Polyherbal and Multimodal Treatments: Kaempferol- and Quercetin-Rich Herbs Alleviate Symptoms of Alzheimer’s Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Hallmarks of Alzheimer’s Disease
4. Anti-AD Mechanisms of Quercetin and Kaempferol
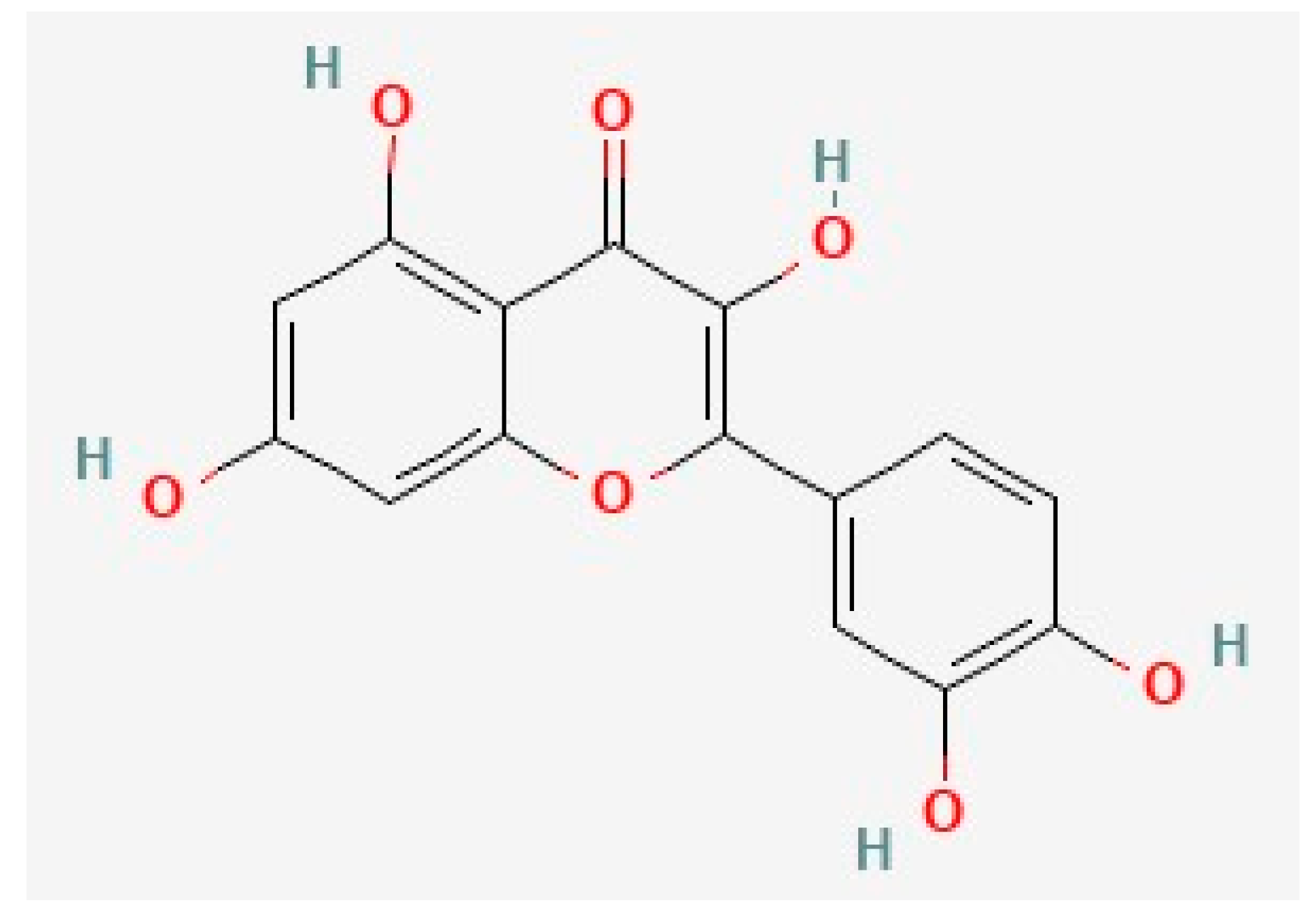
4.1. Quercetin
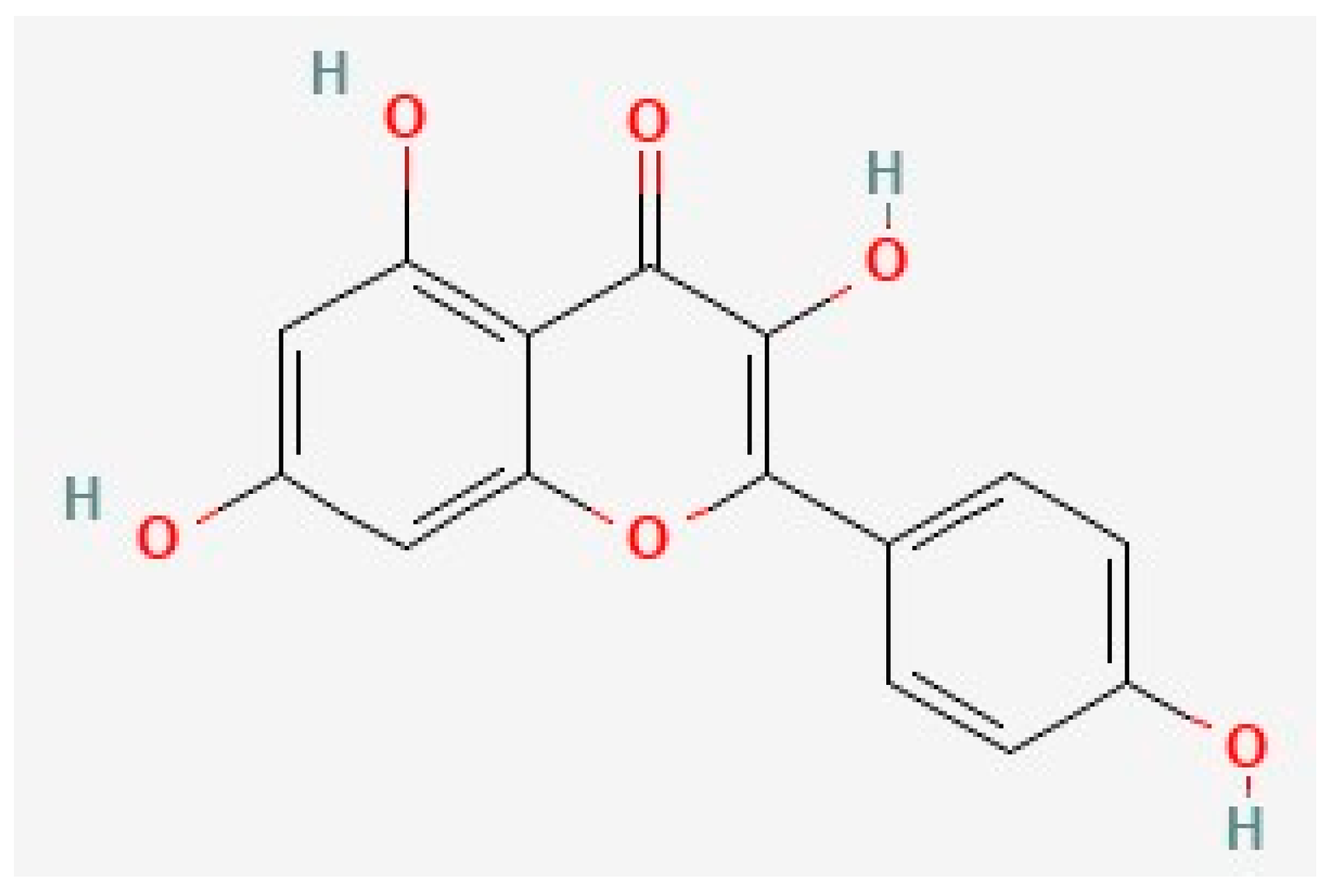
4.2. Kaempferol
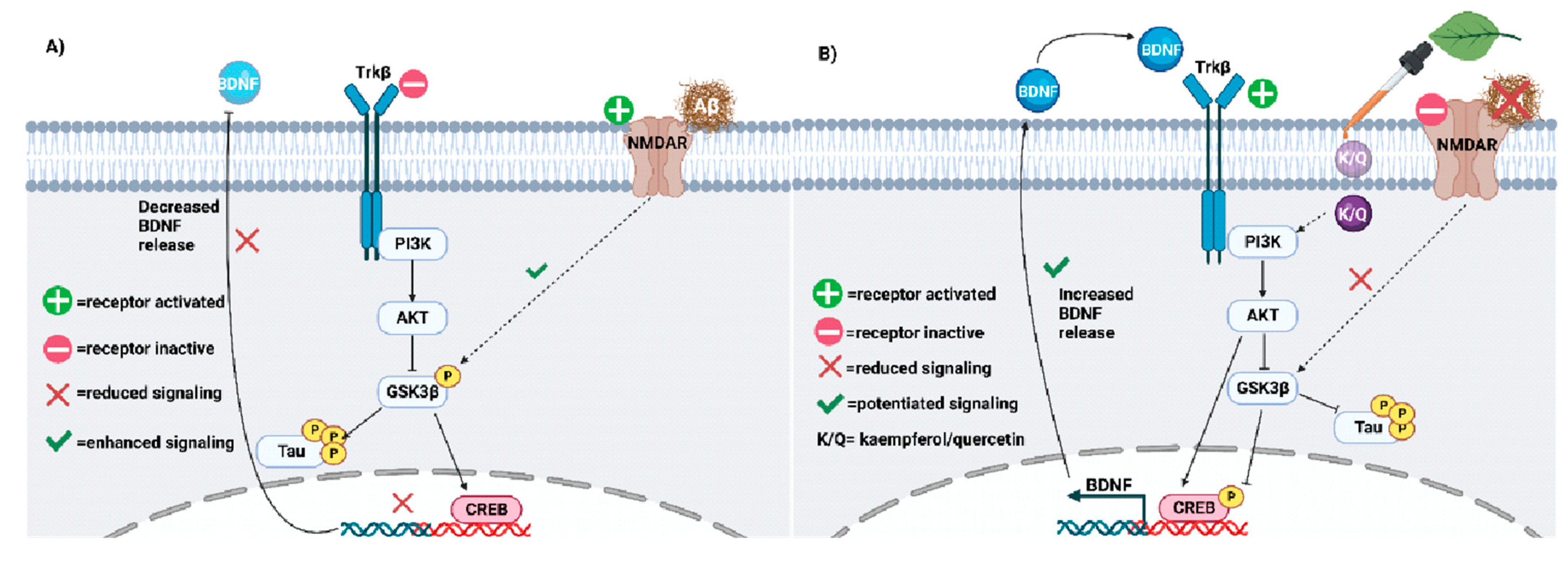
5. Kaempferol, Quercetin, and Neuroplasticity
5.1. Neuroplasticity Deficits in AD
5.2. Quercetin and Kaempferol Resolve AD-Related Plasticity Deficits
6. Quercetin and Kaempferol in Common Herbs
| Species Name | Kaempferol | Quercetin | Example Health Effects | Reference |
|---|---|---|---|---|
| Ginkgo biloba | + | + | Memory and cognition improvement | [285,296,307,308] |
| Camellia sinensis | + | + | Improved memory and antioxidant effects | [297,298,299] |
| Maesa membranacea | + | + | Neuroprotective | [175,188,225,278] |
| Schima wallichii Korth | + | − | Neuroprotective | [175,187,225,278] |
| Carthamus tinctorius | + | + | Neuroprotective | [175,187,225,278,309] |
| Panax ginseng | + | + | Neuroprotective | [175,187,225,278,310] |
| Morenga oleifera | + | + | Memory improvement | [300,301,302] |
| Cuscuta chinensis | + | + | Memory improving, Neuroprotective, Hepatoprotective, Immunomodulatory | [311] |
| Allium cepa | + | + | Anti-inflammatory | [312,313] |
| Hippophae rhamnoides L. | + | + | Anti-inflammatory | [294,295] |
| Litchi chinensis | + | + | Neuroprotective | [303,314] |
| Prakia roxburghii | - | + | Neuroprotective | [304] |
| Radix astragali | + | + | Neuroprotective | [213] |
| Fagopyrum tataricum (L.) | + | + | Decrease neurotoxicity | [251] |
| Carthami flos | + | + | Anti-ischemic | [213] |
| Punica granatum | + | + | Anti-inflammatory | [264,315] |
| Cyperi rhizoma | + | + | Antidepressant | [257] |
7. Limitations of Kaempferol and Quercetin Treatment
7.1. Bioavailability
7.2. Adverse Health Effects and Other Limitations
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Herbs Manuscript Abbreviations
| Aβ | amyloid beta |
| AChEIs | acetylcholinesterase inhibitors |
| AChE | acetylcholinesterase |
| ACh | acetylcholine |
| AD | Alzheimer’s disease |
| AKT | protein Kinase B |
| AMPK | AMP-activated protein kinase |
| APP | amyloid precursor protein |
| BACE1 | beta-site APP cleaving enzyme 1 |
| Bax | bcl-2-like protein 4 |
| BBB | blood-brain barrier |
| BDNF | brain-derived neurotrophic factor |
| Cdk5 | cyclin-dependent kinase 5 |
| p-Cdk5 | phosphorylated forms of Cdk5 |
| CNS | central nervous system |
| COX-1 | cyclooxygenase-1 |
| COX-2 | cyclooxygenase-2 |
| CREB | cAMP response element-binding protein |
| CSS | Chaihu shugan san |
| EGCG | epigallocatechin-3-gallate |
| EPM | elevated plus-maze test |
| ERK1/2 | extracellular receptor signal-regulated kinase 1&2 |
| GLUT4 | glucose transporter type 4 |
| GSH | glutathione |
| GSK3β | glycogen synthase kinase-3 beta |
| GPx | glutathione peroxidase |
| HT22 | immortalized mouse hippocampal cell line |
| HO-1 | heme oxygenase-1 |
| HQSJDZ | Huangqi Sijunzi |
| H2O2 | hydrogen peroxide |
| IDE | insulin-degrading enzyme |
| IFN-γ | interferon gamma |
| IL-1β | interleukin-1β |
| IL-2 | interleukin-2 |
| iNOS | inducible nitric oxide synthase |
| ICR | strain of Swiss mice produced at the Institute of Cancer Research |
| IR | insulin resistance |
| I/R | cerebral ischemia/reperfusion |
| IRS1 | insulin response substrate-1 |
| JNK | c-Jun N-terminal kinase |
| KAG | kaempferol 3-O-(6″-acetyl)-β-glucopyranoside |
| KAT | lysine acetylase |
| KDAC | lysine deacetylase |
| K-3-Rh | kaempferol-3-O-rhamnoside |
| K/Q | kaempferol and quercetin co-treatment |
| LPS | lipopolysaccharide |
| PGE2 | prostaglandin E2 |
| PI3K | phosphoinositide 3-kinases |
| PI3K/AKT/GSK-3β | phosphoinositide 3-kinase/protein kinase B/glycogen synthase kinase-3 beta signaling pathway |
| PKC | protein kinase C |
| PP2A | protein phosphatase 2 |
| PSD-95 | postsynaptic density protein 95 |
| MAP | microtubule-associated protein |
| MAPK | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| MLK2 | mixed lineage kinase 2 |
| NF-kB | nuclear factor kappa B |
| NFTs | neurofibrillary tangles |
| NMDARs | N-methyl-d-aspartate receptors |
| NO | nitric oxide |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NR2B | N-methyl d-aspartate receptor subtype 2B |
| PET | positron emission tomography |
| PON2 | paroxonase 2 |
| ROS | reactive oxygen species |
| SIRT1 | Sirtuin 1 |
| Ser | serine |
| Ser9 | serine 9 |
| SOD | superoxide dismutase |
| STZ | streptozotocin |
| TCM | traditional Chinese medicine |
| Thr | threonine |
| TLRs | toll-like receptors |
| TLR2 | toll-like receptor 2 |
| TLR4 | toll-like receptor 4 |
| TLR9 | toll-like receptor 9 |
| TNF-α | tumor necrosis factor-α |
| Trkβ | tropomycin-related kinase β |
| 3 × Tg AD mice | triple transgenic Alzheimer’s disease mice |
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef] [PubMed]
- Riche, K.; Lenard, N.R. Quercetin’s Effects on Glutamate Cytotoxicity. Molecules 2022, 27, 7620. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J. Amyloid-β: A double agent in Alzheimer’s disease? Biomed. Pharmacother. 2021, 139, 111575. [Google Scholar] [CrossRef] [PubMed]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Minter, M.R.; Taylor, J.M.; Crack, P.J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem. 2016, 136, 457–474. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimer's Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef]
- Bhaskar, K.; Miller, M.; Chludzinski, A.; Herrup, K.; Zagorski, M.; Lamb, B.T. The PI3K-Akt-mTOR pathway regulates Abeta oligomer induced neuronal cell cycle events. Mol. Neurodegener. 2009, 4, 14. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel'nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T. Oxidative Stress in Alzheimer’s Disease: Molecular Hallmarks of Underlying Vulnerability. In Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease; Ashraf, G., Alexiou, A., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.T.; Witting, P.K. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci. Rep. 2018, 8, 11553. [Google Scholar] [CrossRef]
- Gao, W.; Wang, W.; Peng, Y.; Deng, Z. Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/β-catenin cascade. Metab. Brain Dis. 2019, 34, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Alquezar, C.; Arya, S.; Kao, A.W. Tau Post-translational Modifications: Dynamic Transformers of Tau Function, Degradation, and Aggregation. Front. Neurol. 2021, 11, 595532. [Google Scholar] [CrossRef] [PubMed]
- Merino-Serrais, P.; Benavides-Piccione, R.; Blazquez-Llorca, L.; Kastanauskaite, A.; Rábano, A.; Avila, J.; DeFelipe, J. The influence of phospho-tau on dendritic spines of cortical pyramidal neurons in patients with Alzheimer’s disease. Brain 2013, 136, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Spittaels, K.; Haute, C.V.D.; Van Dorpe, J.; Geerts, H.; Mercken, M.; Bruynseels, K.; Lasrado, R.; Vandezande, K.; Laenen, I.; Boon, T.; et al. Glycogen synthase kinase-3β phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J. Biol. Chem. 2000, 275, 41340–41349. [Google Scholar] [CrossRef]
- Tatebayashi, Y.; Haque, N.; Tung, Y.-C.; Iqbal, K.; Grundke-Iqbal, I. Role of tau phosphorylation by glycogen synthase kinase-3β in the regulation of organelle transport. J. Cell Sci. 2004, 117, 1653–1663. [Google Scholar] [CrossRef]
- Jaworski, T.; Kügler, S.; van Leuven, F. Modeling of tau-mediated synaptic and neuronal degeneration in Alzheimer’s disease. Int. J. Alzheimer's Dis. 2010, 2010, 573138. [Google Scholar] [CrossRef]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.-L.; et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef]
- Thies, E.; Mandelkow, E.-M. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J. Neurosci. 2007, 27, 2896–2907. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2013, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Joe, E.; Ringman, J.M. Cognitive symptoms of Alzheimer’s disease: Clinical management and prevention. BMJ 2019, 367, l6217. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.A.; Antimisiaris, D.; O’Brien, J. Drugs for Alzheimer’s disease: Are they effective? Pharm. Ther. 2010, 35, 208–211. [Google Scholar]
- Francis, P.T. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectrums 2005, 10 (Suppl. S18), 6–9. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimer's Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Kouhestani, S.; Jafari, A.; Babaei, P. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regen. Res. 2018, 13, 1827–1832. [Google Scholar] [CrossRef]
- Farlow, M.R.; Miller, M.L.; Pejovic, V. Treatment options in Alzheimer’s disease: Maximizing benefit, managing expectations. Dement. Geriatr. Cogn. Disord. 2008, 25, 408–422. [Google Scholar] [CrossRef]
- Tian, J.; Shi, J.; Zhang, X.; Wang, Y. Herbal therapy: A new pathway for the treatment of Alzheimer’s disease. Alzheimer's Res. Ther. 2010, 2, 30. [Google Scholar] [CrossRef]
- Lee, J.; Jin, C.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Kwon, S. Herbal medicine treatment for Alzheimer disease: A protocol for a systematic review and meta-analysis. Medicine 2020, 99, e21745. [Google Scholar] [CrossRef]
- Chen, M.M.; Yin, Z.Q.; Zhang, L.Y.; Liao, H. Quercetin promotes neurite growth through enhancing intracellular cAMP level and GAP-43 expression. Chin. J. Nat. Med. 2015, 13, 667–672. [Google Scholar] [CrossRef]
- Zhang, X.W.; Chen, J.Y.; Ouyang, D.; Lu, J.H. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 493. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Sreenivasmurthy, S.G.; Liu, J.Y.; Song, J.X.; Yang, C.B.; Malampati, S.; Wang, Z.Y.; Huang, Y.Y.; Li, M. Neurogenic traditional Chinese medicine as a promising strategy for the treatment of Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 272. [Google Scholar] [CrossRef]
- Khan, A.; Ali, T.; Rehman, S.U.; Khan, M.S.; Alam, S.I.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M.O. Neuroprotective Effect of Quercetin Against the Detrimental Effects of LPS in the Adult Mouse Brain. Front. Pharmacol. 2018, 9, 1383. [Google Scholar] [CrossRef]
- Testa, G.; Gamba, P.; Badilli, U.; Gargiulo, S.; Maina, M.; Guina, T.; Calfapietra, S.; Biasi, F.; Cavalli, R.; Poli, G.; et al. Loading into nanoparticles improves quercetin’s efficacy in preventing neuroinflammation induced by oxysterols. PLoS ONE 2014, 9, e96795. [Google Scholar] [CrossRef]
- Ma, Z.X.; Zhang, R.Y.; Rui, W.J.; Wang, Z.Q.; Feng, X. Quercetin alleviates chronic unpredictable mild stress-induced depressive-like behaviors by promoting adult hippocampal neurogenesis via FoxG1/CREB/ BDNF signaling pathway. Behav. Brain Res. 2021, 406, 113245. [Google Scholar] [CrossRef]
