Distribution, Effect, and Control of Exotic Plants in Republic of Korea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
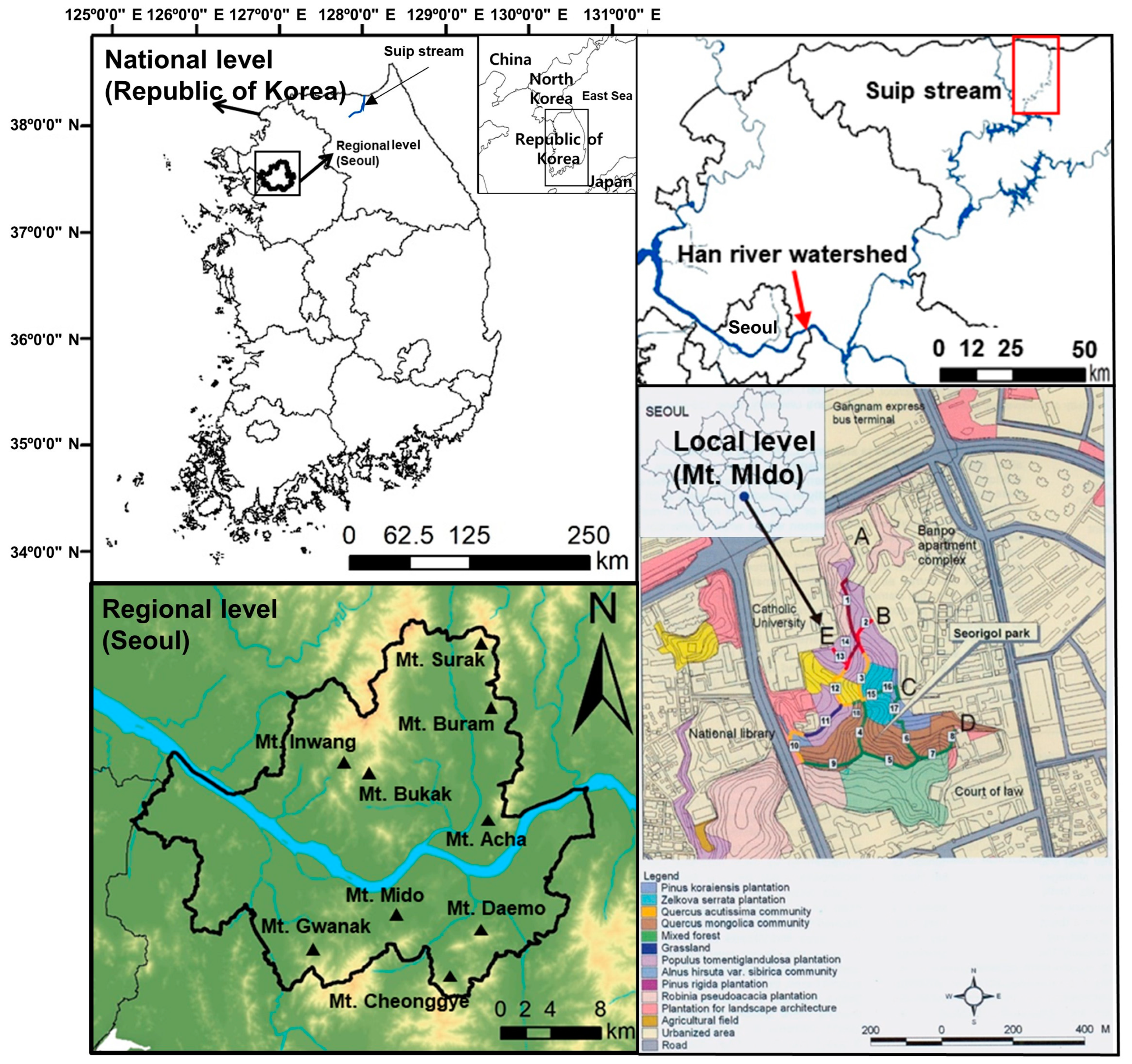
2.1. Study Area
2.2. Survey on Forest Vegetation
2.3. Survey on the Riparian Vegetation
2.4. Statistics Analysis
3. Results
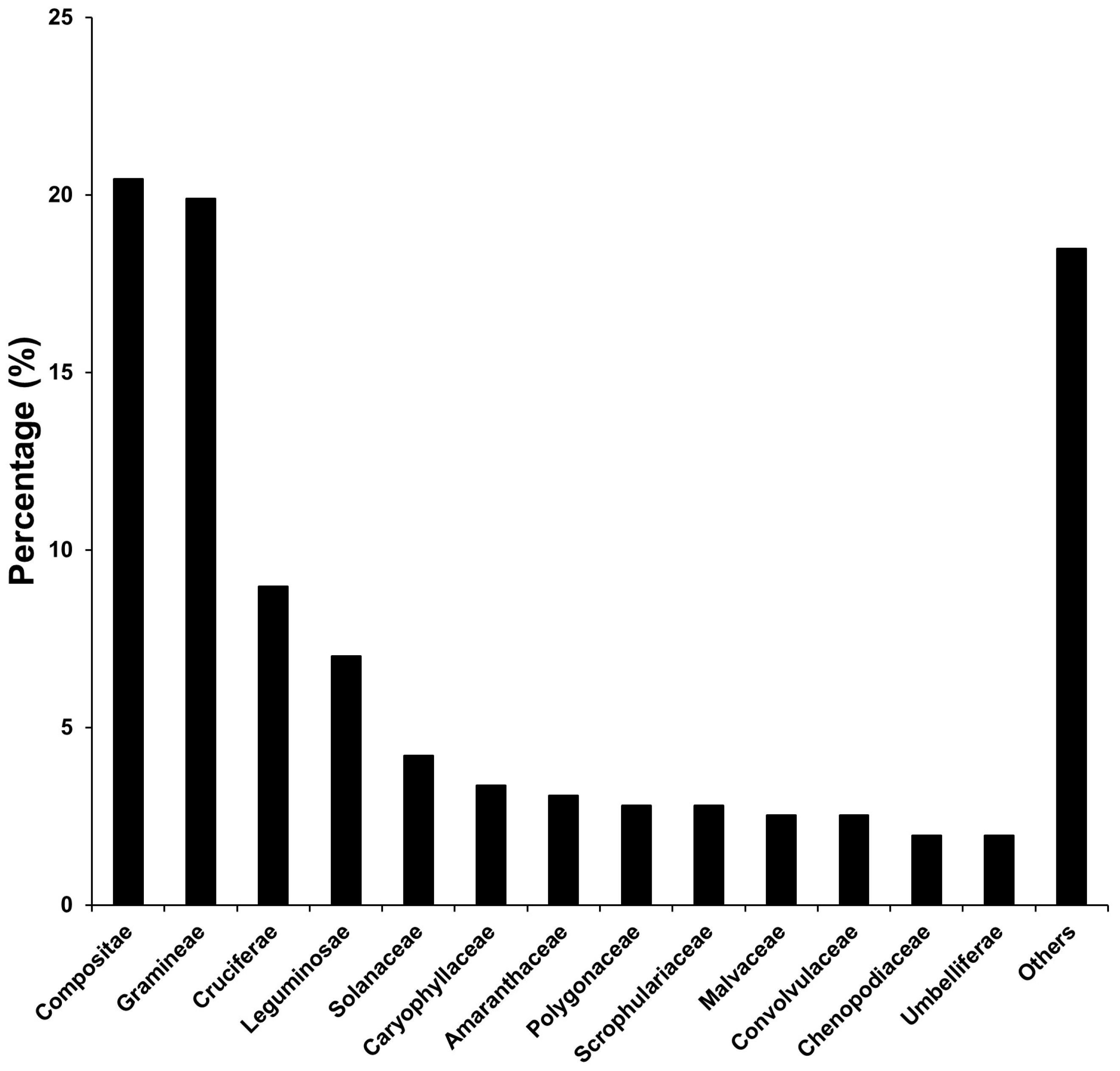
3.1. A Comparison among Taxa of Exotic Plants
3.2. Life-Form Composition of Exotic Plants
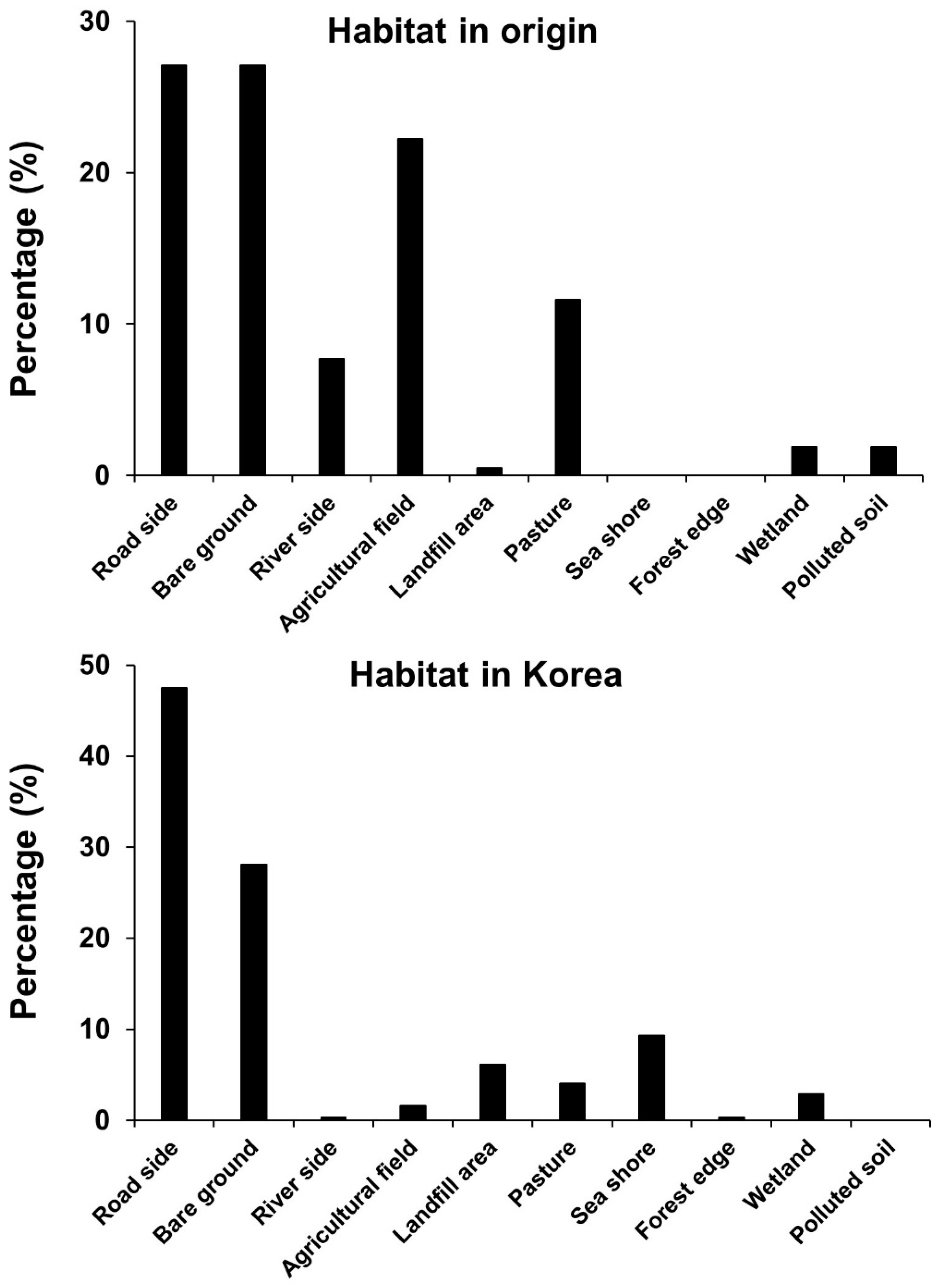
3.3. Habitat Types of Exotic Plants
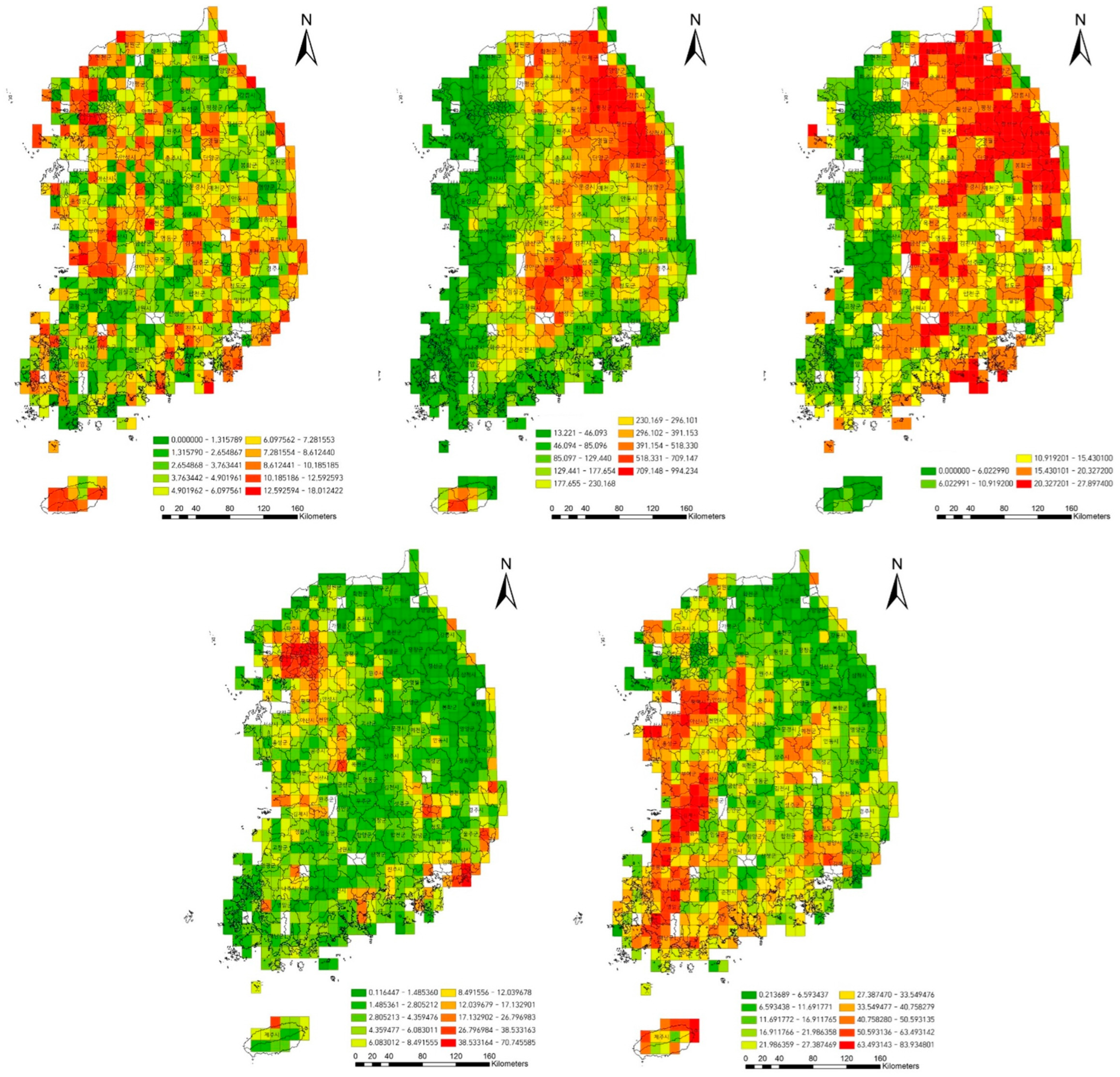
3.4. Spatial Distribution of Exotic Plants at the National Level
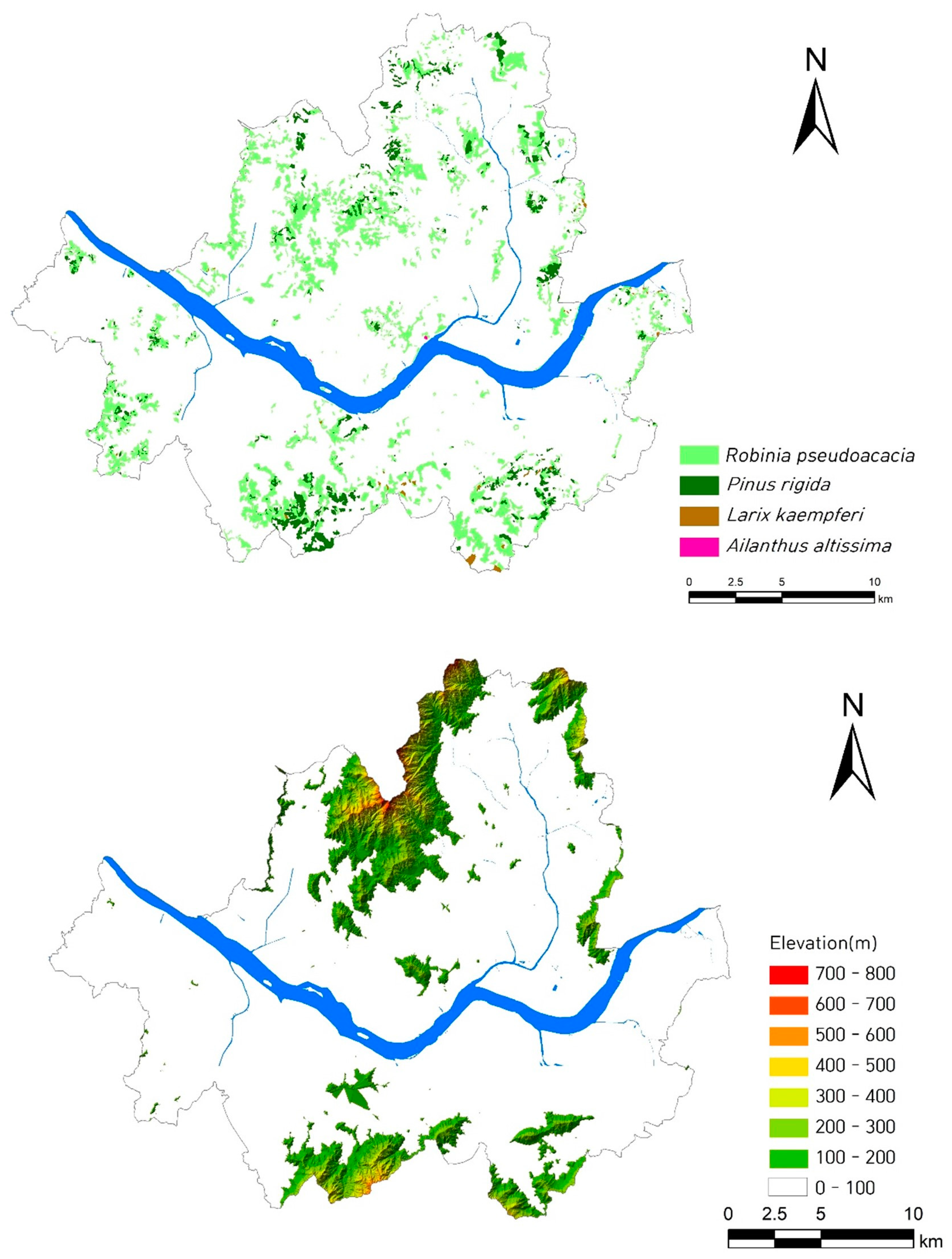
3.5. Spatial Distribution of Exotic Plants at the Regional Level
3.6. Spatial Distribution of A. altissima as an Exotic Plant
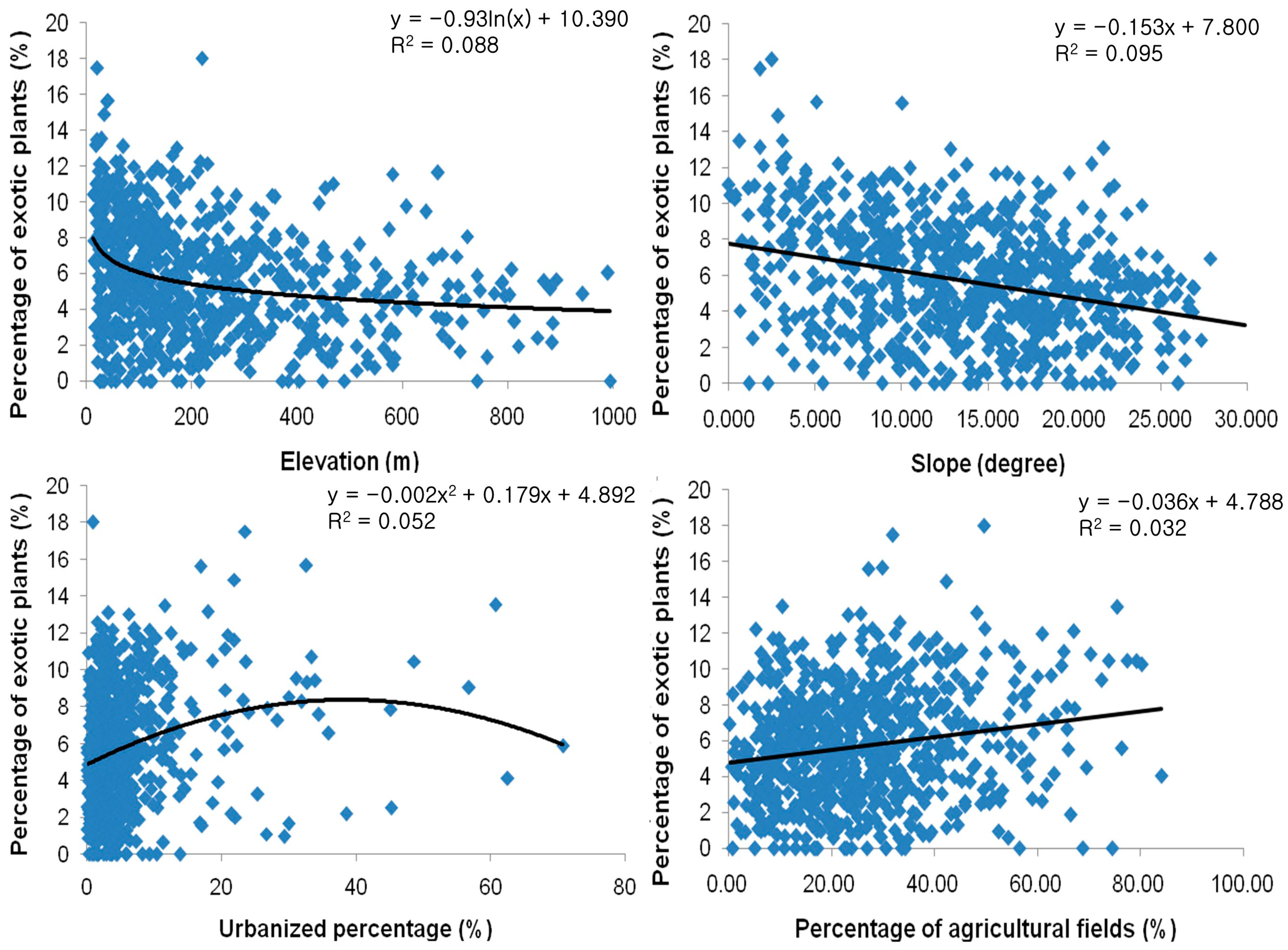
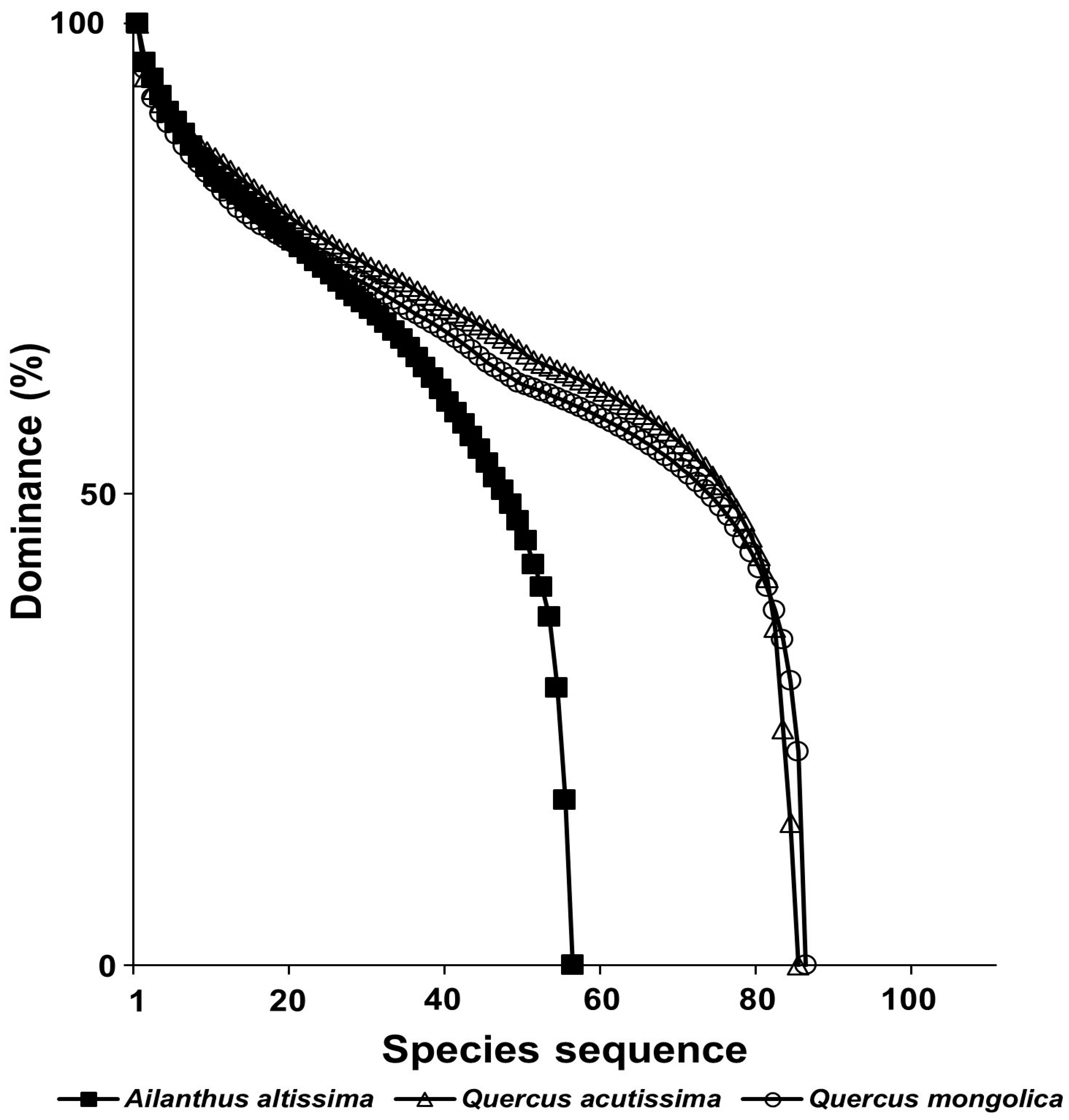
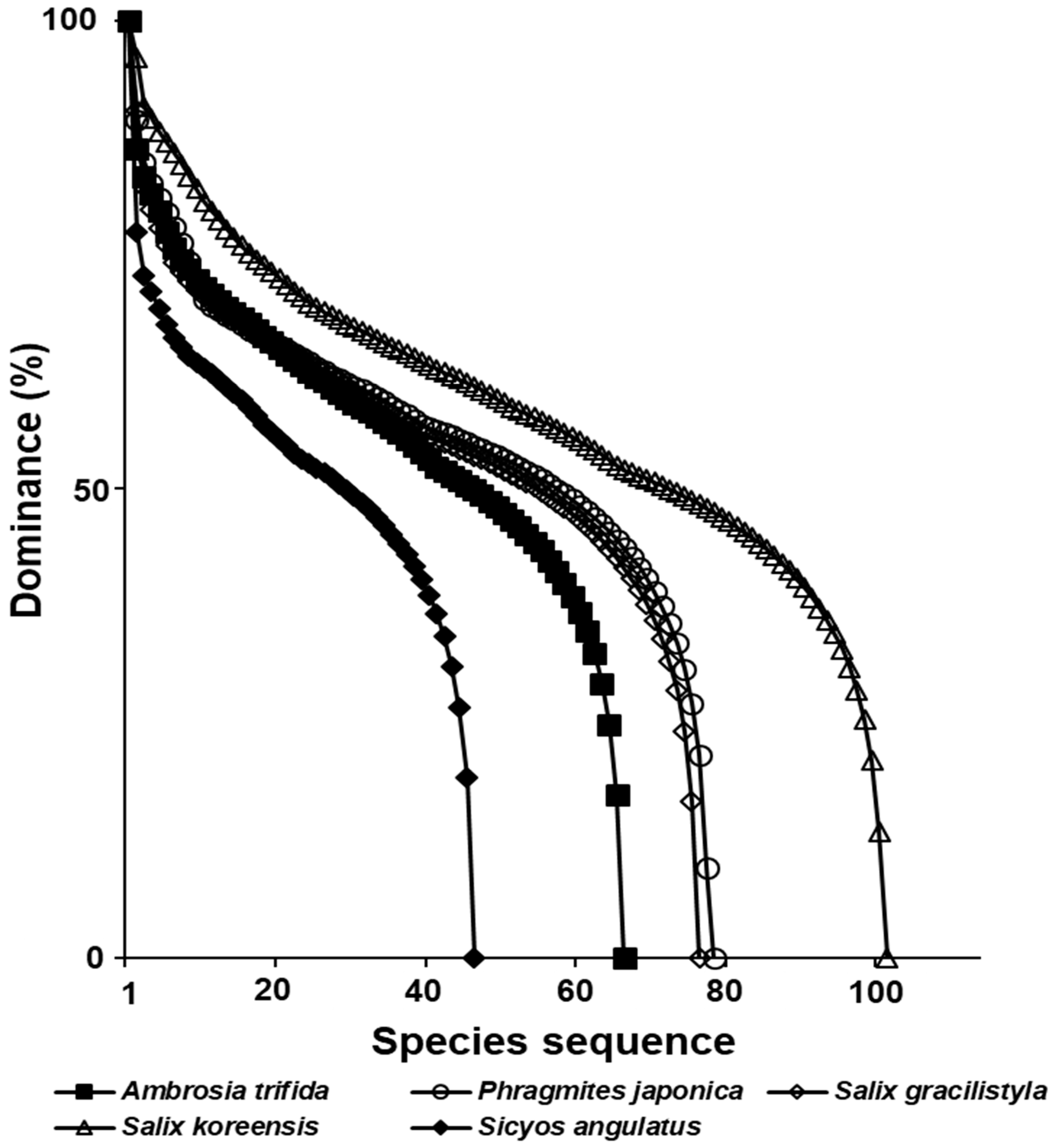
3.7. The Ecological Effects of Exotic Plant Infection
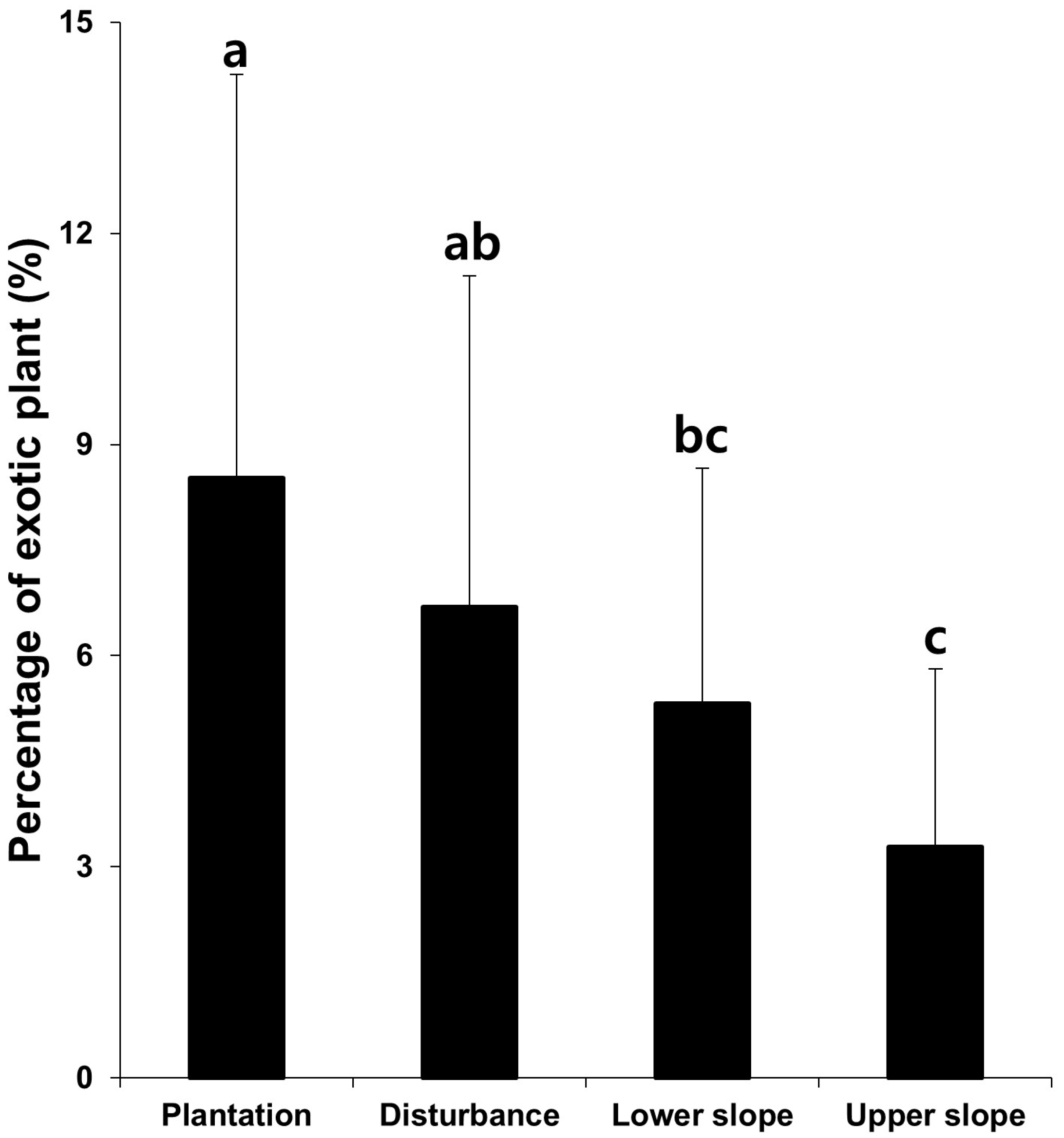
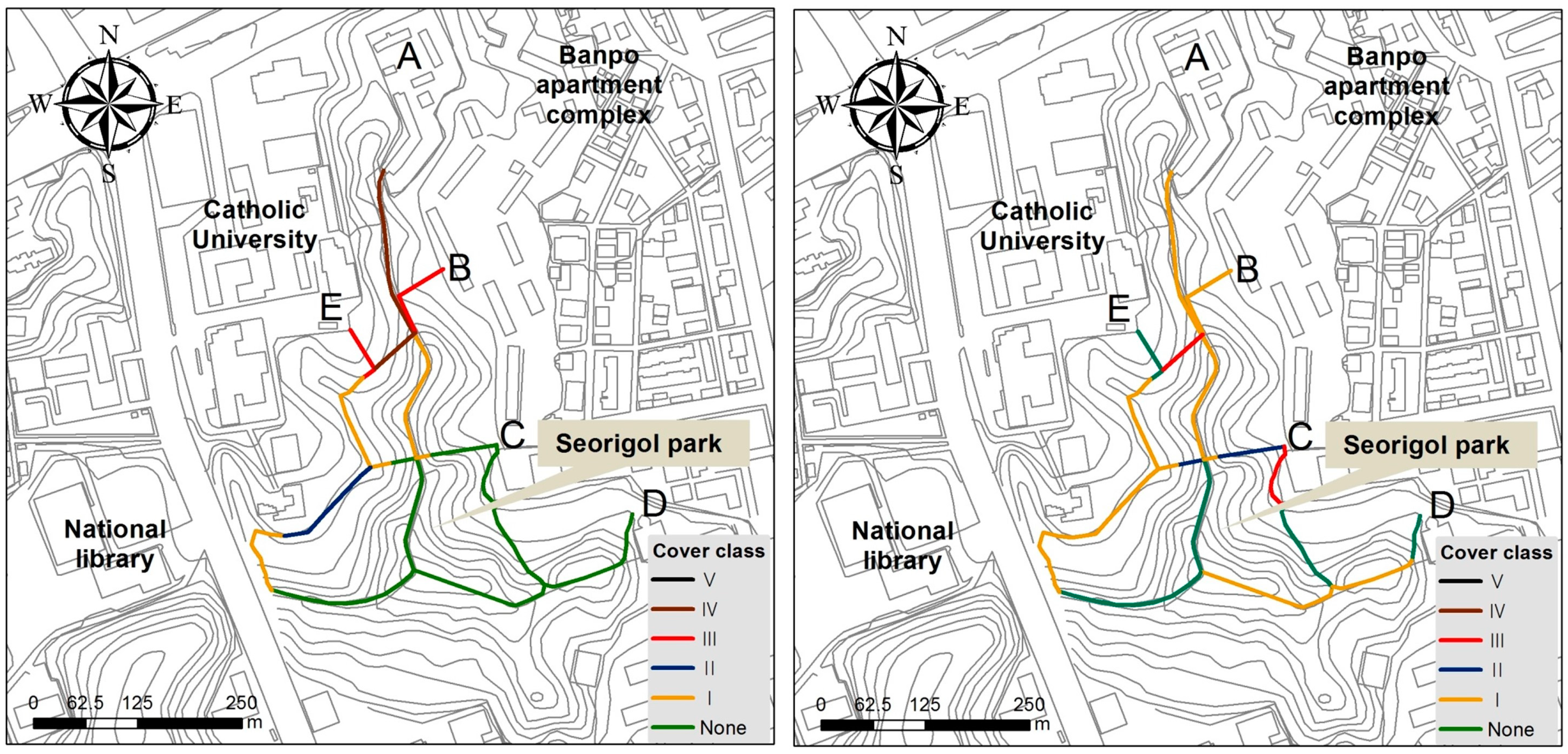
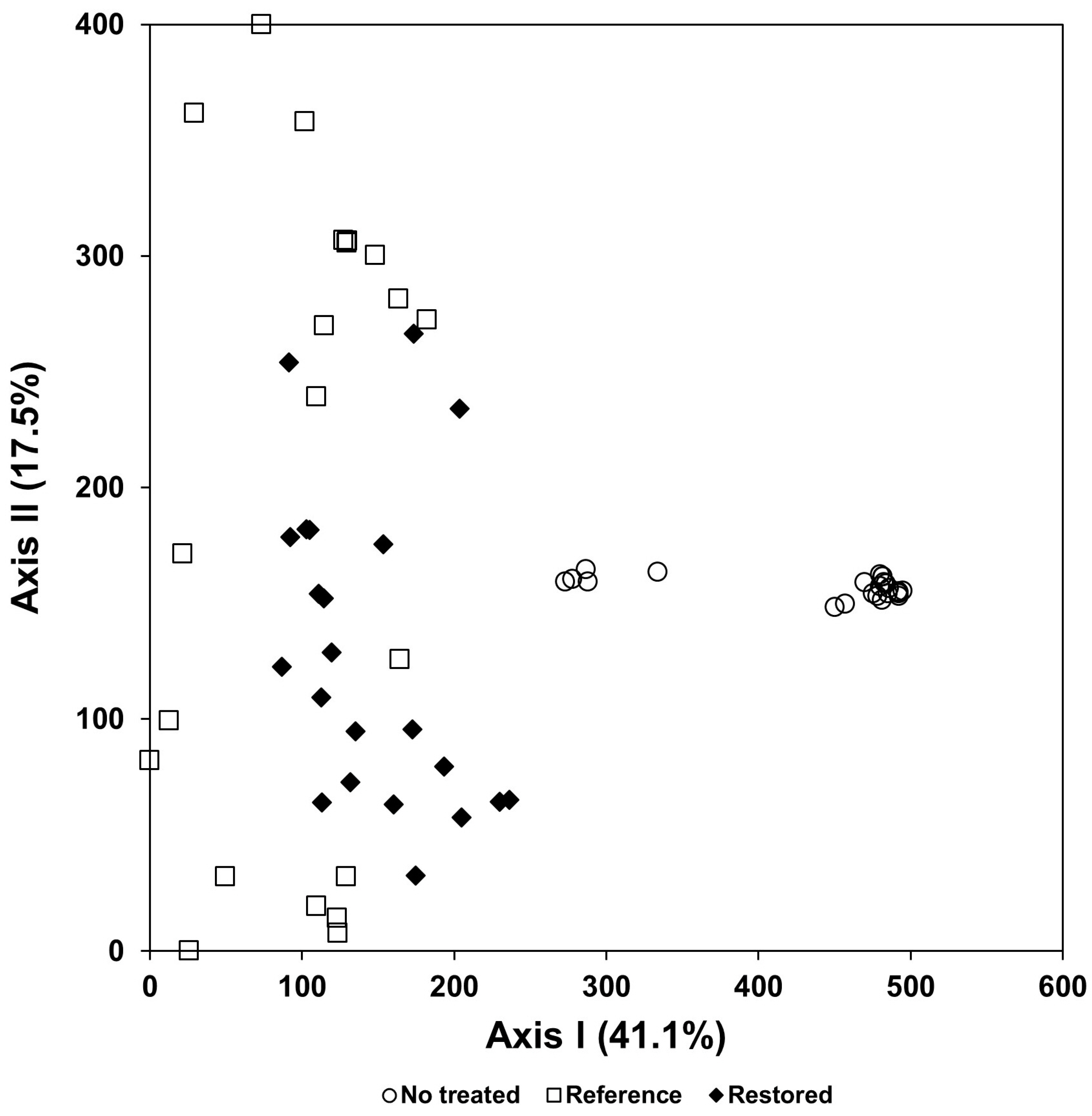
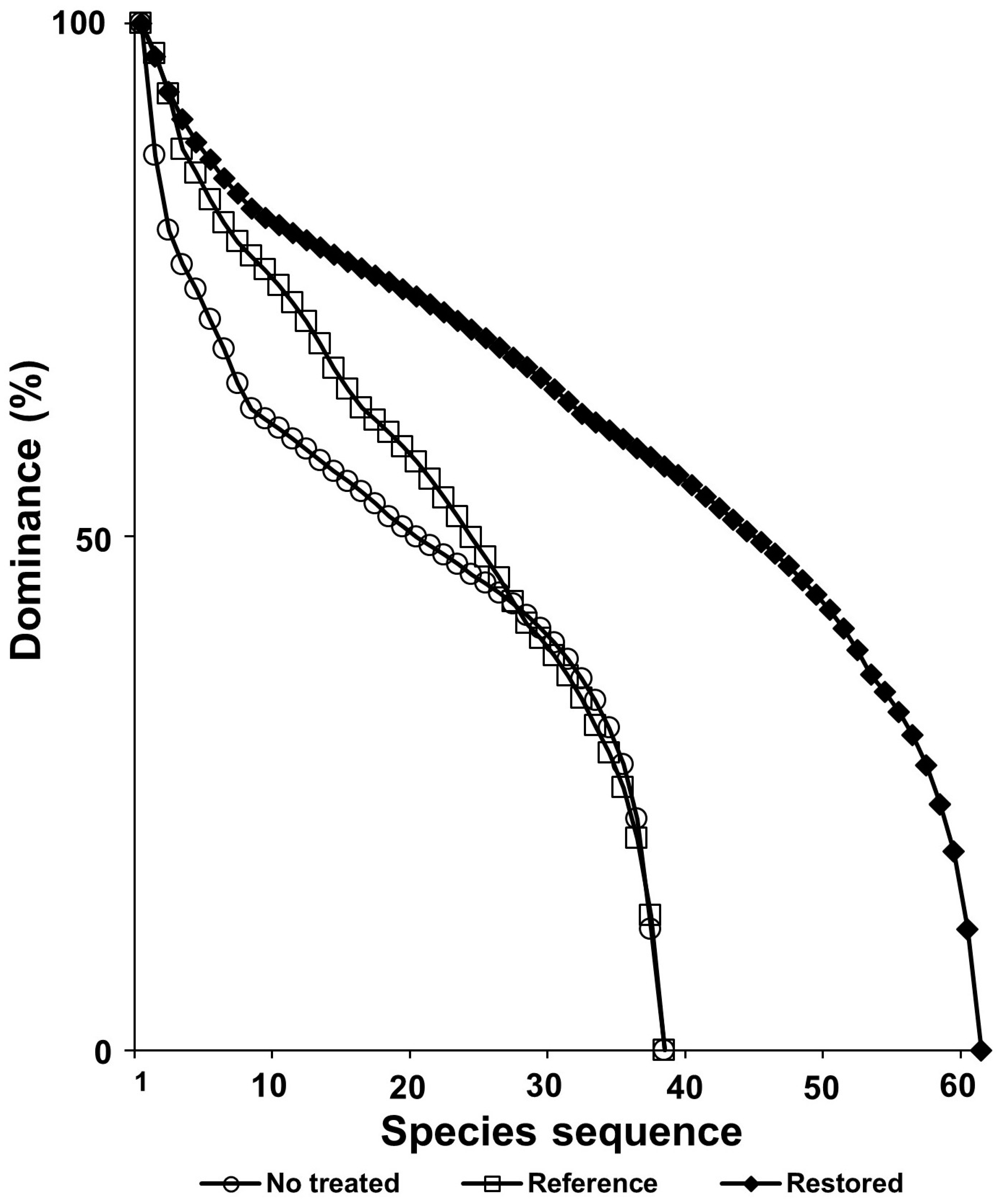
3.8. The Effects of Ecological Restoration for Control of Exotic Plant Species
4. Discussion
4.1. Which Taxa Invade?
4.2. How Fast Do They Invade?
4.3. What Types of Ecosystems Are Susceptible to Exotic Plant Species and Their Impacts?
4.4. What Is the Ecological Impact of Exotic Species Invasion?
4.5. Restoration as a Tool to Inhibit Invasion and Expansion of Invasive Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. A List of Exotic Plants Investigated in Republic of Korea
| Family Name | Scientific Name | Origin |
| Amaranthaceae | Amaranthus albus | Am |
| Amaranthaceae | Amaranthus arenicola | Am |
| Amaranthaceae | Amaranthus blitum | Eu |
| Amaranthaceae | Amaranthus hybridus | Am |
| Amaranthaceae | Amaranthus palmeri | Am |
| Amaranthaceae | Amaranthus patulus | Am |
| Amaranthaceae | Amaranthus powellii | Am |
| Amaranthaceae | Amaranthus retroflexus | Am |
| Amaranthaceae | Amaranthus spinosus | Am |
| Amaranthaceae | Amaranthus viridis | Am |
| Amaranthaceae | Celosia argentea | Am |
| Amaryllidaceae | Zephyranthes candida | Am |
| Boraginaceae | Amsinckia lycopsoides | Am |
| Boraginaceae | Asperugo procumbens | Eu–As–Af |
| Boraginaceae | Symphytum officinale | Eu |
| Campanulaceae | Triodanis perfoliata | Am |
| Cannabaceae | Cannabis sativa | As |
| Caryophyllaceae | Cerastium glomeratum | Eu |
| Caryophyllaceae | Dianthus armeria | Eu |
| Caryophyllaceae | Holosteum umbellatum | Eu–As |
| Caryophyllaceae | Saponaria officinalis | Eu |
| Caryophyllaceae | Scleranthus annuus | Eu |
| Caryophyllaceae | Silene alba | Eu |
| Caryophyllaceae | Silene antirrhina | Am |
| Caryophyllaceae | Silene armeria | Eu |
| Caryophyllaceae | Silene gallica | Eu–As |
| Caryophyllaceae | Spergula arvensis | Eu |
| Caryophyllaceae | Spergularia rubra | Eu–As |
| Caryophyllaceae | Vaccaria vulgaris | Eu |
| Chenopodiaceae | Atriplex hastata | Eu |
| Chenopodiaceae | Chenopodium album | Eu–As |
| Chenopodiaceae | Chenopodium ambrosioides | Am |
| Chenopodiaceae | Chenopodium ficifolium | Eu |
| Chenopodiaceae | Chenopodium glaucum | Eu |
| Chenopodiaceae | Chenopodium hybridum | Eu–As |
| Chenopodiaceae | Chenopodium pumilio | Au |
| Commelinaceae | Commelina benghalensis | As–Af |
| Commelinaceae | Commelina diffusa | etc. |
| Commelinaceae | Tradescantia reflexa | Am |
| Compositae | Achillea millefolium | Eu |
| Compositae | Ageratum conyzoides | Am |
| Compositae | Ambrosia artemisiifolia | Am |
| Compositae | Ambrosia trifida f. integrifolia | Am |
| Compositae | Ambrosia trifida | Am |
| Compositae | Anthemis arvensis | Eu |
| Compositae | Anthemis cotula | Eu |
| Compositae | Aster novi-belgii | Am |
| Compositae | Aster pilosus | Am |
| Compositae | Aster subulatus | Am |
| Compositae | Aster subulatus var. sandwicensis | Am |
| Compositae | Bidens frondosa | Am |
| Compositae | Bidens pilosa | Am |
| Compositae | Bidens pilosa var. minor | Am |
| Compositae | Bidens polylepis | Am |
| Compositae | Bidens subalternans | Am |
| Compositae | Carduus crispus f. albus | Eu–As |
| Compositae | Carduus crispus | Eu–As |
| Compositae | Carduus nutans | Eu |
| Compositae | Centaurea cyanus | Eu |
| Compositae | Chrysanthemum leucanthemum | Eu |
| Compositae | Cirsium arvense | Eu |
| Compositae | Cirsium vulgare | Eu |
| Compositae | Conyza bonariensis | Am |
| Compositae | Conyza canadensis | Am |
| Compositae | Conyza parva | Am |
