Simple Summary
Adoptive cell therapy (ACT) is an innovative approach to combat cancer and infectious diseases using specialised immune cells, such as chimeric antigen receptor T-cells (CAR-Ts), tumour-infiltrating lymphocytes (TILs) and virus-specific T-cells (VSTs). Such therapies are manufactured individually for each patient and can be negatively affected by poor cell quality, often impaired by prior treatments, age, and complex manufacturing. To overcome this, this field is assessing the potential of creating cell therapies from “fit” donors to provide off-the-shelf treatment options. Induced pluripotent cells (iPSCs) have renewable characteristics and offer a solution towards off-the-shelf therapy. iPSCs can be used as an unlimited source to derive different immune cells, including natural killer (NK) cells and T-cells. iPSCs can be further genetically modified and used create different ACTs. In this review, we describe the methodologies for generating such cell therapies from iPSCs and discuss the current advances and challenges with a focus on CAR-T/NK-, TIL- and VST-based therapies.
Abstract
Adoptive cell therapy (ACT) has transformed the treatment landscape for cancer and infectious disease through the investigational use of chimeric antigen receptor T-cells (CAR-Ts), tumour-infiltrating lymphocytes (TILs) and viral-specific T-cells (VSTs). Whilst these represent breakthrough treatments, there are subsets of patients who fail to respond to autologous ACT products. This is frequently due to impaired patient T-cell function or “fitness” as a consequence of prior treatments and age, and can be exacerbated by complex manufacturing protocols. Further, the manufacture of autologous, patient-specific products is time-consuming, expensive and non-standardised. Induced pluripotent stem cells (iPSCs) as an allogeneic alternative to patient-specific products can potentially overcome the issues outlined above. iPSC technology provides an unlimited source of rejuvenated iPSC-derived T-cells (T-iPSCs) or natural killer (NK) cells (NK-iPSCs), and in the context of the growing field of allogeneic ACT, iPSCs have enormous potential as a platform for generating off-the-shelf, standardised, “fit” therapeutics for patients. In this review, we evaluate current and future applications of iPSC technology in the CAR-T/NK, TIL and VST space. We discuss current and next-generation iPSC manufacturing protocols, and report on current iPSC-based adoptive therapy clinical trials to elucidate the potential of this technology as the future of ACT.
1. Introduction
Adoptive cell therapy (ACT) using chimeric antigen receptor (CAR) modified T-cells/natural killer (NK) cells, tumour-infiltrating lymphocytes (TILs) and virus-specific T-cells (VSTs) has revolutionised the treatment of a number of cancers and infectious diseases.
CARs are synthetic receptors designed to redirect T-cell or NK-cell effector functions against surface antigens found on cancer cells in a major histocompatibility complex (MHC) independent manner. The CAR structure consists of an extracellular single chain variable fragment (scFv) antigen-binding domain, an extracellular spacer region, a transmembrane domain and an intracellular signalling domain, comprising a T-cell receptor (TCR)-derived CD3z sequence and associated co-stimulatory domains [1]. CARs have been developed and tested against many cancer targets, but to date have demonstrated the greatest success within the clinic against CD19, a cell surface antigen which is highly expressed on B-cell-derived leukaemia and lymphoma. This led to the Food and Drug Administration (FDA) approval of four CD19-targeting CAR-T therapies for these indications, namely Tisagenlecleucel (Kymriah) [2], Axicabtagene ciloleucel (Yescarta) [3], Brexucabtagene autoleucel (Tecartus) [4] and Lisocabtagene maraleucel (Breyanzi) [5]. BCMA-targeting CARs have also been FDA-approved for use in multiple myeloma [6,7]. In contrast to chimeric antigen receptor T-cells (CAR-T), CAR-NK cells have not yet been FDA-approved but are the subject of ongoing clinical trials [8]. CAR NK cells have several potential biological advantages over CAR-Ts, namely a lack of MHC restriction, broader cytotoxic potential (via both the CAR and NK-specific innate pathways) and lower cytokine release upon antigen binding, potentially reducing the risk of cytokine release syndrome (CRS) and associated neurotoxicity frequently observed in patients undergoing CAR-T therapy [9].
In contrast to CAR therapies, TIL and VST therapies do not require a gene engineering step to recognise and bind target antigens; rather, they utilise the endogenous TCR to direct cytotoxicity against cancer and viral infection, respectively. Whilst progress towards the licensing and widespread application of TIL and VST therapeutics has been less rapid than for CAR-T therapy, these products have shown impressive responses against a range of solid tumours [10,11] and viral infections [12,13].
CAR-T/NK manufacture requires patient or healthy donor leukapheresis (in some cases, cord blood can be used), viral vector transduction and cell expansion, and the manufacturing time varies from <10 days to >30 days [14,15,16]. TIL/VST manufacture is often more time-consuming and complex due to the low frequency of initiating tumour/virus-reactive T-cells obtained from the starting material, and as such, manufacture can take between 5 and 10 weeks per product [17,18]. TIL manufacture requires the fragmentation or enzymatic digestion of excised patient-derived tumours, the enrichment of tumour-reactive TILs through IFN-γ-based selection following stimulation with autologous tumours, and a rapid expansion protocol (REP) in the presence of IL-2 and anti-CD3 antibodies to achieve target cell doses for patient treatment [17,19]. As an alternative to IFN-γ-based selection, other groups have explored the use of PD-1 (CD279) and 41BB (CD137) T-cell activation marker-based selection to enrich more specifically for tumour-reactive TILs [11,20,21]. For further stringent tumour-specific TIL enrichment, whole-exome sequencing and improvements in the generation of tandem minigene (TMG) libraries permit the identification of neoantigen-specific TILs for downstream expansion [11,17]. These products are currently being tested in clinical trials [22]. To expedite TIL manufacture, some groups have eliminated the IFN-γ-based TIL enrichment step, instead proceeding directly to a REP following tumour fragmentation/digestion [23,24].
VSTs can similarly be manufactured through T-cell expansion following direct viral peptide stimulation or co-culture with peptide-pulsed antigen-presenting cells (APCs), where VSTs can be isolated and enriched using multimer and IFN-γ-based selection [18].
Whilst ACTs have demonstrated impressive results within the clinic, there are several formidable challenges in the widespread delivery of products to the number of patients who need them. Firstly, the majority of ACT products are autologous therapies, bespoke to each patient, which increases cost and limits product standardisation, mainly due to patient-specific factors such as age-related immunosenescence, prior chemotherapy and underlying disease. Secondly, lengthy manufacturing times often result in delayed patient treatment, leaving patients vulnerable to disease progression and death before they can receive the product [25,26,27]. For these reasons, allogenic ACT approaches are gaining traction in what is a growing effort to generate a truly standardised universal ACT, manufactured at scale and batched for all patients [28,29,30]. Frequently, healthy donor T-cells are used as the starting material for allogeneic ACT products, and gene editing of the TCR and other cell surface molecules is often required to reduce the risks of graft-versus-host disease (GvHD) and graft rejection, respectively [29,30,31]. Despite clear potential advantages of the allogeneic approach, the manufacture of off-the-shelf therapies can be complex and expensive, requiring multiple healthy donors for each batch of ACT product, with a requirement to repeat the donor harvest and manufacture process when each batch is exhausted [29,30].
The ability to generate induced pluripotent stem cells (iPSCs) from any somatic cell through the introduction of selective transcription factors paved the way for groundbreaking scientific advances. iPSCs harbour self-renewal capabilities, accompanied with the potential to differentiate into cell types of all three germ layers, making them an invaluable resource for use in disease modelling and cellular therapeutics. Such characteristics of iPSCs similarly offer potential solutions to some of the problems with allogenic ACTs described above. As a renewable cell source, iPSCs eliminate the requirement for multiple healthy donor-derived batches. iPSCs have been successfully shown to generate functional NK/T-cells [32,33] and, in the ACT space, have the distinct advantage of limitless clonal replicative potential, which can be coupled with gene-editing approaches to provide a standardised source of NK/T-cells [34,35]. Through this review, we will explore the use of iPSCs in CAR, TIL and VST ACT manufacturing.
2. Discovery, Generation and Characterisation of iPSCs
In 2006, Shinya Yamanaka made a revolutionary scientific advancement by successfully reprogramming mouse and human somatic cells into iPSCs [36,37]. Such reprogramming was initially achieved by introducing a combination of four reprogramming transcription factors, namely Oct 3/4, Klf4, Sox2 and c-Myc, known as “OKSM-Yamanaka factors” into adult human fibroblasts. The resulting iPSCs displayed marked similarities to human embryonic stem cells (ESCs) in relation to their morphology, proliferation rate, surface antigens, gene expression, epigenetic profiles of pluripotency-related genes, telomerase activity and their ability to differentiate into cell types of the three germ layers in vitro and form teratomas in vivo [36,37]. This breakthrough allowed for the reprogramming of differentiated cells back to a pluripotent state, bypassing the ethical concerns associated with the derivation of ESCs derived from the inner cell mass of a blastocyst. Subsequent studies showed that Klf4 and c-Myc can be replaced by the transcription factors L-Myc [38], Nanog and Lin28 [39] for the efficient reprogramming of human somatic cells. Methods eliminating the use of c-Myc and Lin28 are thought to reduce neoplastic risk associated with iPSC reprogramming [40]. iPSCs can be successfully generated from a range of somatic cells including cord blood [41], dermal fibroblasts [42] and peripheral blood mononuclear cells (PBMCs) [43]. With their potential to differentiate into various cell types, the use of iPSCs is invaluable for disease modelling, drug screening and tissue regeneration, and has opened new avenues in regenerative medicine and personalised therapeutics.