- Das, D.; Biswal, S.; Barhwal, K.K.; Chaurasia, O.P.; Hota, S.K. Kaempferol Inhibits Extra-synaptic NMDAR-Mediated Downregulation of TRkβ in Rat Hippocampus During Hypoxia. Neuroscience 2018, 392, 77–91. [Google Scholar] [CrossRef]
- Hussein, R.M.; Mohamed, W.R.; Omar, H.A. A neuroprotective role of kaempferol against chlorpyrifos-induced oxidative stress and memory deficits in rats via GSK3β-Nrf2 signaling pathway. Pestic. Biochem. Physiol. 2018, 152, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, C.; Wang, L.F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.R. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-κB and STAT3 in transient focal stroke. PLoS ONE 2013, 8, e55839. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Jakaria, M.; Kim, I.S.; Kim, J.; Haque, M.E.; Choi, D.K. Regulation of Toll-Like Receptor (TLR) Signaling Pathway by Polyphenols in the Treatment of Age-Linked Neurodegenerative Diseases: Focus on TLR4 Signaling. Front. Immunol. 2019, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Aboukhatwa, M.A.; Lei, D.L.; Manaye, K.; Khan, I.; Luo, Y. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology 2010, 58, 911–920. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.Y.; Cho, E.J. Protective effects of kaempferol, quercetin, and its glycosides on amyloid beta-induced neurotoxicity in C6 glial cell. J. Appl. Biol. Chem. 2019, 62, 327–332. [Google Scholar] [CrossRef]
- Zeng, K.; Li, M.; Hu, J.; Mahaman, Y.A.R.; Bao, J.; Huang, F.; Xia, Y.; Liu, X.; Wang, Q.; Wang, J.Z.; et al. Ginkgo biloba Extract EGb761 Attenuates Hyperhomocysteinemia-induced AD Like Tau Hyperphosphorylation and Cognitive Impairment in Rats. Curr. Alzheimer Res. 2018, 15, 89–99. [Google Scholar] [CrossRef]
- Ly, P.T.; Wu, Y.; Zou, H.; Wang, R.; Zhou, W.; Kinoshita, A.; Zhang, M.; Yang, Y.; Cai, F.; Woodgett, J.; et al. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Investig. 2013, 123, 224–235. [Google Scholar] [CrossRef]
- Latta, C.H.; Brothers, H.M.; Wilcock, D.M. Neuroinflammation in Alzheimer’s disease; A source of heterogeneity and target for personalized therapy. Neuroscience 2015, 302, 103–111. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Madathil, S.K.; Pandey, M.; Haobam, R.; Rajamma, U.; Mohanakumar, K.P. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 2013, 236, 136–148. [Google Scholar] [CrossRef]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; Walker, R.; McGeer, E.G. Patterns of gliosis in Alzheimer’s disease and aging cerebrum. Glia 1989, 2, 420–436. [Google Scholar] [CrossRef]
- Delacourte, A. General and dramatic glial reaction in Alzheimer brains. Neurology 1990, 40, 33. [Google Scholar] [CrossRef] [PubMed]
- Arends, Y.M.; Duyckaerts, C.; Rozemuller, J.M.; Eikelenboom, P.; Hauw, J.J. Microglia, amyloid and dementia in alzheimer disease. A correlative study. Neurobiol. Aging 2000, 21, 39–47. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, S.J.; Cho, H.Y.; Hwang, H.J.; Kim, Y.J.; Lim, S.T.; Kim, C.J.; Kim, H.K.; Peterson, S.; Shin, D.H. Protective effects of kaempferol (3,4’,5,7-tetrahydroxyflavone) against amyloid beta peptide (Abeta)-induced neurotoxicity in ICR mice. Biosci. Biotechnol. Biochem. 2010, 74, 397–401. [Google Scholar] [CrossRef]
- Shen, X.Y.; Luo, T.; Li, S.; Ting, O.Y.; He, F.; Xu, J.; Wang, H.Q. Quercetin inhibits okadaic acid-induced tau protein hyperphosphorylation through the Ca2+-calpain-p25-CDK5 pathway in HT22 cells. Int. J. Mol. Med. 2018, 41, 1138–1146. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Sperling, R.A.; Mormino, E.C.; Schultz, A.P.; Betensky, R.A.; Papp, K.V.; Amariglio, R.E.; Hanseeuw, B.J.; Buckley, R.; Chhatwal, J.; Hedden, T.; et al. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann. Neurol. 2019, 85, 181–193. [Google Scholar] [CrossRef]
- Pievani, M.; de Haan, W.; Wu, T.; Seeley, W.W.; Frisoni, G.B. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011, 10, 829–843. [Google Scholar] [CrossRef]
- Sakakibara, R.; Kawai, T. Cerebrospinal fluid oxidative stress markers in Alzheimer’s disease. Neurol. Clin. Neurosci. 2020, 8, 232–240. [Google Scholar] [CrossRef]
- Willette, A.A.; Li, T.; Willette, S.A.; Larsen, B.A.; Pollpeter, A.; Klinedinst, B.S.; Moody, S.; Barnett, N.; Parvin, M.; Pappas, C.; et al. Oxidative stress biomarkers and longitudinal changes in human brain imaging across the Alzheimer’s disease continuum. Alzheimer’s Dement. 2022, 18, e068364. [Google Scholar] [CrossRef]
- Kim, H.G.; Ju, M.S.; Shim, J.S.; Kim, M.C.; Lee, S.H.; Huh, Y.; Kim, S.Y.; Oh, M.S. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson’s disease models. Br. J. Nutr. 2010, 104, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Yang, E.J.; Kim, G.S.; Kim, J.A. Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. Pharmacogn. Mag. 2013, 9, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, S.M.; Connolly, C.N. Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology 2007, 53, 891–898. [Google Scholar] [CrossRef]
- Mattson, M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000, 1, 120–130. [Google Scholar] [CrossRef]
- Simpson, D.S.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Di Filippo, M.; Sarchielli, P.; Picconi, B.; Calabresi, P. Neuroinflammation and synaptic plasticity: Theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol. Sci. 2008, 29, 402–412. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S.; et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Paris, D.; Mathura, V.; Ait-Ghezala, G.; Beaulieu-Abdelahad, D.; Patel, N.; Bachmeier, C.; Mullan, M. Flavonoids lower Alzheimer’s Aβ production via an NFκB dependent mechanism. Bioinformation 2011, 6, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. NeuroMolecular Med. 2011, 13, 223–250. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, P.; Seo, S.W.; Roh, J.H.; Oh, M.; Oh, J.S.; Oh, S.J.; Kim, J.S.; Jeong, Y. Comparison of Amyloid β and Tau Spread Models in Alzheimer’s Disease. Cereb. Cortex 2019, 29, 4291–4302. [Google Scholar] [CrossRef]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: A longitudinal PET study. J. Neuroinflammation 2020, 17, 151. [Google Scholar] [CrossRef]
- Wang, D.M.; Li, S.Q.; Wu, W.L.; Zhu, X.Y.; Wang, Y.; Yuan, H.Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem. Res. 2014, 39, 1533–1543. [Google Scholar] [CrossRef]
- Cha, M.Y.; Han, S.H.; Son, S.M.; Hong, H.S.; Choi, Y.J.; Byun, J.; Mook Jung, I. Mitochondria-specific accumulation of amyloid beta induces mitochondrial dysfunction leading to apoptotic cell death. PLoS ONE 2012, 7, e34929. [Google Scholar] [CrossRef]
- Moreira, P.I.; Santos, M.S.; Moreno, A.; Rego, A.C.; Oliveira, C. Effect of amyloid beta-peptide on permeability transition pore: A comparative study. J. Neurosci. Res. 2002, 69, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Mitochondria take centre stage in aging and neurodegeneration. Ann. Neurol. 2005, 58, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rusinek, H.; Butler, T.; Glodzik, L.; Pirraglia, E.; Babich, J.; Mozley, P.D.; Nehmeh, S.; Pahlajani, S.; Wang, X.; et al. Decreased CSF clearance and increased brain amyloid in Alzheimer’s disease. Fluids Barriers CNS 2022, 19, 21. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Suzuki, H.; Yoshiyama, T.; Lobello, K.; Peng, Y.; Liu, E.; Ketter, N.; Margolin, R.; Jackson, N.; Fujimoto, Y. Safety, tolerability and immunogenicity of an immunotherapeutic vaccine (vanutide cridificar [ACC-001]) and the QS-21 adjuvant in Japanese individuals with mild-to-moderate Alzheimer’s disease: A phase IIa, multicenter, randomized, adjuvant and placebo clinical trial. Alzheimer’s Dement. 2013, 9, 282. [Google Scholar]
- Doody, R.S.; Raman, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; He, F.; Sun, X.; Thomas, R.G.; et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013, 369, 341–350. [Google Scholar] [CrossRef]
- Doody, R.S.; Farlow, M.; Aisen, P.S. Alzheimer’s Disease Cooperative Study Data Analysis and Publication Committee. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 1460. [Google Scholar]
- Li, S.; Selkoe, D.J. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. J. Neurochem. 2020, 154, 583–597. [Google Scholar] [CrossRef]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Stancu, I.C.; Vasconcelos, B.; Terwel, D.; Dewachter, I. Models of β-amyloid induced Tau-pathology: The long and “folded” road to understand the mechanism. Mol. Neurodegener. 2014, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Takashima, A.; Honda, T.; Yasutake, K.; Michel, G.; Murayama, O.; Murayama, M.; Ishiguro, K.; Yamaguchi, H. Activation of tau protein kinase I/glycogen synthase kinase-3beta by amyloid beta peptide (25–35) enhances phosphorylation of tau in hippocampal neurons. Neurosci. Res. 1998, 31, 317–323. [Google Scholar] [CrossRef]
- Ferreira, A.; Lu, Q.; Orecchio, L.; Kosik, K.S. Selective phosphorylation of adult tau isoforms in mature hippocampal neurons exposed to fibrillar A beta. Mol. Cell. Neurosci. 1997, 9, 220–234. [Google Scholar] [CrossRef]
- Zheng, W.H.; Bastianetto, S.; Mennicken, F.; Ma, W.; Kar, S. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 2002, 115, 201–211. [Google Scholar] [CrossRef]
- Ma, Q.L.; Lim, G.P.; Harris-White, M.E.; Yang, F.; Ambegaokar, S.S.; Ubeda, O.J.; Glabe, C.G.; Teter, B.; Frautschy, S.A.; Cole, G.M. Antibodies against beta-amyloid reduce Abeta oligomers, glycogen synthase kinase-3beta activation and tau phosphorylation in vivo and in vitro. J. Neurosci. Res. 2006, 83, 374–384. [Google Scholar] [CrossRef]
- Tackenberg, C.; Grinschgl, S.; Trutzel, A.; Santuccione, A.C.; Frey, M.C.; Konietzko, U.; Grimm, J.; Brandt, R.; Nitsch, R.M. NMDA receptor subunit composition determines beta-amyloid-induced neurodegeneration and synaptic loss. Cell Death Dis. 2013, 4, e608. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.W.; Iturria-Medina, Y.; Strandberg, O.T.; Smith, R.; Levitis, E.; Evans, A.C.; Hansson, O.; Alzheimer’s Disease Neuroimaging Initiative; Swedish BioFinder Study. Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat. Commun. 2020, 11, 2612, Erratum in Nat. Commun. 2021, 12, 4862. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E.; Gold, G. Alzheimer disease therapy—Moving from amyloid-β to tau. Nat. Rev. Neurol. 2013, 9, 677–686. [Google Scholar] [CrossRef]
- Amaral, A.C.; Perez-Nievas, B.G.; Chong, M.S.T.; Gonzalez-Martinez, A.; Argente-Escrig, H.; Rubio-Guerra, S.; Commins, C.; Muftu, S.; Eftekharzadeh, B.; Hudry, E.; et al. Isoform-selective decrease of glycogen synthase kinase-3-beta (GSK-3β) reduces synaptic tau phosphorylation, transcellular spreading, and aggregation. Iscience 2021, 24, 102058. [Google Scholar] [CrossRef]
