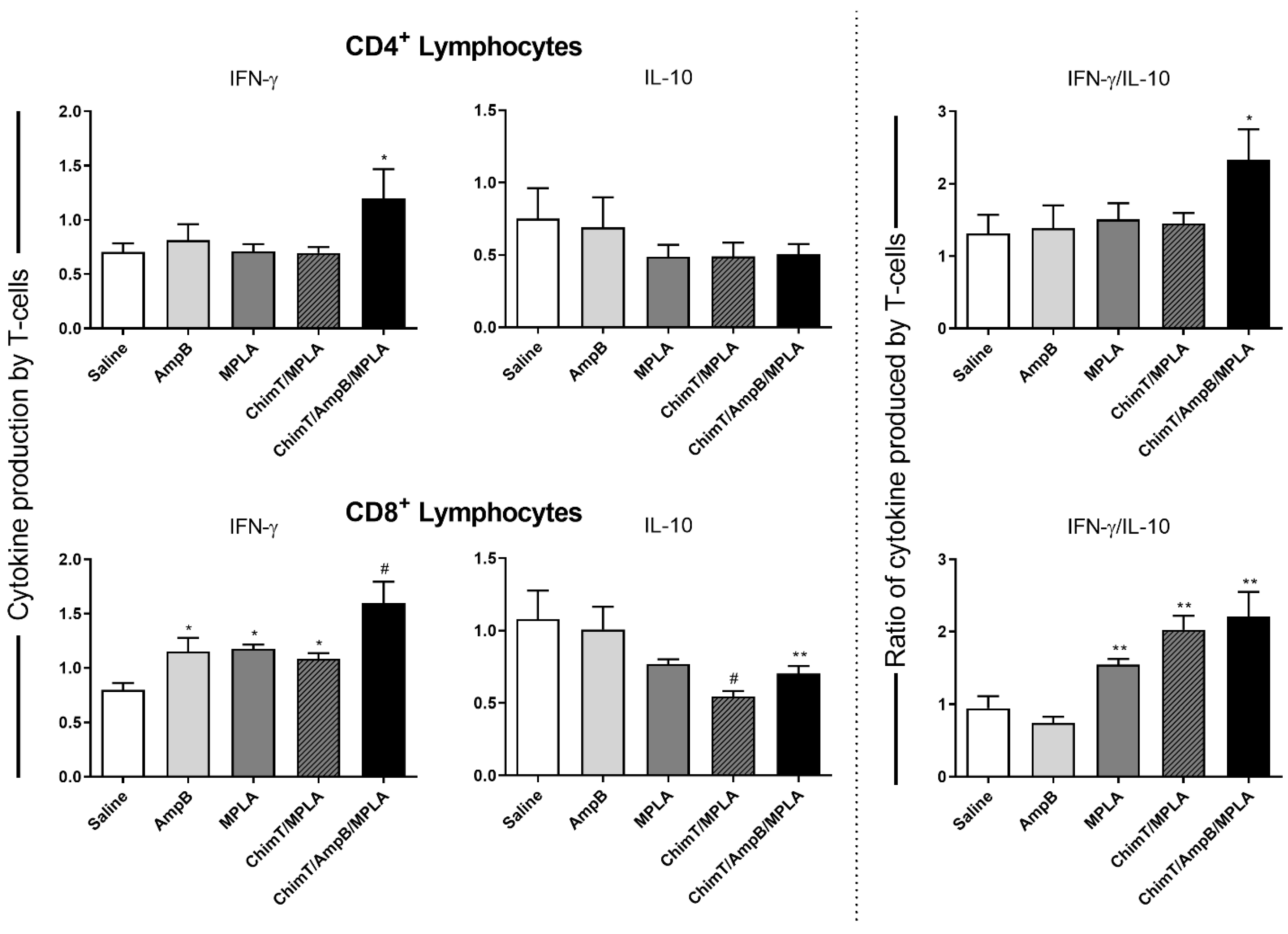
3.3. Mice Receiving ChimT/MPLA/AmpB Develop a Th1-Type Cellular Immune Response
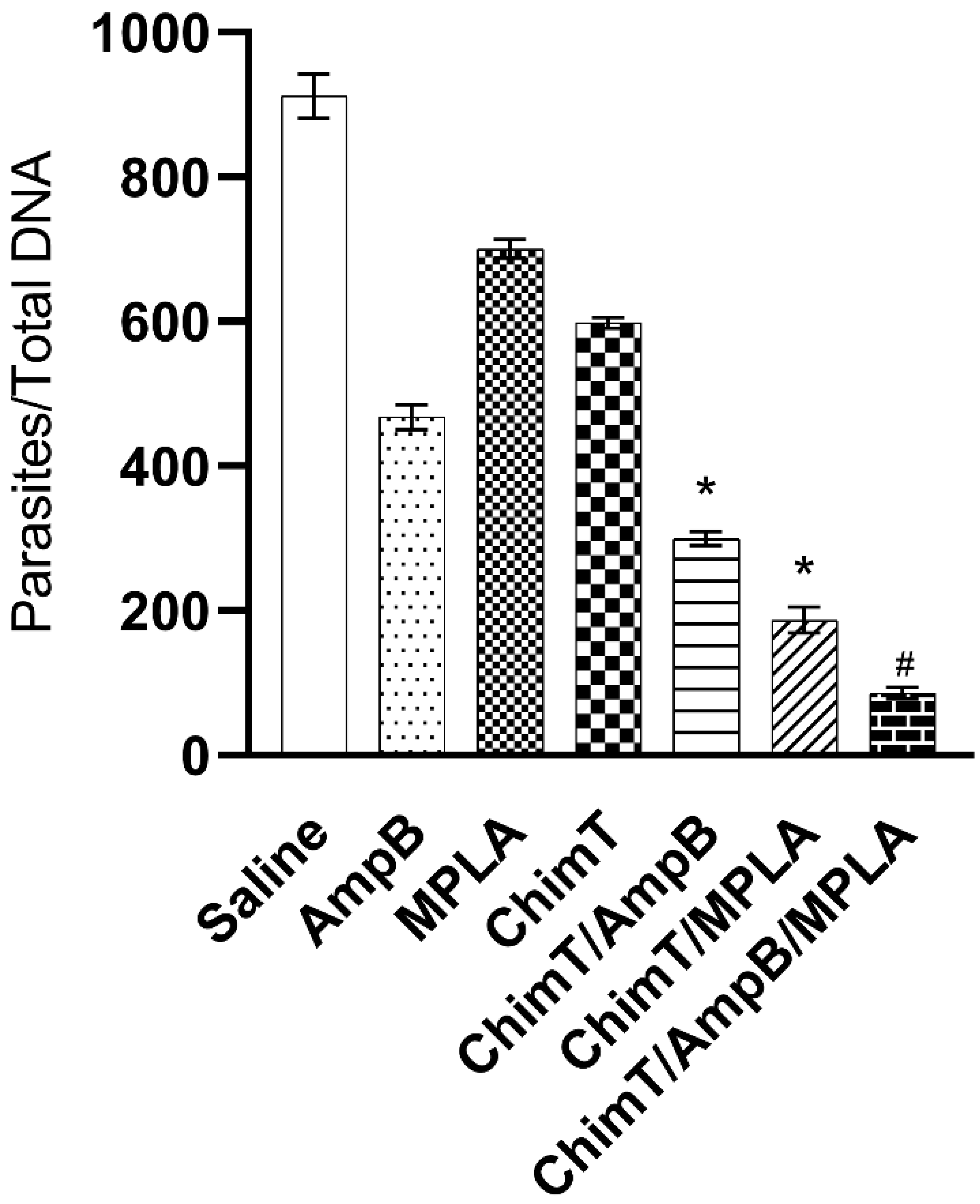
3.4. Treatment with ChimT/MPLA/AmpB Reduces Parasitism in Treated Mice
3.5. ChimT Stimulates In Vitro Infected Macrophages to Kill Parasites and to Produce Th1-Type Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- World-Health-Organisation. Available online: http://www.who.int/topics/leishmaniasis/en/ (accessed on 30 March 2023).
- Grimaldi, G.; Tesh, R.B. Leishmaniases of the New World: Current concepts and implications for future research. Clin. Microbiol. Rev. 1993, 6, 230–250. [Google Scholar] [CrossRef] [PubMed]
- Ejazi, S.A.; Ali, N. Developments in diagnosis and treatment of visceral leishmaniasis during the last decade and future prospects. Expert Rev. Anti-Infect. Ther. 2013, 11, 79–98. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, O.P. Molecular Diagnosis of Visceral Leishmaniasis. Mol. Diagn. Ther. 2018, 22, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; de Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kumar, V.; Tiwari, R.K.; Ravidas, V.; Pandey, K.; Kumar, A. Amphotericin B: A drug of choice for Visceral Leishmaniasis. Acta Trop. 2022, 235, 106661. [Google Scholar] [CrossRef]
- Chávez-Fumagalli, M.A.; Ribeiro, T.G.; Castilho, R.O.; Fernandes, S.O.A.; Cardoso, V.N.; Coelho, C.S.P.; Mendonça, D.V.C.; Soto, M.; Tavares, C.A.P.; Faraco, A.A.G.; et al. New delivery systems for amphotericin B applied to the improvement of leishmaniasis treatment. Rev. Soc. Bras. Med. Trop. 2015, 48, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mosimann, V.; Neumayr, A.; Paris, D.H.; Blum, J. Liposomal amphotericin B treatment of Old World cutaneous and mucosal leishmaniasis: A literature review. Acta Trop. 2018, 182, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Silva-Carvalho, R.; Fidalgo, J.; Melo, K.; Queiroz, M.; Leal, S.; Rocha, H.; Cruz, T.; Parpot, P.; Tomás, A.; Gama, M. Development of dextrin-amphotericin B formulations for the treatment of Leishmaniasis. Int. J. Biol. Macromol. 2020, 153, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Silva-Carvalho, R.; Fidalgo, J.; Melo, K.; Queiroz, M.; Leal, S.; Rocha, H.; Cruz, T.; Parpot, P.; Tomás, A.; Gama, M. Corrigendum to “Development of dextrin-amphotericin B formulations for the treatment of Leishmaniasis” [Int. J. Biol. Macromol., 15 (2020) 276–288]. Int. J. Biol. Macromol. 2021, 166, 1619. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- McQuarrie, S.; Kasper, K.; Moffatt, D.C.; Marko, D.; Keynan, Y. Relapse of Visceral Leishmaniasis in an HIV-Infected Patient Successfully Treated with a Combination of Miltefosine and Amphotericin B. Can. J. Infect. Dis. Med. Microbiol. 2015, 26, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, C.D.P.; Kushawaha, P.K.; Sangwan, R.S.; Mandal, C.; Misra-Bhattacharya, S.; Dube, A. Withania somnifera chemotype NMITLI 101R significantly increases the efficacy of antileishmanial drugs by generating strong IFN-γ and IL-12 mediated immune responses in Leishmania donovani infected hamsters. Phytomedicine 2017, 24, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Oryan, A.; Hatam, G. Immunotherapy in treatment of leishmaniasis. Immunol. Lett. 2021, 233, 80–86. [Google Scholar] [CrossRef]
