Heteroplasmy and Individual Mitogene Pools: Characteristics and Potential Roles in Ecological Studies
Simple Summary
Abstract
1. Introduction
2. Characteristics of mtGenomic Heteroplasmy
3. The Influence of Heteroplasmy on Cellular Functions
4. Dynamics of Heteroplasmy
4.1. Generation of Variants during Ontogenesis
4.2. Tolerance to Heteroplasmy and Elimination
4.3. Dynamics of Heteroplasmy in an Individual and across Generations
5. Perspectives for Wildlife Research
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, W.M.; Prager, E.M.; Wang, A.; Wilson, A.C. Mitochondrial DNA sequences of primates: Tempo and mode of evolution. J. Mol. Evol. 1982, 18, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.G. Animal mitochondrial DNA as a genetic marker in population and evolutionary biology. Trends Ecol. Evol. 1989, 4, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Solignac, M.; Monnerot, M.; Mounolou, J.C. Mitochondrial DNA heteroplasmy in Drosophila mauritiana. Proc. Natl. Acad. Sci. USA 1983, 80, 6942–6946. [Google Scholar] [CrossRef] [PubMed]
- Melton, T. Mitochondrial DNA Heteroplasmy. Forensic Sci. Rev. 2004, 16, 1. [Google Scholar]
- Parakatselaki, M.E.; Ladoukakis, E.D. mtDNA Heteroplasmy: Origin, Detection, Significance, and Evolutionary Consequences. Life 2021, 11, 633. [Google Scholar] [CrossRef]
- Zheng, W.; Khrapko, K.; Coller, H.A.; Thilly, W.G.; Copeland, W.C. Origins of human mitochondrial point mutations as DNA polymerase gamma-mediated errors. Mutat. Res. 2006, 599, 11–20. [Google Scholar] [CrossRef]
- Shokolenko, I.; Venediktova, N.; Bochkareva, A.; Wilson, G.L.; Alexeyev, M.F. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009, 37, 2539–2548. [Google Scholar] [CrossRef]
- Fontana, G.A.; Gahlon, H.L. Mechanisms of replication and repair in mitochondrial DNA deletion formation. Nucleic Acids Res. 2020, 48, 11244–11258. [Google Scholar] [CrossRef]
- Rand, D.M. The Units of Selection on Mitochondrial DNA. Annu. Rev. Ecol. Syst. 2001, 32, 415–448. [Google Scholar] [CrossRef]
- Zhang, R.; Nakahira, K.; Choi, A.M.K.; Gu, Z. Heteroplasmy concordance between mitochondrial DNA and RNA. Sci. Rep. 2019, 9, 12942. [Google Scholar] [CrossRef]
- Gong, S.; Wang, X.; Meng, F.; Cui, L.; Yi, Q. Overexpression of mitochondrial histidyl-tRNA synthetase restores mitochondrial dysfunction caused by a deafness-associated tRNA(His) mutation. J. Biol. Chem. 2020, 295, 940–954. [Google Scholar] [CrossRef]
- Gong, S.; Peng, Y.; Jiang, P.; Wang, M.; Fan, M.; Wang, X.; Zhou, H.; Li, H.; Yan, Q.; Huang, T.; et al. A deafness-associated tRNAHis mutation alters the mitochondrial function, ROS production and membrane potential. Nucleic Acids Res. 2014, 42, 8039–8048. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowska, J.; Malinska, D.; Wieckowski, M.R.; Duszynski, J. Effect of mtDNA point mutations on cellular bioenergetics. Biochim. Biophys. Acta 2012, 1817, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, N.J.; Allendorf, F.W. Mitochondrial mutations may decrease population viability. Trends Ecol. Evol. 2001, 16, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, N.; Aita, T.; Motooka, D.; Nakamura, S.; Yomo, T. Periodic Pattern of Genetic and Fitness Diversity during Evolution of an Artificial Cell-Like System. Mol. Biol. Evol. 2015, 32, 3205–3214. [Google Scholar] [CrossRef]
- Li, M.; Rothwell, R.; Vermaat, M.; Wachsmuth, M.; Schröder, R.; Laros, J.F.; van Oven, M.; de Bakker, P.I.; Bovenberg, J.A.; van Duijn, C.M.; et al. Transmission of human mtDNA heteroplasmy in the Genome of the Netherlands families: Support for a variable-size bottleneck. Genome Res. 2016, 26, 417–426. [Google Scholar] [CrossRef]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef]
- Jones, C.K. The Distribution of Fitness Effects of Spontaneous Mutations in Vibrio Fischeri. 2014. Available online: https://www.proquest.com/dissertations-theses/rate-spectrum-effects-spontaneous-mutation/docview/1803262712/se-2?accountid=28189 (accessed on 6 October 2023).