| Compositae | Conyza sumatrensis | Am |
| Compositae | Coreopsis lanceolata | Am |
| Compositae | Coreopsis tinctoria | Am |
| Compositae | Cosmos bipinnatus | Am |
| Compositae | Cosmos sulphureus | Am |
| Compositae | Crassocephalum crepidioides | Af |
| Compositae | Crepis tectorum | Eu |
| Compositae | Dracopis amplexicaulis | Am |
| Compositae | Eclipta alba var. erecta | Am |
| Compositae | Erechtites hieracifolia | Am |
| Compositae | Erigeron annuus | Am |
| Compositae | Erigeron philadelphicus | Am |
| Compositae | Erigeron strigosus | Eu |
| Compositae | Eupatorium rugosum | Am |
| Compositae | Euthamia graminifolia | Am |
| Compositae | Galinsoga ciliata | Am |
| Compositae | Galinsoga parviflora | Am |
| Compositae | Gamochaeta pensylvanica | Am |
| Compositae | Gnaphalium calviceps | Am |
| Compositae | Gnaphalium purpureum | Am |
| Compositae | Helianthus debilis | Am |
| Compositae | Helianthus tuberosus | Am |
| Compositae | Hieracium caespitosum | Eu |
| Compositae | Hypochaeris radicata | Eu |
| Compositae | Lactuca scariola | Eu |
| Compositae | Lapsana communis | Eu |
| Compositae | Matricaria inodora | Eu |
| Compositae | Matricaria matricariodes | As |
| Compositae | Parthenium hysterophorus | Am |
| Compositae | Rudbeckia bicolor | Am |
| Compositae | Rudbeckia hirta | Am |
| Compositae | Rudbeckia laciniata var. hortensis | Am |
| Compositae | Senecio inaequidens | Af |
| Compositae | Senecio scandens | As |
| Compositae | Senecio vulgaris | Eu |
| Compositae | Solidago altissima | Am |
| Compositae | Solidago serotina | Am |
| Compositae | Sonchus asper | Eu |
| Compositae | Sonchus oleraceus | Eu |
| Compositae | Tagetes minuta | Am |
| Compositae | Taraxacum laevigatum | Eu |
| Compositae | Taraxacum officinale | Eu |
| Compositae | Tragopogon dubius | Eu |
| Compositae | Verbesina alternifolia | Am |
| Compositae | Xanthium canadense | Am |
| Compositae | Xanthium italicum | Am |
| Compositae | Xanthium strumarium | As |
| Convolvulaceae | Convolvulus arvensis | Eu |
| Convolvulaceae | Cuscuta pentagona | Am |
| Convolvulaceae | Ipomoea hederacea | Am |
| Convolvulaceae | Ipomoea hederacea var. integriuscula | Am |
| Convolvulaceae | Ipomoea lacunosa | Am |
| Convolvulaceae | Ipomoea purpurea | Am |
| Convolvulaceae | Ipomoea triloba | Am |
| Convolvulaceae | Jacquemontia tamnifolia | Am |
| Convolvulaceae | Quamoclit coccinea | Am |
| Crassulaceae | Sedum mexicanum | Am |
| Cruciferae | Alliaria petiolata | Eu–As |
| Cruciferae | Barbarea verna | Eu |
| Cruciferae | Barbarea vulgaris | Eu |
| Cruciferae | Brassica juncea | As |
| Cruciferae | Cakile edentula | Am |
| Cruciferae | Camelina microcarpa | Eu |
| Cruciferae | Cardaria draba | Eu |
| Cruciferae | Chorispora tenella | Eu–As |
| Cruciferae | Coronopus didymus | Eu |
| Cruciferae | Descurainia pinnata | Am |
| Cruciferae | Diplotaxis muralis | Eu |
| Cruciferae | Erucastrum gallicum | Eu |
| Cruciferae | Lepidium apetalum | Am |
| Cruciferae | Lepidium bonariense | Am |
| Cruciferae | Lepidium campestre | Eu |
| Cruciferae | Lepidium latifolium | Eu–As |
| Cruciferae | Lepidium perfoliatum | Eu–As |
| Cruciferae | Lepidium ruderale | Eu |
| Cruciferae | Lepidium virginicum | Am |
| Cruciferae | Myagrum perfoliatum | Eu |
| Cruciferae | Nasturtium officinale | Eu |
| Cruciferae | Neslia paniculata | Eu |
| Cruciferae | Raphanus raphanistrum | Eu |
| Cruciferae | Rapistrum rugosum | Eu |
| Cruciferae | Rorippa sylvestris | Eu |
| Cruciferae | Sinapis arvensis | Eu |
| Cruciferae | Sinapis arvensis var. orientalis | Eu |
| Cruciferae | Sisymbrium altissimum | Eu |
| Cruciferae | Sisymbrium officinale | Eu |
| Cruciferae | Sisymbrium officinale var. leiocarpum | Eu |
| Cruciferae | Sisymbrium orientale | Eu |
| Cruciferae | Thlaspi arvense | Eu |
| Cucurbitaceae | Sicyos angulatus | Am |
| Cyperaceae | Carex scoparia | Am |
| Euphorbiaceae | Euphorbia dentata | Am |
| Euphorbiaceae | Euphorbia heterophylla | Am |
| Euphorbiaceae | Euphorbia hirta | Am |
| Euphorbiaceae | Euphorbia maculata | Am |
| Euphorbiaceae | Euphorbia prostrata | Am |
| Euphorbiaceae | Euphorbia supina | Am |
| Fumariaceae | Fumaria officinalis | Eu |
| Geraniaceae | Erodium cicutarium | Eu |
| Geraniaceae | Geranium carolinianum | Am |
| Geraniaceae | Geranium dissectum | Eu |
| Gramineae | Aegilops cylindrica | Eu |
| Gramineae | Agropyron repens | Eu |
| Gramineae | Agropyron repens f. aristatum | Eu |
| Gramineae | Aira caryophyllea | Eu |