- Mazire, P.H.; Saha, B.; Roy, A. Immunotherapy for visceral leishmaniasis: A trapeze of balancing counteractive forces. Int. Immunopharmacol. 2022, 110, 108969. [Google Scholar] [CrossRef]
- Nylén, S.; Sacks, D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007, 28, 378–384. [Google Scholar] [CrossRef]
- Bacellar, O.; Brodskyn, C.; Guerreiro, J.; Barral-Netto, M.; Costa, C.H.; Coffman, R.L.; Johnson, W.D.; Carvalho, E.M. Interleukin-12 Restores Interferon- Production and Cytotoxic Responses in Visceral Leishmaniasis. J. Infect. Dis. 1996, 173, 1515–1518. [Google Scholar] [CrossRef]
- Ramos, I.; Alonso, A.; Marcen, J.; Peris, A.; Castillo, J.; Colmenares, M.; Larraga, V. Heterologous prime-boost vaccination with a non-replicative vaccinia recombinant vector expressing LACK confers protection against canine visceral leishmaniasis with a predominant Th1-specific immune response. Vaccine 2008, 26, 333–344. [Google Scholar] [CrossRef]
- Dinc, R. Leishmania Vaccines: The Current Situation with Its Promising Aspect for the Future. Korean J. Parasitol. 2022, 60, 379–391. [Google Scholar] [CrossRef]
- Deak, E.; Jayakumar, A.; Cho, K.W.; Goldsmith-Pestana, K.; Dondji, B.; Lambris, J.D.; McMahon-Pratt, D. Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur. J. Immunol. 2010, 40, 1355–1368. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Ali, N. Involvement and interactions of different immune cells and their cytokines in human visceral leishmaniasis. Rev. Soc. Bras. Med. Trop. 2013, 46, 128–134. [Google Scholar] [CrossRef]
- Le Rutte, E.A.; Coffeng, L.E.; Malvolti, S.; Kaye, P.M.; de Vlas, S.J. The potential impact of human visceral leishmaniasis vaccines on population incidence. PLoS Negl. Trop. Dis. 2020, 14, e0008468. [Google Scholar] [CrossRef]
- Poot, J.; Janssen, L.; van Kasteren-Westerneng, T.; van der Heijden-Liefkens, K.; Schijns, V.; Heckeroth, A. Vaccination of dogs with six different candidate leishmaniasis vaccines composed of a chimerical recombinant protein containing ribosomal and histone protein epitopes in combination with different adjuvants. Vaccine 2009, 27, 4439–4446. [Google Scholar] [CrossRef]
- Martins, V.T.; Duarte, M.C.; Lage, D.P.; Costa, L.E.; Carvalho, A.M.R.S.; Mendes, T.A.O.; Roatt, B.M.; Menezes-Souza, D.; Soto, M.; Coelho, E.A.F. A recombinant chimeric protein composed of human and mice-specific CD4+ and CD8+ T-cell epitopes protects against visceral leishmaniasis. Parasite Immunol. 2017, 39, e12359. [Google Scholar] [CrossRef]