Efficient reprogramming relies on the delivery of OKSM/Nanog/Lin28 transcription factors via integrating or non-integrating approaches. Integrating approaches using retroviral or lentiviral vectors are highly efficient gene transfer methods, permitting stable transgene expression, but the random nature of the integration event into the host genome carries a risk of insertional mutagenesis and oncogene-activation-led tumorigenesis [44]. To avoid this, transcription factor delivery using non-integrating viral vectors, such as Sendai virus [38,45] and adeno-associated virus [46], and other non-integrating approaches, such as episomal plasmids [47,48], messenger RNA [49], transposon [50] and minicircle [51]-based methods have been developed. Such non-integrating methodologies offer a cGMP clinically applicable method of iPSC generation.
Following the reprogramming of somatic cells, iPSCs must undergo stringent characterisation to ensure pluripotency and genome stability. Initially, emerging iPSCs can be identified using an alkaline phosphatase (AP) live stain (high expression in ESCs/iPSCs), followed by the characterisation of pluripotent colonies using surface (TRA-1-60, TRA-1-81, SSEA3, SSEA4) and intracellular (Oct3/4, Sox2, Nanog) staining via flow cytometry or immunofluorescence microscopy [52]. iPSCs can then be maintained under lineage-specific culture conditions and tested for their ability to generate all three germ layers using markers specific for the ectoderm (PAX-6, Nestin), endoderm (CXCR4, Sox17, FOXA2) and mesoderm (Brachyury, NCAM). Trilineage differentiation can further be identified through teratoma formation following iPSC injection into immunocompromised mice [53]. Lastly, genome stability can be confirmed through G-banding karyotyping, whole-genome sequencing (WGS) and single-nucleotide polymorphism (SNP) arrays to ensure the absence of chromosomal aberrations, point mutations and copy number variations. This is common in iPSCs due to existing aberrations in somatic cells, or aberrations introduced by reprogramming or long term culture [44,54].
3. PBMC Reprogramming
The optimisation of reprogramming efficiency was initially conducted in fibroblasts, which are adherent cells with a high long-term proliferative capacity, i.e., characteristics that favour reprogramming. Whilst efficiency is largely dependent on the reprogramming method used, fibroblasts have shown average reprogramming efficiencies of >0.01% [46,55,56] (range, 0.0002%–>10%). Whole PBMCs or isolated B-cells, CD34+ cells and T-cells can be reprogrammed as mixed or independent populations. The reprogramming of PBMCs is less efficient than that of fibroblasts (on average, <0.001% (ranging from <0.0001% to 0.1%), which is in part due to them being suspension cell cultures and thus vulnerable to cell loss during the frequent media changes required during reprogramming. The serial seeding of cord blood-derived PBMCs onto vitronectin-coated plates with centrifugation has been shown to minimise cell loss and increase iPSC yield [43].
With a focus on PBMCs, T-cells require specific consideration of their differentiation status prior to iPSC reprogramming. To increase reprogramming efficiency, studies have shown that T-cell activation is critical [57], using methods such as plate-bound/soluble CD3/CD28 antibodies [57,58], Phytohaemagglutin (PHA) and DynabeadsTM [32]. Additionally, T-cells harbour a range of differentiation states, including naïve T (Tn), central memory (Tcm), effector (Te) and terminal (Tte) subsets, with a progressive loss of proliferative potential [59]. Strong or protracted T-cell activation can drive T-cell differentiation towards Te/Tte-enriched subsets, such as those commonly observed in TIL and VST cultures. Reprogramming Te/Tte subsets is challenging due to activation-induced cell death (AICD) and reduced proliferative potential, but feasibility has been demonstrated using Sendai virus vectors encoding OKSM [32,57,58]. In one study, peripheral blood-derived Tte cells activated with plate-bound CD3 antibodies were able to generate iPSCs, with efficiencies up to 0.1%, with increasing virus multiplicity of infection (MOI) [58]. Similarly, following two consecutive rounds of CD3/CD28 antibody stimulation, melanoma-derived TILs generated iPSCs at efficiencies of 0.01–0.05%. In parallel, control PBMCs with a greater Tcm (CCR7+) profile generated iPSCs at an efficiency of 0.1% [57]. Further, a range of antigen-specific T-cells targeting HIV-1, Nef, CMV pp65, GAD and α-GalCer were successfully reprogrammed following DynabeadTM and PHA stimulation at lower efficiencies from 0.000002% to >0.003% [32].
Senescent cells lacking proliferative ability are more likely to fail reprogramming [60]. The transduction of these cells with SV40 large T antigen (the function of which is to disable retinoblastoma (Rb) and p53 tumour suppressor pathways towards enhanced proliferation [61]) or short hairpin RNA (shRNA) against p53 [62] alongside the OKSM factors [32] can improve reprogramming efficiency. Similarly, episomal plasmid-based PBMC reprogramming with Oct3/4, Sox2, Klf4, L-Myc, Lin28 and shRNA against p53 and EBNA1 protein (to permit the high and persistent expression of transcription factors) resulted in reprogramming efficiencies of 0.1% [47]. A summary of the PBMC/T-cell reprogramming methods is illustrated in Figure 1.
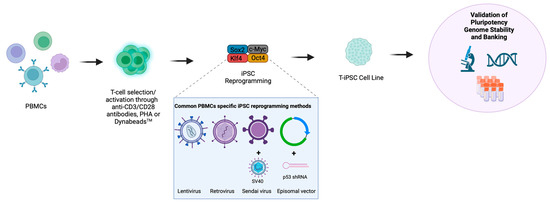
Figure 1.
Methods of T-cell reprogramming. T-cells can be reprogrammed directly from bulk peripheral blood mononuclear cells (PBMCs) or following a T-cell selection step. T-cells are primarily activated via anti-CD3/CD28 antibodies, Phytohemagglutinin (PHA) or DynabeadsTM, and subsequently reprogrammed into iPSCs through the introduction of transcription factors, which most commonly include, OCT4, SOX2, KLF4 and MYC (OSKM). The most common methods for the delivery of OSKM transcription factors into T-cells include the use of lentivirus, retrovirus, Sendai virus or episomal vectors where Sendai virus and episomal vectors have been supplemented with SV40 large T antigen or a p53-targeting shRNA, respectively. The resulting T-cell-derived iPSC colonies (T-iPSCs) must undergo stringent characterisation to confirm pluripotency and genome stability prior to banking and use in downstream differentiations. Created with BioRender.com.
4. Methods of iPSC-to-T-Cell Differentiation
Figure 2 summarises the iPSC-to-T-cell differentiation steps. The generation of T-cells from iPSCs is a lengthy (35 days/longer) two-step process comprising the generation of haematopoietic stem cells (HSCs) and subsequent T-cell lineage commitment [32,63,64,65,66,67,68]. HSC generation can be achieved through a number of methods involving the co-culture of iPSCs with mouse embryonic stromal cell lines (e.g., OP9 [63,64,65] or C3H10T1/2 [32,66]) or without stromal cells through embryoid body (EB) formation on low-attachment plates under variable media and cytokine compositions [67,68,69]. HSCs (CD34+) are then committed to T-cell lineage following the activation of Notch signalling, which plays a critical role in haematopoiesis [70]. OP9 cells, engineered to express the notch ligands delta-like ligand 1 (DLL1) and the more potent DLL4 under a range of media and cytokine compositions, can successfully generate mature T-cells from iPSCs [71,72]. Some studies suggest that DLL4 is better for the generation of T-cells [73,74], but this may vary depending on the parental iPSC source. T-cell-derived iPSCs (T-iPSCs) show early T-cell receptor (TCR) expression and may require more potent notch signalling with DLL4, whereas DLL1 may be sufficient for ESCs, fibroblast-derived iPSCs and T-iPSCs with TCR knockout [69].
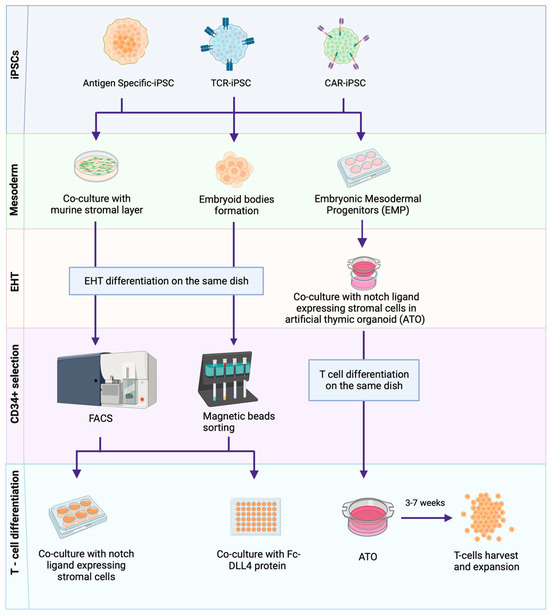
Figure 2.
Methods of iPSC–to–T-cell differentiation. T-cell-derived iPSCs are guided through a multi-step redifferentiation process to acquire antigen-specific T-cells. This process starts with the differentiation of iPSCs into mesoderm lineages and subsequent endothelial-to-hematopoietic transition (EHT), achieved through various methods, including co-culturing iPSCs with murine stromal layers (OP9 or C3H10T1/2), the formation of stroma-free embryoid bodies (EBs) and the use of cytokine cocktails to obtain hematopoietic stem cells (HSCs) and embryonic mesoderm progenitors (EMPs). Upon the induction of HSCs, T-cell differentiation is facilitated through methods including co-culture with murine stromal layers (OP9-DLL1/4), the use of Fc-DLL4 (Delta like ligand 4) protein or through a 3D artificial thymic organoid (ATO) system. A cultivation period of approximately 3–7 weeks results in the production of expanded T-cells. Created with BioRender.com. Key: Antigen-specific T-cell-derived iPSCs (Antigen-specific-iPSCs), T-cell receptor transduced iPSCs (TCR-iPSCs), Chimeric antigen receptor knock-in/transduced iPSCs (CAR-iPSCs).