- Lin, J. Characterizing the Frequency of Heteroplasmy in Mitochondrial DNA of Tissues Using Next-Generation Sequencing. Master’s Thesis, University of California, Davis, CA, USA, 2016. [Google Scholar]
- Yamato, K.T.; Newton, K.J. Heteroplasmy and homoplasmy for maize mitochondrial mutants: A rare homoplasmic nad4 deletion mutant plant. J. Hered. 1999, 90, 369–373. [Google Scholar] [CrossRef]
- Kmiec, B.; Woloszynska, M.; Janska, H. Heteroplasmy as a common state of mitochondrial genetic information in plants and animals. Curr. Genet. 2006, 50, 149–159. [Google Scholar] [CrossRef]
- de la Providencia, I.E.; Nadimi, M.; Beaudet, D.; Morales, G.R.; Hijri, M. Detection of a transient mitochondrial DNA heteroplasmy in the progeny of crossed genetically divergent isolates of arbuscular mycorrhizal fungi. New Phytol. 2013, 200, 211–221. [Google Scholar] [CrossRef]
- Kunkel, T.A.; Bebenek, K. DNA replication fidelity. Annu. Rev. Biochem. 2000, 69, 497–529. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, M.; Bebenek, K. Multiple functions of DNA polymerases. Crit. Rev. Plant Sci. 2007, 26, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C.; Chalkia, D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a021220. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Van Dyken, C.; Darby, H.; Mikhalchenko, A.; Marti-Gutierrez, N.; Koski, A.; Liang, D.; Li, Y.; Tippner-Hedges, R.; Kang, E.; et al. Germline transmission of donor, maternal and paternal mtDNA in primates. Hum. Reprod. 2021, 36, 493–505. [Google Scholar] [CrossRef]
- Li, M.; Schröder, R.; Ni, S.; Madea, B.; Stoneking, M. Extensive tissue-related and allele-related mtDNA heteroplasmy suggests positive selection for somatic mutations. Proc. Natl. Acad. Sci. USA 2015, 112, 2491–2496. [Google Scholar] [CrossRef]
- Krjutškov, K.; Koltšina, M.; Grand, K.; Võsa, U.; Sauk, M.; Tõnisson, N.; Salumets, A. Tissue-specific mitochondrial heteroplasmy at position 16,093 within the same individual. Curr. Genet. 2014, 60, 11–16. [Google Scholar] [CrossRef]
- Gu, X.; Kang, X.; Liu, J. Mutation signatures in germline mitochondrial genome provide insights into human mitochondrial evolution and disease. Hum. Genet. 2019, 138, 613–624. [Google Scholar] [CrossRef]
- Naue, J.; Hörer, S.; Sänger, T.; Strobl, C.; Hatzer-Grubwieser, P.; Parson, W.; Lutz-Bonengel, S. Evidence for frequent and tissue-specific sequence heteroplasmy in human mitochondrial DNA. Mitochondrion 2015, 20, 82–94. [Google Scholar] [CrossRef]
- Neupane, J.; Ghimire, S.; Vandewoestyne, M.; Lu, Y.; Gerris, J.; Van Coster, R.; Deroo, T.; Deforce, D.; Vansteelandt, S.; De Sutter, P.; et al. Cellular Heterogeneity in the Level of mtDNA Heteroplasmy in Mouse Embryonic Stem Cells. Cell Rep. 2015, 13, 1304–1309. [Google Scholar] [CrossRef]
- Ma, H.; Lee, Y.; Hayama, T.; Van Dyken, C.; Marti-Gutierrez, N.; Li, Y.; Ahmed, R.; Koski, A. Germline and somatic mtDNA mutations in mouse aging. PLoS ONE 2018, 13, e0201304. [Google Scholar] [CrossRef]
