Water Hardness Improves the Antioxidant Response of Zinc-Exposed Goldfish (Carassius auratus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Fishes
2.2. Zinc and Water Hardness Treatment and Sampling
2.3. Total RNA Extraction and Complementary DNA Synthesis
2.4. Real-Time Polymerase Chain Reaction
2.5. In Situ Hybridization Detection of SOD mRNA
2.6. Analysis of Plasma Parameter
2.7. Comet Assays
2.8. Statistical Analysis
3. Results
3.1. Water Hardness
3.2. Changes SOD and CAT Activity in Plasma
3.3. SOD mRNA Expression in Liver Using In Situ Hybridization
3.4. Levels of Hydrogen Peroxide and Lipid Peroxidation in Plasma
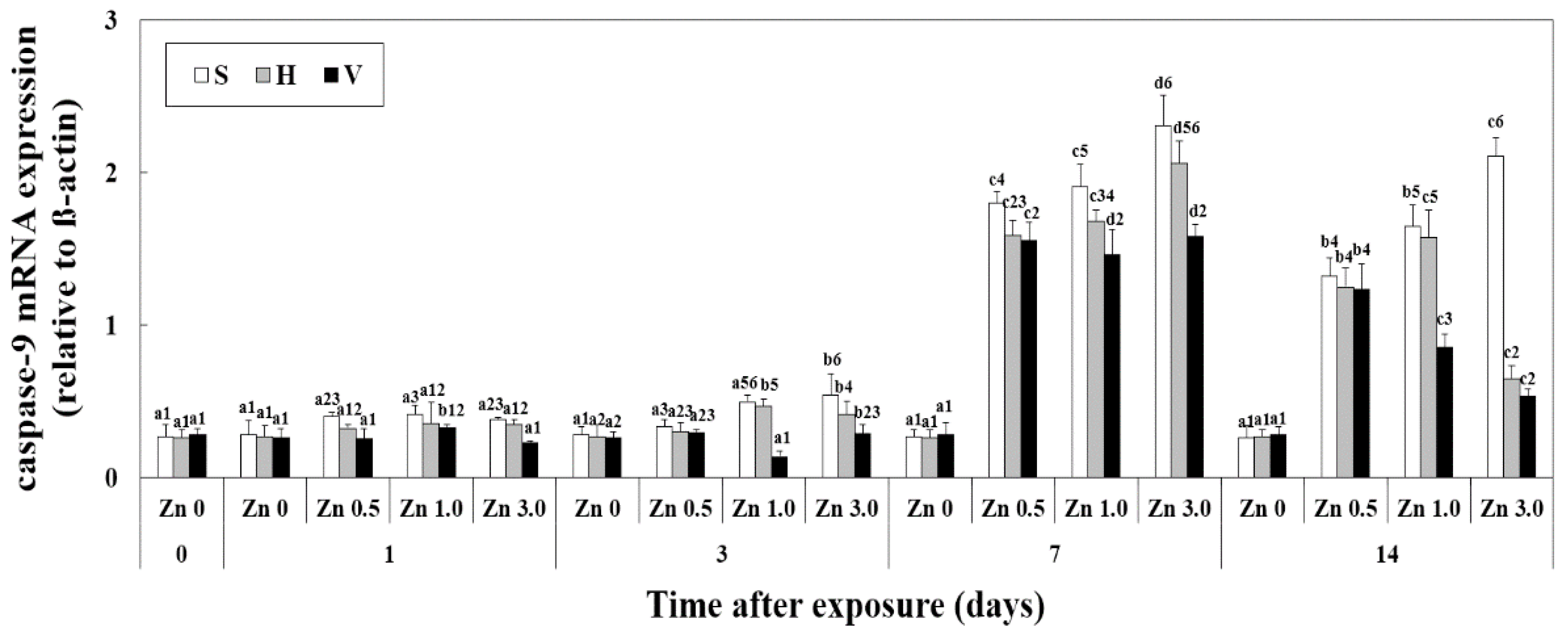
3.5. Changes of Caspase-9 mRNA Expression in Liver
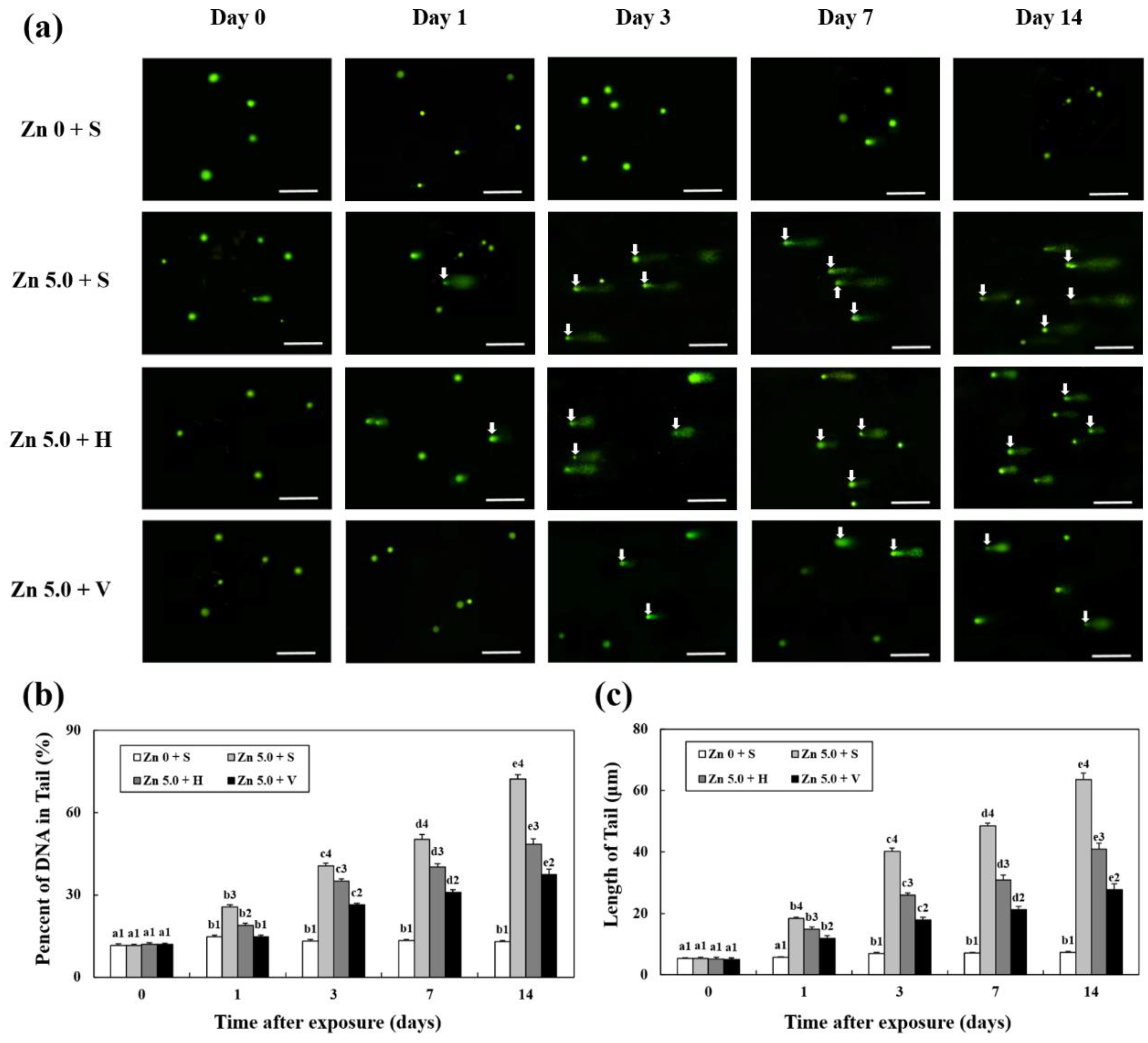
3.6. Analysis of DNA Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uysal, K.; Köse, E.; Bülbül, M.; Dönmez, M.; Erdoğan, Y.; Koyun, M.; Ömeroğlu, Ç.; Özmal, F. The comparison of heavy metal accumulation ratios of some fish species in Enne Dame Lake (Kütahya/Turkey). Environ. Monit. Assess. 2009, 157, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Sloman, K.A. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 2004, 68, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Li, X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Pandey, L.K.; Kumar, D.; Yadav, A.; Rai, J.; Gaur, J.P. Morphological abnormalities in periphytic diatoms as a tool for biomonitoring of heavy metal pollution in a river. Ecol. Indic. 2014, 36, 272–279. [Google Scholar] [CrossRef]