Alternative T-cell differentiation methods to OP9-DLL1/4 include the use of murine foetal thymic lobes and artificial thymic organoids (ATOs). In the foetal thymic lobe setting, iPSC-derived immature T-cells cultured on OP9-DLL1 stromal cells are seeded in a hanging drop plate 3D culture with murine foetal thymic lobes [75]. In this ATO system, mesoderm induction is initiated in feeder-free conditions under a cytokine cocktail, where human embryonic mesoderm progenitors (hEMPs) (CD326−/CD56+) are aggregated into organoids using the MS5-DLL4 mouse stromal cell line on a porous membrane [76].
For clinical applicability, differentiation methods that do not require murine stromal cell lines, namely the addition of exogenous DLL4 protein, have been developed. Here, iPSC-derived CD34+ cells are cultured with DLL4-coated streptavidin polystyrene beads [77], or EB-derived CD34mid/CD43+ populations are cultured on DLL4 protein-coated retronectin plates. This DLL4 protein-based method has been shown to be highly efficient, such that 3 × 105 iPSCs can generate 6.2 × 108 CD8αβ+ T-cells [68].
Directly comparing the differentiation efficiency of each method is difficult, confounded by the different culture conditions used between studies, as summarised in Table 1. Most protocols demonstrate progressive T-cell commitment akin to that seen during native T-cell development in the thymus. At the outset, CD4−/CD8− double-negative (DN) cells predominate, but progressive differentiation leads to CD4+ intermediate single-positive (ISP) populations and then CD4+/CD8+ double-positive (DP) states. To generate CD8αβ+ single-positive T-cells from CD4+/CD8+ DP subsets, most protocols incorporate a T-cell activation step using CD3/CD28 antibodies/PHA/peptide simulation or co-culture with antigen presenting cells (APCs) engineered with costimulatory domains (e.g., 4-1BB) [32,64,65,66,67,68].
The emergence of erroneous CD8 T-cell populations has also been reported during iPSC-to-T-cell differentiation. Whilst the classical emergence of CD8αβ+ T-cells, representing mature T-cells, is desired, CD8αα+ homodimers have been reported. These homodimers are classically found on innate immune cells with impaired TCR signalling and strong TCR-independent cytotoxicity [67,76,78]. A study attributed the stimulation of CD4−/CD8− DN subsets to the generation of CD8αα+ T-cells, whereas the stimulation of CD4+/CD8+ DP subsets gives rise to CD8αβ+ [79]. Some iPSC-derived T-cells have further been shown to lack T-cell-specific surface antigens such as CD2/CD28/CD5, and have a high expression of innate cell markers such as CD56 [32,67].
Current protocols convincingly demonstrate that functional CD8 T-cells can be derived from iPSCs. To date, there is limited data to guide iPSC differentiation into CD4 T-helper subsets, with the exception of the ATO method, which has demonstrated the differentiation of iPSCs into functional CD4+ SP T-cells, productive of cytokines, such as IL-2/IFN-γ/IL-4 [76]. The incorporation of gene editing in the ATO system to knock out TBX21 (encoding T-bet) or IL4 (Th2 regulator) genes in iPSCs resulted in the generation of CD4 SP T-cells, exclusively productive of IFN-γ and IL-4, recapitulating Th1 and Th2 subsets, respectively [80].

Table 1.
Summary of reported primary culture components used for HSC induction, and T-cell differentiation, maturation and expansion from iPSC cell lines across studies.
Table 1.
Summary of reported primary culture components used for HSC induction, and T-cell differentiation, maturation and expansion from iPSC cell lines across studies.
| Mesoderm/HSC Induction | |||
| Methods | Embryoid Bodies | Co-Culture with OP9/C3H10T1/2 | Artificial Thymic Organoids (ATOs) |
| Primary Culture Components | CHIR99021 10 µM | VEGF (15–50 ng/mL) | rhActivin A (10 ng/mL) |
| SB431542 6 µM | SCF (50 ng/mL) | rhBMP4 (10 ng/mL) | |
| BMP-4 (10–50 ng/mL) | FLT3L (10 ng/mL) | rhVEGF (10 ng/mL) | |
| bFGF (5–50 ng/mL) | rhFGF (10 ng/mL) | ||
| VEGF (15–50 ng/mL) | |||
| FLT3L (10 ng/mL) | |||
| SCF (50 ng/mL) | |||
| TPO(30 ng/mL) | |||
| References | [67,68,74] | [32,65,81] | [76] |
| T-cell differentiation | |||
| Methods | Fc-DLL4/Retronectin (stroma free) | Co-culture with OP9−DLL1/4 | Artificial thymic organoids (ATOs) |
| Primary Culture Components | rhFLT-3L (50 ng/mL) | rhFLT-3L (5–10 ng/mL) | rhFLT-3L (5 ng/mL) |
| rhIL-7 (50 ng/mL) | rhIL-7 (1–10 ng/mL) | rhIL-7, first 7 days (5 ng/mL) | |
| rhSCF (50 ng/mL) | rhSCF (5–10 ng/mL) | rhSCF, first 7 days, (10 ng/mL) | |
| rhTPO (100 ng/mL) | rhSCF, after 7 days (50 ng/mL) | ||
| rhSDF-1α (30 nM) | rhTPO, after 7 days, (5 ng/mL) | ||
| SB203580 (15 µM) | |||
| References | [68] | [32,65,66,67,79,81,82] | [76] |
| T-cell Maturation/Expansion | |||
| Methods | anti-CD3/CD28 mAbs | anti-CD3 | Phytohemagglutinin (PHA) |
| Primary Culture Components | hIL-7 (2–5 ng/mL) | hIL-7 (10 ng/mL) | hIL-7 (10 ng/mL) |
| hFlt-3L (0–5 ng/mL) | rhIL-2 (10 ng/mL) | IL-15 (5 ng/mL) | |
| hSCF (0–10 ng/mL) | dexamethasone 10 nM | PHA 2 μg/mL | |
| anti-human CD3 (50–5000 ng/mL) | Anti-human CD3 (OKT3) 500 ng/mL | ||
| anti-human CD28 (1000–2000 ng/mL) | |||
| hIL-2 (2 ng/mL/200 U/mL) | |||
| References | [74] | [68] | [64,82,83] |
5. Methods of iPSC-to-NK Cell Differentiation
Similar to T-cells, NK cells can also be successfully derived from iPSCs, and the process is outlined in Figure 3. Initial HSC generation is similar to T-cell protocols where mouse stromal cells such as M2-10B4/S17 and spin EB generation have been used [83,84]. Unlike T-cells, differentiation into NK cells does not require Notch signalling, but instead requires specific cytokine cocktails and mouse bone marrow stromal cell lines, such as AFT024 and EL08-1D2 [83,85].
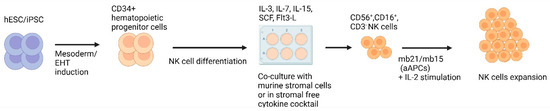
Figure 3.
Methods for generating iPSC−derived NK cells. This figure illustrates the methods employed to derive NK cells from iPSCs. First, hematopoietic cells are produced from iPSCs. HSCs are subsequently cultivated on murine stromal cells or in a stromal-free cytokine cocktail for the development of NK cells, commonly characterised by the presence of CD56+, CD16+ and CD3− markers. Generated NK cells can undergo additional expansion through co-culture with artificial antigen presenting cells (aAPCs) engineered to express membrane-bound (mb) IL-21/IL-15 in the presence of IL-2. Created with BioRender.com.
In parallel, cGMP protocols towards NK cell differentiation have been developed for clinical application that eliminate serum- and mouse-derived stromal cells [83]. Robust NK cell expansion is then achieved using exogenous IL-2 and 41BB/membrane-bound IL-21 engineered APCs [83,86,87]. Membrane-bound IL-21 can expand NK cells by 60-fold and is superior to membrane-bound IL-15 [87]. Phenotyping demonstrates the expression of mature NK cell markers (CD56, CD16, CD94), NK activating receptors (NKG2D, DNAM-1), NK cytotoxic receptors (NKp46, NKp44, KIRs) and cell death ligands (FasL, TRAIL) [83,84,85,88]. iPSC-derived NK cells demonstrate cytotoxicity through cytokine/chemokine, death receptor and antibody-dependent cellular cytotoxicity (ADCC) mechanisms [89].
6. Use of iPSCs in Adoptive Cell Therapy
6.1. CAR T-Cell Therapy
One early example of iPSC-derived CD19-targeting CAR-T therapy was developed by Themeli et al. [67], who showed that T-iPSC cell lines can be transduced with a CAR cassette using lentiviral vectors, and CAR-T-iPSC cell lines can then be successfully differentiated into CAR-Ts. The resulting products were CD8αα+, which lacked CD5 and expressed the NK marker CD161, matching a phenotypic profile commonly seen with (innate) γδ T-cells. The CAR-Ts elicited robust anti-tumour responses in an in vivo Burkitt’s Lymphoma murine model [67].
The potential benefits of allogeneic iPSC-derived CAR-T therapies include the development of standardised products and rapid access to an off-the-shelf product for patients. A substantial risk of allogenic T-cell therapy is the risk of human leukocyte antigen (HLA) mismatch between the donor/recipient leading to graft rejection or fatal GvHD. A potential mitigation strategy is to bank multiple HLA homozygous CAR-iPSC cells lines, such that HLA-typed patients could receive “best-matched” CAR-iPSCs. In terms of scale, a bank of 50–175 iPSC lines has been estimated to be sufficient to cover >70% of Japanese and UK patient populations, respectively [89,90,91].
To mitigate for the risk of GvHD from an iPSC-derived allogeneic CAR product, an alternative strategy to the HLA-diverse CAR-iPSC bank approach outlined above is to use iPSC gene editing to delete the TCR. A particular advantage of iPSCs is that their clonal growth characteristics permit the stringent selection of muti-edited cell lines, even at a low editing efficiency [92]. Multiple edited clones can be further screened for genome stability and off-target genomic toxicity to improve the safety profile of resulting iPSC-CAR products. CRISPR Cas9 has been used for multiplex iPSC editing to not only delete the TCR, but to delete the HLA-I and HLA-II genes and thus reduce the risk of immunological rejection and GvHD. However, additional modifications were added to prevent NK-mediated rejection, which has been reported when HLA is disrupted, namely the knockout of the NK-activating receptor DNAM-1 and transduction with HLA-E to inhibit NK-mediated lysis. T-cells differentiated from this multi-edited iPSC cell line demonstrated immune escape against allogeneic immune cells in vitro and in vivo [34].
