World Spread of Tropical Soda Apple (Solanum viarum) under Global Change: Historical Reconstruction, Niche Shift, and Potential Geographic Distribution
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Records and Reconstruction of Invasion
2.2. Climate Variables
2.3. Land–Use Harmonization Data
2.4. Model Settings and Evaluation
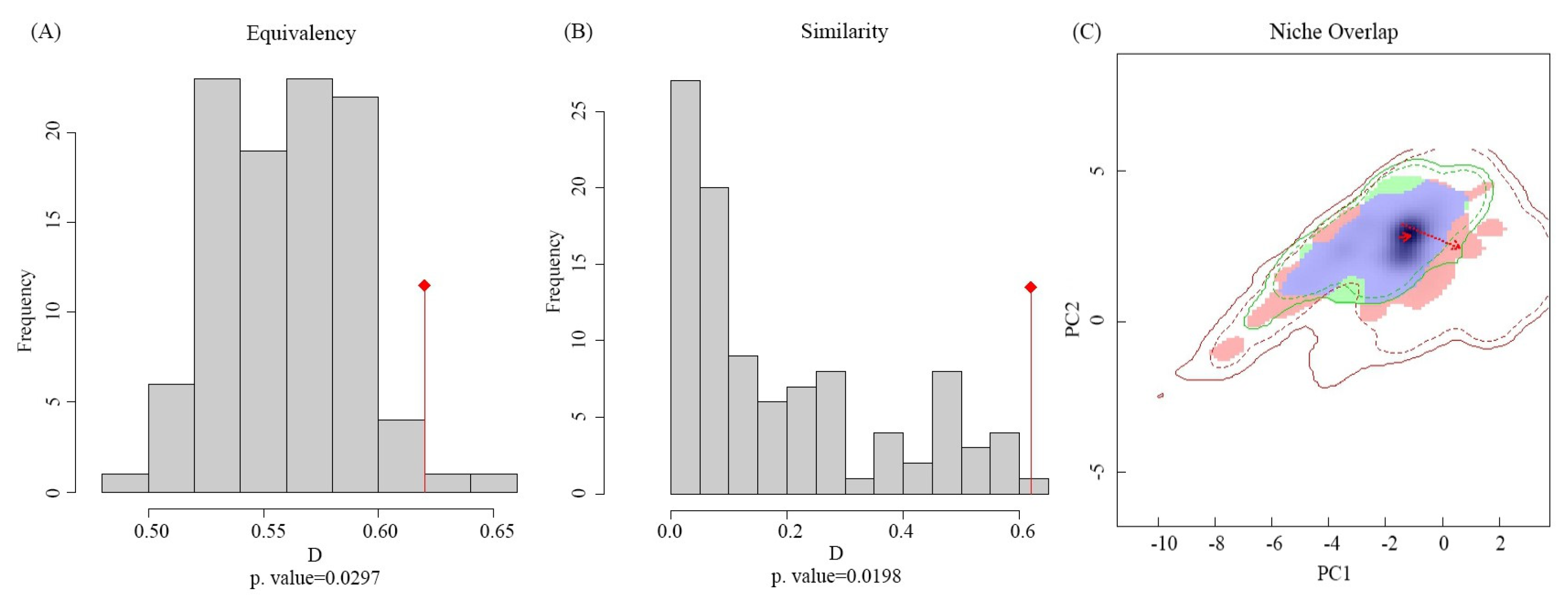
2.5. Ecological Niche Comparison Method
3. Results
3.1. Reconstruction of Invasion
3.2. Global Variation in the Ecological Niche of Solanum viarum
3.3. Model Performance and Significant Variables
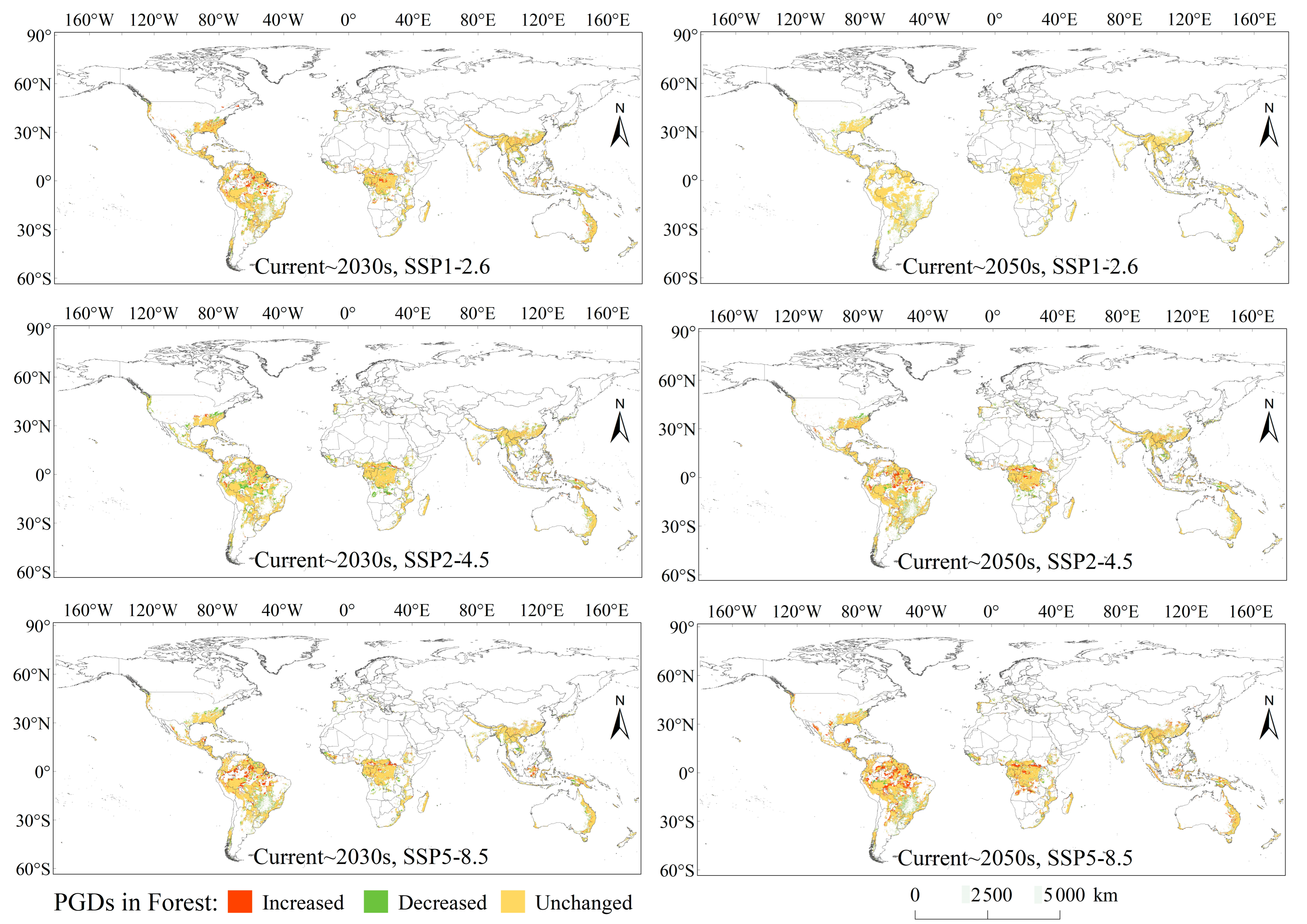
3.4. PGDs in Forest, Grassland, Cropland, and Urban Ecosystems
3.5. Variations of PGDs in Forest, Grassland, Cropland, and Urban Ecosystems
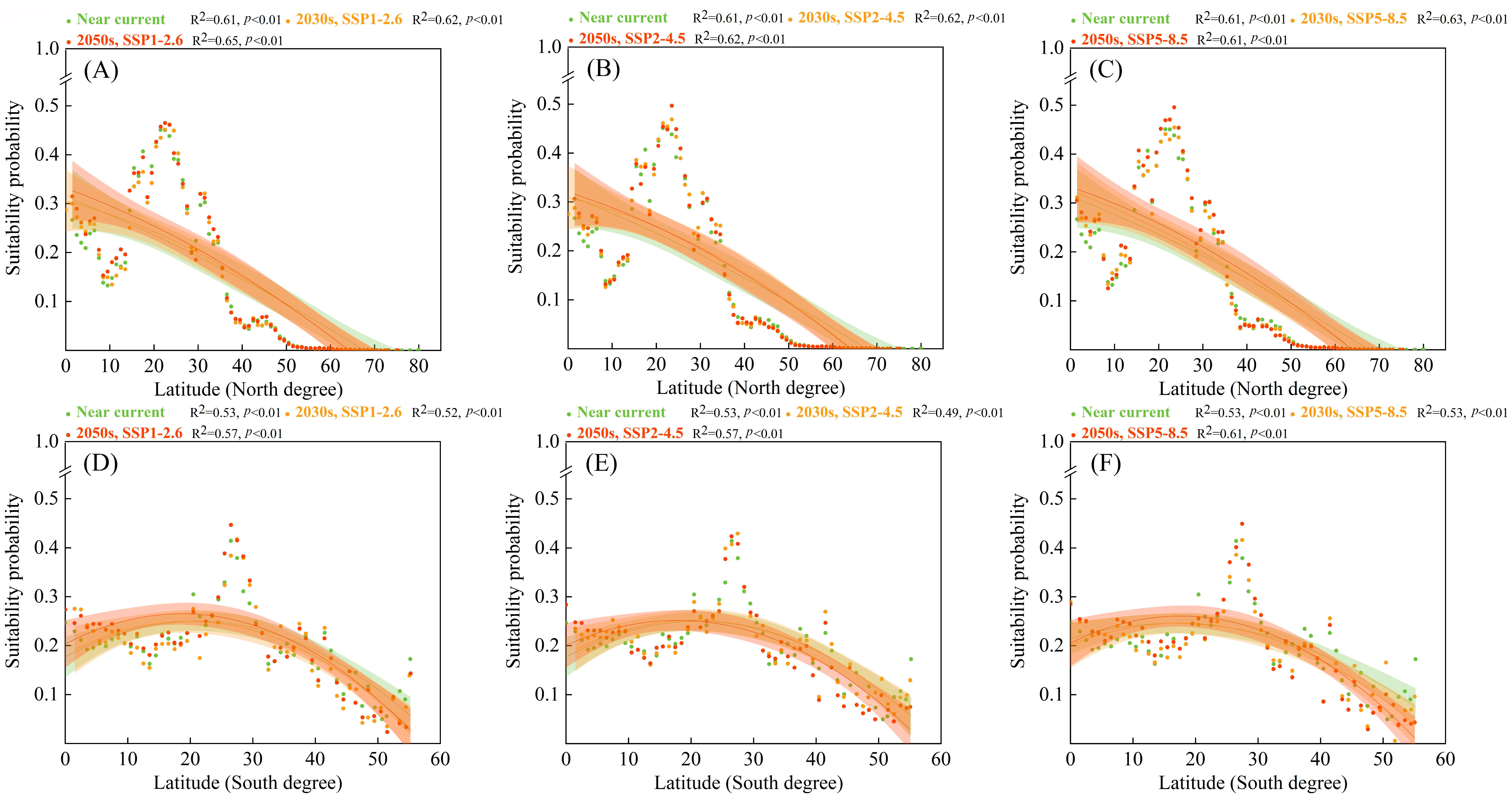
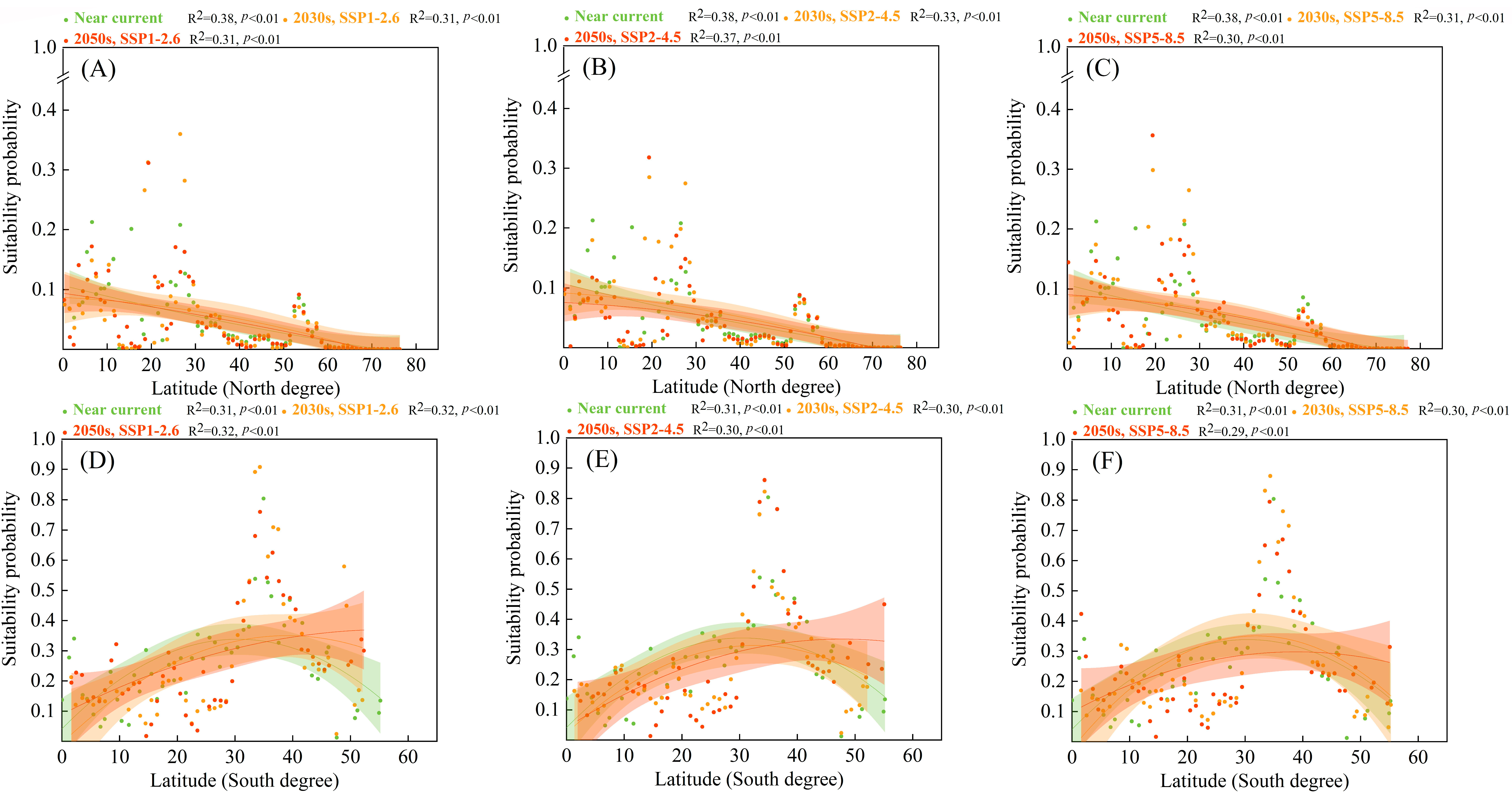
3.6. Suitability Probabilities along Latitudinal Gradients
4. Discussion
4.1. Reconstruction of Invasion History