- Ostolin, T.L.V.D.P.; Gusmão, M.R.; Mathias, F.A.S.; Cardoso, J.M.D.O.; Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Ruiz, J.C.; Resende, D.D.M.; de Brito, R.C.F.; Reis, A.B. A chimeric vaccine combined with adjuvant system induces immunogenicity and protection against visceral leishmaniasis in BALB/c mice. Vaccine 2021, 39, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Baldricka, P.; Richardson, D.; Elliottc, G.; Wheeler, A.W. Safety Evaluation of Monophosphoryl Lipid A (MPL): An Immunostimulatory Adjuvant. Regul. Toxicol. Pharmacol. 2002, 35, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.S.; Chilton, P.M.; Ward, J.R.; Evans, J.T.; Mitchell, T.C. The low-toxicity versions of LPS, MPL® adjuvant and RC529, are efficient adjuvants for CD4+ T cells. J. Leukoc. Biol. 2005, 78, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, R.; Zhu, N. Enhancement of humoral and cellular immune responses by monophosphoryl lipid A (MPLA) as an adjuvant to the rabies vaccine in BALB/c mice. Immunobiology 2013, 218, 1524–1528. [Google Scholar] [CrossRef]
- Blanco-Pérez, F.; Goretzki, A.; Wolfheimer, S.; Schülke, S. The vaccine adjuvant MPLA activates glycolytic metabolism in mouse mDC by a JNK-dependent activation of mTOR-signaling. Mol. Immunol. 2019, 106, 159–169. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, C.; Li, R.; Wang, Z.; Yuan, Y.; Li, H.; Fu, Z.; Zhou, M.; Zhao, L. Monophosphoryl-Lipid A (MPLA) is an Efficacious Adjuvant for Inactivated Rabies Vaccines. Viruses 2019, 11, 1118. [Google Scholar] [CrossRef]
- Yang, D.; Luo, X.; Lian, Q.; Gao, L.; Wang, C.; Qi, X.; Zhang, R.; Liu, Z.; Liao, G. Fully synthetic Tn-based three-component cancer vaccine using covalently linked TLR4 ligand MPLA and iNKT cell agonist KRN-7000 as built-in adjuvant effectively protects mice from tumor development. Acta Pharm. Sin. B 2022, 12, 4432–4445. [Google Scholar] [CrossRef] [PubMed]
- Lage, D.P.; Ribeiro, P.A.; Dias, D.S.; Mendonça, D.V.; Ramos, F.F.; Carvalho, L.M.; Steiner, B.T.; Tavares, G.S.; Martins, V.T.; Machado, A.S.; et al. Liposomal Formulation of ChimeraT, a Multiple T-Cell Epitope-Containing Recombinant Protein, Is a Candidate Vaccine for Human Visceral Leishmaniasis. Vaccines 2020, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.A.F.; Tavares, C.A.P.; Carvalho, F.A.A.; Chaves, K.F.; Teixeira, K.N.; Rodrigues, R.C.; Charest, H.; Matlashewski, G.; Gazzinelli, R.T.; Fernandes, A.P. Immune Responses Induced by the Leishmania (Leishmania) donovani A2 Antigen, but Not by the LACK Antigen, Are Protective against Experimental Leishmania (Leishmania) amazonensis Infection. Infect. Immun. 2003, 71, 3988–3994. [Google Scholar] [CrossRef] [PubMed]
- Lage, D.P.; Vale, D.L.; Linhares, F.P.; Freitas, C.S.; Machado, A.S.; Cardoso, J.M.O.; de Oliveira, D.; Galvani, N.C.; de Oliveira, M.P.; Oliveira-Da-Silva, J.A.; et al. A Recombinant Chimeric Protein-Based Vaccine Containing T-Cell Epitopes from Amastigote Proteins and Combined with Distinct Adjuvants, Induces Immunogenicity and Protection against Leishmania infantum Infection. Vaccines 2022, 10, 1146. [Google Scholar] [CrossRef]