- Chung, S.Y.; Venkatramanan, S.; Park, N.; Ramkumar, T.; Sujitha, S.B.; Jonathan, M.P. Evaluation of physico-chemical parameters in water and total heavy metals in sediments at Nakdong River Basin, Korea. Environ. Earth Sci. 2016, 75, 50. [Google Scholar] [CrossRef]
- Gupta, A.; Rai, D.K.; Pandey, R.S.; Sharma, B. Analysis of some heavy metals in the riverine water, sediments and fish from river Ganges at Allahabad. Environ. Monit. Assess. 2009, 157, 449–458. [Google Scholar] [CrossRef]
- Pandey, L.K.; Park, J.; Son, D.H.; Kim, W.; Islam, M.S.; Choi, S.; Lee, H.; Han, T. Assessment of metal contamination in water and sediments from major rivers in South Korea from 2008 to 2015. Sci. Total Environ. 2019, 651, 323–333. [Google Scholar] [CrossRef]
- McRae, N.K.; Gaw, S.; Glover, C.N. Mechanisms of zinc toxicity in the galaxiid fish, Galaxias maculatus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 179, 184–190. [Google Scholar] [CrossRef]
- Bishop, G.M.; Dringen, R.; Robinson, S.R. Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes. Free Radic. Biol. Med. 2007, 42, 1222–1230. [Google Scholar] [CrossRef]
- Bergamini, C.M.; Gambetti, S.; Dondi, A.; Cervellati, C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharm. Des. 2004, 10, 1611–1626. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Husak, V.V.; Mosiichuk, N.M.; Kubrak, O.I.; Matviishyn, T.M.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Acute exposure to copper induces variable intensity of oxidative stress in goldfish tissues. Fish Physiol. 