4.2. Ecological Niches and PGDs in Different Ecosystem
4.3. Early Warning and Prevention Efforts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sitzia, T.; Campagnaro, T.; Kotze, D.J.; Nardi, S.; Ertani, A. The invasion of abandoned fields by a major alien tree filters understory plant traits in novel forest ecosystems. Sci. Rep. 2018, 8, 8410. [Google Scholar] [CrossRef] [PubMed]
- Skubel, S.A.; Su, X.; Poulev, A.; Foxcroft, L.C.; Dushenkov, V.; Raskin, I. Metabolomic differences between invasive alien plants from native and invaded habitats. Sci. Rep. 2020, 10, 9749. [Google Scholar] [CrossRef] [PubMed]
- Vilà, M.; Pujadas, J. Land–use and socio–economic correlates of plant invasions in European and North African countries. Biol. Conserv. 2001, 100, 397–401. [Google Scholar] [CrossRef]
- Capinha, C.; Essl, F.; Seebens, H.; Moser, D.; Pereira, H.M. The dispersal of alien species redefines biogeography in the Anthropocene. Science 2015, 348, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Lu, X.; Siemann, E.; Shao, X.; Wei, H.; Ding, J. Climate warming affects biological invasions by shifting interactions of plants and herbivores. Glob. Chang. Biol. 2013, 19, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Brothers, T.S.; Spingarn, A. Forest fragmentation and alien plant invasion of central Indiana old–growth forests. Conserv. Biol. 1992, 6, 91–100. [Google Scholar] [CrossRef]
- Carlson, B.S.; Rotics, S.; Nathan, R.; Wikelski, M.; Jetz, W. Individual environmental niches in mobile organisms. Nat. Commun. 2021, 12, 4572. [Google Scholar] [CrossRef] [PubMed]
- Janssens, C.; Havlik, P.; Krisztin, T.; Baker, J.; Frank, S.; Hasegawa, T.; Leclere, D.; Ohrel, S.; Ragnauth, S.; Schmid, E.; et al. International trade is a key component of climate change adaptation. Nat. Clim. Chang. 2021, 11, 915–916. [Google Scholar] [CrossRef]