- Machado, A.S.; Lage, D.P.; Vale, D.L.; Freitas, C.S.; Linhares, F.P.; Cardoso, J.M.; Pereira, I.A.; Ramos, F.F.; Tavares, G.S.; Ludolf, F.; et al. A recombinant Leishmania amastigote-specific protein, rLiHyG, with adjuvants, protects against infection with Leishmania infantum. Acta Trop. 2022, 230, 106412. [Google Scholar] [CrossRef]
- Giulietti, A.; Overbergh, L.; Valckx, D.; Decallonne, B.; Bouillon, R.; Mathieu, C. An Overview of Real-Time Quantitative PCR: Applications to Quantify Cytokine Gene Expression. Methods 2001, 25, 386–401. [Google Scholar] [CrossRef]
- Freitas, C.S.; Lage, D.P.; Machado, A.S.; Vale, D.L.; Martins, V.T.; Cardoso, J.M.; Oliveira-Da-Silva, J.A.; Reis, T.A.; Tavares, G.S.; Ramos, F.F.; et al. Exploring drug repositioning for leishmaniasis treatment: Ivermectin plus polymeric micelles induce immunological response and protection against tegumentary leishmaniasis. Cytokine 2023, 164, 156143. [Google Scholar] [CrossRef]
- Duthie, M.S.; Favila, M.; Hofmeyer, K.A.; Tutterrow, Y.L.; Reed, S.J.; Laurance, J.D.; Picone, A.; Guderian, J.; Bailor, H.R.; Vallur, A.C.; et al. Strategic evaluation of vaccine candidate antigens for the prevention of Visceral Leishmaniasis. Vaccine 2016, 34, 2779–2786. [Google Scholar] [CrossRef]
- Okwor, I.; Uzonna, J.E. Immunotherapy as a strategy for treatment of leishmaniasis: A review of the literature. Immunotherapy 2009, 1, 765–776. [Google Scholar] [CrossRef]
- Gonçalves, A.A.M.; Leite, J.C.; Resende, L.A.; Mariano, R.M.D.S.; Silveira, P.; Melo-Júnior, O.A.D.O.; Ribeiro, H.S.; de Oliveira, D.S.; Soares, D.F.; Santos, T.A.P.; et al. An Overview of Immunotherapeutic Approaches Against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy. Front. Cell. Infect. Microbiol. 2019, 9, 427. [Google Scholar] [CrossRef]
- Ostolin, T.L.V.D.P.; Gusmão, M.R.; Mathias, F.A.S.; Cardoso, J.M.D.O.; Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Ruiz, J.C.; Resende, D.D.M.; de Brito, R.C.F.; Reis, A.B. A specific Leishmania infantum polyepitope vaccine triggers Th1-type immune response and protects against experimental visceral leishmaniasis. Cell. Immunol. 2022, 380, 104592. [Google Scholar] [CrossRef]
- Gupta, R.; Kushawaha, P.K.; Samant, M.; Jaiswal, A.K.; Baharia, R.K.; Dube, A. Treatment of Leishmania donovani-infected hamsters with miltefosine: Analysis of cytokine mRNA expression by real-time PCR, lymphoproliferation, nitrite production and antibody responses. J. Antimicrob. Chemother. 2012, 67, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Coler, R.N.; Goto, Y.; Bogatzki, L.; Raman, V.; Reed, S.G. Leish-111f, a Recombinant Polyprotein Vaccine That Protects against Visceral Leishmaniasis by Elicitation of CD4+ T Cells. Infect. Immun. 2007, 75, 4648–4654. [Google Scholar] [CrossRef] [PubMed]