With the goal of preventing GvHD and inducing more physiological expression of CAR (akin to the expression of native TCR), a further study of CAR-iPSCs utilised CRISPR-mediated homology-directed repair (HDR) to insert a CD19-targeting CAR construct [93] into the T-cell receptor α constant (TRAC) gene locus encoding TCR. This simultaneously disrupts TCR expression whilst inducing CAR expression under the control of the physiological TCR promoter. These edited iPSC cell lines were able to derive functional CD8αβ+ CAR T-cells with anti-tumour activity in an in vivo leukaemia model without inducing GvHD in mice [69].
Another study assessed the CRISPR-mediated knockout of diacylglycerol (DAG) isoforms α/ζ to enhance RAS/ERK signalling in a glypican-3 (GPC-3)-targeting CAR iPSC cell lines, and the resulting CAR-iPSCs underwent retroviral transduction with membrane-bound IL-15/IL-15α. These edits were found to enhance anti-tumour responses in comparison to non-edited iPSC-derived CAR T-cells [66].
Together, these studies demonstrate that the multiplexed gene editing of iPSCs to generate allogeneic CAR-T products is feasible. Clinical testing is underway, with Fate Therapeutics leading a phase I dose escalation of iPSC-derived CD19 CAR T-cells where the CAR is traduced into a TRAC locus (FT819) against B-cell lymphoma and leukaemia. The interim results demonstrate safety and anti-tumour activity [94]. Other CAR-iPSC T-cell therapies in clinical development are highlighted in Table 2, and a summary of the use of iPSCs in adoptive cell therapy is illustrated in Figure 4.

Table 2.
Registered iPSC−derived NK and T-cell therapy clinical trials. This clinical trial list was compiled from those registered on ClinicalTrial.gov and the WHO Clinical Trials Registry.
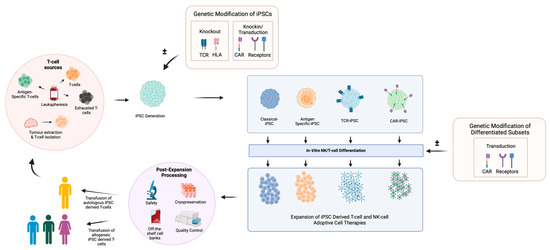
Figure 4.
Generation of adoptive cell therapies from iPSCs. T-cell subsets including antigen-specific T-cells and exhausted T-cells, which can be obtained from patient leukapheresis and tumour-infiltrating lymphocytes (TILs), are sourced following tumour dissociation. These diverse T-cell populations are reprogrammed into iPSCs and tailored through genetic modification to induce the knockout of genes such as T-cell receptors (TCRs) or human leukocyte antigens (HLAs) to mitigate alloreactivity. Elements such as chimeric antigen receptors (CARs), tumour antigen-specific TCR receptors and the HLA-E complex can be introduced for anti-tumour functionality and to mitigate graft rejection. The generated classical/modified iPSC cell lines undergo NK/T-cell-specific differentiation, where resulting cells can be further modified to augment functionality. For use as cell therapeutics, derived products must be expanded to sufficient treatment doses and cryopreserved. Rigorous safety and quality control assessments are required prior to clinical treatment where differentiated T-cells can then be infused as allogeneic or autologous T-cell therapies. This process can be utilised to generate a variety of CAR T-cell, CAR NK, TIL and VST ACTs. Created with BioRender.com. Key: Unmodified T-cell-derived iPSCs (Classical iPSCs), Antigen-specific T-cell-derived iPSCs (Antigen-specific-iPSCs), T-cell receptor transduced iPSCs (TCR-iPSCs) and Chimeric antigen receptor knock-in/transduced iPSCs (CAR-iPSCs).
6.2. CAR NK Cell Therapy
Due to the lack of MHC restriction and the ease of NK cell generation from iPSCs, iPSC-derived CAR NK therapy is an attractive allogenic treatment option. Early studies of CAR NKs utilised the same CAR design as T-cells [95], but Li et al. [96] subsequently showed that using NK-specific activation and co-stimulatory domains could improve the efficacy of CAR-NKs in vitro and in vivo. Specifically, combining the NKG2D transmembrane domain, the 2B4 co-stimulatory domain and the CD3z signalling domain resulted in powerful activation and anti-tumour responses from iPSC-derived CAR-NKs in an in vivo ovarian cancer model. The iPSC-derived CAR-NK cells demonstrated anti-tumour activity in vivo, in keeping with classical CAR T-cells, but with less toxicity [96]. Fate Therapeutics are paving the way for the use of iPSC-derived NK cells, having initiated numerous trials testing both classical iPSC-derived NK cells (FT500) [97] and NK cells with function-enhancing edits against a range of cancers. The iPSC cell lines used to derive the NK cell therapies are edited with a non-cleavable CD16 module that can bind to the Fc portion of co-infused anti-tumour monoclonal antibodies to enhance ADCC (FT516) [98]. Others edits include the addition of non-cleavable CD16 with an IL-15 receptor fusion to enhance persistence, the addition an anti-CD19 CAR NK-specific construct (FT596) [35] and the knockout of CD38 to limit CD38-mediated fratricide when given in combination with a CD38-targeting antibody (FT536/FT576) [99,100]. Trial results from FT516 against a range of solid tumours in combination with Avelumab, as well as FT596 against B-cell lymphoma in combination with Rituximab, demonstrate efficacy and a favourable safety profile [35,98]. All trials incorporating iPSC-derived NK cell therapy are listed in Table 2, and a summary of the use of iPSCs in adoptive cell therapy is illustrated in Figure 4.
6.3. TIL Therapy
TIL therapy relies on anti-tumour TCR specificity, and an important consideration in the TIL-iPSC field is whether T-cells reprogrammed to iPSCs faithfully retain the same TCR when differentiated back into T-cells. During T-cell development in the thymus, TCRβ and TCRα genes are rearranged between the late CD4−/CD8− DN and the CD4+/CD8+ DP stage through recombination, activating genes RAG1 and RAG2 [101]. Studies have demonstrated that MART-1 [75] and LMP2 [79] epitope-specific, antigen-specific T-cells could be reprogrammed into iPSC cell lines and re-differentiated into CD8 T-cells with retained TCR specificity. Whilst 90% of iPSC-derived re-differentiated MART-1 T-cells retained the original epitope [75], a 4.6% proportion of iPSC-derived re-differentiated LMP2 CD8 T-cells lost epitope specificity following stimulation, attributed to TCRα rearrangement [79]. Subsequently, it has been demonstrated that TCR specificity can be stabilised through the knockout of the RAG protein complex required for TCRα/TCRβ rearrangement. As an exemplar, iPSCs were generated from GPC-3-specific CD8 T-cells and RAG was knocked out at the iPSC stage using CRISPR. Differentiated CD8 T-cells retained GPC-3 TCR specificity in RAG knockout iPSCs, whereas wild-type RAG-derived T-cells lost 40% of their antigen specificity [81]. Similar TCR stability was seen following RAG knockout in monocyte-derived iPSCs transduced to express TCRs against GPC-3 and WT1. The resulting differentiated CD8 T-cells demonstrated preserved monoclonal expression of the transduced TCRs [81].
Whilst technically promising, studies primarily demonstrate feasibility in antigen-specific T-cells; however, these are single-antigen-targeting treatments restricted by HLA, thus limiting the number of patients that can be treated. True TIL therapy would require the autologous generation of polyclonal tumour-reactive T-cells from iPSCs. The feasibility of such a process was demonstrated by Ito et al., where TILs isolated from colorectal tumours were expanded and co-cultured with autologous tumour spheroids. Reactive TILs were sorted based on CD107a/41BB markers where TCR specificity was confirmed through HLA class 1 blocking; these TILs were subsequently reprogrammed to iPSCs. Using a feeder-free protocol, functional multiclonal tumour-specific CD8 T-cells were generated with the absence of additional TCR rearrangements [102]. Although feasible, a key limitation of utilising iPSCs in autologous TIL therapy is the reprogramming efficiency. Current protocols are low in efficiency, which greatly narrows the TCR repertoire that can be achieved. Moreso, the manufacturing time and costs, as well as the ability to quality control such a bespoke therapy, will inform its potential as a clinical therapeutic. A summary of the use of iPSCs in adoptive cell therapy is illustrated in Figure 4.
6.4. VST Therapy
Like TIL therapy, VST therapy relies on TCR specificity. Researchers have similarly demonstrated that iPSC cell lines can be generated from virus-specific T-cells and differentiated back into functional CD8 T-cells with retained TCR specificity in HIV [32], human papilloma virus type 16 (HPV16) [103] and Epstein–Barr virus (EBV) [79,104] models. Apart from HIV, HPV16- and EBV-specific T-cells have been investigated in the context of HPV/EBV-associated cancer [32,79,103,104]. Whilst Nishimura et al. demonstrate that Cytomegalovirus (CMV)-reactive T-cells specific for the pp65 CMV antigen could be successfully reprogrammed [32], the use of iPSCs in classical VST therapy against CMV, EBV and Adenovirus (Adv) has not been studied. Adoptive cell therapy against viral infection can be used to clear infection or as a prophylactic against viral reactivation in immunocompromised individuals, and has shown remarkable efficacy. Manufacturing such VSTs can take several weeks and involves the expansion of VSTs through direct stimulation with pooled viral peptides or simulation with peptide-pulsed/viral transduction of dendritic cells with virus-specific antigens. VSTs can be manufactured as both autologous and allogeneic therapies [18]. In the allogenic setting, it is suggested that VSTs with defined TCR specificity eliminates alloreactivity, leading to GvHD [105]. This requires the stringent selection of viral antigen-reactive T-cells. Current methods to enrich VSTs include the use of an IFN-γ capture system following peptide/antigen stimulation which lacks purity [106]. Alternatively, VSTs can be selected through the use of multimers, where HLA monomers loaded with viral peptides bind virus-specific TCRs [107]. Although stringent, this method is limited to single viral epitopes and is HLA-restricted, where only a subset of HLA-targeting multimers are commercially available. As such, deriving VSTs from iPSCs is an attractive approach, where the clonal replicative potential of iPSCs would enable the stringent selection of viral reactive clones. Coupled with the ability to generate HLA-specific iPSC cell lines, this could permit differentiation into polyclonal viral-reactive T-cells and is worthy of further study. A summary of the use of iPSCs in adoptive cell therapy is illustrated in Figure 4.