- Mandelkow, E.-M.; Mandelkow, E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2012, 2, a006247. [Google Scholar] [CrossRef] [PubMed]
- Roberson, E.D.; Scearce-Levie, K.; Palop, J.J.; Yan, F.; Cheng, I.H.; Wu, T.; Gerstein, H.; Yu, G.Q.; Mucke, L. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 2007, 316, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R. Mitochondria and calcium: From cell signaling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Squìer, M.K.; Miller, A.C.; Malkinson, A.M.; Cohen, J.J. Calpain activation in apoptosis. J. Cell. Physiol. 1994, 159, 229–237. [Google Scholar] [CrossRef]
- Maher, P.; Schubert, D. Signaling by reactive oxygen species in the nervous system. Cell. Mol. Life Sci. 2000, 57, 1287–1305. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Uversky, V.N. Intrinsic disorder and posttranslational modifications: The darker side of the biological dark matter. Front. Genet. 2018, 9, 158. [Google Scholar] [CrossRef]
- Barber, K.W.; Rinehart, J. The ABCs of PTMs. Nat. Chem. Biol. 2018, 14, 188–192. [Google Scholar] [CrossRef]
- Buee, L.; Bussiere, T.; Buee-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef]
- Xia, C.; Makaretz, S.J.; Caso, C.; McGinnis, S.; Gomperts, S.N.; Sepulcre, J.; Gomez-Isla, T.; Hyman, B.T.; Schultz, A.; Vasdev, N.; et al. Association of in vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol. 2017, 74, 427–436. [Google Scholar] [CrossRef]
- Bejanin, A.; Schonhaut, D.R.; La Joie, R.; Kramer, J.H.; Baker, S.L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimeras disease. Brain 2017, 140, 3286–3300. [Google Scholar] [CrossRef]
- Fleeman, R.M.; Proctor, E.A. Astrocytic Propagation of Tau in the Context of Alzheimer’s Disease. FFront. Cell. Neurosci. 2021, 15, 645233. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, S.; Bennett, R.E.; Delorme, L.; Robbins, A.B.; Hu, M.; MacKenzie, D.; Kirk, M.J.; Schiantarelli, J.; Tunio, N.; Amaral, A.C.; et al. Experimental evidence for the age dependence of tau protein spread in the brain. Sci. Adv. 2019, 5, eaaw6404. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, M.; Wang, D. The propagation mechanisms of extracellular tau in Alzheimer’s disease. J. Neurol. 2022, 269, 1164–1181. [Google Scholar] [CrossRef]
- Kazim, S.F.; Sharma, A.; Saroja, S.R.; Seo, J.H.; Larson, C.S.; Ramakrishnan, A.; Wang, M.; Blitzer, R.D.; Shen, L.; Peña, C.J.; et al. Chronic Intermittent Hypoxia Enhances Pathological Tau Seeding, Propagation, and Accumulation and Exacerbates Alzheimer-like Memory and Synaptic Plasticity Deficits and Molecular Signatures. Biol. Psychiatry 2022, 91, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. Int. Rev. J. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Feskens, E.J.M.; Hollman, P.C.H.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Anhê, G.F.; Okamoto, M.M.; Kinote, A.; Sollon, C.; Lellis-Santos, C.; Anhê, F.F.; Lima, G.A.; Hirabara, S.M.; Velloso, L.A.; Bordin, S.; et al. Quercetin decreases inflammatory response and increases insulin action in skeletal muscle of ob/ob mice and in L6 myotubes. Eur. J. Pharmacol. 2012, 689, 285–293. [Google Scholar] [CrossRef]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef]
- Hanaki, M.; Murakami, K.; Akagi, K.; Irie, K. Structural insights into mechanisms for inhibiting amyloid β42 aggregation by non-catechol-type flavonoids. Bioorganic Med. Chem. 2016, 24, 304–313. [Google Scholar] [CrossRef]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37. [Google Scholar] [CrossRef]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: Implications for the prevention and therapeutics of Alzheimer’s disease. J. Neurochem. 2003, 87, 172–181. [Google Scholar] [CrossRef]
- Jiménez-Aliaga, K.; Bermejo-Bescós, P.; Benedí, J.; Martín-Aragón, S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011, 89, 939–945. [Google Scholar] [CrossRef]
- Sato, M.; Murakami, K.; Uno, M.; Nakagawa, Y.; Katayama, S.; Akagi, K.; Masuda, Y.; Takegoshi, K.; Irie, K. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 2013, 288, 23212–23224. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Mu, X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. BioMed Res. Int. 2020, 2020, 8210578. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Krishnakumar, V.G.; Morya, V.; Gupta, S.; Datta, B. Nanobiocatalyst facilitated aglycosidic quercetin as a potent inhibitor of tau protein aggregation. Int. J. Biol. Macromol. 2019, 138, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Yang, H.; Tang, C.; Yao, G.; Kong, L.; He, H.; Zhou, Y. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int. Immunopharmacol. 2015, 28, 744–750. [Google Scholar] [CrossRef]
- Peng, J.; Li, Q.; Li, K.; Zhu, L.; Lin, X.; Lin, X.; Shen, Q.; Li, G.; Xie, X. Quercetin Improves Glucose and Lipid Metabolism of Diabetic Rats: Involvement of Akt Signaling and SIRT1. J. Diabetes Res. 2017, 2017, 3417306. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Xu, M.; Huang, H.; Mo, X.; Zhu, Y.; Chen, X.; Li, X.; Peng, X.; Xu, Z.; Chen, L.; Rong, S.; et al. Quercetin-3-O-Glucuronide Alleviates Cognitive Deficit and Toxicity in Aβ1-42 -Induced AD-Like Mice and SH-SY5Y Cells. Mol. Nutr. Food Res. 2021, 65, e2000660. [Google Scholar] [CrossRef]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef]
- Babaei, F.; Mirzababaei, M.; Nassiri-Asl, M. Quercetin in Food: Possible Mechanisms of Its Effect on Memory. J. Food Sci. 2018, 83, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Nishihira, J.; Nishimura, M.; Kurimoto, M.; Kagami-Katsuyama, H.; Hattori, H.; Nakagawa, T.; Muro, T.; Kobori, M. The effect of 24-week continuous intake of quercetin-rich onion on age-related cognitive decline in healthy elderly people: A randomized, double-blind, placebo-controlled, parallel-group comparative clinical trial. J. Clin. Biochem. Nutr. 2021, 69, 203–215. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Lim, B.O. Quercetin Is An Active Agent in Berries against Neurodegenerative Diseases Progression through Modulation of Nrf2/HO1. Nutrients 2022, 14, 5132. [Google Scholar] [CrossRef]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Neuropharmacology 2022, 12, 665031. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Naeem, A.; Zou, J.; Yu, C.; Wang, Y.; Chen, J.; Ping, Y. Isolation of Phenolic Compounds from Raspberry Based on Molecular Imprinting Techniques and Investigation of Their Anti-Alzheimer’s Disease Properties. Molecules 2022, 27, 6893. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Xiao, L.; Luo, G.; Tang, Y.; Yao, P. Quercetin and iron metabolism: What we know and what we need to know. Food Chem. Toxicol. 2018, 114, 190–203. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1. [Google Scholar]
- Nakagawa, T.; Ohta, K. Quercetin Regulates the Integrated Stress Response to Improve Memory. Int. J. Mol. Sci. 2019, 20, 2761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Liu, X.L.; Kong, L.; Zhang, M.Y.; Chen, Y.J.; Zhu, X.; Hao, Y.C. Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation. Biomed. Pharmacother. 2019, 109, 2145–2154. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef]
- Wei, C.; Li, S.; Zhu, Y.; Chen, W.; Li, C.; Xu, R. Network pharmacology identify intersection genes of quercetin and Alzheimer’s disease as potential therapeutic targets. Front. Aging Neurosci. 2022, 14, 902092. [Google Scholar] [CrossRef] [PubMed]
- García-Mediavilla, M.V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Jeong, W.S.; Jun, M. Inhibition of β-amyloid peptide-induced neurotoxicity by kaempferol 3-O-(6″-acetyl)-β-glucopyranoside from butterbur (Petasites japonicus) leaves in B103 cells. Food Sci. Biotechnol. 2012, 21, 845–851. [Google Scholar] [CrossRef]
- Kaypee, S.; Singh, S.; Swarnkar, S.; Kundu, T.K. Emerging epigenetic therapies—Lysine acetyltransferase inhibitors. In Epigenetic Cancer Therapy; Academic Press: Cambridge, MA, USA, 2023; pp. 459–505. [Google Scholar]
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS ONE 2011, 6, e22934. [Google Scholar] [CrossRef]
- Pei, Y.; Parks, J.S.; Kang, H.W. Quercetin alleviates high-fat diet-induced inflammation in brown adipose tissue. J. Funct. Foods 2021, 85, 104614. [Google Scholar] [CrossRef]
- Son, S.M.; Park, S.J.; Fernandez-Estevez, M.; Rubinsztein, D.C. Autophagy regulation by acetylation-implications for neurodegenerative diseases. Exp. Mol. Med. 2021, 53, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, F.; Mai, A.; Rotili, D. Lysine Acetyltransferase Inhibitors From Natural Sources. Front. Pharmacol. 2020, 11, 1243. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Mai, X.; Wu, X.; Hu, X.; Luo, X.; Zhang, G. Exploring the Inhibition of Quercetin on Acetylcholinesterase by Multispectroscopic and In Silico Approaches and Evaluation of Its Neuroprotective Effects on PC12 Cells. Molecules 2022, 27, 7971. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.; Birch, D.J.; Vyshemirsky, V.; Rolinski, O.J. Impact of the Flavonoid Quercetin on β-Amyloid Aggregation Revealed by Intrinsic Fluorescence. J. Phys. Chem. B 2022, 126, 7229–7237. [Google Scholar] [CrossRef]
- Ho, C.L.; Kao, N.J.; Lin, C.I.; Cross, T.L.; Lin, S.H. Quercetin Increases Mitochondrial Biogenesis and Reduces Free Radicals in Neuronal SH-SY5Y Cells. Nutrients 2022, 14, 3310. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Wang, J.; Pang, X.; Liu, H. Protective Effect of Quercetin against Oxidative Stress-Induced Cytotoxicity in Rat Pheochromocytoma (PC-12) Cells. Molecules 2017, 22, 1122. [Google Scholar] [CrossRef]
- Jiang, W.; Luo, T.; Li, S.; Zhou, Y.; Shen, X.-Y.; He, F.; Xu, J.; Wang, H.Q. Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons. PLoS ONE 2016, 11, e0152371. [Google Scholar] [CrossRef]
- Paula, P.C.; Maria, S.G.; Luis, C.H.; Patricia, C.G. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules 2019, 24, 2287. [Google Scholar] [CrossRef]
- Molaei, A.; Hatami, H.; Dehghan, G.; Sadeghian, R.; Khajehnasiri, N. Synergistic effects of quercetin and regular exercise on the recovery of spatial memory and reduction of parameters of oxidative stress in animal model of Alzheimer’s disease. EXCLI J. 2020, 19, 596–612. [Google Scholar] [CrossRef]
- Dhawan, S.; Kapil, R.; Singh, B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J. Pharm. Pharmacol. 2011, 63, 342–351. [Google Scholar] [CrossRef]
- Chen, J.; Deng, X.; Liu, N.; Li, M.; Liu, B.; Fu, Q.; Qu, R.; Ma, S. Quercetin attenuates tau hyperphosphorylation and improves cognitive disorder via suppression of ER stress in a manner dependent on AMPK pathway. J. Funct. Foods 2016, 22, 463–476. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280343, Quercetin. Retrieved 9 November 2023. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Quercetin (accessed on 6 October 2023).