- Baharia, R.K.; Tandon, R.; Sharma, T.; Suthar, M.K.; Das, S.; Siddiqi, M.I.; Saxena, J.K.; Sunder, S.; Dube, A. Recombinant NAD-dependent SIR-2 Protein of Leishmania donovani: Immunobiochemical Characterization as a Potential Vaccine against Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0003557. [Google Scholar] [CrossRef]
- Oliveira-Da-Silva, J.A.; Lage, D.P.; Ramos, F.F.; Machado, A.S.; Tavares, G.S.; Mendonça, D.V.; Pereira, I.A.; Martins, V.T.; Carvalho, L.M.; Ludolf, F.; et al. Leishmania infantum pyridoxal kinase evaluated in a recombinant protein and DNA vaccine to protects against visceral leishmaniasis. Mol. Immunol. 2020, 124, 161–171. [Google Scholar] [CrossRef]
- Joshi, J.; Malla, N.; Kaur, S. A comparative evaluation of efficacy of chemotherapy, immunotherapy and immunochemotherapy in visceral leishmaniasis-an experimental study. Parasitol. Int. 2014, 63, 612–620. [Google Scholar] [CrossRef]
- Sukhbir, K.; Tejinder, K.; Jyoti, J. Immunogenicity and protective efficacy of DNA vaccine against visceral leishmaniasis in BALB/c mice. J. Biomed. Res. 2016, 30, 304–313. [Google Scholar] [CrossRef]
- Balodi, D.C.; Anand, A.; Ramalingam, K.; Yadav, S.; Goyal, N. Dipeptidylcarboxypeptidase of Leishmania donovani: A potential vaccine molecule against experimental visceral leishmaniasis. Cell. Immunol. 2022, 375, 104529. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Costa, M.A.F.; Fumagalli, M.A.C.; Valadares, D.G.; Duarte, M.C.; Costa, L.E.; Martins, V.T.; Gomes, R.F.; Melo, M.N.; Soto, M.; et al. Evaluation of parasitological and immunological parameters of Leishmania chagasi infection in BALB/c mice using different doses and routes of inoculation of parasites. Parasitol. Res. 2012, 110, 1277–1285. [Google Scholar] [CrossRef]
- Carrion, J.; Nieto, A.; Iborra, S.; Iniesta, V.; Soto, M.; Folgueira, C.; Abanades, D.R.; Requena, J.M.; Alonso, C. Immunohistological features of visceral leishmaniasis in BALB/c mice. Parasite Immunol. 2006, 28, 173–183. [Google Scholar] [CrossRef]
- Duarte, M.C.; Lage, L.M.D.R.; Lage, D.P.; Martins, V.T.; Carvalho, A.M.R.S.; Roatt, B.M.; Menezes-Souza, D.; Tavares, C.A.P.; Alves, R.J.; Barichello, J.M.; et al. Treatment of murine visceral leishmaniasis using an 8-hydroxyquinoline-containing polymeric micelle system. Parasitol. Int. 2016, 65, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Jawed, J.J.; Das, M.C.; Parveen, S.; Ghosh, C.; Majumdar, S.; Saha, B.; Bhattacharjee, S. Lupeol and amphotericin B mediate synergistic anti-leishmanial immunomodulatory effects in Leishmania donovani-infected BALB/c mice. Cytokine 2021, 137, 155319. [Google Scholar] [CrossRef] [PubMed]
- Keyhani, A.; Sharifi, I.; Salarkia, E.; Khosravi, A.; Oliaee, R.T.; Babaei, Z.; Almani, P.G.N.; Hassanzadeh, S.; Kheirandish, R.; Mostafavi, M.; et al. In vitro and in vivo therapeutic potentials of 6-gingerol in combination with amphotericin B for treatment of Leishmania major infection: Powerful synergistic and multifunctional effects. Int. Immunopharmacol. 2021, 101, 108274. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Dose/Mouse |