7. Potential and Challenges of iPSC-Derived Adoptive Cell Therapies
It is important to weigh the complexity and challenges of generating iPSC-based therapies against classical autologous and healthy-donor-derived allogeneic adoptive cell therapies. The manufacture of iPSC-derived adoptive cell therapies is costly/lengthy and does not shorten the time to treatment in the autologous setting. iPSCs hold more promise in the allogenic setting for their ability to generate multiple cell batches from single iPSC cell lines. However, can deriving cell therapies from iPSCs produce functionally superior cells to truly justify their use as autologous therapeutics?
The production of classical NK cell therapies is challenging. NK cell therapies utilise NKs from peripheral/cord blood, where the proportion of NK cells is low at 10–20% and requires ex vivo expansion to obtain clinical doses [108]. Despite the ability to expand these cell populations, the cell yields are not sufficient for multiple patient dosing [109]. Moreso, the production of NK-CARs with multiple gene edits, as discussed above, introduces a number of technical challenges; namely, the transduction efficiency of primary and expanded NK cells can be highly variable and low [110,111]. Although the use of NK cell lines such as NK-92 can overcome some of these transduction and cell expansion challenges in vitro, for safety, they require irradiation prior to infusion, which restricts in vivo expansion/persistence, and clinical responses remain subpar [112]. In this setting, deriving NK cell therapies from iPSCs offers many advantages. The development of simple xenogeneic feeder-free manufactures consistently produces NK cells from iPSCs with comparable functionality to classical NK cells. This, accompanied by the clonal properties of iPSCs and their amenability to gene editing, offer an elegant solution for the standardised production of iPSC-derived NK cell therapies. There are many clinical trials underway to assess the safety and efficacy of multi-edited NK cell therapies, as per Table 2, which will inform their clinical potential.
Unlike NK cells, the differentiation of T-cells from iPSCs is more complex. The current iPSC-to-T-cell differentiation methods involve several phases, as per Table 1 and the resulting cells vary considerably, which can lead to the emergence of erroneous/inconsistent T-cell populations with variable functions [32,66,67,68,76,81]. A priority for the field will be to improve standardisation and reduce the variability observed in these emerging iPSC-derived T-cell products. Despite the variable differentiation methods, studies demonstrate that T-cells derived from iPSCs appear rejuvenated. iPSC-derived HIV-specific T-cells were found to expand better after repeat stimulation and expressed markers of less differentiated Tcm subsets, including CCR7, CD27 and CD28, with elongated telomeres compared to paternal HIV-specific T-cells; however, their cytotoxicity was not compared to the parental cytotoxicity [32]. Similarly, in a feeder-free differentiation protocol, iPSC-derived TILs were found to have upregulated expression of Tcm markers, including CD62L, CD28 and TCF-7, increased telomere length, improved metabolic profile and enhanced expansion in vitro/in vivo against cancer spheroids when compared to parental TILs. iPSC-derived TILs demonstrate comparable in vitro cytotoxicity and inhibited spheroid engraftment in vivo compared to parental TILs; however, iPSC TILs also demonstrated non-specific cytotoxicity in non-cancer spheroids [102]. This rejuvenated profile, however, is not consistent across all studies; some demonstrate CD27/CD28 expression with the absence/low expression of CCR7/CD62L Tcm markers, whilst others lack expression at all [67,68,79]. Similarly, tumour control is either similar to parental therapies or lacking [67,68,102], where complete tumour regression was seen in parental CAR-Ts, but iPSC-derived CAR T-cells were only able to delay tumour regression [67]. It must be noted that not all iPSC-derived ACT studies compare their function to respective therapies manufactured via classical methods [34,79]. Moreso, particularly in the iPSC-derived CAR T-cell setting, in vivo models are established with far fewer tumour cells and larger T-cells doses accompanied, by the co-infusion of supportive cytokines including IL-2 and IL-15 [67,68]. Although IL-2 supplementation is frequently used in TIL therapy [113], it is not common practice in conventional CAR T-cell in vivo models or clinical therapy [93,114]. Such inconsistencies between studies and difficulty modelling long-term persistence in immunocompromised mouse models make it challenging to ascertain the true benefits of deriving T-cells from iPSCs versus the conventional manufacture of autologous CAR-T products for patients.
The classical manufacture of adoptive T-cell therapies incorporating viral transduction and gene editing has been optimised over the years to produce highly functional T-cell therapies. This leads to the question of whether deriving T-cells from iPSCs provides additional advantages to justify their complex manufacture, and how tangible their potential towards clinical translation is. Whilst there have been significant advances in the development of GMP-appropriate, clinically applicable protocols for iPSC-based cell therapy manufacture that eliminate the use of xenogeneic feeder layers and serum [68,83], several unique challenges for clinical scalability remain. Firstly, such diverse/lengthy manufacturing harbours a significant cost burden. Secondly, tight control over the rapid generation, characterisation and long-term maintenance/stability of iPSC cell lines in clinical manufacturing is an area that still requires more development. Thirdly—and this is a critical quality control issue—is how the quantitative, qualitative and functional consistency of the cell populations derived between differentiation batches can be tested and controlled, as even simple variations in seeding density have been shown to affect lineage skew [115]. Lastly, several cell therapeutic approaches utilise genome-editing steps during the generation of ACTs, which poses further challenges with respect to product safety, as it is imperative that the field evolves towards nimble and low-cost approaches towards the identification of off-target effects and genome stability between batches.
Some advances have been made towards the clinical translation of iPSC-derived cell therapeutics through the development of closed and automated bioreactor systems to support the controlled and large-scale manufacture of iPSC banks [116,117], as well as to support large scale differentiation [118]. Ultimately, significant collaboration between scientists, industry leaders and regulatory bodies is required to optimise clinically relevant protocols, develop resources to support the automation and standardisation of iPSC banking/differentiation, and develop regulatory pipelines for quality control and safety assessments of such iPSC-derived therapies.
Nonetheless, iPSCs possess huge potential, particularly in the allogeneic ACT setting where there are several iPSC-derived NK/CAR T-cell therapies currently in clinal trials, as illustrated in Table 2. Whilst the clinical efficacy of iPSC-derived T-cell therapies and their use, even in autologous ACT, remains to be determined, early phase I studies of iPSC-derived NK cell therapies have demonstrated clinical scalability and efficacy against a range of cancers [35,98], and their off-the-shelf nature makes them highly desirable in the ACT space. Despite progress, there remains a significant need for further development to optimize, enhance and standardise current iterations of iPSC-derived cell therapeutics; however, the use of iPSCs has already begun to pave a critical path as a source of next-generation adoptive cell therapies.