- Dimitrić Marković, J.M.; Milenković, D.; Amić, D.; Popović-Bijelić, A.; Mojović, M.; Pašti, I.A.; Marković, Z.S. Energy requirements of the reactions of kaempferol and selected radical species in different media: Towards the prediction of the possible radical scavenging mechanisms. Struct. Chem. 2014, 25, 1795–1804. [Google Scholar] [CrossRef]
- Wang, L.; Tu, Y.C.; Lian, T.W.; Hung, J.T.; Yen, J.H.; Wu, M.J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Sapkota, K.; Kim, H.; Kim, S.J. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br. J. Pharmacol. 2011, 164, 1008–1025. [Google Scholar] [CrossRef]
- Olszewska, M. Separation of quercetin, sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC determination of flavonoid aglycones in inflorescences, leaves and fruits of three Sorbus species. J. Pharm. Biomed. Anal. 2008, 48, 629–635. [Google Scholar] [CrossRef]
- Kiziltaş, H. Comprehensive evaluation of Reseda lutea L. (Wild Mignonette) and 7 isolated flavonol glycosides: Determination of antioxidant activity, anti-Alzheimer, antidiabetic and cytotoxic effects with in vitro and in silico methods. Turk. J. Chem. 2022, 46, 1185–1198. [Google Scholar] [CrossRef]
- Sulfahri; Wardhani, R.; Makatita, F.A.; Iskandar, I.W. Utilization of Nypa fruit in Alzheimer’s Disease: An In Silico Approach. J. Phys. Conf. Ser. 2019, 1341, 022003. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef]
- Uysal, M.; Celikten, M.; Beker, M.; Polat, N.; Huseyinbas, O.; Terzioglu-Usak, S.; Elibol, B. Kaempferol treatment ameliorates memory impairments in STZ-induced neurodegeneration by acting on reelin signaling. Acta Neurobiol. Exp. (Wars) 2023, 83, 236–245. [Google Scholar] [CrossRef]
- Beg, T.; Jyoti, S.; Naz, F.; Rahul, X.; Ali, F.; Ali, S.K.; Reyad, A.M.; Siddique, Y.H. Protective Effect of Kaempferol on the Transgenic Drosophila Model of Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2018, 17, 421–429. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, H.; Wang, Y.; Yao, Y.; Liu, G.; Lei, X.; Sun, H.; Wu, X.; Li, J. Protective mechanism of kaempferol against Aβ25-35-mediated apoptosis of pheochromocytoma (PC-12) cells through the ER/ERK/MAPK signalling pathway. Arch. Med Sci. 2020, 17, 406–416. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Hu, L.; Yan, J. K-3-Rh Protects Against Cerebral Ischemia/Reperfusion Injury by Anti-Apoptotic Effect Through PI3K-Akt Signaling Pathway in Rat. Neuropsychiatr. Dis. Treat. 2020, 16, 1217–1227. [Google Scholar] [CrossRef]
- Al-Brakati, A.; Albarakati, A.J.A.; Lokman, M.S.; Theyab, A.; Algahtani, M.; Menshawi, S.; AlAmri, O.D.; Al Omairi, N.E.; Essawy, E.A.; Kassab, R.B.; et al. Possible Role of Kaempferol in Reversing Oxidative Damage, Inflammation, and Apoptosis-Mediated Cortical Injury Following Cadmium Exposure. Neurotox. Res. 2021, 39, 198–209. [Google Scholar] [CrossRef]
- Ai, R.; Zhuang, X.X.; Anisimov, A.; Lu, J.H.; Fang, E.F. A synergized machine learning plus cross-species wet-lab validation approach identifies neuronal mitophagy inducers inhibiting Alzheimer disease. Autophagy 2022, 18, 939–941. [Google Scholar] [CrossRef]
- Zarei, M.; Mohammadi, S.; Komaki, A.; Golipour Choshali, Z. Antidepressant-like Effects of Intra-cerebroventricular Microinjection of Kaempferol in Male Rats: Involvement of 5-HT2 Receptors. Avicenna J. Neuro Psycho Physiol. 2022, 9, 23–30. [Google Scholar]
- Rita, L.; Neumann, N.R.; Laponogov, I.; Gonzalez, G.; Veselkov, D.; Pratico, D.; Aalizadeh, R.; Thomaidis, N.S.; Thompson, D.C.; Vasiliou, V.; et al. Alzheimer’s disease: Using gene/protein network machine learning for molecule discovery in olive oil. Hum. Genom. 2023, 17, 57. [Google Scholar] [CrossRef]
- Karunakaran, K.B.; Thiyagaraj, A.; Santhakumar, K. Novel insights on acetylcholinesterase inhibition by Convolvulus pluricaulis, scopolamine and their combination in zebrafish. Nat. Prod. Bioprospecting 2022, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, B.O.; Ojo, O.A.; Okesola, M.A.; Akinyemi, A.J.; Talabi, J.Y.; Idowu, O.T.; Fadaka, A.O.; Boligon, A.A.; de Campos, M.M.A. In vitro antioxidant activities and inhibitory effects of phenolic extract of Senecio biafrae (Oliv and Hiern) against key enzymes linked with type II diabetes mellitus and Alzheimer’s disease. Food Sci. Nutr. 2018, 6, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Shabir, I.; Pandey, V.K.; Shams, R.; Dar, A.H.; Dash, K.K.; Khan, S.A.; Bashir, I.; Jeevarathinam, G.; Rusu, A.V.; Esatbeyoglu, T.; et al. Promising bioactive properties of quercetin for potential food applications and health benefits: A review. Front. Nutr. 2022, 9, 999752. [Google Scholar] [CrossRef]
- Álvarez-Berbel, I.; Espargaró, A.; Viayna, A.; Caballero, A.B.; Busquets, M.A.; Gámez, P.; Luque, F.J.; Sabaté, R. Three to Tango: Inhibitory Effect of Quercetin and Apigenin on Acetylcholinesterase, Amyloid-β Aggregation and Acetylcholinesterase-Amyloid Interaction. Pharmaceutics 2022, 14, 2342. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, J.; Wang, R.; Li, S.; Wu, B.; Yuan, Y. Kaempferol Protects Against Cerebral Ischemia Reperfusion Injury Through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front. Pharmacol. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhou, S.; Nao, J. Kaempferol as a therapeutic agent in Alzheimer’s disease: Evidence from preclinical studies. Ageing Res. Rev. 2023, 87, 101910. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Cheng, X.; Yang, Y.L.; Liu, M.; Zhang, S.S.; Wang, Y.H.; Du, G.H. Kaempferol attenuates neuroinflammation and blood brain barrier dysfunction to improve neurological deficits in cerebral ischemia/reperfusion rats. Brain Res. 2019, 1722, 146361. [Google Scholar] [CrossRef]
- El-Kott, A.F.; Abd-Lateif, A.-E.M.; Khalifa, H.S.; Morsy, K.; Ibrahim, E.H.; Bin-Jumah, M.; Abdel-Daim, M.M.; Aleya, L. Kaempferol protects against cadmium chloride-induced hippocampal damage and memory deficits by activation of silent information regulator 1 and inhibition of poly (ADP-Ribose) polymerase-1. Sci. Total. Environ. 2020, 728, 138832. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Zhao, J.; Lin, Z. Protective effect of kaempferol against cognitive and neurological disturbances induced by d-galactose and aluminum chloride in mice. J. Funct. Foods 2023, 100, 105385. [Google Scholar] [CrossRef]
- Selvi, R.B.; Swaminathan, A.; Chatterjee, S.; Shanmugam, M.K.; Li, F.; Ramakrishnan, G.B.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; et al. Inhibition of p300 lysine acetyltransferase activity by luteolin reduces tumor growth in head and neck squamous cell carcinoma (HNSCC) xenograft mouse model. Oncotarget 2015, 6, 43806–43818. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Li, G.C. Kaempferol protects cell damage in in vitro ischemia reperfusion model in rat neuronal PC12 cells. BioMed Res. Int. 2020, 2020, 2461079. [Google Scholar] [CrossRef]
- Kadioglu, O.; Nass, J.; Saeed, M.E.; Schuler, B.; Efferth, T. Kaempferol Is an Anti-Inflammatory Compound with Activity towards NF-κB Pathway Proteins. Anticancer Res. 2015, 35, 2645–2650. [Google Scholar]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef] [PubMed]
- Sharoar, G.; Thapa, A.; Shahnawaz, M.; Ramasamy, V.S.; Woo, E.-R.; Shin, S.Y.; Park, I.-S. Keampferol-3-O-rhamnoside abrogates amyloid beta toxicity by modulating monomers and remodeling oligomers and fibrils to non-toxic aggregates. J. Biomed. Sci. 2012, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; Ko, H.J.; Lee, H.; Aminul Haque, M.; Park, I.S.; Lee, D.S.; Woo, E.R. Oleanane triterpenoids from Akebiae Caulis exhibit inhibitory effects on Aβ42 induced fibrillogenesis. Arch. Pharm. Res. 2017, 40, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sebastian, L.; Sopher, B.L. Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid-β peptide toxicity. J. Neurochem. 1999, 72, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free. Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Miranda, S.; Opazo, C.; Larrondo, L.F.; Munoz, F.J. The role of oxidative stress in the toxicity induced by amyloid β-peptide in Alzheimer’s disease. Prog. Neurobiol. 2000, 62, 633–648. [Google Scholar] [CrossRef]
- Jafari, A.; Babaei, P.; Rohampour, K.; Rashtiani, S. The Effect of Kaempferol on Autophagy and Nrf-2 Signaling in a Rat Model of Aβ1-42-induced Alzheimer’s Disease. Casp. J. Neurol. Sci. 2022, 8, 7–16. [Google Scholar] [CrossRef]
- Xie, C.; Zhuang, X.X.; Niu, Z.; Ai, R.; Lautrup, S.; Zheng, S.; Jiang, Y.; Han, R.; Gupta, T.S.; Cao, S.; et al. Amelioration of Alzheimer’s disease pathology by mitophagy inducers identified via machine learning and a cross-species workflow. Nat. Biomed. Eng. 2022, 6, 76–93. [Google Scholar] [CrossRef]
- Kaempferol: National Center for Biotechnology Information. PubChem Compound Summary for CID 5280863, Kaempferol. Retrieved 9 November 2023. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Kaempferol (accessed on 6 October 2023).