|---|---|
| Saline | 50 µL/mouse |
| AmpB | 1 mg/kg/weight dose/mouse |
| MPLA | 20 µg dose/mouse |
| ChimT | 20 µg per dose/mouse |
| ChimT/AmpB | 20 µg ChimT and 1 mg/kg/weight AmpB dose/mouse |
| ChimT/MPLA | 20 µg ChimT and 20 µg MPLA dose/mouse |
| ChimT/MPLA/AmpB | 20 µg ChimT, 20 µg MPLA, 1 mg/kg/weight AmpB dose/mouse |
| Urea | |||||
| 1 Day | 30 Days | ||||
| Groups | Mean | Std. Deviation | Mean | Std. Deviation | Paired t Test |
| Naive | 13.3 | 1.7 | 19.5 | 1.3 | 0.8729 |
| Saline | 24.0 | 1.8 | 26.8 | 1.7 | 0.1627 |
| AmpB | 28.8 | 3.8 | 31.0 | 1.8 | 0.1697 |
| MPLA | 22.3 | 1.7 | 24.5 | 1.3 | 0.1697 |
| ChimT | 19.8 | 2.6 | 19.5 | 1.3 | 0.8543 |
| ChimT/AmpB | 20.5 | 1.3 | 17.8 | 1.3 | 0.0222 |
| ChimT/MPLA | 16.3 | 2.2 | 15.5 | 1.3 | 0.6807 |
| ChimT/AmpB/ MPLA | 17.3 | 1.7 | 14.5 | 1.8 | 0.1152 |
| Creatinine | |||||
| 1 day | 30 days | ||||
| Groups | Mean | Std. Deviation | Mean | Std. Deviation | Paired t test |
| Naive | 1.1 | 0.2 | 2.0 | 0.1 | 0.4228 |
| Saline | 2.9 | 0.3 | 2.9 | 0.2 | 0.3910 |
| AmpB | 3.2 | 0.4 | 3.3 | 0.3 | 0.4740 |
| MPLA | 2.6 | 0.2 | 2.6 | 0.2 | 0.2152 |
| ChimT | 1.7 | 0.2 | 2.0 | 0.1 | 0.1817 |
| ChimT/AmpB | 1.8 | 0.2 | 1.8 | 0.2 | 0.8088 |
| ChimT/MPLA | 1.5 | 0.3 | 1.5 | 0.2 | 0.3910 |
| ChimT/AmpB/ MPLA | 1.6 | 0.3 | 1.4 | 0.3 | 0.3542 |
| Alanine aminotransferase (ALT) | |||||
| 1 day | 30 days | ||||
| Groups | Mean | Std. Deviation | Mean | Std. Deviation | Paired t test |
| Naive | 9.0 | 0.8 | 9.8 | 1.0 | 0.3910 |
| Saline | 26.0 | 1.8 | 29.0 | 2.2 | 0.0577 |
| AmpB | 30.5 | 1.3 | 31.3 | 3.3 | 0.6714 |
| MPLA | 23.0 | 1.8 | 25.3 | 1.3 | 0.2007 |
| ChimT | 20.0 | 2.2 | 23.3 | 1.7 | 0.1438 |
| ChimT/AmpB | 21.5 | 2.7 | 19.5 | 1.3 | 0.3081 |
| ChimT/MPLA | 12.5 | 1.3 | 15.5 | 1.3 | 0.0805 |
| ChimT/AmpB/ MPLA | 13.0 | 2.2 | 14.0 | 1.8 | 0.5943 |
| Aspartate transaminase (ALT) | |||||
| 1 day | 30 days | ||||
| Groups | Mean | Std. Deviation | Mean | Std. Deviation | Paired t test |
| Naive | 11.3 | 2.2 | 10.8 | 1.7 | 0.6042 |
| Saline | 24.8 | 1.8 | 24.8 | 1.9 | 0.3910 |
| AmpB | 28.0 | 2.2 | 29.3 | 1.7 | 0.1411 |
| MPLA | 21.8 | 2.6 | 21.0 | 1.8 | 0.7177 |
| ChimT | 18.5 | 2.1 | 18.0 | 1.9 | 0.6376 |
| ChimT/AmpB | 19.0 | 1.9 | 19.0 | 2.2 | 1.0000 |
| ChimT/MPLA | 14.3 | 1.3 | 15.0 | 1.8 | 0.3910 |
| ChimT/AmpB/ MPLA | 15.0 | 1.7 | 13.8 | 2.5 | 0.5367 |
| Products | Concentration (µg/mL) | Percentage of Infected Macrophages after Treatment | ||
| L. amazonensis | L. donovani | L. infantum | ||
| ChimT | 20.0 | 24.5 ± 2.7 | 19.8 ± 3.3 | 13.2 ± 1.7 |
| 10.0 | 40.5 ± 3.3 | 35.4 ± 4.3 | 26.7 ± 2.9 | |
| 5.0 | 53.4 ± 2.8 | 49.8 ± 2.8 | 42.3 ± 3.3 | |
| 2.5 | 70.5 ± 3.7 | 65.5 ± 3.7 | 59.8 ± 4.4 | |
| Amphotericin B | 5.0 | 19.8 ± 3.0 | 16.7 ± 2.0 | 10.4 ± 1.0 |
| 2.0 | 35.4 ± 2.8 | 28.7 ± 2.5 | 21.3 ± 1.8 | |
| 1.0 | 48.7 ± 3.6 | 40.8 ± 4.1 | 37.8 ± 4.1 | |
| 0.5 | 68.7 ± 5.0 | 58.7 ± 3.8 | 54.3 ± 2.7 | |