- Dalmazzone, S.; Giaccaria, S. Economic drivers of biological invasions: A worldwide, bio–geographic analysis. Ecol. Econ. 2014, 105, 154–165. [Google Scholar] [CrossRef]
- Randall, R.P. A Global Compendium of Weeds; RP Randall: South Perth, Australia, 2017. [Google Scholar]
- Nee, M. Synopsis of Solanum in the New World. In Solanaceae IV: Advances in Biology and Utilization; Royal Botanic Gardens, Kew: Richmond, UK, 1999; pp. 285–333. [Google Scholar]
- CABI Database. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.50562 (accessed on 20 June 2023).
- Medal, J.; Bustamante, N.; Overholt, W.; Diaz, R.; Stansly, P.; Roda, A.; Amalin, D.; Hibbard, K.; Gaskalla, R.; Sellers, B.; et al. Biological control of Tropical soda apple (Solanaceae) in Florida: Post–release evaluation. Fla Entomol. 2010, 93, 130–132. [Google Scholar] [CrossRef]
- Singha, H.R.; Sinha, S.; Sinha, R.K. Karyomorphology of Solanum viarum Dunal–an ethnomedicinal species of Tripura. Vegetos 2016, 29, 65–68. [Google Scholar] [CrossRef]
- Welman, W.G. The genus Solanum (Solanaceae) in southern Africa: Subgenus Leptostemonum, the introduced sections Acanthophora and Torva. Bothalia 2003, 33, 1–18. [Google Scholar] [CrossRef]
- Bryson, C.T.; Reddy, K.N.; Byrd, J.D., Jr. Growth, development, and morphological differences among native and nonnative prickly nightshades (Solanum spp.) of the southeastern United States. Invasive Plant Sci. Manag. 2012, 5, 341–352. [Google Scholar] [CrossRef]
- Medal, J.C.; Charudattan, R.; Mullahey, J.J.; Pitelli, R.A. An exploratory insect survey of tropical soda apple in Brazil and Paraguay. Fla Entomol. 1996, 79, 70–73. [Google Scholar] [CrossRef]
- USDA Natural Resources Conservation Service. The Plants Database. Available online: http://plants.usda.gov/ (accessed on 20 June 2023).
- EPPO Global Database. Available online: https://gd.eppo.int/taxon/SOLVI/categorization (accessed on 20 June 2023).