- Kouhestani, S.; Zare, S.; Babaei, P. Effects of pure flavonoid of medlar leaves on passive avoidance learning and memory in Alzheimer model of ovariectomized rats. J. Guilan Univ. Med. Sci. 2017, 26, 62–71. [Google Scholar]
- Krishnaveni, M. Flavonoid in enhancing memory function. J. Pharm. Res. 2012, 5, 3870–3874. [Google Scholar]
- Spencer, J.P. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010, 104, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Zhen, Y.; Guo, T.; Wang, C.; Shen, L.; Li, W. Quercetin inhibits cytotoxicity of PC12 cells induced by amyloid-beta 25–35 via stimulating estrogen receptor α, activating ERK1/2, and inhibiting apoptosis. Open Life Sci. 2022, 17, 230–242. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, L.; Wang, L. Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: From chemistry to medicine. Biomed. Pharmacother. 2023, 165, 115215. [Google Scholar] [CrossRef]
- Damirchi, A.; Hosseini, F.; Babaei, P. Mental Training Enhances Cognitive Function and BDNF More Than Either Physical or Combined Training in Elderly Women With MCI: A Small-Scale Study. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Fukumoto, H.; Orne, J.; Klucken, J.; Raju, S.; Vanderburg, C.R.; Irizarry, M.C.; Hyman, B.T.; Ingelsson, M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp. Neurol. 2005, 194, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; He, B.; Xu, M.; Wu, B.; Xiao, F.; Bi, K.; Jia, Y. Kaempferide prevents cognitive decline via attenuation of oxidative stress and enhancement of brain-derived neurotrophic factor/tropomyosin receptor kinase B/cAMP response element-binding signaling pathway. Phytotherapy Res. 2019, 33, 1065–1073. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Walton, M.R.; Dragunow, M. Is CREB a key to neuronal survival? Trends Neurosci. 2000, 23, 48–53. [Google Scholar] [CrossRef]
- Gao, Q.; Tian, D.; Han, Z.; Lin, J.; Chang, Z.; Zhang, D.; Ma, D. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of buyang huanwu decoction in the treatment of ischemic stroke. Evid. -Based Complement. Altern. Med. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.Z.; Ye, K. Deficiency in BDNF/TrkB neurotrophic activity stimulates δ-secretase by upregulating C/EBPβ in Alzheimer’s disease. Cell Rep. 2019, 28, 655–669. [Google Scholar] [CrossRef]
- Connor, B.; Young, D.; Yan, Q.; Faull, R.L.M.; Synek, B.; Dragunow, M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Mol. Brain Res. 1997, 49, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Levenga, J.; Wong, H.; Milstead, R.; LaPlante, L.; Hoeffer, C.A. Immunohistological Examination of AKT Isoforms in the Brain: Cell-Type Specificity That May Underlie AKT’s Role in Complex Brain Disorders and Neurological Disease. Cereb. Cortex Commun. 2021, 2, tgab036. [Google Scholar] [CrossRef] [PubMed]
- Zarneshan, S.N.; Fakhri, S.; Khan, H. Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: A mechanistic approach. Pharmacol. Res. 2022, 177, 106099. [Google Scholar] [CrossRef] [PubMed]
- Pak, M.E.; Yang, H.J.; Li, W.; Kim, J.K.; Go, Y. Yuk-Gunja-Tang attenuates neuronal death and memory impairment via ERK/CREB/BDNF signaling in the hippocampi of experimental Alzheimer’s disease model. Front. Pharmacol. 2022, 13, 1014840. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Baitharu, I.; Prasad, D.; Ilavazhagan, G. Enriched environment prevents hypobaric hypoxia induced memory impairment and neurodegeneration: Role of BDNF/PI3K/GSK3β pathway coupled with CREB activation. PLoS ONE 2013, 8, e62235. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Rada, P.; Rojo, A.I.; Chowdhry, S.; McMahon, M.; Hayes, J.D.; Cuadrado, A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011, 31, 1121–1133. [Google Scholar] [CrossRef]
- Kume, T.; Kouchiyama, H.; Kaneko, S.; Maeda, T.; Kaneko, S.; Akaike, A.; Shimohama, S.; Kihara, T.; Kimura, J.; Wada, K.; et al. BDNF prevents NO mediated glutamate cytotoxicity in cultured cortical neurons. Brain Res. 1997, 756, 200–204. [Google Scholar] [CrossRef]
- Mercado-Gómez, O.; Hernández-Fonseca, K.; Villavicencio-Queijeiro, A.; Massieu, L.; Chimal-Monroy, J.; Arias, C. Inhibition of Wnt and PI3K signaling modulates GSK-3beta activity and induces morphological changes in cortical neurons: Role of tau phosphorylation. Neurochem. Res. 2008, 33, 1599–1609. [Google Scholar] [CrossRef]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef]
- Jantas, D.; Malarz, J.; Le, T.N.; Stojakowska, A. Neuroprotective Properties of Kempferol Derivatives from Maesa membranacea against Oxidative Stress-Induced Cell Damage: An Association with Cathepsin D Inhibition and PI3K/Akt Activation. Int. J. Mol. Sci. 2021, 22, 10363. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, H.; Chu, T.; Jiang, P.; Li, S.T. Neuregulin-1β attenuates sepsis-induced diaphragm atrophy by activating the PI3K/Akt signaling pathway. J. Muscle Res. Cell Motil. 2019, 40, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kandezi, N.; Mohammadi, M.; Ghaffari, M.; Gholami, M.; Motaghinejad, M.; Safari, S. Novel Insight to Neuroprotective Potential of Curcumin: A Mechanistic Review of Possible Involvement of Mitochondrial Biogenesis and PI3/Akt/GSK3 or PI3/Akt/CREB/BDNF Signaling Pathways. Int. J. Mol. Cell. Med. 2020, 9, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Tanqueiro, S.R.; Ramalho, R.M.; Rodrigues, T.M.; Lopes, L.V.; Sebastião, A.M.; Diógenes, M.J. Inhibition of NMDA Receptors Prevents the Loss of BDNF Function Induced by Amyloid β. Front. Pharmacol. 2018, 9, 237. [Google Scholar] [CrossRef]
- Garzon, D.J.; Fahnestock, M. Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. J. Neurosci. 2007, 27, 2628–2635. [Google Scholar] [CrossRef]
- Tong, L.; Thornton, P.L.; Balazs, R.; Cotman, C.W. β-amyloid-(1–42) impairs activity-dependent cAMP-response element-binding protein signaling in neurons at concentrations in which cell survival is not compromised. J. Biol. Chem. 2001, 276, 17301–17306. [Google Scholar] [CrossRef]
- Cowansage, K.K.; LeDoux, J.E.; Monfils, M.H. Brain-derived neurotrophic factor: A dynamic gatekeeper of neural plasticity. Curr. Mol. Pharmacol. 2010, 3, 12–29. [Google Scholar] [CrossRef]
- Rosa, E.; Fahnestock, M. CREB expression mediates amyloid β-induced basal BDNF downregulation. Neurobiol. Aging 2015, 36, 2406–2413. [Google Scholar] [CrossRef]
- DaRocha-Souto, B.; Coma, M.; Perez-Nievas, B.; Scotton, T.; Siao, M.; Sánchez-Ferrer, P.; Hashimoto, T.; Fan, Z.; Hudry, E.; Barroeta, I. Activation of glycogen synthase kinase-3 beta mediates β-amyloid induced neuritic damage in Alzheimer’s disease. Neurobiol. Dis. 2012, 45, 425–437. [Google Scholar] [CrossRef]
- Barco, A.; Pittenger, C.; Kandel, E.R. CREB, memory enhancement and the treatment of memory disorders: Promises, pitfalls and prospects. Expert Opin. Ther. Targets 2003, 7, 101–114. [Google Scholar] [CrossRef]
- Christensen, R.; Marcussen, A.B.; Wörtwein, G.; Knudsen, G.; Aznar, S. Aβ (1–42) injection causes memory impairment, lowered cortical and serum BDNF levels, and decreased hippocampal 5-HT2A levels. Exp. Neurol. 2008, 210, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, A.; Salani, F.; Bizzoni, F.; Orfei, M.D.; Langella, R.; Angelucci, F.; Spalletta, G.; Taddei, A.R.; Caltagirone, C.; Bossù, P. The stimulation of dendritic cells by amyloid beta 1–42 reduces BDNF production in Alzheimer’s disease patients. Brain Behav. Immun. 2013, 32, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Zussy, C.; Brureau, A.; Keller, E.; Marchal, S.; Blayo, C.; Delair, B.; Ixart, G.; Maurice, T.; Givalois, L. Alzheimer’s disease related markers, cellular toxicity and behavioral deficits induced six weeks after oligomeric amyloid-β peptide injection in rats. PLoS ONE 2013, 8, e53117. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, D.; Jiang, H.; Xiong, Y.; Qu, C.; Li, B.; Mahmood, A.; Zhou, D.; Chopp, M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/AKT pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J. Neurotrauma 2008, 25, 130–139. [Google Scholar] [CrossRef]
- Hu, Y.S.; Long, N.; Pigino, G.; Brady, S.T.; Lazarov, O. Molecular mechanisms of environmental enrichment: Impairments in AKT/GSK3β, neurotrophin-3 and CREB signaling. PLoS ONE. 2013, 8, e64460. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol. 2004, 3, 519–527. [Google Scholar] [CrossRef]
- Caricasole, A.; Copani, A.; Caruso, A.; Caraci, F.; Iacovelli, L.; Sortino, M.A.; Terstappen, G.C.; Nicoletti, F. The Wnt pathway, cell-cycle activation and beta-amyloid: Novel therapeutic strategies in Alzheimer’s disease? Trends Pharmacol. Sci. 2003, 24, 233–238. [Google Scholar] [CrossRef]
- Lee, C.W.; Lau, K.F.; Miller, C.C.; Shaw, P.C. Glycogen synthase kinase-3 beta-mediated tau phosphorylation in cultured cell lines. Neuroreport 2003, 14, 257–260. [Google Scholar] [CrossRef]
- Yao, R.Q.; Qi, D.S.; Yu, H.L.; Liu, J.; Yang, L.H.; Wu, X.X. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem. Res. 2012, 37, 2777–2786. [Google Scholar] [CrossRef]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Cardone, M.H.; Roy, N.; Stennicke, H.R.; Salvesen, G.S.; Franke, T.F.; Stanbridge, E.; Frisch, S.; Reed, J.C. Regulation of cell death protease caspase-9 by phosphorylation. Science 1998, 282, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Martinez, A.A.; Morado, J.; Scofield, V.; Roberts, J.L.; Maffi, S.K. Retinoic acid protects against proteasome inhibition associated cell death in SH-SY5Y cells via the AKT pathway. Neurochem. Int. 2013, 62, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Koh, S.H.; Noh, M.Y.; Park, K.W.; Lee, Y.J.; Kim, S.H. Glycogen synthase kinase-3beta activity plays very important roles in determining the fate of oxidative stress-inflicted neuronal cells. Brain Res. 2007, 1129, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, X.; Xu, A.; Chen, W.; Liu, K.; Wu, L.; Mo, S.; Hu, Y.; Liu, M.; Luo, Q. Protein-ligand binding affinity prediction with edge awareness and supervised attention. iScience 2022, 26, 105892. [Google Scholar] [CrossRef]
- Jain, A.N. Surflex: Fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef]
- Simayi, J.; Bayinsang; Nuermaimaiti, M.; Hailati, S.; Han, M.; Reheman, Z.; Wumaier, A.; Zhou, W. A Network Pharmacology-Based Study on the Mechanism of Dibutyl Phthalate of Ocimum basilicum L. against Alzheimer’s Disease through the AKT/GSK-3β Pathway. BioMed Res. Int. 2022, 2022, 9494548. [Google Scholar] [CrossRef]
- Rahmatkar, S.N.; Rana, A.K.; Kumar, R.; Singh, D. Fagopyrum tataricum (L.) Gaertn interacts with Gsk-3β/Nrf-2 signalling to protect neurotoxicity in a zebrafish model. J. Ethnopharmacol. 2023, 319, 117187. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Teng, Y.S.; Chen, C.M.; Sun, Y.C.; Hsieh-Li, H.M.; Chang, K.H.; Lee-Chen, G.J. A Neuroprotective Action of Quercetin and Apigenin through Inhibiting Aggregation of Aβ and Activation of TRKB Signaling in a Cellular Experiment. Biomol. Ther. 2023, 31, 285–297. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, S.; Chakraborty, S. Scope of β-secretase (bace1)-targeted therapy in alzheimer’s disease: Emphasizing the flavonoid based natural scaffold for bace1 inhibition. CS Chem. Neurosci. 2020, 11, 3510–3522. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 819–825. [Google Scholar] [CrossRef]