| Gramineae | Alopecurus japonicus | As |
| Gramineae | Alopecurus myosuroides | Eu–As |
| Gramineae | Alopecurus pratensis | Eu–As |
| Gramineae | Andropogon virginicus | Am |
| Gramineae | Anthoxanthum odoratum | Eu–As |
| Gramineae | Arrhenatherum elatius | Eu |
| Gramineae | Arrhenatherum elatius var. bulbosum | Eu |
| Gramineae | Avena fatua | Eu–As |
| Gramineae | Avena sativa | Eu–As |
| Gramineae | Briza minor | Eu |
| Gramineae | Bromus carinatus | Eu–Am |
| Gramineae | Bromus inermis | Eu |
| Gramineae | Bromus mollis | Eu |
| Gramineae | Bromus racemosus | Eu |
| Gramineae | Bromus rigidus | Eu |
| Gramineae | Bromus secalinus | Eu |
| Gramineae | Bromus sterilis | Eu |
| Gramineae | Bromus tectorum | Eu |
| Gramineae | Bromus tectorum var. glabratus | Eu–As |
| Gramineae | Bromus unioloides | Am |
| Gramineae | Catapodium rigidum | Eu |
| Gramineae | Cenchrus longispinus | Am |
| Gramineae | Chloris virgata | Am |
| Gramineae | Coix lacryma-jobi | As |
| Gramineae | Dactylis glomerata | Eu–As |
| Gramineae | Dactyloctenium aegyptium | As |
| Gramineae | Dichanthelium acuminatum | Am |
| Gramineae | Eragrostis curvula | Af |
| Gramineae | Eremochloa ophiuroides | As |
| Gramineae | Festuca arundinacea | Eu |
| Gramineae | Festuca megalura | Am |
| Gramineae | Festuca myuros | Eu |
| Gramineae | Festuca pratensis | Eu |
| Gramineae | Glyceria declinata | Eu |
| Gramineae | Holcus lanatus | Eu |
| Gramineae | Hordeum jubatum | Eu |
| Gramineae | Hordeum murinum | Eu–As |
| Gramineae | Hordeum pusillum | Am |
| Gramineae | Leptochloa fusca | As |
| Gramineae | Lolium multiflorum | Eu |
| Gramineae | Lolium multiflorum var. ramosum | Eu |
| Gramineae | Lolium perenne | Eu |
| Gramineae | Lolium rigidum | Med |
| Gramineae | Lolium temulentum | Eu |
| Gramineae | Panicum dichotomiflorum | Am |
| Gramineae | Panicum miliaceum | As |
| Gramineae | Panicum virgatum | Am |
| Gramineae | Parapholis incurva | Eu |
| Gramineae | Paspalum dilatatum | Am |
| Gramineae | Paspalum distichum | As |
| Gramineae | Paspalum distichum var. indutum | Am |
| Gramineae | Paspalum notatum | Am |
| Gramineae | Paspalum urvillei | Am |
| Gramineae | Pennisetum flaccidum | As |
| Gramineae | Phalaris canariensis | Eu–As |
| Gramineae | Phalaris minor | Eu–As |
| Gramineae | Phleum paniculatum | Eu |
| Gramineae | Phleum pratense | Eu |
| Gramineae | Poa bulbosa var. vivipara | Eu |
| Gramineae | Poa compressa | Eu |
| Gramineae | Poa pratensis | Eu |
| Gramineae | Puccinellia distans | Eu |
| Gramineae | Rottboellia cochinchinensis | etc. |
| Gramineae | Saccharum arundinaceum | As |
| Gramineae | Sorghum halepense | Eu |
| Gramineae | Sorghum halepense f. muticum | Eu |
| Gramineae | Spartina anglica | Eu |
| Guttiferae | Hypericum perforatum | Eu |
| Iridaceae | Sisyrinchium angustifolium | Am |
| Iridaceae | Tritonia crocosmaeflora | Eu |
| Juglandaceae | Pterocarya stenoptera | As |
| Labiatae | Lamium purpureum | Eu–As |
| Labiatae | Lamium purpureum var. hybridum | Eu |
| Labiatae | Scutellaria baicalensis | As |
| Leguminosae | Amorpha fruticosa | Am |
| Leguminosae | Astragalus sinicus | As |
| Leguminosae | Lespedeza davidii | As |
| Leguminosae | Lespedeza floribunda | As |
| Leguminosae | Lespedeza lichiyuniae | As |
| Leguminosae | Lotus corniculatus | Eu |
| Leguminosae | Lotus uliginosus | Eu–Af |
| Leguminosae | Lupinus angustifolius | Eu |
| Leguminosae | Medicago lupulina | Eu |
| Leguminosae | Medicago minima | Eu |
| Leguminosae | Medicago polymorpha | Eu |
| Leguminosae | Medicago sativa | Med |
| Leguminosae | Melilotus alba | As |
| Leguminosae | Melilotus suaveolens | As |
| Leguminosae | Robinia pseudoacacia | Am |
| Leguminosae | Securigera varia | Eu–As |
| Leguminosae | Trifolium campestre | Eu |
| Leguminosae | Trifolium dubium | Eu–As |
| Leguminosae | Trifolium hybridum | Eu–As |
| Leguminosae | Trifolium incarnatum | Eu |
| Leguminosae | Trifolium pratense | Eu |
| Leguminosae | Trifolium repens | Eu–Af |
| Leguminosae | Trifolium resupinatum | Eu–As |
| Leguminosae | Vicia dasycarpa | Eu |
| Leguminosae | Vicia villosa | Eu |
| Lythraceae | Ammannia coccinea | Am |
| Magnoliaceae | Magnolia obovata | As |
| Malvaceae | Abutilon theophrasti | As |
| Malvaceae | Hibiscus trionum | Eu |
| Malvaceae | Malva neglecta | Eu–As |
| Malvaceae | Malva parviflora | Eu |
| Malvaceae | Malva pusilla | Eu |
| Malvaceae | Malva sylvestris var. mauritiana | Eu |
| Malvaceae | Modiola caroliniana | Am |
| Malvaceae | Sida rhombifolia | Am |