Author Contributions
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| aAPC | Artificial antigen-presenting cell |
| ACT | Adoptive cell therapy |
| ADCC | Antibody-dependent cellular cytotoxicity |
| Adv | Adenovirus |
| AICD | Activation-induced cell death |
| AP | Alkaline phosphatase |
| APC | Antigen-presenting cell |
| ATO | Artificial thymic organoids |
| BCMA | B-cell maturation antigen |
| bFGF | Basic fibroblast growth factor |
| BMP-4 | Bone morphogenetic protein 4 |
| CAR-T | Chimeric antigen receptor T-Cell |
| CMV | Cytomegalovirus |
| CRS | Cytokine release syndrome |
| CXCR | Chemokine receptor |
| DAG | Diacylglycerol |
| DLL | Delta-like ligand |
| DN | Double negative |
| DP | Double positive |
| EB | Embryoid body |
| EBV | Epstein–Barr virus |
| EHT | Endothelial-to-hematopoietic transition |
| EMO | Embryonic mesodermal progenitor |
| ESC | Embryonic stem cell |
| FDA | Food and Drug Administration |
| FLT3L | Fms-related tyrosine kinase 3 ligand |
| GPC-3 | Glypican-3 |
| GvHD | Graft-versus-host disease |
| HDR | Homology-directed repair |
| hEMP | Human embryonic mesoderm progenitor |
| HIV | Human immunodeficiency virus |
| HLA | Human leukocyte antigen |
| HPV16 | Human papilloma virus type 16 |
| HSC | Hematopoietic stem cell |
| IFN | Interferon |
| IL | Interleukin |
| iPSC | Induced pluripotent stem cell |
| ISP | Intermediate single positive |
| mb | Membrane-bound |
| MHC | Major histocompatibility complex |
| MM | Multiple myeloma |
| MOI | Multiplicity of infection |
| NK | Natural killer |
| Oct | Octamer-binding transcription factor |
| PBMC | Peripheral blood mononuclear cells |
| PD-1 | Programmed cell death protein 1 |
| PHA | Phytohemagglutinin |
| RAG | Recombination-activating gene |
| Rb | Retinoblastoma |
| REP | Rapid expansion protocol |
| SCF | Stem cell factor |
| scFv | Single-chain variable fragment |
| SDF-1α | Stromal cell-derived factor 1 alpha |
| shRNA | Short hairpin RNA |
| SNP | Single-nucleotide polymorphism |
| Tcm | Central memory T-cell |
| TCR | T-cell receptor |
| Te | Effector T-cell |
| Th | T helper |
| TIL | Tumour-infiltrating lymphocyte |
| TMG | Tandem minigene |
| Tn | Naïve T-cell |
| TPO | Thrombopoietin |
| TRAC | T-cell receptor α constant |
| Tte | Terminal T-cell |
| VEGF | Vascular endothelial growth factor |
| VST | Viral-specific T-cell |
| WGS | Whole-genome sequencing |
References
- Ghorashian, S.; Pule, M.; Amrolia, P. CD19 Chimeric Antigen Receptor T Cell Therapy for Haematological Malignancies. Br. J. Haematol. 2015, 169, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.T.; Grupp, S.A.; Maude, S.L.; Levine, J.E.; Pulsipher, M.A.; Boyer, M.W.; August, K.J.; Myers, G.D.; Tam, C.S.; Jaeger, U.; et al. Tisagenlecleucel Immunogenicity in Relapsed/Refractory Acute Lymphoblastic Leukemia and Diffuse Large B-Cell Lymphoma. Blood Adv. 2021, 5, 4980–4991. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.R.; Oluwole, O.O.; Kersten, M.J.; Miklos, D.B.; Perales, M.-A.; Ghobadi, A.; Rapoport, A.P.; Sureda, A.; Jacobson, C.A.; Farooq, U.; et al. Survival with Axicabtagene Ciloleucel in Large B-Cell Lymphoma. N. Engl. J. Med. 2023, 389, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jain, P.; Locke, F.L.; Maurer, M.J.; Frank, M.J.; Munoz, J.L.; Dahiya, S.; Beitinjaneh, A.M.; Jacobs, M.T.; Mcguirk, J.P.; et al. Brexucabtagene Autoleucel for Relapsed or Refractory Mantle Cell Lymphoma in Standard-of-Care Practice: Results from the US Lymphoma CAR T Consortium. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 2594–2606. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Hoda, D.; Riedell, P.A.; Ghosh, N.; Hamadani, M.; Hildebrandt, G.C.; Godwin, J.E.; Reagan, P.M.; Wagner-Johnston, N.; Essell, J.; et al. Lisocabtagene Maraleucel as Second-Line Therapy in Adults with Relapsed or Refractory Large B-Cell Lymphoma Who Were Not Intended for Haematopoietic Stem Cell Transplantation (PILOT): An Open-Label, Phase 2 Study. Lancet Oncol. 2022, 23, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J. Clin. Oncol. 2023, 41, 1265–1274. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Feng, X.; Han, Z. CAR-NK Cells for Cancer Immunotherapy: From Bench to Bedside. Biomark. Res. 2022, 10, 12. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Borch, T.H.; van den Berg, J.H.; Met, Ö.; Kessels, R.; Geukes Foppen, M.H.; Stoltenborg Granhøj, J.; Nuijen, B.; Nijenhuis, C.; Jedema, I.; et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2022, 387, 2113–2125. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, J.; Rao, S.; Guo, S.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; et al. Tumor Infiltrating Lymphocyte (TIL) Therapy for Solid Tumor Treatment: Progressions and Challenges. Cancers 2022, 14, 4160. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Tzannou, I.; Wu, M.; Ramos, C.; Sasa, G.; Martinez, C.; Lulla, P.; Krance, R.A.; Scherer, L.; Ruderfer, D.; et al. Posoleucel, an Allogeneic, Off-the-Shelf Multivirus-Specific T-Cell Therapy, for the Treatment of Refractory Viral Infections in the Post-HCT Setting. Clin. Cancer Res. 2023, 29, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.D.; Bollard, C.M. Virus-Specific T-Cell Therapies for Patients with Primary Immune Deficiency. Blood 2020, 135, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Tumaini, B.; Lee, D.W.; Lin, T.; Castiello, L.; Stroncek, D.F.; Mackall, C.; Wayne, A.; Sabatino, M. Simplified Process for the Production of Anti-CD19-CAR Engineered T Cells. Cytotherapy 2013, 15, 1406–1415. [Google Scholar] [CrossRef]
- Roddie, C.; O’Reilly, M.; Dias Alves Pinto, J.; Vispute, K.; Lowdell, M. Manufacturing Chimeric Antigen Receptor T Cells: Issues and Challenges. Cytotherapy 2019, 21, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Vormittag, P.; Gunn, R.; Ghorashian, S.; Veraitch, F.S. A Guide to Manufacturing CAR T Cell Therapies. Curr. Opin. Biotechnol. 2018, 53, 164–181. [Google Scholar] [CrossRef]
- Hopewell, E.L.; Cox, C.; Pilon-Thomas, S.; Kelley, L.L. Tumor Infiltrating Lymphocytes Streamlining a Complex Manufacturing Process. Cytotherapy 2019, 21, 307–314. [Google Scholar] [CrossRef]
- Shafat, M.S.; Mehra, V.; Peggs, K.S.; Roddie, C. Cellular Therapeutic Approaches to Cytomegalovirus Infection Following Allogeneic Stem Cell Transplantation. Front. Immunol. 2020, 11, 1694. [Google Scholar] [CrossRef]
- Rohaan, M.W.; van den Berg, J.H.; Kvistborg, P.; Haanen, J.B.A.G. Adoptive Transfer of Tumor-Infiltrating Lymphocytes in Melanoma: A Viable Treatment Option. J. ImmunoTherapy Cancer 2018, 6, 102. [Google Scholar] [CrossRef]
- Inozume, T.; Hanada, K.; Wang, Q.J.; Ahmadzadeh, M.; Wunderlich, J.R.; Rosenberg, S.A.; Yang, J.C. Selection of CD8+PD-1+ Lymphocytes in Fresh Human Melanomas Enriches for Tumor-Reactive T-Cells. J. Immunother. 2010, 33, 956–964. [Google Scholar] [CrossRef]
- Ye, Q.; Song, D.-G.; Poussin, M.; Yamamoto, T.; Best, A.; Li, C.; Coukos, G.; Powell, D.J. CD137 Accurately Identifies and Enriches for Naturally Occurring Tumor-Reactive T Cells in Tumor. Clin. Cancer Res. 2014, 20, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Cadieux, E.L.; Salgado, R.; Bakir, M.A.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanić, M.; Reading, J.L.; Joshi, K.; et al. Neoantigen-Directed Immune Escape in Lung Cancer Evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef]
- Donia, M.; Junker, N.; Ellebaek, E.; Andersen, M.H.; Straten, P.T.; Svane, I.M. Characterization and Comparison of “standard” and “Young” Tumour-Infiltrating Lymphocytes for Adoptive Cell Therapy at a Danish Translational Research Institution. Scand. J. Immunol. 2012, 75, 157–167. [Google Scholar] [CrossRef]
- Tran, K.Q.; Zhou, J.; Durflinger, K.H.; Langhan, M.M.; Shelton, T.E.; Wunderlich, J.R.; Robbins, P.F.; Rosenberg, S.A.; Dudley, M.E. Minimally Cultured Tumor-Infiltrating Lymphocytes Display Optimal Characteristics for Adoptive Cell Therapy. J. Immunother. 2008, 31, 742–751. [Google Scholar] [CrossRef]