- Li, E.; Yan, K.; Zhang, R.; Zou, P.; Li, S.; Ma, Q.; Liao, B. Kaempferol Protects Against Apoptosis in PC12 Cells Exposed to Hydrogen Peroxide by Activating Akt1. Nat. Prod. Commun. 2023, 18, 1934578X231170448. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Chen, W.; Shi, W.; Zhao, Q.; Zhao, J.; Li, L. Network Pharmacology and Experimental Evidence: PI3K/AKT Signaling Pathway is Involved in the Antidepressive Roles of Chaihu Shugan San. Drug Des. Dev. Ther. 2021, 15, 3425–3441. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, M.; Zhao, Z.; Pan, J.; Huang, S. Network pharmacology and molecular docking analyses of mechanisms underlying effects of the cyperi rhizoma-chuanxiong rhizoma herb pair on depression. Evid. -Based Complement. Altern. Med. 2021, 2021, 5704578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Liu, M.; Liu, D.N.; Shang, Y.F.; Du, G.H.; Wang, Y.H. Network pharmacology analysis and experimental validation of kaempferol in the treatment of ischemic stroke by inhibiting apoptosis and regulating neuroinflammation involving neutrophils. Int. J. Mol. Sci. 2022, 23, 12694. [Google Scholar] [CrossRef] [PubMed]
- Touati, I.; Abdalla, M.; Ali, N.H.; AlRuwaili, R.; Alruwaili, M.; Britel, M.R.; Maurady, A. Constituents of Stachys plants as potential dual inhibitors of AChE and NMDAR for the treatment of Alzheimer’s disease: A molecular docking and dynamic simulation study. J. Biomol. Struct. Dyn. 2023, 1–17. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini Ghazvini, S.M.B.; Yarani, R.; Altıntaş, A.; Jooneghani, S.G.N.; Ramalho, T.C. Deciphering inhibitory activity of flavonoids against tau protein kinases: A coupled molecular docking and quantum chemical study. J. Biomol. Struct. Dyn. 2022, 40, 411–424. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (β-secretase, Cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Chukwuma, I.F.; Ezeorba, T.P.C.; Nworah, F.N.; Apeh, V.O.; Khalid, M.; Sweilam, S.H. Bioassay-guided identification of potential Alzheimer’s disease therapeutic agents from Kaempferol-Enriched fraction of Aframomum melegueta seeds using in vitro and chemoinformatics approaches. Arab. J. Chem. 2023, 16, 105089. [Google Scholar] [CrossRef]
- Pandey, D.; Pal, T.; Sharma, A. Phytochemicals as Potential Anti-Alzheimer’s Agents- An In-Silico Evidence. J. Dis. Markers 2022, 7, 1047. [Google Scholar]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Gong, P.; Wang, D.; Cui, D.; Yang, Q.; Wang, P.; Yang, W.; Chen, F. Anti-aging function and molecular mechanism of Radix Astragali and Radix Astragali preparata via network pharmacology and PI3K/Akt signaling pathway. Phytomedicine 2021, 84, 153509. [Google Scholar] [CrossRef]
- Sadighparvar, S.; Darband, S.G.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Mobaraki, K.; Majidinia, M. Combination of quercetin and exercise training attenuates depression in rats with 1,2-dimethylhydrazine-induced colorectal cancer: Possible involvement of inflammation and BDNF signalling. Exp. Physiol. 2020, 105, 1598–1609. [Google Scholar] [CrossRef]
- Khan, H.; Singh, A.; Thapa, K.; Garg, N.; Grewal, A.K.; Singh, T.G. Therapeutic modulation of the phosphatidylinositol 3-kinases (PI3K) pathway in cerebral ischemic injury. Brain Res. 2021, 1761, 147399. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.J.; Park, H.R.; Kim, M.E.; Piao, S.; Lee, E.; Jo, D.G.; Chung, H.Y.; Ha, N.C.; Mattson, M.P.; Lee, J. Morin attenuates tau hyperphosphorylation by inhibiting GSK3beta. Neurobiol. Dis. 2011, 44, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Miyai, S.; Yamaguchi, A.; Iwasaki, T.; Shamsa, F.; Ohtsuki, K. Biochemical characterization of epigallocatechin-3-gallate as an effective stimulator for the phosphorylation of its binding proteins by glycogen synthase kinase-3beta in vitro. Biol. Pharm. Bull. 2010, 33, 1932–1937. [Google Scholar] [CrossRef]
- Ashrafpour, M.; Parsaei, S.; Sepehri, H. Quercetin improved spatial memory dysfunctions in rat model of intracerebroventricular streptozotocin-induced Alzheimer’s disease. Natl. J. Physiol. Pharm. Pharmacol. 2015, 5, 411. [Google Scholar] [CrossRef]
- Tong-un, T.; Muchimapura, S.; Phachonpai, W.; Wattanathorn, J. Effects of quercetin encapsulated liposomes via nasal administration: A novel cognitive enhancer. Am. J. Appl. Sci. 2010, 7, 906–913. [Google Scholar] [CrossRef]
- Sriraksa, N.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-enhancing effect of quercetin in a rat model of Parkinson’s disease induced by 6-hyrodoxydopamine. J. Evid. Based Complement. Alternat. Med. 2011, 2012, 823206. [Google Scholar] [CrossRef]
- Elreedy, H.A.; Elfiky, A.M.; Ahmed Mahmoud, A.; Ibrahim, K.S.; Ghazy, M.A. Effect Of Quercetin As Therapeutic And Protective Agent In Aluminum Chloride-Induced Alzheimer’s Disease Rats. Egypt. J. Chem. 2022, 65, 633–641. [Google Scholar] [CrossRef]
- Elfiky, A.M.; Mahmoud, A.A.; Elreedy, H.A.; Ibrahim, K.S.; Ghazy, M.A. Quercetin stimulates the non-amyloidogenic pathway via activation of ADAM10 and ADAM17 gene expression in aluminum chloride-induced Alzheimer’s disease rat model. Life Sci. 2021, 285, 119964. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Garabadu, D. Quercetin Exhibits α7nAChR/Nrf2/HO-1-Mediated Neuroprotection Against STZ-Induced Mitochondrial Toxicity and Cognitive Impairments in Experimental Rodents. Neurotox. Res. 2021, 39, 1859–1879. [Google Scholar] [CrossRef]
- Parent, M.; Chitturi, J.; Santhakumar, V.; Hyder, F.; Sanganahalli, B.G.; Kannurpatti, S.S. Kaempferol Treatment after Traumatic Brain Injury during Early Development Mitigates Brain Parenchymal Microstructure and Neural Functional Connectivity Deterioration at Adolescence. J. Neurotrauma 2020, 37, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lv, M.; Shi, Y.; Mu, Y.; Yao, Z.; Yang, Z. Network Pharmacology-Based Study of the Underlying Mechanisms of Huangqi Sijunzi Decoction for Alzheimer’s Disease. Evid. Based Complement. Altern. Med. 2021, 2021, 6480381. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, E.S.; Shin, R.W.; Billingsley, M.L.; van de Voorde, A.; O’Connor, M.; Trojanowski, J.Q.; Lee, V.M. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer’s disease paired helical filament tau. Neuron 1994, 13, 989–1002. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.X. Tau and neurodegenerative disease: The story so far. Nat. Rev. Neurol. 2016, 12, 15–27. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, R.; Chen, R.; Tian, Q.; Zeng, K.; Hu, J.; Liu, X.; Wang, Q.; Wang, P.; Wang, X.C.; et al. Novel multipotent AChEI-CCB attenuates hyperhomocysteinemia-induced memory deficits and Neuropathologies in rats. J. Alzheimer's Dis. 2014, 42, 1029–1039. [Google Scholar] [CrossRef]
- Russo, M.; Milito, A.; Spagnuolo, C.; Carbone, V.; Rosén, A.; Minasi, P.; Lauria, F.; Russo, G.L. CK2 and PI3K are direct molecular targets of quercetin in chronic lymphocytic leukaemia. Oncotarget 2017, 8, 42571–42587. [Google Scholar] [CrossRef]
- Images Created. Available online: BioRender.com (accessed on 6 October 2023).
- Fang, J.; Wang, L.; Wu, T.; Yang, C.; Gao, L.; Cai, H.; Liu, J.; Fang, S.; Chen, Y.; Tan, W.; et al. Network pharmacology-based study on the mechanism of action for herbal medicines in Alzheimer treatment. J. Ethnopharmacol. 2017, 196, 281–292. [Google Scholar] [CrossRef]
- Luo, Y.; Smith, J.V.; Paramasivam, V.; Burdick, A.; Curry, K.J.; Buford, J.P.; Khan, I.; Netzer, W.J.; Xu, H.; Butko, P. Inhibition of amyloid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA 2002, 99, 12197–12202. [Google Scholar] [CrossRef]
- Longpré, F.; Garneau, P.; Christen, Y.; Ramassamy, C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: Involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free. Radic. Biol. Med. 2006, 41, 1781–1794. [Google Scholar] [CrossRef]
- Tchantchou, F.; Xu, Y.; Wu, Y.; Christen, Y.; Luo, Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007, 21, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.J.; Lee, E.J.; Cho, K.S.; Cho, D.H.; Shin, C.Y.; Han, S.H. Ginkgo biloba extract (Egb761) attenuates zinc-induced tau phosphorylation at Ser262 by regulating GSK3β activity in rat primary cortical neurons. Food Funct. 2015, 6, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Lejri, I.; Grimm, A.; Eckert, A. Ginkgo biloba extract increases neurite outgrowth and activates the Akt/mTOR pathway. PLoS ONE. 2019, 14, e0225761. [Google Scholar] [CrossRef] [PubMed]
- Rhein, V.; Song, X.; Wiesner, A.; Ittner, L.M.; Baysang, G.; Meier, F.; Ozmen, L.; Bluethmann, H.; Dröse, S.; Brandt, U.; et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc. Natl. Acad. Sci. USA 2009, 106, 20057–20062. [Google Scholar] [CrossRef]
- Muller, W.E.; Heiser, J.; Leuner, K. Effects of the standardized Ginkgo biloba extract EGb 761(R) on neuroplasticity. Int. Psychogeriatrics 2012, 24 (Suppl. S1), S21–S24. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, C.; Pang, C.; Christen, Y.; Luo, Y. Restoration of impaired phosphorylation of cyclic AMP response element-binding protein (CREB) by EGb 761 and its constituents in Abeta-expressing neuroblastoma cells. Eur. J. Neurosci. 2007, 26, 2931–2939. [Google Scholar] [CrossRef]
- Tchantchou, F.; Lacor, P.N.; Cao, Z.; Lao, L.; Hou, Y.; Cui, C.; Klein, W.L.; Luo, Y. Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. J. Alzheimer's Dis. 2009, 18, 787–798. [Google Scholar] [CrossRef]
- Tian, R.; Wang, H.; Xiao, Y.; Hu, P.; Du, R.; Shi, X.; Wang, Z.; Xie, Y. Fabrication of nanosuspensions to improve the oral bioavailability of total flavones from Hippophae rhamnoides L. and their comparison with an inclusion complex. AAPS Pharmscitech 2020, 21, 249. [Google Scholar] [CrossRef]
- Xia, C.X.; Gao, A.X.; Zhu, Y.; Dong, T.T.; Tsim, K.W. Flavonoids from Seabuckthorn (Hippophae rhamnoides L.) restore CUMS-induced depressive disorder and regulate the gut microbiota in mice. Food Funct. 2023, 14, 7426–7438. [Google Scholar] [CrossRef]
- Liu, H.; Ye, M.; Guo, H. An Updated Review of Randomized Clinical Trials Testing the Improvement of Cognitive Function of Ginkgo biloba Extract in Healthy People and Alzheimer’s Patients. Front. Pharmacol. 2020, 10, 1688. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, B.; Punyasiri, P.A.; Kottawa-Arachchi, J.D.; Ranatunga, M.A.; Abeysinghe, I.S.; Gunasekare, M.T.; Bandara, B.M. Genetic Variation of Flavonols Quercetin, Myricetin, and Kaempferol in the Sri Lankan Tea (Camellia sinensis L.) and Their Health-Promoting Aspects. Int. J. Food Sci. 2016, 2016, 6057434. [Google Scholar] [CrossRef] [PubMed]
- Ishan, T.; Lalit, S.; Girdhari, G.; Gupta, G.L. Synergistic antioxidant activity of three medicinal plants Hypericum perforatum, Bacopa monnieri, and Camellia sinensis. Indo Am. J. Pharm. Res. 2014, 4, 2563–2568. [Google Scholar]
- Mahmoodzadeh, T.; Kashani, M.H.K.; Ramshini, H.; Moslem, A.; Mohammad-Zadeh, M. Effect of Camellia sinensis on Spatial Memory in a Rat Model of Alzheimer’s Disease. J. Biomed. 2016, 1, e5340. [Google Scholar] [CrossRef]
- Onasanwo, S.A.; Adamaigbo, V.O.; Adebayo, O.G.; Eleazer, S.E. Moringa oleifera-supplemented diet protect against cortico-hippocampal neuronal degeneration in scopolamine-induced spatial memory deficit in mice: Role of oxido-inflammatory and cholinergic neurotransmission pathway. Metab. Brain Dis. 2021, 36, 2445–2460. [Google Scholar] [CrossRef]
- Sermkaew, N.; Plyduang, T. Self-microemulsifying drug delivery systems of Moringa oleifera extract for enhanced dissolution of kaempferol and quercetin. Acta Pharm. 2020, 70, 77–88. [Google Scholar] [CrossRef]
- Liu, W.L.; Wu, B.F.; Shang, J.H.; Wang, X.F.; Zhao, Y.L.; Huang, A.X. Moringa oleifera seed ethanol extract and its active component kaempferol potentiate pentobarbital-induced sleeping behaviours in mice via a GABAergic mechanism. Pharm. Biol. 2022, 60, 810–824. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, A.; Li, X.; Qin, D.; Jin, B.; Liu, J.; Tang, Y.; Wu, J.; Yu, C. The seed of Litchi chinensis fraction ameliorates hippocampal neuronal injury in an Aβ25-35-induced Alzheimer’s disease rat model via the AKT/GSK-3β pathway. Pharm. Biol. 2020, 58, 35–43. [Google Scholar] [CrossRef]
- El Gizawy, H.A.; Abo-Salem, H.M.; Ali, A.A.; Hussein, M.A. Phenolic Profiling and Therapeutic Potential of Certain Isolated Compounds from Parkia roxburghii against AChE Activity as well as GABAA α5, GSK-3β, and p38α MAP-Kinase Genes. ACS Omega 2021, 6, 20492–20511. [Google Scholar] [CrossRef]