- Tostes, K.; Dos Santos, A.C. Autophagy deficiency abolishes liver mitochondrial DNA segregation. Autophagy 2022, 18, 2397–2408. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xu, L.; Xu, C.; Cui, Y.; Jiang, S.; Dong, J.; Liao, L. The Mutations and Clinical Variability in Maternally Inherited Diabetes and Deafness: An Analysis of 161 Patients. Front. Endocrinol. 2021, 12, 728043. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Freire, M.; Moore, A.Z.; Peterson, C.A.; Kosmac, K.; McDermott, M.M.; Sufit, R.L.; Guralnik, J.M.; Polonsky, T.; Tian, L.; Kibbe, M.R.; et al. Associations of Peripheral Artery Disease With Calf Skeletal Muscle Mitochondrial DNA Heteroplasmy. J. Am. Heart Assoc. 2020, 9, e015197. [Google Scholar] [CrossRef] [PubMed]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Ye, K.; Picard, M.; Gu, Z. Independent impacts of aging on mitochondrial DNA quantity and quality in humans. BMC Genom. 2017, 18, 890. [Google Scholar] [CrossRef]
- Nicholls, T.J.; Minczuk, M. In D-loop: 40 years of mitochondrial 7S DNA. Exp. Gerontol. 2014, 56, 175–181. [Google Scholar] [CrossRef]
- Froman, D.P.; Pizzari, T.; Feltmann, A.J.; Castillo-Juarez, H.; Birkhead, T.R. Sperm mobility: Mechanisms of fertilizing efficiency, genetic variation and phenotypic relationship with male status in the domestic fowl, Gallus gallus domesticus. Proc. Biol. Sci. 2002, 269, 607–612. [Google Scholar] [CrossRef]
- Froman, D.P.; Kirby, J.D. Sperm mobility: Phenotype in roosters (Gallus domesticus) determined by mitochondrial function. Biol. Reprod. 2005, 72, 562–567. [Google Scholar] [CrossRef]
- Kretzschmar, C.; Roolf, C.; Timmer, K.; Sekora, A.; Knübel, G.; Murua Escobar, H.; Fuellen, G.; Ibrahim, S.M.; Tiedge, M.; Baltrusch, S.; et al. Polymorphisms of the murine mitochondrial ND4, CYTB and COX3 genes impact hematopoiesis during aging. Oncotarget 2016, 7, 74460–74472. [Google Scholar] [CrossRef]
- Farge, G.; Touraille, S.; Debise, R.; Alziari, S. The respiratory chain complex thresholds in mitochondria of a Drosophila subobscura mutant strain. Biochimie 2002, 84, 1189–1197. [Google Scholar] [CrossRef]
- Pérez-Amado, C.J.; Bazan-Cordoba, A.; Hidalgo-Miranda, A. Mitochondrial Heteroplasmy Shifting as a Potential Biomarker of Cancer Progression. Int. J. Mol. Sci. 2021, 22, 7369. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Austin, S.; St-Pierre, J. PGC1α and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Virbasius, J.V.; Scarpulla, R.C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 1309–1313. [Google Scholar] [CrossRef]
- Gemmell, N.J.; Metcalf, V.J.; Allendorf, F.W. Mother’s curse: The effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 2004, 19, 238–244. [Google Scholar] [CrossRef]
- Stewart, J.B.; Chinnery, P.F. The dynamics of mitochondrial DNA heteroplasmy: Implications for human health and disease. Nat. Rev. Genet. 2015, 16, 530–542. [Google Scholar] [CrossRef]