- Aikio, S.; Duncan, R.P.; Hulme, P.E. Lag–phases in alien plant invasions: Separating the facts from the artefacts. Oikos 2010, 119, 370–378. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef]
- Pili, A.N.; Tingley, R.; Sy, E.Y.; Diesmos, M.L.L.; Diesmos, A.C. Niche shifts and environmental non–equilibrium undermine the usefulness of ecological niche models for invasion risk assessments. Sci. Rep. 2020, 10, 7972. [Google Scholar] [CrossRef]
- Urvois, T.; Auger–Rozenberg, M.A.; Roques, A.; Rossi, J.P.; Kerdelhue, C. Climate change impact on the potential geographical distribution of two invading Xylosandrus ambrosia beetles. Sci. Rep. 2021, 11, 1339. [Google Scholar] [CrossRef]
- Cao, J.; Xu, J.; Pan, X.; Monaco, T.A.; Zhao, K.; Wang, D.; Rong, Y. Potential impact of climate change on the global geographical distribution of the invasive species, Cenchrus spinifex (Field sandbur, Gramineae). Ecol. Indic. 2021, 131, 108204. [Google Scholar] [CrossRef]
- Puchalka, R.; Dyderski, M.K.; Vitkova, M.; Sadlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T.; et al. Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe under changing climate. Glob. Chang. Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.V.; Stokes, K.E.; van Klinken, R.D. Predicting the potential distribution of a riparian invasive plant: The effects of changing climate, flood regimes and land–use patterns. Glob. Chang. Biol. 2012, 18, 1738–1753. [Google Scholar] [CrossRef]
- Dyderski, M.; Zarnowiec, J.; Stebel, A.; Chmura, D. Propagule pressure and land–use are more important than climate for invasive bryophytes regional distributions. Landsc. Ecol. 2022, 37, 1871–1884. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Brown, J.L.; Hill, D.J.; Dolan, A.M.; Carnaval, A.C.; Haywood, A.M. PaleoClim, high spatial resolution paleoclimate surfaces for global land areas. Sci. Data 2018, 5, 180254. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1–km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Frieler, K.; Lange, S.; Piontek, F.; Reyer, C.P.O.; Schewe, J.; Warszawski, L.; Zhao, F.; Chini, L.; Denvil, S.; Emanuel, K.; et al. Assessing the impacts of 1.5 °C global warming—Simulation protocol of the Inter–Sectoral Impact Model Intercomparison Project (ISIMIP2b). Geosci. Model Dev. 2017, 10, 4321–4345. [Google Scholar] [CrossRef]
- Hurtt, G.C.; Chini, L.; Sahajpal, R.; Frolking, S.; Bodirsky, B.L.; Calvin, K.; Doelman, J.C.; Fisk, J.; Fujimori, S.; Goldewijk, K.K.; et al. Harmonization of global land use change and management for the period 850–2100 (LUH2) for CMIP6. Geosci. Model Dev. 2020, 13, 5425–5464. [Google Scholar] [CrossRef]
- Javidan, N.; Kavian, A.; Pourghasemi, H.R.; Conoscenti, C.; Jafarian, Z.; Rodrigo–Comino, J. Evaluation of multi–hazard map produced using MaxEnt machine learning technique. Sci. Rep. 2021, 11, 6496. [Google Scholar] [CrossRef]
- Betts, M.G.; Yang, Z.Q.; Hadley, A.S.; Smith, A.C.; Rousseau, J.S.; Northrup, J.M.; Nocera, J.J.; Gorelick, N.; Gerber, B.D. Forest degradation drives widespread avian habitat and population declines. Nat. Ecol. Evol. 2022, 6, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio–Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.X.; Xian, X.Q.; Zhao, Z.H.; Zhang, G.F.; Liu, W.X.; Wan, F.H. Climate change increases the expansion risk of Helicoverpa zea in China According to potential geographical distribution estimation. Insects 2022, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Hill, M.P.; Terblanche, J.S. Niche Overlap of Congeneric Invaders Supports a Single–Species Hypothesis and Provides Insight into Future Invasion Risk: Implications for Global Management of the Bactrocera dorsalis Complex. PLoS ONE 2014, 9, e90121. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.P.; Gallardo, B.; Terblanche, J.S. A global assessment of climatic niche shifts and human influence in insect invasions. Glob. Ecol. Biogeogr. 2017, 26, 679–689. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2011, 62, 2868–2883. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Marchioro, C.A.; Krechemer, F.S. Reconstructing the biological invasion of Tuta absoluta: Evidence of niche shift and its consequences for invasion risk assessment. J. Pest Sci. 2023, 1, 1–15. [Google Scholar] [CrossRef]