| ChimT/MPLA/AmpB | 15.0 | 9.8 ± 3.3 | 8.6 ± 2.3 | 4.5 ± 1.8 |
| 10.0 | 21.3 ± 4.4 | 16.5 ± 3.4 | 10.8 ± 2.6 | |
| 5.0 | 36.5 ± 6.2 | 28.7 ± 6.3 | 21.4 ± 2.7 | |
| 2.5 | 55.6 ± 5.7 | 43.4 ± 5.0 | 38.7 ± 4.8 | |
| Control | (-) | 97.6 ± 4.4 | 96.3 ± 3.6 | 95.2 ± 5.0 |
| Products | Concentration (µg/mL) | Number of recovered amastigotes | ||
| L. amazonensis | L. donovani | L. infantum | ||
| ChimT | 20.0 | 2.0 ± 0.8 | 1.6 ± 0.7 | 1.2 ± 0.6 |
| 10.0 | 3.1 ± 1.2 | 2.8 ± 0.6 | 2.2 ± 0.7 | |
| 5.0 | 4.6 ± 1.1 | 4.1 ± 0.8 | 3.8 ± 0.9 | |
| 2.5 | 6.7 ± 0.9 | 6.0 ± 1.1 | 5.6 ± 1.4 | |
| Amphotericin B | 5.0 | 1.5 ± 0.5 | 1.0 ± 0.3 | 0.9 ± 0.3 |
| 2.0 | 2.4 ± 0.7 | 1.9 ± 0.6 | 1.9 ± 0.6 | |
| 1.0 | 3.9 ± 0.8 | 2.9 ± 0.8 | 3.1 ± 0.9 | |
| 0.5 | 5.9 ± 1.4 | 3.8 ± 0.8 | 5.0 ± 1.1 | |
| ChimT/MPLA/AmpB | 15.0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| 10.0 | 0.7 ± 0.2 | 0.6 ± 0.3 | 0.8 ± 0.3 | |
| 5.0 | 1.9 ± 0.5 | 1.5 ± 0.4 | 1.8 ± 0.5 | |
| 2.5 | 4.1 ± 0.5 | 2.9 ± 0.6 | 3.6 ± 0.7 | |
| Control | (-) | 8.7 ± 0.9 | 7.9 ± 1.2 | 8.3 ± 1.7 |
| Cytokine Levels in the Culture Supernatant from Treated and L. amazonensis-Infected Macrophages (pg/mL) | |||||
| Products | Concentration (µg/mL) | IFN-γ | IL-4 | IL-10 | IL-12 |
| ChimT | 20.0 | 231.5 ± 9.8 | 51.2 ± 2.6 | 50.6 ± 5.6 | 149.6 ± 3.4 |
| 10.0 | 152.6 ± 7.6 | 48.3 ± 4.0 | 42.3 ± 2.7 | 100.2 ± 3.6 | |
| 5.0 | 99.7 ± 4.0 | 41.2 ± 2.7 | 39.8 ± 3.0 | 74.5 ± 3.6 | |
| 2.5 | 56.5 ± 2.6 | 31.3 ± 3.5 | 35.4 ± 3.7 | 44.7 ± 4.0 | |
| Amphotericin B | 5.0 | 53.3 ± 4.6 | 48.7 ± 5.4 | 51.2 ± 2.7 | 56.7 ± 3.3 |
| 2.0 | 45.6 ± 3.4 | 41.2 ± 3.0 | 43.3 ± 5.4 | 50.7 ± 3.6 | |
| 1.0 | 41.2 ± 2.6 | 32.4 ± 2.7 | 37.6 ± 3.6 | 42.2 ± 3.9 | |
| 0.5 | 34.5 ± 2.2 | 29.4 ± 3.3 | 32.2 ± 2.0 | 35.3 ± 2.4 | |
| ChimT/MPLA/AmpB | 15.0 | 405.4 ± 17.6 | 48.7 ± 3.8 | 45.4 ± 3.8 | 174.3 ± 2.5 |
| 10.0 | 265.3 ± 13.9 | 40.2 ± 3.5 | 40.2 ± 4.6 | 143.4 ± 8.7 | |
| 5.0 | 189.8 ± 11.2 | 34.4 ± 2.7 | 31.4 ± 3.5 | 111.5 ± 7.6 | |
| 2.5 | 98.5 ± 6.7 | 26.4 ± 1.5 | 26.5 ± 1.7 | 76.6 ± 4.7 | |
| Control | (-) | 32.4 ± 2.3 | 25.4 ± 1.6 | 30.2 ± 3.3 | 31.2 ± 4.1 |
| Cytokine Levels in the Culture Supernatant from Treated and L. donovani-Infected Macrophages (pg/mL) | |||||
| Products | Concentration (µg/mL) | IFN-γ | IL-4 | IL-10 | IL-12 |
| ChimT | 20.0 | 197.8 ± 7.8 | 50.8 ± 1.8 | 58.7 ± 3.7 | 134.5 ± 4.6 |
| 10.0 | 143.2 ± 8.0 | 45.6 ± 3.7 | 51.1 ± 3.0 | 97.6 ± 5.0 | |
| 5.0 | 87.8 ± 3.7 | 39.8 ± 3.0 | 44.5 ± 2.8 | 68.7 ± 4.5 | |
| 2.5 | 49.8 ± 2.0 | 31.3 ± 3.5 | 36.5 ± 2.6 | 40.9 ± 3.5 | |
| Amphotericin B | 5.0 | 58.7 ± 2.7 | 63.3 ± 3.8 | 59.8 ± 3.0 | 60.9 ± 4.3 |
| 2.0 | 49.8 ± 4.4 | 59.7 ± 2.7 | 51.6 ± 3.6 | 51.7 ± 4.6 | |
| 1.0 | 46.5 ± 3.0 | 50.6 ± 3.3 | 45.5 ± 4.0 | 41.4 ± 2.9 | |
| 0.5 | 32.3 ± 3.1 | 41.2 ± 2.6 | 38.7 ± 2.7 | 34.5 ± 2.7 | |
| ChimT/MPLA/AmpB | 15.0 | 28.7 ± 10.9 | 49.8 ± 5.3 | 49.8 ± 4.5 | 198.7 ± 12.1 |
| 10.0 | 198.4 ± 16.7 | 47.6 ± 2.7 | 41.2 ± 3.5 | 156.4 ± 8.7 | |