- Singh, S.; Singh, T.G.; Mahajan, K.; Dhiman, S. Medicinal plants used against various inflammatory biomarkers for the management of rheumatoid arthritis. J. Pharm. Pharmacol. 2020, 72, 1306–1327. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Q.; Li, A.; Li, K.; Qin, X. Mechanisms exploration of herbal pair of HuangQi-DanShen on cerebral ischemia based on metabonomics and network pharmacology. J. Ethnopharmacol. 2020, 253, 112688. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, M.; Tominaga, E.; Kito, K.; Nakagawa, R.; Kapoor, M.P.; Matsumiya, Y.; Fukuhara, T.; Kobayashi, J.; Satomoto, K.; Yamagata, H.; et al. Bioavailability of Flavonoids in Ginkgo biloba Extract-γ-Cyclodextrin Complex. Nat. Prod. Commun. 2023, 18, 1934578X231170221. [Google Scholar] [CrossRef]
- Zubčić, K.; Radovanović, V.; Vlainić, J.; Hof, P.R.; Oršolić, N.; Šimić, G.; Jazvinšćak Jembrek, M. PI3K/Akt and ERK1/2 Signalling Are Involved in Quercetin-Mediated Neuroprotection against Copper-Induced Injury. Oxidative Med. Cell. Longev. 2020, 2020, 9834742. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Maddeppungeng, N.M.; Asma, N.; Rahim, A.; Nainu, F.; Bahar, M.A.; Yulianty, R. Validation of HPLC-UV method for simultaneous determination of quercetin and luteolin from chartamus tinctorius L in solid lipid nanoparticles incorporated in floating gel in situ formulation. Microchem. J. 2023, 194, 109373. [Google Scholar] [CrossRef]
- Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Jung, J.G.; Kim, M.J.; Bin Jeong, J.; Jang, S.N.; Lee, G.O.; Sim, H.-S.; Kang, M.J.; et al. Comprehensive Comparison of Chemical Composition and Antioxidant Activity of Panax ginseng Sprouts by Different Cultivation Systems in a Plant Factory. Plants 2022, 11, 1818. [Google Scholar] [CrossRef]
- Lin, M.K.; Lee, M.S.; Huang, H.C.; Cheng, T.J.; Cheng, Y.D.; Wu, C.R. Cuscuta chinensis and C. campestris attenuate scopolamine-induced memory deficit and oxidative damage in mice. Molecules 2018, 23, 3060. [Google Scholar] [CrossRef]
- Marefati, N.; Ghorani, V.; Shakeri, F.; Boskabady, M.; Kianian, F.; Rezaee, R.; Boskabady, M.H. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm. Biol. 2021, 59, 285–300. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Benkeblia, N.; Xiao, J. Onion (Allium cepa L.) bioactives: Chemistry, pharmacotherapeutic functions, and industrial applications. Food Front. 2022, 3, 380–412. [Google Scholar] [CrossRef]
- Tan, S.; Tang, J.; Shi, W.; Wang, Z.; Xiang, Y.; Deng, T.; Gao, X.; Li, W.; Shi, S. Effects of three drying methods on polyphenol composition and antioxidant activities of Litchi chinensis Sonn. Food Sci. Biotechnol. 2019, 29, 351–358. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Gao, C.; Chen, W.; Vong, C.T.; Yao, P.; Yang, Y.; Li, X.; Tang, X.; Wang, S.; et al. Astragali Radix (Huangqi): A promising edible immunomodulatory herbal medicine. J. Ethnopharmacol. 2020, 258, 112895. [Google Scholar] [CrossRef]
- Pool, H.; Mendoza, S.; Xiao, H.; McClements, D.J. Encapsulation and release of hydrophobic bioactive components in nanoemulsion-based delivery systems: Impact of physical form on quercetin bioaccessibility. Food Funct. 2013, 4, 162–174. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2022, 1–25. [Google Scholar] [CrossRef]
- Elahy, M.; Jackaman, C.; Mamo, J.C.; Lam, V.; Dhaliwal, S.S.; Giles, C.; Nelson, D.; Takechi, R. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun. Ageing 2015, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.O.; Toescu, E.C.; Popescu, L.M.; Bajenaru, O.; Muresanu, D.F.; Schultzberg, M.; Bogdanovic, N. Blood-brain barrier alterations in ageing and dementia. J. Neurol. Sci. 2009, 283, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Leoni, V.; Masterman, T.; Patel, P.; Meaney, S.; Diczfalusy, U.; Björkhem, I. Side chain oxidized oxysterols in cerebrospinal fluid and the integrity of blood-brain and blood-cerebrospinal fluid barriers. J. Lipid Res. 2003, 44, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.J.; Frauen, H.D.; Zhang, B.; Nordhues, B.A.; Bijan, S.; Lin, Y.-C.; Zamudio, F.; Hernandez, L.D.; Sabbagh, J.J.; Selenica, M.-L.B.; et al. Tau depletion prevents progressive blood-brain barrier damage in a mouse model of tauopathy. Acta Neuropathol. Commun. 2015, 3, 8. [Google Scholar] [CrossRef]
- Majerova, P.; Michalicova, A.; Cente, M.; Hanes, J.; Vegh, J.; Kittel, A.; Kosikova, N.; Cigankova, V.; Mihaljevic, S.; Jadhav, S.; et al. Trafficking of immune cells across the blood-brain barrier is modulated by neurofibrillary pathology in tauopathies. PLoS ONE 2019, 14, e0217216. [Google Scholar] [CrossRef]
- Moradi-Afrapoli, F.; Oufir, M.; Walter, F.R.; Deli, M.A.; Smiesko, M.; Zabela, V.; Butterweck, V.; Hamburger, M. Validation of UHPLC-MS/MS methods for the determination of kaempferol and its metabolite 4-hydroxyphenyl acetic acid, and application to in vitro blood-brain barrier and intestinal drug permeability studies. J. Pharm. Biomed. Anal. 2016, 128, 264–274. [Google Scholar] [CrossRef]
- Gupta, A.; Kaur, C.D.; Saraf, S.; Saraf, S. Formulation, characterization, and evaluation of ligand-conjugated biodegradable quercetin nanoparticles for active targeting. Artif. Cells Nanomed. Biotechnol. 2016, 44, 960–970. [Google Scholar] [CrossRef]
- Dong, Y.; Tao, B.; Xue, X.; Feng, C.; Ren, Y.; Ma, H.; Zhang, J.; Si, Y.; Zhang, S.; Liu, S.; et al. Molecular mechanism of Epicedium treatment for depression based on network pharmacology and molecular docking technology. BMC Complement. Med. Ther. 2021, 21, 222. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, L.; Zhang, S. Distribution of quercetin in plasma and tissues in rats. Chin. J. Hosp. Pharm. 2008, 28, 1822–1824. [Google Scholar]
- Orhan, I.E. Cholinesterase Inhibitory Potential of Quercetin towards Alzheimer’s Disease—A Promising Natural Molecule or Fashion of the Day?—A Narrowed Review. Curr. Neuropharmacol. 2021, 19, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Thabet, H.K.; Alaqel, S.I.; Alzahrani, A.R.; Abida, A.; Alshammari, M.K.; Kamal, M.; Diwan, A.; Asdaq, S.M.B.; Alshehri, S. The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature. Antioxidants 2022, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Huebbe, P.; Wagner, A.E.; Boesch-Saadatmandi, C.; Sellmer, F.; Wolffram, S.; Rimbach, G. Effect of dietary quercetin on brain quercetin levels and the expression of antioxidant and Alzheimer’s disease relevant genes in mice. Pharmacol. Res. 2010, 61, 242–246. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of Dietary Flavonoids and Phenolic Compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between obesity, diabetes, and alzheimer’s disease: Introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 2020, 62, 101095. [Google Scholar] [CrossRef]
- Pinheiro, R.; Granja, A.; Loureiro, J.; Pereira, M.; Pinheiro, M.; Neves, A.; Reis, S. RVG29-Functionalized Lipid Nanoparticles for Quercetin Brain Delivery and Alzheimer’s Disease. Pharm. Res. 2020, 37, 139. [Google Scholar] [CrossRef]
- Qi, Y.; Yi, P.; He, T.; Song, X.; Liu, Y.; Li, Q.; Zheng, J.; Song, R.; Liu, C.; Zhang, Z.; et al. Quercetin-loaded selenium nanoparticles inhibit amyloid-β aggregation and exhibit antioxidant activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125058. [Google Scholar] [CrossRef]
- Chen, G.; Yang, F.; Fan, S.; Jin, H.; Liao, K.; Li, X.; Liu, G.-B.; Liang, J.; Zhang, J.; Xu, J.F.; et al. Immunomodulatory roles of selenium nanoparticles: Novel arts for potential immunotherapy strategy development. Front. Immunol. 2022, 13, 956181. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on growth and antioxidative system of yeast cells. Mol. Biol. Rep. 2019, 46, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; de Lima Melchiades, G.; Michels, L.R.; Figueiró, F.; Bassani, V.L.; Teixeira, H.F.; Koester, L.S. Solid Dispersion of Kaempferol: Formulation Development, Characterization, and Oral Bioavailability Assessment. AAPS PharmSciTech 2019, 20, 106. [Google Scholar] [CrossRef] [PubMed]
- Alkandahri, M.Y.; Pamungkas, B.T.; Oktoba, Z.; Shafirany, M.Z.; Sulastri, L.; Arfania, M.; Anggraeny, E.N.; Pratiwi, A.; Astuti, F.D.; Indriyani, D.S.Y.; et al. Hepatoprotective Effect of Kaempferol: A Review of the Dietary Sources, Bioavailability, Mechanisms of Action, and Safety. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 1387665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kong, L.; Luo, C.; Li, X.; Zhou, Y. Pharmacokinetic Study of Keampferol in Rabbits. China Pharm. 2014, 25, 4040–4042. [Google Scholar]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Y.; Xie, W.; Huang, A.; Yuan, X.; Zhou, H.; Zhu, X.; Chen, X.; Liu, J.; Liu, J.; et al. Microbubbles in combination with focused ultrasound for the delivery of quercetin-modified sulfur nanoparticles through the blood brain barrier into the brain parenchyma and relief of endoplasmic reticulum stress to treat Alzheimer’s disease. Nanoscale 2020, 12, 6498–6511. [Google Scholar]
- Chandekar, L.; Katgeri, R.; Takke, A. The Potential Clinical Uses and Nanoformulation Strategies of Kaempferol, a Dietary Flavonoid. Rev. Bras. de Farm. 2022, 32, 693–707. [Google Scholar]
- Benameur, T.; Soleti, R.; Porro, C. The Potential Neuroprotective Role of Free and Encapsulated Quercetin Mediated by miRNA against Neurological Diseases. Nutrients 2021, 13, 1318. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Nakagawa, T.; Muro, T.; Sato, Y.; Satoh, H.; Kobori, M.; Nishihira, J. A randomized, double-blind, placebo-controlled study evaluating the effects of quercetin-rich onion on cognitive function in elderly subjects. Funct. Foods Heal. Dis. 2017, 7, 353. [Google Scholar] [CrossRef]
- Broman-Fulks, J.J.; Canu, W.H.; Trout, K.L.; Nieman, D.C. The effects of quercetin supplementation on cognitive functioning in a community sample: A randomized, placebo-controlled trial. Ther. Adv. Psychopharmacol. 2012, 2, 131–138. [Google Scholar] [CrossRef]
- Nakagawa, T.; Itoh, M.; Ohta, K.; Hayashi, Y.; Hayakawa, M.; Yamada, Y.; Akanabe, H.; Chikaishi, T.; Nakagawa, K.; Itoh, Y.; et al. Improvement of memory recall by quercetin in rodent contextual fear conditioning and human early-stage Alzheimer’s disease patients. Neuroreport 2016, 27, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H.; Yin, T.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J. Colloid Interface Sci. 2019, 552, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Rananaware, P.; Pandit, P.; Naik, S.; Mishra, M.; Keria, R.S.; Brahmkhatri, V.P. Anti-amyloidogenic property of gold nanoparticle decorated querc. RSC Adv. 2022, 12, 23661–23674. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, Y.; Li, X.; Chen, X.; Lin, X.; Wu, X. Develop potential multi-target drugs by self-assembly of quercetin with amino acids and metal ion to achieve significant efficacy in anti-Alzheimer’s disease. Nano Res. 2022, 15, 5173–5182. [Google Scholar]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- Karthika, C.; Appu, A.P.; Akter, R.; Rahman, H.; Tagde, P.; Ashraf, G.M.; Abdel-Daim, M.M.; Ul Hassan, S.S.; Abid, A.; Bungau, S. Potential innovation against Alzheimer’s disorder: A tricomponent combination of natural antioxidants (vitamin E, quercetin, and basil oil) and the development of its intranasal delivery. Environ. Sci. Pollut. Res. 2022, 29, 10950–10965. [Google Scholar]
- Alaqeel, N.K.; AlSheikh, M.H.; Al-Hariri, M.T. Quercetin Nanoemulsion Ameliorates Neuronal Dysfunction in Experimental Alzheimer’s Disease Model. Antioxidants 2022, 11, 19866. [Google Scholar] [CrossRef]
- Center for Food Safety and Applied Nutrition. (n.d.). Gras Notice Inventory—Agency Response Letter Gras Notice no. GRN 000341. Archive. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=341 (accessed on 6 October 2023).