| Malvaceae | Sida spinosa | Am |
| Molluginaceae | Mollugo verticillata | Am |
| Onagraceae | Oenothera biennis | Am |
| Onagraceae | Oenothera erythrosepala | Am |
| Onagraceae | Oenothera laciniata | Am |
| Onagraceae | Oenothera rosea | Am |
| Onagraceae | Oenothera striata | Am |
| Oxalidaceae | Oxalis articulata | Am |
| Oxalidaceae | Oxalis corymbosa | Am |
| Papaveraceae | Papaver dubium | Eu |
| Papaveraceae | Papaver hybridum | Eu |
| Papaveraceae | Papaver rhoeas | Eu |
| Phytolaccaceae | Phytolacca americana | Am |
| Phytolaccaceae | Phytolacca esculenta | As |
| Plantaginaceae | Nuttallanthus canadensis | Am |
| Plantaginaceae | Plantago aristata | Am |
| Plantaginaceae | Plantago lanceolata | Eu |
| Plantaginaceae | Plantago virginica | Am |
| Polygonaceae | Fallopia convolvulus | Eu–As |
| Polygonaceae | Fallopia dentatoalata | Eu |
| Polygonaceae | Fallopia dumetorum | Eu |
| Polygonaceae | Persicaria capitata | As |
| Polygonaceae | Persicaria orientalis | As |
| Polygonaceae | Persicaria wallichii | As |
| Polygonaceae | Rumex acetosella | Eu |
| Polygonaceae | Rumex crispus | Eu |
| Polygonaceae | Rumex nipponicus | As |
| Polygonaceae | Rumex obtusifolius | Eu–As |
| Ranunculaceae | Ranunculus arvensis | Eu |
| Ranunculaceae | Ranunculus muricatus | Eu |
| Rosaceae | Potentilla amurensis | Eu |
| Rosaceae | Potentilla supina | Eu |
| Rosaceae | Rubus fruticosus | Eu |
| Rosaceae | Sanguisorba minor | Eu |
| Rubiaceae | Diodia teres var. hirsutior | Am |
| Rubiaceae | Diodia teres | Am |
| Rubiaceae | Diodia virginiana | Am |
| Rubiaceae | Oldenlandia corymbosa | As–Af |
| Rubiaceae | Sherardia arvensis | Eu |
| Saururaceae | Houttuynia cordata | As |
| Scrophulariaceae | Cymbalaria muralis | Eu |
| Scrophulariaceae | Gratiola officinalis | Eu |
| Scrophulariaceae | Lindernia anagallidea | Am |
| Scrophulariaceae | Lindernia dubia | Am |
| Scrophulariaceae | Verbascum thapsus | Eu |
| Scrophulariaceae | Veronica americana | Am |
| Scrophulariaceae | Veronica arvensis | Eu–As |
| Scrophulariaceae | Veronica hederaefolia | Eu |
| Scrophulariaceae | Veronica persica | Eu–As |
| Scrophulariaceae | Veronica serpyllifolia | Eu |
| Simaroubaceae | Ailanthus altissima | As |
| Solanaceae | Datura meteloides | Am |
| Solanaceae | Datura stramonium | As |
| Solanaceae | Datura stramonium var. chalybea | Am |
| Solanaceae | Nicandra physalodes | Am |
| Solanaceae | Physalis angulata | Am |
| Solanaceae | Physalis wrightii | Am |
| Solanaceae | Solanum americanum | Am |
| Solanaceae | Solanum carolinense | Am |
| Solanaceae | Solanum elaeagnifolium | Am |
| Solanaceae | Solanum nigrum var. humile | Eu |
| Solanaceae | Solanum photeinocarpum | Am |
| Solanaceae | Solanum rostratum | Am |
| Solanaceae | Solanum sarachoides | Am |
| Solanaceae | Solanum sisymbriifolium | Am |
| Solanaceae | Solanum viarum | Am |
| Umbelliferae | Anthriscus caucalis | Eu |
| Umbelliferae | Apium leptophyllum | Am |
| Umbelliferae | Bifora radians | Med |
| Umbelliferae | Chaerophyllum tainturieri | Am |
| Umbelliferae | Conium maculatum | Eu |
| Umbelliferae | Foeniculum vulgare | Eu |
| Umbelliferae | Lisaea heterocarpa | As |
| Valerianaceae | Valerianella olitoria | Eu |
| Verbenaceae | Verbena bonariensis | Am |
| Verbenaceae | Verbena brasiliensis | Am |
| Violaceae | Viola arvensis | Eu |
| Violaceae | Viola papilionacea | Am |
References
- Tóth, E.G.; Tremblay, F.; Housset, J.M.; Bergeron, Y.; Carcaillet, C. Geographic isolation and climatic variability contribute to genetic differentiation in fragmented populations of the long-lived subalpine conifer Pinus cembra L. in the western Alps. BMC Evol. Biol. 2019, 19, 190. [Google Scholar] [CrossRef]
- Dambros, C.; Zuquim, G.; Moulatlet, G.M.; Costa, F.R.C.; Tuomisto, H.; Ribas, C.C.; Azevedo, R.; Baccaro, F.; Bobrowiec, P.E.D.; Dias, M.S.; et al. The role of environmental filtering, geographic distance and dispersal barriers in shaping the turnover of plant and animal species in Amazonia. Biodivers. Conserv. 2020, 29, 3609–3634. [Google Scholar] [CrossRef]
- Rincón-Barrado, M.; Olsson, S.; Villaverde, T.; Moncalvillo, B.; Pokorny, L.; Forrest, A.; Riina, R.; Sanmartín, I. Ecological and geological processes impacting speciation modes drive the formation of wide-range disjunctions within tribe Putorieae (Rubiaceae). J. Syst. Evol. 2021, 59, 915–934. [Google Scholar] [CrossRef]