- Ding, Y.; Xia, B.-H.; Liu, Q.; Li, M.-Y.; Huang, S.-X.; Zhuo, G.-C. Allele-specific PCR for detecting the deafness-associated mitochondrial 12S rRNA mutations. Gene 2016, 591, 148–152. [Google Scholar] [CrossRef]
- Xiang, Y.-B.; Tang, S.-H.; Li, H.-Z.; Xu, C.-Y.; Chen, C.; Xu, Y.-Z.; Ding, L.-R.; Xu, X.-Q. Mutation analysis of common deafness-causing genes among 506 patients with nonsyndromic hearing loss from Wenzhou city, China. Int. J. Pediatr. Otorhinolaryngol. 2019, 122, 185–190. [Google Scholar] [CrossRef]
- Dai, P.; Huang, L.-H.; Wang, G.-J.; Gao, X.; Qu, C.-Y.; Chen, X.-W.; Ma, F.-R.; Zhang, J.; Xing, W.-L.; Xi, S.-Y.; et al. Concurrent Hearing and Genetic Screening of 180,469 Neonates with Follow-up in Beijing, China. Am. J. Hum. Genet. 2019, 105, 803–812. [Google Scholar] [CrossRef]
- Mutai, H.; Watabe, T.; Kosaki, K.; Ogawa, K.; Matsunaga, T. Mitochondrial mutations in maternally inherited hearing loss. BMC Med. Genet. 2017, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Zarrouk-Mahjoub, S. Infantile-onset deafness in m.7445A>G carriers may be multicausal. Int. J. Pediatr. Otorhinolaryngol. 2018, 111, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Mitochondrial Cardiomyopathy Due to the MT-TI Variant m.4300A>G Requires Comprehensive Clinical and Genetic Workup. Radiol. Cardiothorac. Imaging 2023, 5, e230144. [Google Scholar] [CrossRef]
- Perli, E.; Giordano, C.; Tuppen, H.A.L.; Montopoli, M.; Montanari, A.; Orlandi, M.; Pisano, A.; Catanzaro, D.; Caparrotta, L.; Musumeci, B.; et al. Isoleucyl-tRNA synthetase levels modulate the penetrance of a homoplasmic m.4277T>C mitochondrial tRNAIle mutation causing hypertrophic cardiomyopathy. Hum. Mol. Genet. 2012, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, X.; Zhao, L.; Shi, Y.; Zhou, S.; Wang, Y. Clinical Profile and Outcome of Pediatric Mitochondrial Myopathy in China. Front. Neurol. 2020, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Ma, M.-M.; Pang, M.; Yang, L.; Li, G.; Song, J.; Zhang, J.-W. Broadening the phenotype of m.5703G>A mutation in mitochondrial tRNAAsn gene from mitochondrial myopathy to myoclonic epilepsy with ragged red fibers syndrome. Chin. Med. J. 2019, 132, 865–867. [Google Scholar] [CrossRef]
- Souilem, S.; Chebel, S.; Mancuso, M.; Petrozzi, L.; Siciliano, G.; FrihAyed, M.; Hentati, F.; Amouri, R. A novel mitochondrial tRNAIle point mutation associated with chronic progressive external ophthalmoplegia and hyperCKemia. J. Neurol. Sci. 2011, 300, 187–190. [Google Scholar] [CrossRef]
- Mimaki, M.; Hatakeyama, H.; Komaki, H.; Yokoyama, M.; Arai, H.; Kirino, Y.; Suzuki, T.; Nishino, I.; Nonaka, I.; Goto, Y.-i. Reversible Infantile Respiratory Chain Deficiency: A Clinical and Molecular Study. Ann. Neurol. 2010, 68, 845–854. [Google Scholar] [CrossRef]