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J. Biol. Chem. 2003, 278, 34783–34793. [Google Scholar] [CrossRef]
- Kimoto, H.; Fujiwara, S.; Koyama, N.; Uesugi, T. Genotoxicity and subchronic toxicity of a kaempferol aglycone-rich product produced from horseradish leaves. Fundam. Toxicol. Sci. 2022, 9, 71–83, 2189-115X. [Google Scholar] [CrossRef]
- Yangzom, P.; Amruthanand, S.; Sharma, M.; Mahajan, S.; Lingaraju, M.C.; Parida, S.; Sahoo, M.; Kumar, D.; Singh, T.U. Subacute 28 days oral toxicity study of kaempferol and biochanin-A in the mouse model. J. Biochem. Mol. Toxicol. 2022, 36, e23090. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Mizokami, T.; Ito, H.; Ikeda, Y. A randomized, placebo-controlled trial evaluating the safety of excessive administration of kaempferol aglycone. Food Sci. Nutr. 2023, 11, 5427–5437. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.J.; Tako, E.; Wiesinger, J.; Glahn, R.P. Polyphenolic Profiles of Yellow Bean Seed Coats and Their Relationship with Iron Bioavailability. J. Agric. Food Chem. 2020, 68, 769–778. [Google Scholar] [CrossRef]
- Vaez, S.; Parivr, K.; Amidi, F.; Rudbari, N.H.; Moini, A.; Amini, N. Quercetin and polycystic ovary syndrome; inflammation, hormonal parameters and pregnancy outcome: A randomized clinical trial. Am. J. Reprod. Immunol. 2023, 89, e13644. [Google Scholar] [CrossRef]
- Thakur, K.; Zhu, Y.Y.; Feng, J.Y.; Zhang, J.G.; Hu, F.; Prasad, C.; Wei, Z.J. Morin as an imminent functional food ingredient: An update on its enhanced efficacy in the treatment and prevention of metabolic syndromes. Food Funct. 2020, 11, 8424–8443. [Google Scholar] [CrossRef]
- Carmona, V.; Martín-Aragón, S.; Goldberg, J.; Schubert, D.; Bermejo-Bescós, P. Several targets involved in Alzheimer’s disease amyloidogenesis are affected by morin and isoquercitrin. Nutr. Neurosci. 2020, 23, 575–590. [Google Scholar] [CrossRef]
- Gur, C.; Kandemir, F.M.; Darendelioglu, E.; Caglayan, C.; Kucukler, S.; Kandemir, O.; Ileriturk, M. Morin protects against acrylamide-induced neurotoxicity in rats: An investigation into different signal pathways. Environ. Sci. Pollut. Res. 2021, 28, 49808–49819. [Google Scholar] [CrossRef]
- Mohammadi, N.; Asle-Rousta, M.; Rahnema, M.; Amini, R. Morin attenuates memory deficits in a rat model of Alzheimer’s disease by ameliorating oxidative stress and neuroinflammation. Eur. J. Pharmacol. 2021, 910, 174506. [Google Scholar] [CrossRef]
- Soubh, A.A.; El-Gazar, A.A.; Mohamed, E.A.; Awad, A.S.; El-Abhar, H.S. Further insights for the role of Morin in mRTBI: Implication of non-canonical Wnt/PKC-α and JAK-2/STAT-3 signaling pathways. Int. Immunopharmacol. 2021, 100, 108123. [Google Scholar] [CrossRef]
- Thabet, N.M.; Moustafa, E.M. Protective effect of rutin against brain injury induced by acrylamide or gamma radiation: Role of PI3K/AKT/GSK-3β/NRF-2 signalling pathway. Arch. Physiol. Biochem. 2018, 124, 185–193. [Google Scholar] [CrossRef]
- Çelik, H.; Kandemir, F.M.; Caglayan, C.; Özdemir, S.; Çomaklı, S.; Kucukler, S.; Yardım, A. Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol. Biol. Rep. 2020, 47, 2023–2034. [Google Scholar] [CrossRef]
- Ahmad, S.; Jo, M.H.; Ikram, M.; Khan, A.; Kim, M.O. Deciphering the potential neuroprotective effects of luteolin against Aβ1–42-Induced alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 9583. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, X. Luteolin Attenuates Cognitive Dysfunction Induced By Chronic Cerebral Hypoperfusion Through the Modulation of The PI3K/Akt Pathway in Rats. J. Vet. Res. 2021, 65, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Sawmiller, D.; Li, S.; Shahaduzzaman; Smith, A.J.; Obregon, D.; Giunta, B.; Borlongan, C.V.; Sanberg, P.R.; Tan, J. Luteolin reduces Alzheimer’s disease pathologies induced by traumatic brain injury. Int. J. Mol. Sci. 2014, 15, 895–904. [Google Scholar] [CrossRef]
- Zeng, P.; Su, H.F.; Ye, C.Y.; Qiu, S.W.; Shi, A.; Wang, J.Z.; Zhou, X.W.; Tian, Q. A Tau Pathogenesis-Based Network Pharmacology Approach for Exploring the Protections of Chuanxiong Rhizoma in Alzheimer’s Disease. Front. Pharmacol. 2022, 13, 877806. [Google Scholar] [CrossRef]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxidative Med. Cell. Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef] [PubMed]
- Michala, A.S.; Pritsa, A. Quercetin: A Molecule of Great Biochemical and Clinical Value and Its Beneficial Effect on Diabetes and Cancer. Diseases 2022, 10, 37. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Y.L.; Yang, H.; Wang, Y.H.; Du, G.H. Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 release and down-regulating TLR4/MyD88 pathway. Int. Immunopharmacol. 2018, 56, 29–35. [Google Scholar] [CrossRef]
- Yang, Y.L.; Cheng, X.; Li, W.H.; Liu, M.; Wang, Y.H.; Du, G.H. Kaempferol Attenuates LPS-Induced Striatum Injury in Mice Involving Anti-Neuroinflammation, Maintaining BBB Integrity, and Down-Regulating the HMGB1/TLR4 Pathway. Int. J. Mol. Sci. 2019, 20, 491. [Google Scholar] [CrossRef]
- Silva Dos Santos, J.; Gonçalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef] [PubMed]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; El-Aleem, S.A.; El-Tahawy, N.F. Neuroprotective effect of quercetin nanoparticles: A possible prophylactic and therapeutic role in Alzheimer’s disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Pantsar, T.; Poso, A. Binding Affinity via Docking: Fact and Fiction. Molecules 2018, 23, 1899. [Google Scholar] [CrossRef]
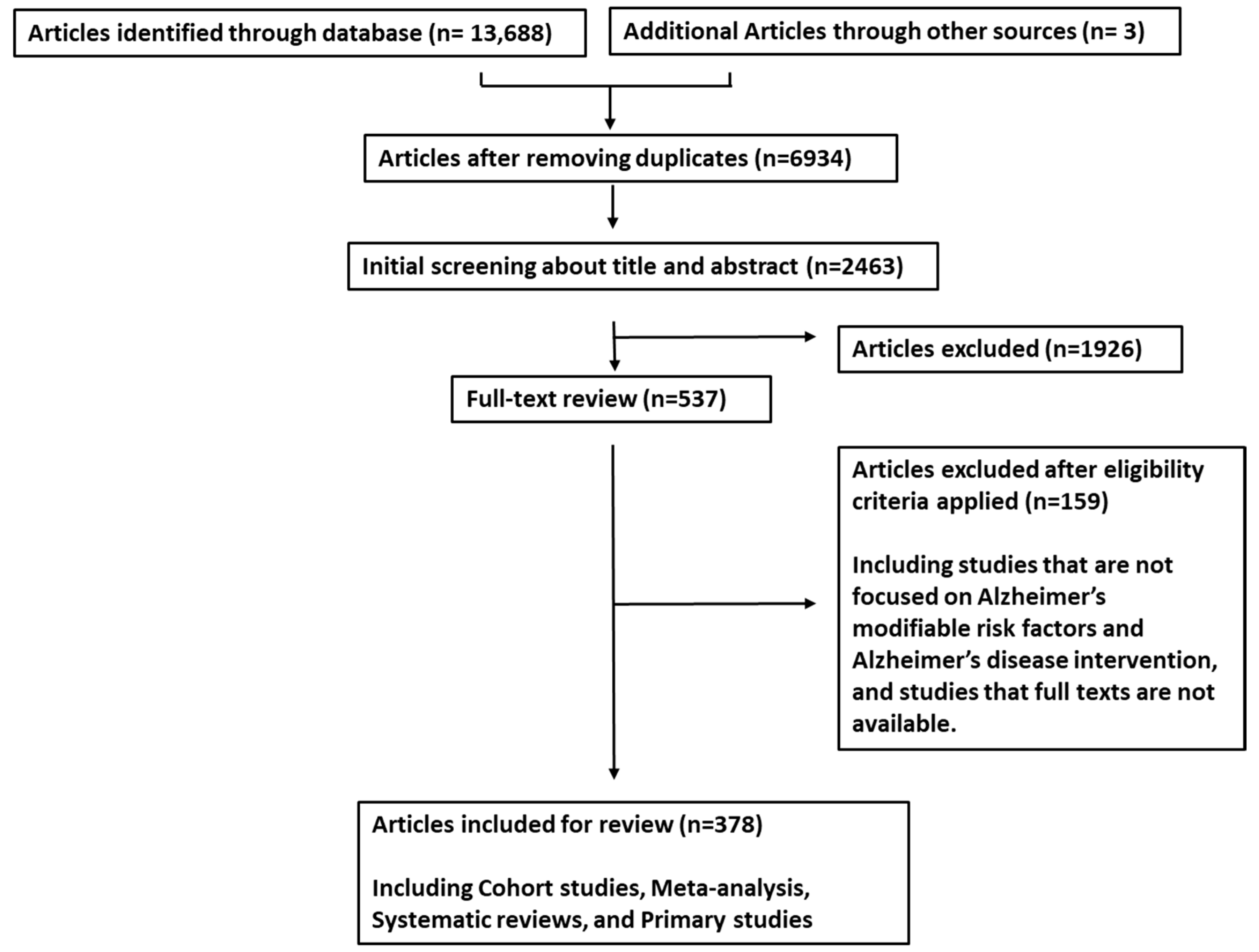
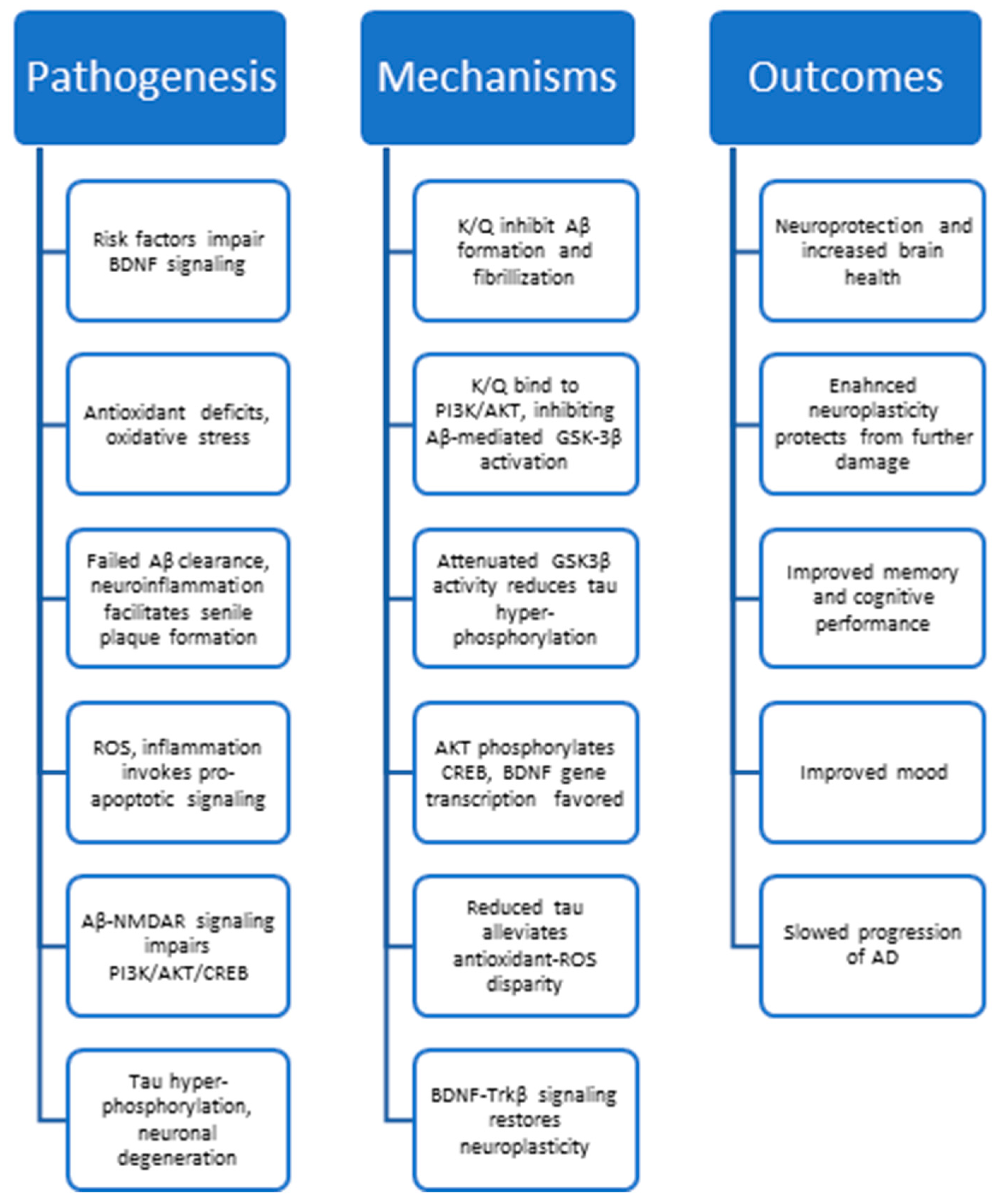
| Molecular Target | Phytochemical | Mechanism | Affinity (DS, BE, or IC50) | References |
|---|---|---|---|---|
| GSK-3β | Kaempferol | Inhibit | 4.6 (DS, mice); −7.9 kcal/mol (human brain docking) −9.2 kcal/mol (zebrafish) | [243,250,251] |
| Quercetin | Inhibit | 5.64 (DS); −8.8 kcal/mol (human brain docking) −9.0 kcal/mol (zebrafish) | [243,250,251] | |
| Aβ | Kaempferol | Inhibit | Indirect | [171] |
| Quercetin | Inhibit | Indirect | [252] | |
| BACE1 | Kaempferol | Inhibit | IC50 = 14.7 µM | [253,254] |
| Quercetin | Inhibit | IC50 = 5.4 µM | [253,254] | |
| Tau | Kaempferol | Inhibit hyperactivation | Indirect | [47] |
| Quercetin | Inhibit hyperactivation | Indirect | [255] | |
| PI3K | Kaempferol | Activate | 5.19 (DS, neurons) | [256] |
| Quercetin | Activate | 7.04 (MD, neurons) | [256] | |
| AKT1 | Kaempferol | Activate | 5.13 (MD, neurons); −9.3 kcal/mol | [256,257] |
| Quercetin | Activate | 5.03 (MD, neurons), −9.4 kcal/mol; −7.96 kcal/mol | [213,256,257] | |
| BDNF | Kaempferol | Upregulate | Indirect | [258] |
| Quercetin | Upregulate | Indirect | [252] | |
| CREB | Kaempferol | Activate | Indirect | [211] |
| Quercetin | Activate | Indirect | [252] | |
| NMDAR | Kaempferol | Reverse Aβ binding | −10.84 kcal/mol | [259] |
| Quercetin | Reverse Aβ binding | Indirect | [255,260] | |
| HDAC | Kaempferol | Activate | Not Found | [188,189] |
| Quercetin | Activate | IC50 = 105.1 µM | [261] | |
| AChE | Kaempferol | Inhibit | −10.26 kcal/mol; between −8.6 and −9.22 kcal/mol | [259,262,263] |
| Quercetin | Inhibit | −7.9 kcal/mol; IC50 = 4.59 ± 0.27 µM | [155,263] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexander, C.; Parsaee, A.; Vasefi, M. Polyherbal and Multimodal Treatments: Kaempferol- and Quercetin-Rich Herbs Alleviate Symptoms of Alzheimer’s Disease. Biology 2023, 12, 1453. https://doi.org/10.3390/biology12111453
Alexander C, Parsaee A, Vasefi M. Polyherbal and Multimodal Treatments: Kaempferol- and Quercetin-Rich Herbs Alleviate Symptoms of Alzheimer’s Disease. Biology. 2023; 12(11):1453. https://doi.org/10.3390/biology12111453
Chicago/Turabian StyleAlexander, Claire, Ali Parsaee, and Maryam Vasefi. 2023. "Polyherbal and Multimodal Treatments: Kaempferol- and Quercetin-Rich Herbs Alleviate Symptoms of Alzheimer’s Disease" Biology 12, no. 11: 1453. https://doi.org/10.3390/biology12111453
APA StyleAlexander, C., Parsaee, A., & Vasefi, M. (2023). Polyherbal and Multimodal Treatments: Kaempferol- and Quercetin-Rich Herbs Alleviate Symptoms of Alzheimer’s Disease. Biology, 12(11), 1453. https://doi.org/10.3390/biology12111453