- Grove, R.H.; Burdon, J.J. Ecology of Biological Invasions; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Hedgpeth, J.W. Foreign invaders. Science 1993, 261, 34–35. [Google Scholar] [CrossRef]
- Cronk, Q.C.; Fuller, J.L. Plant Invaders. People and Plants Conservation Manual; Earthscan: London, UK, 1995. [Google Scholar]
- Mooney, H.A. Invasive alien species: The nature of the problem. In Invasive Alien Species; Island Press: Washington, DC, USA, 2005; Volume 63, pp. 1–15. [Google Scholar]
- Rawlins, K.; Griffin, J.; Moorhead, D.; Bargeron, C.; Evans, C. EDDMapS: Invasive Plant Mapping Handbook; Center for Invasive Species and Ecosystem Health, The University of Georgia: Athens, GA, USA, 2011. [Google Scholar]
- World Wide Fund (WWF). Living Planet Report—2020: Bending the Curve of Biodiversity Loss; World Wide Fund (WWF): Gland, Switzerland, 2020. [Google Scholar]
- Ellis, E.C. Land Use and Ecological Change: A 12,000-Year History. Annu. Rev. Environ. Resour. 2021, 46, 1–33. [Google Scholar] [CrossRef]
- Johnstone, I.M. Plant invasion windows: A time-based classification of invasion potential. Biol. Rev. 1986, 61, 369–394. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Huenneke, L.F. Disturbance, Diversity, and Invasion: Implications for Conservation. Conserv. Biol. 1992, 6, 324–337. [Google Scholar] [CrossRef]
- Mayfield, A.E.; Seybold, S.J.; Haag, W.R.; Johnson, M.T.; Kerns, B.K.; Kilgo, J.C.; Larkin, D.J.; Lucardi, R.D.; Moltzan, B.D.; Pearson, D.E.; et al. Impacts of Invasive Species in Terrestrial and Aquatic Systems in the United States. In Invasive Species in Forests and Rangelands of the United States: A Comprehensive Science Synthesis for the United States Forest Sector; Poland, T.M., Patel-Weynand, T., Finch, D.M., Miniat, C.F., Hayes, D.C., Lopez, V.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 5–39. [Google Scholar]
- Mooney, H.A.; Drake, J.A. Ecology of Biological Invasions of North America and Hawaii; Springer: New York, NY, USA, 1986. [Google Scholar]
- Vitousek, P.M. Biological Invasions and Ecosystem Properties: Can Species Make a Difference? In Ecology of Biological Invasions of North America and Hawaii; Mooney, H.A., Drake, J.A., Eds.; Springer: New York, NY, USA, 1986; pp. 163–176. [Google Scholar]
- Schofield, E.K. Effects of Introduced Plants and Animals on Island Vegetation: Examples from Galápagos Archipelago. Conserv. Biol. 1989, 3, 227–239. [Google Scholar] [CrossRef]
- Simberloff, D.; Schmitz, D.C.; Brown, T.C. Strangers in Paradise: Impact and Management of Nonindigenous Species in Florida; Island Press: Washington, DC, USA, 1997. [Google Scholar]
- Catford, J.A.; Bode, M.; Tilman, D. Introduced species that overcome life history tradeoffs can cause native extinctions. Nat. Commun. 2018, 9, 2131. [Google Scholar] [CrossRef]
- Emery-Butcher, H.E.; Beatty, S.J.; Robson, B.J. The impacts of invasive ecosystem engineers in freshwaters: A review. Freshw. Biol. 2020, 65, 999–1015. [Google Scholar] [CrossRef]
- Ruas, R.d.B.; Costa, L.M.S.; Bered, F. Urbanization driving changes in plant species and communities—A global view. Glob. Ecol. Conserv. 2022, 38, e02243. [Google Scholar] [CrossRef]
- Coblentz, B.E. Exotic Organisms: A Dilemma for Conservation Biology. Conserv. Biol. 1990, 4, 261–265. [Google Scholar] [CrossRef]
- Werren, G.L. Environmental Weeds of the Wet Tropics Bioregion: Risk Assessment & Priority Ranking; Citeseer: Cairns, Australia, 2001. [Google Scholar]
- Convention on Biological Diversity (CBD). Review of the Status and Trends of, and Major Threats to, Forest Biological Diversity; Convention on Biological Diversity (CBD): Cairns, Australia, 2002; p. 164. [Google Scholar]
- Pereira, H.M.; Navarro, L.M.; Martins, I.S. Global Biodiversity Change: The Bad, the Good, and the Unknown. Annu. Rev. Environ. Resour. 2012, 37, 25–50. [Google Scholar] [CrossRef]
- Morris, R.J. Anthropogenic impacts on tropical forest biodiversity: A network structure and ecosystem functioning perspective. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3709–3718. [Google Scholar] [CrossRef]
- Zietsman, L. Observations on Environmental Change in South Africa; SUN Press: Cape Town, South Africa, 2011. [Google Scholar]
- Chu, E.W.; Karr, J.R. Environmental Impact: Concept, Consequences, Measurement. In Reference Module in Life Sciences; PMC COVID-19 Collection; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- McNeely, J.; Mooney, H.; Neville, L.; Schei, P.; Waage, J. Global Strategy on Invasive Alien Species; IUCN, Gland (Suiza) Global Invasive Species Programme: Cambridge, UK, 2001. [Google Scholar]