- Mezghani, N.; Mkaouar-Rebai, E.; Mnif, M.; Charfi, N.; Rekik, N.; Youssef, S.; Abid, M.; Fakhfakh, F. The heteroplasmic m.14709T>C mutation in the tRNAGlu gene in two Tunisian families with mitochondrial diabetes. J. Diabetes Its Complicat. 2010, 24, 270–277. [Google Scholar] [CrossRef]
- Ballhausen, D.; Guerry, F.; Hahn, D.; Schaller, A.; Nuoffer, J.-M.; Bonafe, L.; Jeannet, P.-Y.; Jacquemont, S. Mitochondrial tRNALeu(UUR) mutation m.3302A>G presenting as childhood-onset severe myopathy: Threshold determination through segregation study. J. Inherit. Metab. Dis. 2010, 33, S219–S226. [Google Scholar] [CrossRef]
- Blakely, E.L.; Alston, C.L.; Lecky, B.; Chakrabarti, B.; Falkous, G.; Turnbull, D.M.; Taylor, R.W.; Gorman, G.S. Distal weakness with respiratory insufficiency caused by the m.8344A>G “MERRF” mutation. Neuromuscul. Disord. 2014, 24, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Yabe, I.; Sudo, A.; Hosoki, K.; Yaguchi, H.; Saitoh, S.; Sasaki, H. MERRF/MELAS overlap syndrome: A double pathogenic mutation in mitochondrial tRNA genes. J. Med. Genet. 2010, 47, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Zarrouk-Mahjoub, S. MELAS/Leigh overlap syndrome due to the ND6 mutation m.10158T>C. Brain Dev. 2017, 39, 724. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Z.A.; Cabrera-Orefice, A.; Brandt, U. In-depth characterization of the impacts of Leigh syndrome-causing mutation m.10191T>C in subunit MT-ND3 on the molecular mechanism of human mitochondrial complex I. Biochim. Biophys. Acta-Bioenerg. 2022, 1863, 17. [Google Scholar] [CrossRef]
- Hechmi, M.; Charif, M.; Kraoua, I.; Fassatoui, M.; Dallali, H.; Desquiret-Dumas, V.; Bris, C.; Goudenege, D.; Drissi, C.; Galai, S.; et al. Next-generation sequencing of Tunisian Leigh syndrome patients reveals novel variations: Impact for diagnosis and treatment. Biosci. Rep. 2022, 42, BSR20220194. [Google Scholar] [CrossRef]
- Yu, D.; Jia, X.; Zhang, A.M.; Guo, X.; Zhang, Y.-P.; Zhang, Q.; Yao, Y.-G. Molecular characterization of six Chinese families with m.3460G>A and Leber hereditary optic neuropathy. Neurogenetics 2010, 11, 349–356. [Google Scholar] [CrossRef]
- Jiang, P.; Liang, M.; Zhang, C.; Zhao, X.; He, Q.; Cui, L.; Liu, X.; Sun, Y.-H.; Fu, Q.; Ji, Y.; et al. Biochemical evidence for a mitochondrial genetic modifier in the phenotypic manifestation of Leber’s hereditary optic neuropathy-associated mitochondrial DNA mutation. Hum. Mol. Genet. 2016, 25, 3613–3625. [Google Scholar] [CrossRef]
- Yu, D.; Jia, X.; Zhang, A.M.; Li, S.; Zou, Y.; Zhang, Q.; Yao, Y.-G. Mitochondrial DNA Sequence Variation and Haplogroup Distribution in Chinese Patients with LHON and m.14484T>C. PLoS ONE 2010, 5, e13426. [Google Scholar] [CrossRef]
- Kaguni, L.S. DNA polymerase gamma, the mitochondrial replicase. Annu. Rev. Biochem. 2004, 73, 293–320. [Google Scholar] [CrossRef]
- Johnson, A.A.; Johnson, K.A. Fidelity of nucleotide incorporation by human mitochondrial DNA polymerase. J. Biol. Chem. 2001, 276, 38090–38096. [Google Scholar] [CrossRef]