- Fraietta, J.A.; Lacey, S.F.; Orlando, E.J.; Pruteanu-Malinici, I.; Gohil, M.; Lundh, S.; Boesteanu, A.C.; Wang, Y.; O’Connor, R.S.; Hwang, W.-T.; et al. Author Correction: Determinants of Response and Resistance to CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy of Chronic Lymphocytic Leukemia. Nat. Med. 2021, 27, 561. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Rao, S. Mechanisms Underlying T Cell Immunosenescence: Aging and Cytomegalovirus Infection. Front. Microbiol. 2016, 7, 2111. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Vernau, L.; Grupp, S.A.; Barrett, D.M. Naïve T-Cell Deficits at Diagnosis and after Chemotherapy Impair Cell Therapy Potential in Pediatric Cancers. Cancer Discov. 2019, 9, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Heipertz, E.L.; Zynda, E.R.; Stav-Noraas, T.E.; Hungler, A.D.; Boucher, S.E.; Kaur, N.; Vemuri, M.C. Current Perspectives on “Off-The-Shelf” Allogeneic NK and CAR-NK Cell Therapies. Front. Immunol. 2021, 12, 732135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin, Q.; Song, Y.; Liu, D. Universal CARs, Universal T Cells, and Universal CAR T Cells. J. Hematol. Oncol. 2018, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. ‘Off-the-Shelf’ Allogeneic CAR T Cells: Development and Challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef]
- Martínez Bedoya, D.; Dutoit, V.; Migliorini, D. Allogeneic CAR T Cells: An Alternative to Overcome Challenges of CAR T Cell Therapy in Glioblastoma. Front. Immunol. 2021, 12, 640082. [Google Scholar] [CrossRef]
- Nishimura, T.; Kaneko, S.; Kawana-Tachikawa, A.; Tajima, Y.; Goto, H.; Zhu, D.; Nakayama-Hosoya, K.; Iriguchi, S.; Uemura, Y.; Shimizu, T.; et al. Generation of Rejuvenated Antigen-Specific T Cells by Reprogramming to Pluripotency and Redifferentiation. Cell Stem Cell 2013, 12, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Euchner, J.; Sprissler, J.; Cathomen, T.; Fürst, D.; Schrezenmeier, H.; Debatin, K.-M.; Schwarz, K.; Felgentreff, K. Natural Killer Cells Generated from Human Induced Pluripotent Stem Cells Mature to CD56brightCD16+NKp80+/-In-Vitro and Express KIR2DL2/DL3 and KIR3DL1. Front. Immunol. 2021, 12, 640672. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Iriguchi, S.; Waseda, M.; Ueda, N.; Ueda, T.; Xu, H.; Minagawa, A.; Ishikawa, A.; Yano, H.; Ishi, T.; et al. Generation of Hypoimmunogenic T Cells from Genetically Engineered Allogeneic Human Induced Pluripotent Stem Cells. Nat. Biomed. Eng. 2021, 5, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Ghobadi, A.; Patel, K.; Park, J.H.; Flinn, I.W.; Shah, P.; Wong, C.; Bickers, C.; Szabo, P.; Wong, L.; et al. Safety and Efficacy of FT596, a First-in-Class, Multi-Antigen Targeted, Off-the-Shelf, IPSC-Derived CD19 CAR NK Cell Therapy in Relapsed/Refractory B-Cell Lymphoma. Blood 2021, 138, 823. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Kunitomi, A.; Hirohata, R.; Arreola, V.; Osawa, M.; Kato, T.M.; Nomura, M.; Kawaguchi, J.; Hara, H.; Kusano, K.; Takashima, Y.; et al. Improved Sendai Viral System for Reprogramming to Naive Pluripotency. Cell Rep. Methods 2022, 2, 100317. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Kamath, A.; Ternes, S.; McGowan, S.; English, A.; Mallampalli, R.; Moy, A.B. Efficient Method to Create Integration-Free, Virus-Free, Myc and Lin28-Free Human Induced Pluripotent Stem Cells from Adherent Cells. Future Sci. OA 2017, 3, FSO211. [Google Scholar] [CrossRef]
- Haase, A.; Olmer, R.; Schwanke, K.; Wunderlich, S.; Merkert, S.; Hess, C.; Zweigerdt, R.; Gruh, I.; Meyer, J.; Wagner, S.; et al. Generation of Induced Pluripotent Stem Cells from Human Cord Blood. Cell Stem Cell 2009, 5, 434–441. [Google Scholar] [CrossRef]
- Lowry, W.E.; Richter, L.; Yachechko, R.; Pyle, A.D.; Tchieu, J.; Sridharan, R.; Clark, A.T.; Plath, K. Generation of Human Induced Pluripotent Stem Cells from Dermal Fibroblasts. Proc. Natl. Acad. Sci. USA 2008, 105, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Rim, Y.A.; Yi, H.; Park, N.; Park, S.-H.; Ju, J.H. The Generation of Human Induced Pluripotent Stem Cells from Blood Cells: An Efficient Protocol Using Serial Plating of Reprogrammed Cells by Centrifugation. Stem Cells Int. 2016, 2016, 1329459. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Benvenisty, N. The Tumorigenicity of Human Embryonic and Induced Pluripotent Stem Cells. Nat. Rev. Cancer 2011, 11, 268–277. [Google Scholar] [CrossRef]
- Fusaki, N.; Ban, H.; Nishiyama, A.; Saeki, K.; Hasegawa, M. Efficient Induction of Transgene-Free Human Pluripotent Stem Cells Using a Vector Based on Sendai Virus, an RNA Virus That Does Not Integrate into the Host Genome. Proc. Jpn. Acad. Ser. B 2009, 85, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Freed, C.R. Adenoviral Gene Delivery Can Reprogram Human Fibroblasts to Induced Pluripotent Stem Cells. Stem Cells 2009, 27, 2667–2674. [Google Scholar] [CrossRef]
- Okita, K.; Yamakawa, T.; Matsumura, Y.; Sato, Y.; Amano, N.; Watanabe, A.; Goshima, N.; Yamanaka, S. An Efficient Nonviral Method to Generate Integration-Free Human-Induced Pluripotent Stem Cells from Cord Blood and Peripheral Blood Cells. Stem Cells 2013, 31, 458–466. [Google Scholar] [CrossRef]
- Yu, J.; Hu, K.; Smuga-Otto, K.; Tian, S.; Stewart, R.; Slukvin, I.I.; Thomson, J.A. Human Induced Pluripotent Stem Cells Free of Vector and Transgene Sequences. Science 2009, 324, 797–801. [Google Scholar] [CrossRef]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.-H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified MRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef]
- Woltjen, K.; Michael, I.P.; Mohseni, P.; Desai, R.; Mileikovsky, M.; Hämäläinen, R.; Cowling, R.; Wang, W.; Liu, P.; Gertsenstein, M.; et al. PiggyBac Transposition Reprograms Fibroblasts to Induced Pluripotent Stem Cells. Nature 2009, 458, 766–770. [Google Scholar] [CrossRef]
- Jia, F.; Wilson, K.D.; Sun, N.; Gupta, D.M.; Huang, M.; Li, Z.; Robbins, R.C.; Kay, M.A.; Longaker, M.T.; Wu, J.C. A Nonviral Minicircle Vector for Deriving Human IPS Cells. Nat. Methods 2010, 7, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Baghbaderani, B.A.; Syama, A.; Sivapatham, R.; Pei, Y.; Mukherjee, O.; Fellner, T.; Zeng, X.; Rao, M.S. Detailed Characterization of Human Induced Pluripotent Stem Cells Manufactured for Therapeutic Applications. Stem Cell Rev. 2016, 12, 394–420. [Google Scholar] [CrossRef]
- Kuang, Y.-L.; Munoz, A.; Nalula, G.; Santostefano, K.E.; Sanghez, V.; Sanchez, G.; Terada, N.; Mattis, A.N.; Iacovino, M.; Iribarren, C.; et al. Evaluation of Commonly Used Ectoderm Markers in IPSC Trilineage Differentiation. Stem Cell Res. 2019, 37, 101434. [Google Scholar] [CrossRef]
- Martins-Taylor, K.; Xu, R.-H. Concise Review: Genomic Stability of Human Induced Pluripotent Stem Cells. Stem Cells 2012, 30, 22–27. [Google Scholar] [CrossRef]
- Kogut, I.; McCarthy, S.M.; Pavlova, M.; Astling, D.P.; Chen, X.; Jakimenko, A.; Jones, K.L.; Getahun, A.; Cambier, J.C.; Pasmooij, A.M.G.; et al. High-Efficiency RNA-Based Reprogramming of Human Primary Fibroblasts. Nat. Commun. 2018, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Rao, M.S. A Review of the Methods for Human IPSC Derivation. Methods Mol. Biol. 2013, 997, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Okita, K.; Fusaki, N.; Sabel, M.S.; Chang, A.E.; Ito, F. Reprogramming of Melanoma Tumor-Infiltrating Lymphocytes to Induced Pluripotent Stem Cells. Stem Cells Int. 2016, 2016, 8394960. [Google Scholar] [CrossRef]
- Seki, T.; Yuasa, S.; Oda, M.; Egashira, T.; Yae, K.; Kusumoto, D.; Nakata, H.; Tohyama, S.; Hashimoto, H.; Kodaira, M.; et al. Generation of Induced Pluripotent Stem Cells from Human Terminally Differentiated Circulating T Cells. Cell Stem Cell 2010, 7, 11–14. [Google Scholar] [CrossRef]
- Gattinoni, L.; Speiser, D.E.; Lichterfeld, M.; Bonini, C. T Memory Stem Cells in Health and Disease. Nat. Med. 2017, 23, 18–27. [Google Scholar] [CrossRef]
- Banito, A.; Rashid, S.T.; Acosta, J.C.; Li, S.; Pereira, C.F.; Geti, I.; Pinho, S.; Silva, J.C.; Azuara, V.; Walsh, M.; et al. Senescence Impairs Successful Reprogramming to Pluripotent Stem Cells. Genes Dev. 2009, 23, 2134–2139. [Google Scholar] [CrossRef]
- Mali, P.; Ye, Z.; Hommond, H.H.; Yu, X.; Lin, J.; Chen, G.; Zou, J.; Cheng, L. Improved Efficiency and Pace of Generating Induced Pluripotent Stem Cells from Human Adult and Fetal Fibroblasts. Stem Cells 2008, 26, 1998–2005. [Google Scholar] [CrossRef]
- Chou, B.-K.; Mali, P.; Huang, X.; Ye, Z.; Dowey, S.N.; Resar, L.M.; Zou, C.; Zhang, Y.A.; Tong, J.; Cheng, L. Efficient Human IPS Cell Derivation by a Non-Integrating Plasmid from Blood Cells with Unique Epigenetic and Gene Expression Signatures. Cell Res. 2011, 21, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Galić, Z.; Kitchen, S.G.; Kacena, A.; Subramanian, A.; Burke, B.; Cortado, R.; Zack, J.A. T Lineage Differentiation from Human Embryonic Stem Cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11742–11747. [Google Scholar] [CrossRef] [PubMed]