| 5.0 | 132.2 ± 11.7 | 40.5 ± 4.3 | 32.2 ± 4.0 | 98.8 ± 3.5 | |
| 2.5 | 78.7 ± 4.5 | 34.3 ± 2.5 | 25.6 ± 2.7 | 65.5 ± 4.5 | |
| Control | (-) | 24.5 ± 1.6 | 31.2 ± 2.5 | 35.6 ± 2.8 | 26.5 ± 1.7 |
| Cytokine Levels in the Culture Supernatant from Treated and L. infantum-Infected Macrophages (pg/mL) | |||||
| Products | Concentration (µg/mL) | IFN-γ | IL-4 | IL-10 | IL-12 |
| ChimT | 20.0 | 268.7 ± 10.4 | 60.5 ± 4.5 | 62.3 ± 2.7 | 157.7 ± 8.9 |
| 10.0 | 177.6 ± 7.8 | 53.4 ± 2.6 | 54.4 ± 2.7 | 115.3 ± 5.8 | |
| 5.0 | 100.2 ± 6.5 | 45.6 ± 4.0 | 49.8 ± 3.6 | 76.6 ± 5.6 | |
| 2.5 | 65.5 ± 4.6 | 37.6 ± 4.4 | 40.5 ± 3.3 | 57.7 ± 3.5 | |
| Amphotericin B | 5.0 | 56.5 ± 3.6 | 58.7 ± 3.7 | 58.7 ± 4.7 | 63.2 ± 4.9 |
| 2.0 | 49.8 ± 4.4 | 52.3 ± 3.5 | 53.2 ± 2.6 | 57.6 ± 2.8 | |
| 1.0 | 40.7 ± 3.5 | 44.3 ± 3.0 | 45.6 ± 2.6 | 50.4 ± 3.7 | |
| 0.5 | 31.3 ± 2.7 | 39.8 ± 2.6 | 39.8 ± 3.4 | 44.3 ± 3.6 | |
| ChimT/MPLA/AmpB | 15.0 | 365.6 ± 21.1 | 43.4 ± 2.7 | 50.4 ± 3.9 | 232.8 ± 15.4 |
| 10.0 | 243.3 ± 16.5 | 38.7 ± 4.3 | 45.4 ± 3.5 | 189.8 ± 13.3 | |
| 5.0 | 167.6 ± 12.2 | 30.2 ± 3.1 | 38.7 ± 3.0 | 114.4 ± 10.9 | |
| 2.5 | 98.8 ± 4.5 | 23.2 ± 2.6 | 31.1 ± 2.9 | 78.7 ± 3.4 | |
| Control | (-) | 25.5 ± 2.5 | 33.2 ± 2.6 | 35.7 ± 2.8 | 40.5 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vale, D.L.; Freitas, C.S.; Martins, V.T.; Moreira, G.J.L.; Machado, A.S.; Ramos, F.F.; Pereira, I.A.G.; Bandeira, R.S.; de Jesus, M.M.; Tavares, G.S.V.; et al. Efficacy of an Immunotherapy Combining Immunogenic Chimeric Protein Plus Adjuvant and Amphotericin B against Murine Visceral Leishmaniasis. Biology 2023, 12, 851. https://doi.org/10.3390/biology12060851
Vale DL, Freitas CS, Martins VT, Moreira GJL, Machado AS, Ramos FF, Pereira IAG, Bandeira RS, de Jesus MM, Tavares GSV, et al. Efficacy of an Immunotherapy Combining Immunogenic Chimeric Protein Plus Adjuvant and Amphotericin B against Murine Visceral Leishmaniasis. Biology. 2023; 12(6):851. https://doi.org/10.3390/biology12060851
Chicago/Turabian StyleVale, Danniele L., Camila S. Freitas, Vívian T. Martins, Gabriel J. L. Moreira, Amanda S. Machado, Fernanda F. Ramos, Isabela A. G. Pereira, Raquel S. Bandeira, Marcelo M. de Jesus, Grasiele S. V. Tavares, and et al. 2023. "Efficacy of an Immunotherapy Combining Immunogenic Chimeric Protein Plus Adjuvant and Amphotericin B against Murine Visceral Leishmaniasis" Biology 12, no. 6: 851. https://doi.org/10.3390/biology12060851
APA StyleVale, D. L., Freitas, C. S., Martins, V. T., Moreira, G. J. L., Machado, A. S., Ramos, F. F., Pereira, I. A. G., Bandeira, R. S., de Jesus, M. M., Tavares, G. S. V., Ludolf, F., Chávez-Fumagalli, M. A., Galdino, A. S., Fujiwara, R. T., Bueno, L. L., Roatt, B. M., Christodoulides, M., Coelho, E. A. F., & Lage, D. P. (2023). Efficacy of an Immunotherapy Combining Immunogenic Chimeric Protein Plus Adjuvant and Amphotericin B against Murine Visceral Leishmaniasis. Biology, 12(6), 851. https://doi.org/10.3390/biology12060851