- Larsson, N.G. Somatic mitochondrial DNA mutations in mammalian aging. Annu. Rev. Biochem. 2010, 79, 683–706. [Google Scholar] [CrossRef] [PubMed]
- Miquel, J.; Economos, A.C.; Fleming, J.; Johnson, J.E. Mitochondrial role in cell aging. Exp. Gerontol. 1980, 15, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Wang, Y. LC-MS/MS Identification and Yeast Polymerase η Bypass of a Novel γ-Irradiation-Induced Intrastrand Cross-Link Lesion G[8−5]C. Biochemistry 2004, 43, 6745–6750. [Google Scholar] [CrossRef]
- Sabouri, N.; Viberg, J.; Goyal, D.K.; Johansson, E.; Chabes, A. Evidence for lesion bypass by yeast replicative DNA polymerases during DNA damage. Nucleic Acids Res. 2008, 36, 5660–5667. [Google Scholar] [CrossRef]
- McCulloch, S.D.; Kokoska, R.J.; Garg, P.; Burgers, P.M.; Kunkel, T.A. The efficiency and fidelity of 8-oxo-guanine bypass by DNA polymerases delta and eta. Nucleic Acids Res. 2009, 37, 2830–2840. [Google Scholar] [CrossRef] [PubMed]
- Matkarimov, B.T.; Saparbaev, M.K. DNA Repair and Mutagenesis in Vertebrate Mitochondria: Evidence for Asymmetric DNA Strand Inheritance. Adv. Exp. Med. Biol. 2020, 1241, 77–100. [Google Scholar] [PubMed]
- Ma, H.; Hayama, T.; Van Dyken, C.; Darby, H.; Koski, A.; Lee, Y.; Gutierrez, N.M.; Yamada, S.; Li, Y.; Andrews, M.; et al. Deleterious mtDNA mutations are common in mature oocytes. Biol. Reprod. 2020, 102, 607–619. [Google Scholar] [CrossRef]
- Copeland, W.C.; Ponamarev, M.V.; Nguyen, D.; Kunkel, T.A.; Longley, M.J. Mutations in DNA polymerase gamma cause error prone DNA synthesis in human mitochondrial disorders. Acta Biochim. Pol. 2003, 50, 155–167. [Google Scholar] [CrossRef]
- Knorre, D.A. Intracellular quality control of mitochondrial DNA: Evidence and limitations. Philos. Trans. R. Soc. B 2020, 375, 20190176. [Google Scholar] [CrossRef]
- Carelli, V.; Maresca, A.; Caporali, L.; Trifunov, S.; Zanna, C.; Rugolo, M. Mitochondria: Biogenesis and mitophagy balance in segregation and clonal expansion of mitochondrial DNA mutations. Int. J. Biochem. Cell Biol. 2015, 63, 21–24. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Nunn, C.J.; Goyal, S. Contingency and selection in mitochondrial genome dynamics. eLife 2022, 11, e76557. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Pellicer, A.; Lechuga-Vieco, A.V.; Johnston, I.G.; Hämäläinen, R.H.; Pellico, J.; Justo-Méndez, R.; Fernández-Toro, J.M.; Clavería, C.; Guaras, A.; Sierra, R.; et al. Regulation of Mother-to-Offspring Transmission of mtDNA Heteroplasmy. Cell Metab. 2019, 30, 1120–1130.e5. [Google Scholar] [CrossRef] [PubMed]
- Jenuth, J.P.; Peterson, A.C.; Fu, K.; Shoubridge, E.A. Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat. Genet. 1996, 14, 146–151. [Google Scholar] [CrossRef]
- Khrapko, K. Two ways to make an mtDNA bottleneck. Nat. Genet. 2008, 40, 134–135. [Google Scholar] [CrossRef]