2018, 44, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Jie, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Sirisena, D.M.K.P.; Perera, N.C.N.; Godahewa, G.I.; Kwon, H.; Yang, H.; Nam, B.H.; Lee, J. A manganese superoxide dismutase (MnSOD) from red lip mullet, Liza haematocheila: Evaluation of molecular structure, immune response, and antioxidant function. Fish Shellfish Immunol. 2019, 84, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Jung, S.J.; Choi, Y.J.; Kim, N.N.; Choi, C.Y.; Kim, J.W. Effects of different light wavelengths from LEDs on oxidative stress and apoptosis in olive flounder (Paralichthys olivaceus) at high water temperatures. Fish Shellfish Immunol. 2016, 55, 460–468. [Google Scholar] [CrossRef]
- Pandey, S.; Parvez, S.; Sayeed, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of oxidative stress: A comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar] [CrossRef]
- Tudek, B.; Zdżalik-Bielecka, D.; Tudek, A.; Kosicki, K.; Fabisiewicz, A.; Speina, E. Lipid peroxidation in face of DNA damage, DNA repair and other cellular processes. Free Radic. Biol. Med. 2017, 107, 77–89. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Rengasamy, K.R.; Biswal, B.K. Plumbagin engenders apoptosis in lung cancer cells via caspase-9 activation and targeting mitochondrial-mediated ROS induction. Arch. Pharm. Res. 2020, 43, 242–256. [Google Scholar] [CrossRef]
- Eisler, R. Zinc Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; R. Eisler: Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1993; p. 7.