- Waheed, M.; Arshad, F.; Majeed, M.; Haq, S.M.; Aziz, R.; Bussmann, R.W.; Ali, K.; Subhan, F.; Jones, D.A.; Zaitouny, A. Potential distribution of a noxious weed (Solanum viarum Du–nal), current status, and future invasion risk based on MaxEnt modeling. Geol. Eco. Landsc. 2023, 12, 1–16. [Google Scholar] [CrossRef]
- Boivin, N.L.; Zeder, M.A.; Fuller, D.Q.; Crowther, A.; Larson, G.; Erlandson, J.M.; Denham, T.; Petraglia, M.D. Ecological consequences of human niche construction: Examining long–term anthropogenic shaping of global species distributions. Proc. Natl. Acad. Sci. USA 2016, 113, 6388–6396. [Google Scholar] [CrossRef]
- Fiacconi, M.; Hunt, C.O. Pollen taphonomy at Shanidar Cave (Kurdish Iraq): An initial evaluation. Rev. Palaeobot. Palynol. 2015, 223, 87–93. [Google Scholar] [CrossRef]
- van Kleunen, M.; Essl, F.; Pergl, J.; Brundu, G.; Carboni, M.; Dullinger, S.; Early, R.; Gonzalez–Moreno, P.; Groom, Q.J.; Hulme, P.E.; et al. The changing role of ornamental horticulture in alien plant invasions. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1421–1437. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Dong, B.C.; Fu, Q.Y.; Yang, Q.; Dai, Z.C.; Luo, F.L.; Gao, J.Q.; Yu, F.H.; van Kleunen, M. Cultivated alien plants with high invasion potential are more likely to be traded online in China. Ecol. Appl. 2023, 11, e2811. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-P.; Redei, D.; Kment, P.; Bu, W.-J. Effect of geographic background and equilibrium state on niche model transferability: Predicting areas of invasion of Leptoglossus occidentalis. Biol. Invas. 2014, 16, 1069–1081. [Google Scholar] [CrossRef]
- Lu, X.; He, M.; Ding, J.; Siemann, E. Latitudinal variation in soil biota: Testing the biotic interaction hypothesis with an invasive plant and a native congener. ISME J. 2018, 12, 2811–2822. [Google Scholar] [CrossRef]
- Mullahey, J.J.; Shilling, D.G.; Mislevy, P.; Akanda, R.A. Invasion of tropical soda apple (Solanum viarum) into the U.S.: Lessons learned. Weed Technol. 1998, 12, 733–736. [Google Scholar] [CrossRef]
- Lustenhouwer, N.; Parker, I.M. Beyond tracking climate: Niche shifts during native range expansion and their implications for novel invasions. J. Biogeogr. 2022, 49, 1481–1493. [Google Scholar] [CrossRef]
- Overholt, W.A.; Diaz, R.; Markle, L.; Medal, J.C. The effect of Gratiana boliviana (Coleoptera: Chrysomelidae) herbivory on growth and population density of tropical soda apple (Solanum viarum) in Florida. Biocontrol Sci. Technol. 2010, 20, 791–807. [Google Scholar] [CrossRef]
- Mullahey, J.J.; Nee, M.; Wunderlin, R.P.; Delaney, K.R. Tropical soda apple (Solanum viarum): A new weed threat in subtropical regions. Weed Technol. 1993, 7, 783–786. [Google Scholar] [CrossRef]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche dynamics in space and time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Pecl, G.T.; Araujo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengard, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well–being. Science 2017, 355, 9. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, S.; Ruiz–Benito, P.; Martinez–Vilalta, J.; Lloret, F.; Kitzberger, T.; Allen, C.D.; Fensham, R.; Laughlin, D.C.; Kattge, J.; Bonisch, G.; et al. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Engler, R.; Randin, C.F.; Thuiller, W.; Dullinger, S.; Zimmermann, N.E.; Araujo, M.B.; Pearman, P.B.; Le Lay, G.; Piedallu, C.; Albert, C.H.; et al. 21st century climate change threatens mountain flora unequally across Europe. Glob. Chang. Biol. 2011, 17, 2330–2341. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Sirami, C.; Caplat, P.; Popy, S.; Clamens, A.; Arlettaz, R.; Jiguet, F.; Brotons, L.; Martin, J.L. Impacts of global change on species distributions: Obstacles and solutions to integrate climate and land use. Glob. Ecol. Biogeogr. 2017, 26, 385–394. [Google Scholar] [CrossRef]