- Cao, L.; Shitara, H.; Horii, T.; Nagao, Y.; Imai, H.; Abe, K.; Hara, T.; Hayashi, J.; Yonekawa, H. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat. Genet. 2007, 39, 386–390. [Google Scholar] [CrossRef]
- Cree, L.M.; Samuels, D.C.; de Sousa Lopes, S.C.; Rajasimha, H.K.; Wonnapinij, P.; Mann, J.R.; Dahl, H.H.; Chinnery, P.F. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat. Genet. 2008, 40, 249–254. [Google Scholar] [CrossRef]
- Zhang, H.; Esposito, M.; Pezet, M.G.; Aryaman, J. Mitochondrial DNA heteroplasmy is modulated during oocyte development propagating mutation transmission. Sci. Adv. Mater. 2021, 7, eabi5657. [Google Scholar] [CrossRef]
- LiLi, X.; Yuan, H. Mitochondrial DNA Replication and Its Regulation. Chin. J. Biochem. Mol. Biol. 2006, 22, 435–441. [Google Scholar]
- Dobson, F.S.; Murie, J.O.; Viblanc, V.A. Fitness Estimation for Ecological Studies: An Evaluation in Columbian Ground Squirrels. Front. Ecol. Evol. 2020, 8, 216. [Google Scholar] [CrossRef]
- Dagilis, A.J.; Matute, D.R. The fitness of an introgressing haplotype changes over the course of divergence and depends on its size and genomic location. PLoS Biol. 2023, 21, e3002185. [Google Scholar] [CrossRef] [PubMed]
- Palculict, M.E.; Zhang, V.W.; Wong, L.J.; Wang, J. Comprehensive Mitochondrial Genome Analysis by Massively Parallel Sequencing. Mitochondrial DNA Methods Protoc. 2016, 1351, 3–17. [Google Scholar]
- Legati, A.; Zanetti, N.; Nasca, A.; Peron, C.; Lamperti, C.; Lamantea, E.; Ghezzi, D. Current and New Next-Generation Sequencing Approaches to Study Mitochondrial DNA. J. Mol. Diagn. JMD 2021, 23, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Filges, S.; Yamada, E.; Ståhlberg, A. Impact of Polymerase Fidelity on Background Error Rates in Next-Generation Sequencing with Unique Molecular Identifiers/Barcodes. Sci. Rep. 2019, 9, 3503. [Google Scholar] [CrossRef]
- González, M.D.M.; Ramos, A.; Aluja, M.P.; Santos, C. Sensitivity of mitochondrial DNA heteroplasmy detection using Next Generation Sequencing. Mitochondrion 2020, 50, 88–93. [Google Scholar] [CrossRef]
- Keraite, I.; Becker, P. A method for multiplexed full-length single-molecule sequencing of the human mitochondrial genome. Nat. Commun. 2022, 13, 5902. [Google Scholar] [CrossRef]
- Just, R.S.; Irwin, J.A.; Parson, W. Mitochondrial DNA heteroplasmy in the emerging field of massively parallel sequencing. Forensic Sci. Int. Genet. 2015, 18, 131–139. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.Y.; Lee, J.H.; Lee, S.D. Assessment of mitochondrial DNA heteroplasmy detected on commercial panel using MPS system with artificial mixture samples. Int. J. Leg. Med. 2018, 132, 1049–1056. [Google Scholar] [CrossRef]
- Marquis, J.; Lefebvre, G.; Kourmpetis, Y.A.I.; Kassam, M.; Ronga, F.; De Marchi, U.; Wiederkehr, A.; Descombes, P. MitoRS, a method for high throughput, sensitive, and accurate detection of mitochondrial DNA heteroplasmy. BMC Genom. 2017, 18, 326. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Sato, H.; Kasai, K. Mitochondrial DNA heteroplasmy among hairs from single individuals. J. Forensic Sci. 2004, 49, 986–991. [Google Scholar] [CrossRef]