- Choi, C.Y.; Li, Z.; Song, J.A.; Park, Y.S. Water Hardness Can Reduce the Accumulation and Oxidative Stress of Zinc in Goldfish, Carassius auratus. Antioxidants 2022, 11, 715. [Google Scholar] [CrossRef]
- Qu, R.; Feng, M.; Wang, X.; Qin, L.; Wang, C.; Wang, Z.; Wang, L. Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquat. Toxicol. 2014, 150, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Saglam, D.; Atli, G.; Canli, M. Investigations on the osmoregulation of freshwater fish (Oreochromis niloticus) following exposures to metals (Cd, Cu) in differing hardness. Ecotoxicol. Environ. Saf. 2013, 92, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Saglam, D.; Atli, G.; Dogan, Z.; Baysoy, E.; Gurler, C.; Eroglu, A.; Canli, M. Response of the antioxidant system of freshwater fish (Oreochromis niloticus) exposed to metals (Cd, Cu) in differing hardness. Turk. J. Fish. Aquat. Sci. 2014, 14, 43–52. [Google Scholar] [CrossRef]
- Zikić, R.V.; Stajn, A.S.; Pavlović, S.Z.; Ognjanović, B.I.; Saićić, Z.S. Activities of superoxide dismutase and catalase in erythrocytes and plasma transaminases of goldfish (Carassius auratus gibelio Bloch.) exposed to cadmium. Physiol. Res. 2001, 50, 105–111. [Google Scholar]
- Atli, G.; Alptekin, Ö.; Tükel, S.; Canli, M. Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 218–224. [Google Scholar] [CrossRef]
- Orbea, A.; Fahimi, H.D.; Cajaraville, M.P. Immunolocalization of four antioxidant enzymes in digestive glands of mollusks and crustaceans and fish liver. Histochem. Cell Biol. 2000, 114, 393–404. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yang, S.G.; Jung, M.M.; Kim, B.S.; Yun, S.G.; Choi, C.Y. Effects of waterborne selenium on toxic and physiological stress response in goldfish, Carassius auratus. Mol. Cell. Toxicol. 2015, 11, 35–46. [Google Scholar] [CrossRef]
- Luzio, A.; Monteiro, S.M.; Fontaínhas-Fernandes, A.A.; Pinto-Carnide, O.; Matos, M.; Coimbra, A.M. Copper induced upregulation of apoptosis related genes in zebrafish (Danio rerio) gill. Aquat. Toxicol. 2013, 128, 183–189. [Google Scholar] [CrossRef]
- Jiao, W.; Han, Q.; Xu, Y.; Jiang, H.; Xing, H.; Teng, X. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish Shellfish Immunol. 2019, 86, 239–245. [Google Scholar] [CrossRef]
- Majtnerová, P.; Roušar, T. An overview of apoptosis assays detecting DNA fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Kousar, S.; Javed, M. Diagnosis of metals induced DNA damage in fish using comet assay. Pak. Vet. J. 2015, 35, 168–172. [Google Scholar]
- Deshpande, V.Y. Zinc Induced Histopathological Alternations in Gill, Liver, and Kidney of Freshwater Fish Gonoproktopterus kolus (Sykes). Adv. Zool. Bot. 2020, 8, 400–407. [Google Scholar] [CrossRef]



| Genes (Accession No.) | Primer | DNA Sequences |
|---|---|---|
| For qPCR | ||
| Caspase-9 (XM026241892) | Forward | 5′-CCA GGA CAT GAT CGA TGA AA-3′ |
| Reverse | 5′-AGT TTC ACG CAG ACA CTC CA-3′ | |
| β-actin (LC382464) | Forward | 5′-TTC CCT TGC TCC TTC CAC CA-3′ |
| Reverse | 5′-TGG AGC CAC CAA TCC AGA CA-3′ | |
| For in situ hybridization | ||
| Mn-SOD (KM065388) | Forward | 5′-AGC ACC ATG CGA CTT ATG TC-3′ |
| Reverse | 5′-CCC AGT TCA CAA CAT TCC AG-3′ |
| Zn 0 | Zn 1.0 | Zn 2.0 | Zn 5.0 | |
|---|---|---|---|---|
| Soft | 90.1 ± 1.6 | 91.6 ± 1.8 | 91.4 ± 1.3 | 91.2 ± 1.3 |
| Hard | 271.1 ± 1.6 | 268.8 ± 4.0 | 269.6 ± 4.4 | 273.2 ± 3.4 |
| Very hard | 448.8 ± 4.2 | 449.2 ± 4.2 | 445.4 ± 5.4 | 447.2 ± 9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, C.Y.; Kim, M.J.; Song, J.A.; Kho, K.H. Water Hardness Improves the Antioxidant Response of Zinc-Exposed Goldfish (Carassius auratus). Biology 2023, 12, 289. https://doi.org/10.3390/biology12020289
Choi CY, Kim MJ, Song JA, Kho KH. Water Hardness Improves the Antioxidant Response of Zinc-Exposed Goldfish (Carassius auratus). Biology. 2023; 12(2):289. https://doi.org/10.3390/biology12020289
Chicago/Turabian StyleChoi, Cheol Young, Min Ju Kim, Jin Ah Song, and Kang Hee Kho. 2023. "Water Hardness Improves the Antioxidant Response of Zinc-Exposed Goldfish (Carassius auratus)" Biology 12, no. 2: 289. https://doi.org/10.3390/biology12020289
APA StyleChoi, C. Y., Kim, M. J., Song, J. A., & Kho, K. H. (2023). Water Hardness Improves the Antioxidant Response of Zinc-Exposed Goldfish (Carassius auratus). Biology, 12(2), 289. https://doi.org/10.3390/biology12020289