- Weidlich, E.W.A.; Flórido, F.G.; Sorrini, T.B.; Brancalion, P.H.S. Controlling invasive plant species in ecological restoration: A global review. J. Appl. Ecol. 2020, 57, 1806–1817. [Google Scholar] [CrossRef]
- Rai, P.K. Environmental Degradation by Invasive Alien Plants in the Anthropocene: Challenges and Prospects for Sustainable Restoration. Anthr. Sci. 2022, 1, 5–28. [Google Scholar] [CrossRef]
- Huston, M.A. Biological Diversity: The Coexistence of Species; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Hull, R.B.; Gobster, P.H. Restoring Forest Ecosystems: The Human Dimension. J. For. 2000, 98, 32–36. [Google Scholar] [CrossRef]
- Mooney, H.A.; Hobbs, R.J. Invasive Species in a Changing World; Island Press: Washington, DC, USA, 2000. [Google Scholar]
- Mills, L.S.; Soule, E.M.; Doak, D.F. The Keystone-Species Concept in Ecology and Conservation. BioScience 1993, 43, 219–224. [Google Scholar] [CrossRef]
- Rejmánek, M.; Randall, J.M. Invasive alien plants in California: 1993 summary and comparison with other areas in North America. Madrono 1994, 41, 161–177. [Google Scholar]
- Williamson, M. Invasive species: Vectors management strategies. In Invasion Vectors: A Conceptual Framework for Management; Ruiz, G.M., Carlton, J.T., Eds.; Island Press: Washington, DC, USA, 2003; pp. 459–504. [Google Scholar]
- Mormul, R.P.; Vieira, D.S.; Bailly, D.; Fidanza, K.; da Silva, V.F.B.; da Graça, W.J.; Pontara, V.; Bueno, M.L.; Thomaz, S.M.; Mendes, R.S. Invasive alien species records are exponentially rising across the Earth. Biol. Invasions 2022, 24, 3249–3261. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Ahmed, D.A.; Cuthbert, R.N.; Stubbington, R.; Domisch, S.; Marquez, J.R.G.; Beidas, A.; Amatulli, G.; Kiesel, J.; Shen, L.Q.; et al. Invasion impacts and dynamics of a European-wide introduced species. Glob. Chang. Biol. 2022, 28, 4620–4632. [Google Scholar] [CrossRef]
- Shine, C.; Williams, N.; Gündling, L. A Guide to Designing Legal and Institutional Frameworks on Alien Invasive Species; IUCN: Gland, Switzerland, 2000. [Google Scholar]
- National Invasive Species Council. Meeting the Invasive Species Challenge: National Invasive Species Management Plan; National Invasive Species Council: Washington DC, USA, 2001.
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Wittenberg, R.; JW Cock, M. Invasive Alien Species: A Toolkit of Best Prevention and Management Practices; CAB International: Oxon, UK, 2001. [Google Scholar]
- Meyer, S.E.; Callaham, M.A.; Stewart, J.E.; Warren, S.D. Invasive Species Response to Natural and Anthropogenic Disturbance. In Invasive Species in Forests and Rangelands of the United States: A Comprehensive Science Synthesis for the United States Forest Sector; Poland, T.M., Patel-Weynand, T., Finch, D.M., Miniat, C.F., Hayes, D.C., Lopez, V.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 85–110. [Google Scholar]
- Dybas, C.L. Harmful algal blooms: Biosensors provide new ways of detecting and monitoring growing threat in coastal waters. BioScience 2003, 53, 918–923. [Google Scholar] [CrossRef]
- Chown, S.L.; Hodgins, K.A.; Griffin, P.C.; Oakeshott, J.G.; Byrne, M.; Hoffmann, A.A. Biological invasions, climate change and genomics. Evol. Appl. 2015, 8, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Dix, M.; Bass, B.; Covell, S. Forest Service National Strategic Framework for Invasive Species Management; USDA: Washington, DC, USA, 2013.
- Derr, J.F. Common Reed (Phragmites australis) Response to Mowing and Herbicide Application. Invasive Plant Sci. Manag. 2008, 1, 12–16. [Google Scholar] [CrossRef]
- Kettenring, K.M.; Adams, C.R. Lessons learned from invasive plant control experiments: A systematic review and meta-analysis. J. Appl. Ecol. 2011, 48, 970–979. [Google Scholar] [CrossRef]
- Guo, Q.; Brockway, D.G.; Larson, D.L.; Wang, D.; Ren, H. Improving Ecological Restoration to Curb Biotic Invasion-A Practical Guide. Invasive Plant Sci. Manag. 2018, 11, 163–174. [Google Scholar] [CrossRef]
- Dahlsten, D.L. Control of Invaders. In Ecology of Biological Invasions of North America and Hawaii; Mooney, H.A., Drake, J.A., Eds.; Springer: New York, NY, USA, 1986; pp. 275–302. [Google Scholar]
- Genovesi, P.; Shine, C. European Strategy on Invasive Alien Species: Convention on the Conservation of European Wildlife and Habitats (Bern Convention); Council of Europe: Strasbourg, France, 2004. [Google Scholar]