- Barrett, A.; Arbeithuber, B.; Zaidi, A.; Wilton, P.; Paul, I.M.; Nielsen, R.; Makova, K.D. Pronounced somatic bottleneck in mitochondrial DNA of human hair. Philos. Trans. R. Soc. 2020, 375, 20190175. [Google Scholar] [CrossRef] [PubMed]
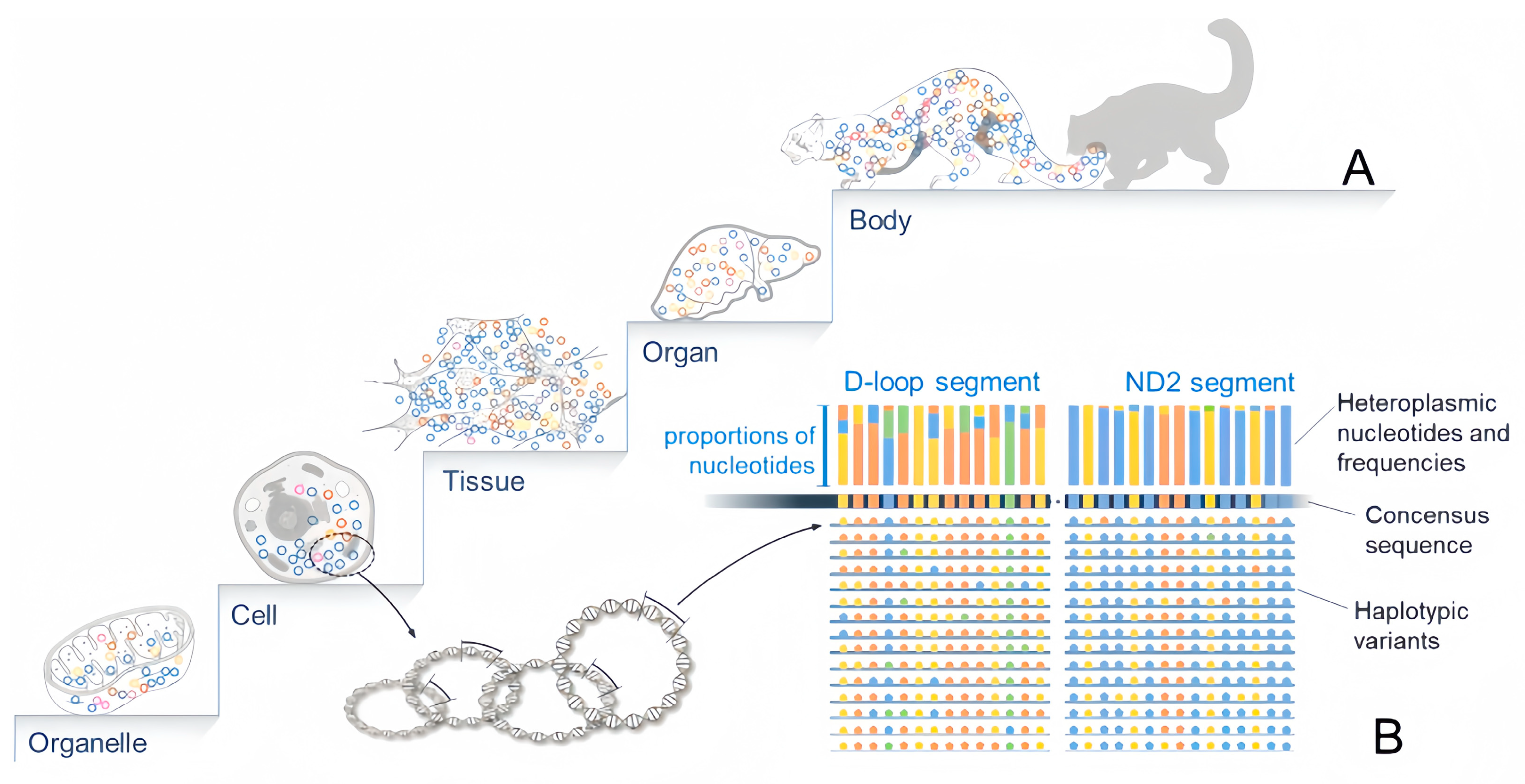
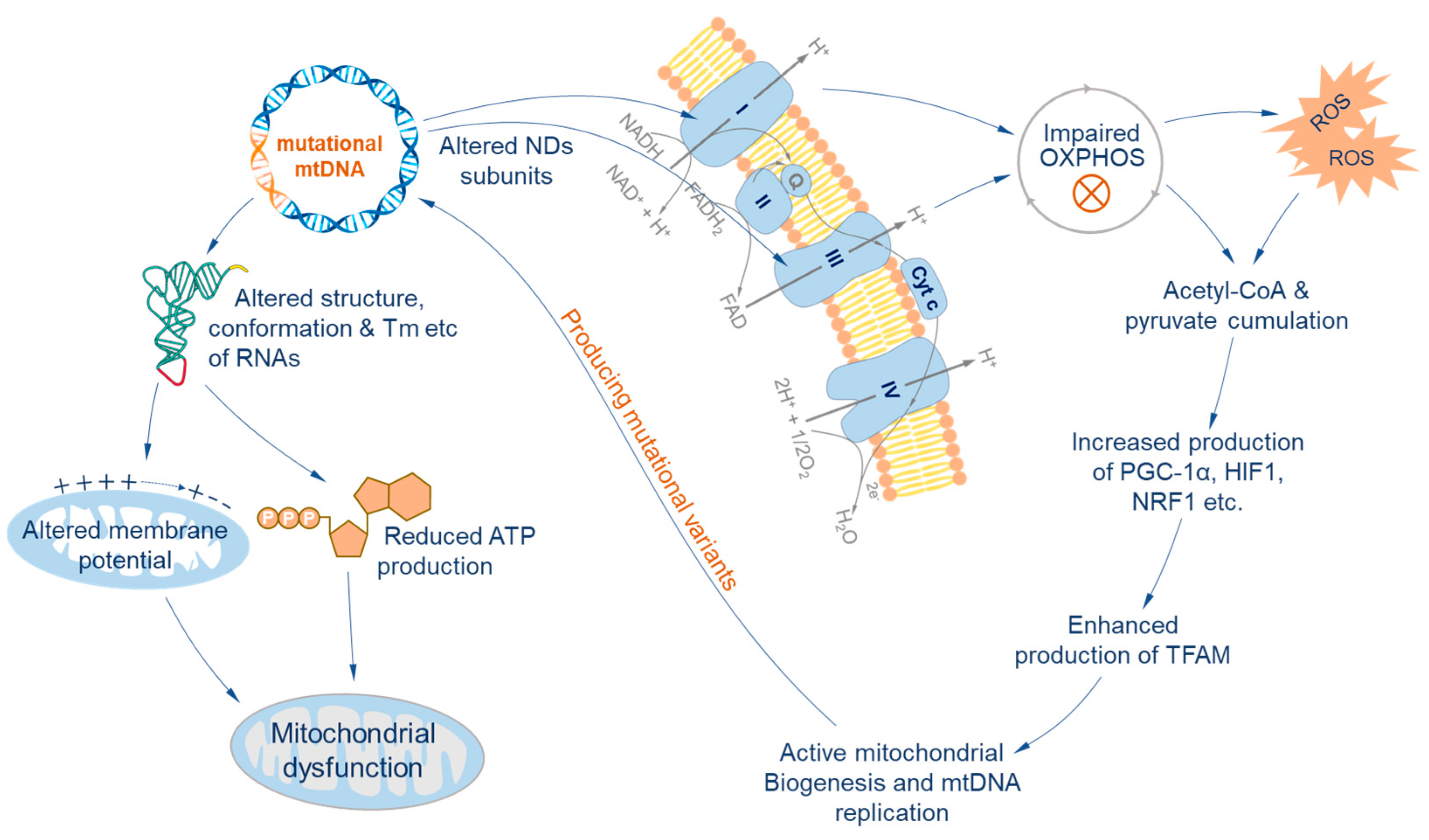

| Mitochondrial Diseases | Mutation Point | Gene | Reference |
|---|---|---|---|
| Deafness | m.1494C>T, m.1555A>G | 12S rRNA | Ding et al., 2016 [50], Xiang et al., 2019 [51], Dai et al., 2019 [52] |
| Deafness | m.1555A>G, m.3243A>G, m.7511T>C, m.7445A>G | tRNAVal, tRNALeu(UUR), tRNASer(UCN) | Mutai et al., 2017 [53], Finsterer and Zarrouk-Mahjoub, 2018 [54] |
| Mitochondrial Cardiomyopathy | m.4300A>G, m.3250T>C, m.3303C>T | tRNAIle, tRNALeu(UUR) | Finsterer, 2023 [55], Perli et al., 2012 [56], Hu et al., 2020 [57] |
| Myopathy | m.5703G>A, m.4308G>A, m.14674T>C, m.14709T>C, m.3302A>G | tRNAAsn, tRNAIle, tRNAGlu, tRNALam(CUN), tRNALeu(UUR), tRNASer(UCN) | Fu et al., 2019 [58], Souilem et al., 2011 [59], Mimaki et al., 2010 [60], Mezghani et al., 2010 [61], Ballhausen et al., 2010 [62] |
| MERRF | m.8344A>G, m.8356T>C | tRNAHis, tRNALys | Blakely et al., 2014 [63], Nakamura et al., 2010 [64], |
| CPEO | m.4308G>A | tRNIle | Souilem et al., 2011 [59] |
| Leigh’s Disease | m.10158T>C, m.10191T>C, m.10197G>A | ND3, ND4, ND5, ND6 | Finsterer and Zarrouk-Mahjoub, 2017 [65], Ahmadi et al., 2022 [66], Hechmi et al., 2022 [67] |
| LHOP | m.3460G>A, m.11778G>A, m.14484T>C | ND1, ND4, ND6 | Yu et al., 2010a [68], Jiang et al., 2016 [69], Yu et al., 2010b [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Lin, L.; Zhang, Q.; Yang, J.; Kamili, E.; Chu, J.; Li, X.; Yang, S.; Xu, Y. Heteroplasmy and Individual Mitogene Pools: Characteristics and Potential Roles in Ecological Studies. Biology 2023, 12, 1452. https://doi.org/10.3390/biology12111452
Wang W, Lin L, Zhang Q, Yang J, Kamili E, Chu J, Li X, Yang S, Xu Y. Heteroplasmy and Individual Mitogene Pools: Characteristics and Potential Roles in Ecological Studies. Biology. 2023; 12(11):1452. https://doi.org/10.3390/biology12111452
Chicago/Turabian StyleWang, Wenhui, Lijun Lin, Qi Zhang, Jincheng Yang, Elizabeth Kamili, Jianing Chu, Xiaoda Li, Shuhui Yang, and Yanchun Xu. 2023. "Heteroplasmy and Individual Mitogene Pools: Characteristics and Potential Roles in Ecological Studies" Biology 12, no. 11: 1452. https://doi.org/10.3390/biology12111452
APA StyleWang, W., Lin, L., Zhang, Q., Yang, J., Kamili, E., Chu, J., Li, X., Yang, S., & Xu, Y. (2023). Heteroplasmy and Individual Mitogene Pools: Characteristics and Potential Roles in Ecological Studies. Biology, 12(11), 1452. https://doi.org/10.3390/biology12111452