- Thebo, A.L.; Drechsel, P.; Lambin, E.F. Global assessment of urban and peri–urban agriculture: Irrigated and rainfed croplands. Environ. Res. Lett. 2014, 9, 9. [Google Scholar] [CrossRef]
- Bren d’Amour, C.B.; Reitsma, F.; Baiocchi, G.; Barthel, S.; Guneralp, B.; Erb, K.H.; Haberl, H.; Creutzig, F.; Seto, K.C. Future urban land expansion and implications for global croplands. Proc. Natl. Acad. Sci. USA 2017, 114, 8939–8944. [Google Scholar] [CrossRef]
- Bonnamour, A.; Gippet, J.M.W.; Bertelsmeier, C. Insect and plant invasions follow two waves of globalisation. Ecol. Lett. 2021, 24, 2418–2426. [Google Scholar] [CrossRef]
- Corson, M.S.; Mondiere, A.; Morel, L.; van der Werf, H.M.G. Beyond agroecology: Agricultural rewilding, a prospect for livestock systems. Agric. Syst. 2022, 199, 103410. [Google Scholar] [CrossRef]
- West, G.G.; Dean, M.G. The Use of Livestock to Control Weeds in New Zealand Forests. FRI Bull. 1990, 155, 128–132. [Google Scholar]
- Mislevy, P.; Martin, F.G. Tropical soda apple control as influenced by frost and herbicides. Soil Crop Sci. Soc. Fla Proc. 1999, 58, 107–109. [Google Scholar]
- Duncan, R.P. Time lags and the invasion debt in plant naturalisations. Ecol. Lett. 2021, 24, 1363–1374. [Google Scholar] [CrossRef] [PubMed]




| Area (×104 km2) | Forest | Grassland | Cropland | Urban | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PGDs | Total | PGDs | Total | PGDs | Total | PGDs | Total | |||||
| Near current | 1467 | 6069 | 24% | 152 | 1450 | 10% | 417 | 2010 | 21% | 15 | 51 | 30% |
| 2030s, SSP1–2.6 | 1512 | 6103 | 25% | 144 | 1363 | 11% | 432 | 1996 | 22% | 23 | 73 | 32% |
| 2030s, SSP2–4.5 | 1602 | 6006 | 27% | 160 | 1436 | 11% | 489 | 2116 | 23% | 23 | 71 | 32% |
| 2030s, SSP5–8.5 | 1475 | 5920 | 25% | 148 | 1403 | 11% | 502 | 2183 | 23% | 24 | 77 | 31% |
| 2050s, SSP1–2.6 | 1748 | 6123 | 29% | 135 | 1267 | 11% | 505 | 2026 | 25% | 25 | 83 | 30% |
| 2050s, SSP2–4.5 | 1456 | 5992 | 24% | 147 | 1385 | 11% | 468 | 2186 | 21% | 26 | 81 | 32% |
| 2050s, SSP5–8.5 | 1557 | 5875 | 27% | 147 | 1403 | 10% | 577 | 2224 | 26% | 31 | 93 | 33% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Xian, X.; Zhao, H.; Yang, M.; Zhang, Y.; Yu, W.; Liu, W. World Spread of Tropical Soda Apple (Solanum viarum) under Global Change: Historical Reconstruction, Niche Shift, and Potential Geographic Distribution. Biology 2023, 12, 1179. https://doi.org/10.3390/biology12091179
Qi Y, Xian X, Zhao H, Yang M, Zhang Y, Yu W, Liu W. World Spread of Tropical Soda Apple (Solanum viarum) under Global Change: Historical Reconstruction, Niche Shift, and Potential Geographic Distribution. Biology. 2023; 12(9):1179. https://doi.org/10.3390/biology12091179
Chicago/Turabian StyleQi, Yuhan, Xiaoqing Xian, Haoxiang Zhao, Ming Yang, Yu Zhang, Wentao Yu, and Wanxue Liu. 2023. "World Spread of Tropical Soda Apple (Solanum viarum) under Global Change: Historical Reconstruction, Niche Shift, and Potential Geographic Distribution" Biology 12, no. 9: 1179. https://doi.org/10.3390/biology12091179
APA StyleQi, Y., Xian, X., Zhao, H., Yang, M., Zhang, Y., Yu, W., & Liu, W. (2023). World Spread of Tropical Soda Apple (Solanum viarum) under Global Change: Historical Reconstruction, Niche Shift, and Potential Geographic Distribution. Biology, 12(9), 1179. https://doi.org/10.3390/biology12091179