- Simmons, M.T. Bullying the Bullies: The Selective Control of an Exotic, Invasive Annual (Rapistrum rugosum) by Oversowing with a Competitive Native Species (Gaillardia pulchella). Restor. Ecol. 2005, 13, 609–615. [Google Scholar] [CrossRef]
- Cuda, J.; Charudattan, R.; Grodowitz, M.; Newman, R.; Shearer, J.; Tamayo, M.; Villegas, B. Recent advances in biological control of submersed aquatic weeds. Aquat. Plant Manag. 2008, 46, 15. [Google Scholar]
- Greipsson, S. Phytoremediation. Nat. Educ. Knowl. 2011, 3, 7. [Google Scholar]
- Buckley, Y.M.; Bolker, B.M.; Rees, M. Disturbance, invasion and re-invasion: Managing the weed-shaped hole in disturbed ecosystems. Ecol. Lett. 2007, 10, 809–817. [Google Scholar] [CrossRef]
- Iannone Iii, B.V.; Galatowitsch, S.M. Altering Light and Soil N to Limit Phalaris arundinacea Reinvasion in Sedge Meadow Restorations. Restor. Ecol. 2008, 16, 689–701. [Google Scholar] [CrossRef]
- Funk, J.L.; Cleland, E.E.; Suding, K.N.; Zavaleta, E.S. Restoration through reassembly: Plant traits and invasion resistance. Trends Ecol. Evol. 2008, 23, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Zedler, J. Ecological restoration: Guidance from theory. San Fr. Estuary Watershed Sci. 2005, 3, 1–31. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Laughlin, D.C. Applying trait-based models to achieve functional targets for theory-driven ecological restoration. Ecol. Lett. 2014, 17, 771–784. [Google Scholar] [CrossRef]
- Korea Meteorological Administration (KMA). Korea Meteorological Data Service. Available online: https://www.weather.go.kr/w/index.do (accessed on 4 May 2023).
- Seoul City. A Survey on Changes of Biological Species Distribution in Seoul; Seoul City: Seoul, Republic of Korea, 1999. [Google Scholar]
- Seoul City. A Survey on Biotope and Establishment of Guideline to Construct Eco-Polis in Seoul; Seoul City: Seoul, Republic of Korea, 2000. [Google Scholar]
- Lee, C.; You, Y.; Robinson, G.R. Secondary Succession and Natural Habitat Restoration in Abandoned Rice Fields of Central Korea. Restor. Ecol. 2002, 10, 306–314. [Google Scholar] [CrossRef]
- Yang, G.Y. A Study on the Forest and the Distribution of Naturalized Plants in Seoul; Chungang University: Seoul, Republic of Korea, 1989. [Google Scholar]
- Seoul City. Statistics of Seoul. Available online: http://data.si.re.kr/2015br10-trendof-population-growth (accessed on 4 May 2023).
- Kim, J.; Choe, S. Seoul: The Making of a Metropolis; Wiley: Hoboken, NJ, USA, 1997; Volume 43. [Google Scholar]
- Lee, C.S.; Cho, Y.C.; Shin, H.C.; Moon, J.S.; Lee, B.C.; Bae, Y.S.; Byun, H.G.; Yi, H.B. Ecological response of streams in Korea under different management regimes. Water Eng. Res. 2005, 6, 131–147. [Google Scholar]
- Lee, C.S.; Cho, Y.C.; Lee, A.N. Restoration Planning for the Seoul Metropolitan Area, Korea. In Ecology, Planning, and Management of Urban Forests: International Perspectives; Carreiro, M.M., Song, Y.C., Wu, J., Eds.; Springer: New York, NY, USA, 2008; pp. 393–419. [Google Scholar]
- Ministry of Environment. Monitoring of Invasive Alien Species Designated by the Wild Life Protection Act; Ministry of Environment: Seoul, Republic of Korea, 2006.
- Korea National Arboretum (KNA). Korean Plant Names Index. Available online: http://www.nature.go.kr/kbi/plant/pilbk/selectPlantPilbkGnrlList.do (accessed on 4 May 2023).
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Charendon Press: Oxford, UK, 1934. [Google Scholar]
- Environmental System Research Institute. Arcview GIS; Environmental System Research Institute: Redlands, CA, USA, 2005. [Google Scholar]
- Seoul City. Seoul Forest Ecosystem Survey Research Report; Seoul City: Seoul, Republic of Korea, 1998. [Google Scholar]
- Braun-Blanquet, J. Pflanzengesellschaft und Biozönose. Pflanzensoziologie 1964, 3, 1–6. [Google Scholar] [CrossRef]
- Hill, M.O. Decorana. A Fortran program for detrended correspondence analysis and reciprocal averaging. Vegetatio 1979, 42, 47–58. [Google Scholar] [CrossRef]
- Kent, M.; Coker, P. Vegetation Description and Analysis: A Practical Approach; WILEY-BLACKWELL: Chichester, UK, 1992. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell: New York, NY, USA, 2004. [Google Scholar]
- Ellenberg, D.; Mueller-Dombois, D. Aims and Methods of Vegetation Ecology; Wiley: New York, NY, USA, 1974. [Google Scholar]
- National Institute of Environmental Research (NIER). Survey for Ecological Impact by Naturalized Organisms (I); National Institute of Environmental: Seoul, Republic of Korea, 1995.
- National Institute of Environmental Research (NIER). Survey for Ecological Impact by Naturalized Organisms (II); National Institute of Environmental: Seoul, Republic of Korea, 1996.
- Kim, J.M.; Yim, Y.J.; Jeon, E.S. Invasive Alien Plants in Korea; Science Books: Seoul, Republic of Korea, 2000. [Google Scholar]
- SoulÉ, M.E. The Onslaught of Alien Species, and Other Challenges in the Coming Decades. Conserv. Biol. 1990, 4, 233–240. [Google Scholar] [CrossRef]
- Cox, G.W. Alien Species and Evolution: The Evolutionary Ecology of Exotic Plants, Animals, Microbes, and Interacting Native Species; Island Press: Washington, DC, USA, 2004. [Google Scholar]
- Richardson, D.M.; Rejmánek, M. Conifers as invasive aliens: A global survey and predictive framework. Divers. Distrib. 2004, 10, 321–331. [Google Scholar] [CrossRef]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: Challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, I. Neophytes in Germany: Quantitative overview, introduction and dispersal pathways, ecological consequences and open questions. Texte des Umweltbundesamtes Berlin 1999, 1, 12–36. [Google Scholar]
- Shiferaw, W.; Demissew, S.; Bekele, T. Invasive alien plant species in Ethiopia: Ecological impacts on biodiversity a review paper. Int. J. Mol. Biol. 2018, 3, 171–178. [Google Scholar] [CrossRef]
- Daly, E.Z.; Chabrerie, O.; Massol, F.; Facon, B.; Hess, M.C.M.; Tasiemski, A.; Grandjean, F.; Chauvat, M.; Viard, F.; Forey, E.; et al. A synthesis of biological invasion hypotheses associated with the introduction–naturalisation–invasion continuum. Oikos 2023, 5, e09645. [Google Scholar] [CrossRef]
- Wiens, J.A.; Nils Chr, S.; Van Horne, B.; Ims, R.A. Ecological Mechanisms and Landscape Ecology. Oikos 1993, 66, 369–380. [Google Scholar] [CrossRef]
- Forman, R.T. Land Mosaics: The Ecology of Landscapes and Regions; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Pickett, S.T.A.; Cadenasso, M.L. Landscape Ecology: Spatial Heterogeneity in Ecological Systems. Science 1995, 269, 331–334. [Google Scholar] [CrossRef]
- Miller, J.H.; Lemke, D.; Coulston, J. The invasion of southern forests by nonnative plants: Current and future occupation, with impacts, management strategies, and mitigation approaches. In The Southern Forest Futures Project: Technical Report; Wear, D.N., Greis, J.G., Eds.; Forest Service: Raleigh, NC, USA, 2013; pp. 397–456. [Google Scholar]
- Hobbs, R.J. Disturbance of a precursor to weed invasion in native vegetation. Plant Prot. Q. 1991, 6, 99–104. [Google Scholar]
- Abensperg-Traun, M.; Atkins, L.; Hobbs, R.; Steven, D. Exotic plant invasion and understorey species richness: A comparison of two types of eucalypt woodland in agricultural Western Australia. Pac. Conserv. Biol. 1998, 4, 21–32. [Google Scholar] [CrossRef]
- Morgan, J.W. Patterns of invasion of an urban remnant of a species-rich grassland in southeastern Australia by non-native plant species. J. Veg. Sci. 1998, 9, 181–190. [Google Scholar] [CrossRef]
- Grzędzicka, E. Assessment of Habitat Selection by Invasive Plants and Conditions with the Best Performance of Invasiveness Traits. Diversity 2023, 15, 333. [Google Scholar] [CrossRef]
- Duggin, J.A.; Gentle, C.B. Experimental evidence on the importance of disturbance intensity for invasion of Lantana camara L. in dry rainforest–open forest ecotones in north-eastern NSW, Australia. For. Ecol. Manag. 1998, 109, 279–292. [Google Scholar] [CrossRef]
- Cadenasso, M.L.; Pickett, S.T.A. Linking forest edge structure to edge function: Mediation of herbivore damage. J. Ecol. 2000, 88, 31–44. [Google Scholar] [CrossRef]
- Lonsdale, W.M. Global patterns of plant invasions and the concept of invasibility. Ecology 1999, 80, 1522–1536. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tempera, F.; Teixeira, H. Mapping the impact of alien species on marine ecosystems: The Mediterranean Sea case study. Divers. Distrib. 2016, 22, 694–707. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J.; Ackerman, J.D.; Marcano-Vega, H.; Willig, M.R. Alien species affect the abundance and richness of native species in tropical forests: The role of adaptive strategies. Ecosphere 2022, 13, e4291. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive Species, Environmental Change and Management, and Health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Ricciardi, A.; Iacarella, J.C.; Aldridge, D.C.; Blackburn, T.M.; Carlton, J.T.; Catford, J.A.; Dick, J.T.A.; Hulme, P.E.; Jeschke, J.M.; Liebhold, A.M.; et al. Four priority areas to advance invasion science in the face of rapid environmental change. Environ. Rev. 2020, 29, 119–141. [Google Scholar] [CrossRef]
- Parendes, L.A.; Jones, J.A. Role of Light Availability and Dispersal in Exotic Plant Invasion along Roads and Streams in the H. J. Andrews Experimental Forest, Oregon. Conserv. Biol. 2000, 14, 64–75. [Google Scholar] [CrossRef]
- Zhu, G.; Gutierrez Illan, J.; Looney, C.; Crowder, D.W. Assessing the ecological niche and invasion potential of the Asian giant hornet. Proc. Natl. Acad. Sci. USA 2020, 117, 24646–24648. [Google Scholar] [CrossRef]
- Jungblut, S.; Beermann, J.; Boos, K.; Saborowski, R.; Hagen, W. Population development of the invasive crab Hemigrapsus sanguineus (De Haan, 1853) and its potential native competitor Carcinus maenas (Linnaeus, 1758) at Helgoland (North Sea) between 2009 and 2014. Aquat. Invasions 2017, 12. [Google Scholar] [CrossRef]
- DeRoy, E.M.; Crookes, S.; Matheson, K.; Scott, R.; McKenzie, C.H.; Alexander, M.E.; Dick, J.T.A.; MacIsaac, H.J. Predatory ability and abundance forecast the ecological impacts of two aquatic invasive species. NeoBiota 2022, 71, 91–112. [Google Scholar] [CrossRef]
- Kangas, P. Ecological Engineering: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Correll, D.L. Principles of planning and establishment of buffer zones. Ecol. Eng. 2005, 24, 433–439. [Google Scholar] [CrossRef]
- Baker, H.G. Patterns of plant invasion in North America. In Ecology of biological invasions of North America and Hawaii; Mooney, H.A., Drake, J.A., Eds.; Springer Science & Business Media: New York, NY, USA, 1986; pp. 44–57. [Google Scholar]
- Berger, J.J. Ecological Restoration and NonIndigenous Plant Species: A Review. Restor. Ecol. 1993, 1, 74–82. [Google Scholar] [CrossRef]
- Aronson, J.; Floret, C.; Le Floc’h, E.; Ovalle, C.; Pontanier, R. Restoration and Rehabilitation of Degraded Ecosystems in Arid and Semi-Arid Lands. I. A View from the South. Restor. Ecol. 1993, 1, 8–17. [Google Scholar] [CrossRef]
- National Research Council. Restoration of Aquatic Ecosystems: Science, Technology, and Public Policy; The National Academies Press: Washington, DC, USA, 1992. [Google Scholar]
- Bradshaw, A.D. Ecological principles and land reclamation practice. Landsc. Plan. 1984, 11, 35–48. [Google Scholar] [CrossRef]
- Pabst, R.; Dias, F.S.; Borda-de-Água, L.; Rodríguez-González, P.M.; Capinha, C. Assessing and predicting the distribution of riparian invasive plants in continental Portugal. Front. Ecol. Evol. Appl. 2022, 10, 875578. [Google Scholar] [CrossRef]
- McDonald, T.; Gann, G.; Jonson, J.; Dixon, K. International Standards for the Practice of Ecological Restoration–Including Principles and Key Concepts; Society for Ecological Restoration: Washington, DC, USA, 2016. [Google Scholar]
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J.; et al. International principles and standards for the practice of ecological restoration. Second edition. Restor. Ecol. 2019, 27, S1–S46. [Google Scholar] [CrossRef]
- Kim, A.R.; Lim, B.S.; Seol, J.; Lee, C.S. Principle of restoration ecology reflected in the process creating the National Institute of Ecology. J. Ecol. Environ. 2021, 45, 12. [Google Scholar] [CrossRef]
- Esler, K.J.; van Wilgen, B.W.; te Roller, K.S.; Wood, A.R.; van der Merwe, J.H. A landscape-scale assessment of the long-term integrated control of an invasive shrub in South Africa. Biol. Invasions 2010, 12, 211–218. [Google Scholar] [CrossRef]
- Gaertner, M.; Biggs, R.; Te Beest, M.; Hui, C.; Molofsky, J.; Richardson, D.M. Invasive plants as drivers of regime shifts: Identifying high-priority invaders that alter feedback relationships. Divers. Distrib. 2014, 20, 733–744. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Richardson, D.M. Invasion ecology and restoreation ecology: Parallel evolution in two fields of endeavour. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton; Richardson, D.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 61–70. [Google Scholar]
- Dulohery, C.J.; Kolka, R.K.; McKevlin, M.R. Effects of a willow overstory on planted seedlings in a bottomland restoration. Ecol. Eng. 2000, 15, S57–S66. [Google Scholar] [CrossRef]
- McLeod, R.W.; Kirkegaard, J.A.; Steel, C.C. Invasion, development, growth and egg laying by Meloidogyne javanica in Brassicaceae crops. Nematology 2001, 3, 463–472. [Google Scholar] [CrossRef]
- Panetta, F.; Hopkins, A. Weeds in corridors: Invasion and management. Nat. Conserv. 1991, 2, 341–351. [Google Scholar]
- Holmes, P.M.; Esler, K.J.; Gaertner, M.; Geerts, S.; Hall, S.A.; Nsikani, M.M.; Richardson, D.M.; Ruwanza, S. Biological Invasions and Ecological Restoration in South Africa. In Biological Invasions in South Africa; van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 665–700. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, B.S.; Seok, J.E.; Lim, C.H.; Kim, G.S.; Shin, H.C.; Lee, C.S. Distribution, Effect, and Control of Exotic Plants in Republic of Korea. Biology 2023, 12, 826. https://doi.org/10.3390/biology12060826
Lim BS, Seok JE, Lim CH, Kim GS, Shin HC, Lee CS. Distribution, Effect, and Control of Exotic Plants in Republic of Korea. Biology. 2023; 12(6):826. https://doi.org/10.3390/biology12060826
Chicago/Turabian StyleLim, Bong Soon, Ji Eun Seok, Chi Hong Lim, Gyung Soon Kim, Hyun Chul Shin, and Chang Seok Lee. 2023. "Distribution, Effect, and Control of Exotic Plants in Republic of Korea" Biology 12, no. 6: 826. https://doi.org/10.3390/biology12060826
APA StyleLim, B. S., Seok, J. E., Lim, C. H., Kim, G. S., Shin, H. C., & Lee, C. S. (2023). Distribution, Effect, and Control of Exotic Plants in Republic of Korea. Biology, 12(6), 826. https://doi.org/10.3390/biology12060826