- Vodyanik, M.A.; Bork, J.A.; Thomson, J.A.; Slukvin, I.I. Human Embryonic Stem Cell–Derived CD34+ Cells: Efficient Production in the Coculture with OP9 Stromal Cells and Analysis of Lymphohematopoietic Potential. Blood 2005, 105, 617–626. [Google Scholar] [CrossRef]
- Nagano, S.; Maeda, T.; Ichise, H.; Kashima, S.; Ohtaka, M.; Nakanishi, M.; Kitawaki, T.; Kadowaki, N.; Takaori-Kondo, A.; Masuda, K.; et al. High Frequency Production of T Cell-Derived IPSC Clones Capable of Generating Potent Cytotoxic T Cells. Mol. Ther. Methods Clin. Dev. 2019, 16, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Shiina, S.; Iriguchi, S.; Terakura, S.; Kawai, Y.; Kabai, R.; Sakamoto, S.; Watanabe, A.; Ohara, K.; Wang, B.; et al. Optimization of the Proliferation and Persistency of CAR T Cells Derived from Human Induced Pluripotent Stem Cells. Nat. Biomed. Eng. 2022, 7, 24–37. [Google Scholar] [CrossRef]
- Themeli, M.; Kloss, C.C.; Ciriello, G.; Fedorov, V.D.; Perna, F.; Gonen, M.; Sadelain, M. Generation of Tumor-Targeted Human T Lymphocytes from Induced Pluripotent Stem Cells for Cancer Therapy. Nat. Biotechnol. 2013, 31, 928–933. [Google Scholar] [CrossRef]
- Iriguchi, S.; Yasui, Y.; Kawai, Y.; Arima, S.; Kunitomo, M.; Sato, T.; Ueda, T.; Minagawa, A.; Mishima, Y.; Yanagawa, N.; et al. A Clinically Applicable and Scalable Method to Regenerate T-Cells from IPSCs for off-the-Shelf T-Cell Immunotherapy. Nat. Commun. 2021, 12, 430. [Google Scholar] [CrossRef]
- van der Stegen, S.J.C.; Lindenbergh, P.L.; Petrovic, R.M.; Xie, H.; Diop, M.P.; Alexeeva, V.; Shi, Y.; Mansilla-Soto, J.; Hamieh, M.; Eyquem, J.; et al. Generation of T-Cell-Receptor-Negative CD8αβ-Positive CAR T Cells from T-Cell-Derived Induced Pluripotent Stem Cells. Nat. Biomed. Eng. 2022, 6, 1284–1297. [Google Scholar] [CrossRef]
- Schwanbeck, R.; Just, U. The Notch Signaling Pathway in Hematopoiesis and Hematologic Malignancies. Haematologica 2011, 96, 1735–1737. [Google Scholar] [CrossRef]
- Montel-Hagen, A.; Crooks, G.M. From Pluripotent Stem Cells to T Cells. Exp. Hematol. 2019, 71, 24–31. [Google Scholar] [CrossRef]
- Leung, A.; Zulick, E.; Skvir, N.; Vanuytsel, K.; Morrison, T.A.; Naing, Z.H.; Wang, Z.; Dai, Y.; Chui, D.H.K.; Steinberg, M.H.; et al. Notch and Aryl Hydrocarbon Receptor Signaling Impact Definitive Hematopoiesis from Human Pluripotent Stem Cells. Stem Cells 2018, 36, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Mohtashami, M.; Shah, D.K.; Nakase, H.; Kianizad, K.; Petrie, H.T.; Zúñiga-Pflücker, J.C. Direct Comparison of Dll1- and Dll4-Mediated Notch Activation Levels Shows Differential Lymphomyeloid Lineage Commitment Outcomes. J. Immunol. 2010, 185, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.; Awong, G.; Sturgeon, C.M.; Ditadi, A.; LaMotte-Mohs, R.; Zúñiga-Pflücker, J.C.; Keller, G. T Lymphocyte Potential Marks the Emergence of Definitive Hematopoietic Progenitors in Human Pluripotent Stem Cell Differentiation Cultures. Cell Rep. 2012, 2, 1722–1735. [Google Scholar] [CrossRef] [PubMed]
- Vizcardo, R.; Masuda, K.; Yamada, D.; Ikawa, T.; Shimizu, K.; Fujii, S.; Koseki, H.; Kawamoto, H. Regeneration of Human Tumor Antigen-Specific T Cells from IPSCs Derived from Mature CD8+ T Cells. Cell Stem Cell 2013, 12, 31–36. [Google Scholar] [CrossRef]
- Montel-Hagen, A.; Seet, C.S.; Li, S.; Chick, B.; Zhu, Y.; Chang, P.; Tsai, S.; Sun, V.; Lopez, S.; Chen, H.-C.; et al. Organoid-Induced Differentiation of Conventional T Cells from Human Pluripotent Stem Cells. Cell Stem Cell 2019, 24, 376–389.e8. [Google Scholar] [CrossRef]
- Trotman-Grant, A.C.; Mohtashami, M.; De Sousa Casal, J.; Martinez, E.C.; Lee, D.; Teichman, S.; Brauer, P.M.; Han, J.; Anderson, M.K.; Zúñiga-Pflücker, J.C. DL4-Μbeads Induce T Cell Lineage Differentiation from Stem Cells in a Stromal Cell-Free System. Nat. Commun. 2021, 12, 5023. [Google Scholar] [CrossRef]
- Vizcardo, R.; Klemen, N.D.; Islam, S.M.R.; Gurusamy, D.; Tamaoki, N.; Yamada, D.; Koseki, H.; Kidder, B.L.; Yu, Z.; Jia, L.; et al. Generation of Tumor Antigen-Specific IPSC-Derived Thymic Emigrants Using a 3D Thymic Culture System. Cell Rep. 2018, 22, 3175–3190. [Google Scholar] [CrossRef]
- Maeda, T.; Nagano, S.; Ichise, H.; Kataoka, K.; Yamada, D.; Ogawa, S.; Koseki, H.; Kitawaki, T.; Kadowaki, N.; Takaori-Kondo, A.; et al. Regeneration of CD8αβ T Cells from T-Cell–Derived IPSC Imparts Potent Tumor Antigen-Specific Cytotoxicity. Cancer Res. 2016, 76, 6839–6850. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Shinohara, T.; Koga, K.; Iriguchi, S.; Miyake, Y.; Song, X.; Tada, M.; Kassai, Y.; Kiyoi, H.; Kaneko, S. Guided Polarization of IPSC-Derived CD4SP Helper T Cells By CRISPR/Cas9-Based Genome-Editing. Blood 2019, 134, 1937. [Google Scholar] [CrossRef]
- Minagawa, A.; Yoshikawa, T.; Yasukawa, M.; Hotta, A.; Kunitomo, M.; Iriguchi, S.; Takiguchi, M.; Kassai, Y.; Imai, E.; Yasui, Y.; et al. Enhancing T Cell Receptor Stability in Rejuvenated IPSC-Derived T Cells Improves Their Use in Cancer Immunotherapy. Cell Stem Cell 2018, 23, 850–858.e4. [Google Scholar] [CrossRef]
- Kawai, Y.; Kawana-Tachikawa, A.; Kitayama, S.; Ueda, T.; Miki, S.; Watanabe, A.; Kaneko, S. Generation of Highly Proliferative, Rejuvenated Cytotoxic T Cell Clones through Pluripotency Reprogramming for Adoptive Immunotherapy. Mol. Ther. 2021, 29, 3027–3041. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.A.; Ni, Z.; Hermanson, D.; Hexum, M.K.; Bendzick, L.; Cooper, L.J.N.; Lee, D.A.; Kaufman, D.S. Clinical-Scale Derivation of Natural Killer Cells from Human Pluripotent Stem Cells for Cancer Therapy. Stem Cells Transl. Med. 2013, 2, 274–283. [Google Scholar] [CrossRef]
- Woll, P.S.; Grzywacz, B.; Tian, X.; Marcus, R.K.; Knorr, D.A.; Verneris, M.R.; Kaufman, D.S. Human Embryonic Stem Cells Differentiate into a Homogeneous Population of Natural Killer Cells with Potent In Vivo Antitumor Activity. Blood 2009, 113, 6094–6101. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Knorr, D.A.; Clouser, C.L.; Hexum, M.K.; Southern, P.; Mansky, L.M.; Park, I.-H.; Kaufman, D.S. Human Pluripotent Stem Cells Produce Natural Killer Cells That Mediate Anti-HIV-1 Activity by Utilizing Diverse Cellular Mechanisms. J. Virol. 2011, 85, 43–50. [Google Scholar] [CrossRef]
- Hermanson, D.L.; Ni, Z.; Kaufman, D.S. Human Pluripotent Stem Cells. In Hematopoietic Differentiation of Human Pluripotent Stem Cells; Cheng, T., Ed.; SpringerBriefs in Stem Cells; Springer: Dordrecht, The Netherlands, 2015; Volume 6, pp. 69–79. ISBN 978-94-017-7311-9. [Google Scholar]
- Denman, C.J.; Senyukov, V.V.; Somanchi, S.S.; Phatarpekar, P.V.; Kopp, L.M.; Johnson, J.L.; Singh, H.; Hurton, L.; Maiti, S.N.; Huls, M.H.; et al. Membrane-Bound IL-21 Promotes Sustained Ex Vivo Proliferation of Human Natural Killer Cells. PLoS ONE 2012, 7, e30264. [Google Scholar] [CrossRef]
- Hermanson, D.L.; Bendzick, L.; Pribyl, L.; McCullar, V.; Vogel, R.I.; Miller, J.S.; Geller, M.A.; Kaufman, D.S. Induced Pluripotent Stem Cell-Derived Natural Killer Cells for Treatment of Ovarian Cancer. Stem Cells 2016, 34, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Nianias, A.; Themeli, M. Induced Pluripotent Stem Cell (IPSC)–Derived Lymphocytes for Adoptive Cell Immunotherapy: Recent Advances and Challenges. Curr. Hematol. Malig. Rep. 2019, 14, 261–268. [Google Scholar] [CrossRef]
- Nakatsuji, N.; Nakajima, F.; Tokunaga, K. HLA-Haplotype Banking and IPS Cells. Nat. Biotechnol. 2008, 26, 739–740. [Google Scholar] [CrossRef]
- Taylor, C.J.; Peacock, S.; Chaudhry, A.N.; Bradley, J.A.; Bolton, E.M. Generating an IPSC Bank for HLA-Matched Tissue Transplantation Based on Known Donor and Recipient HLA Types. Cell Stem Cell 2012, 11, 147–152. [Google Scholar] [CrossRef]
- Yumlu, S.; Stumm, J.; Bashir, S.; Dreyer, A.-K.; Lisowski, P.; Danner, E.; Kühn, R. Gene Editing and Clonal Isolation of Human Induced Pluripotent Stem Cells Using CRISPR/Cas9. Methods 2017, 121–122, 29–44. [Google Scholar] [CrossRef]
- Feucht, J.; Sun, J.; Eyquem, J.; Ho, Y.-J.; Zhao, Z.; Leibold, J.; Dobrin, A.; Cabriolu, A.; Hamieh, M.; Sadelain, M. Calibration of CAR Activation Potential Directs Alternative T Cell Fates and Therapeutic Potency. Nat. Med. 2019, 25, 82–88. [Google Scholar] [CrossRef]
- Mehta, A.; Farooq, U.; Chen, A.; McGuirk, J.P.; Ly, T.; Wong, L.; Cooley, S.; Valamehr, B.; Elstrom, R.; Chu, Y.-W.; et al. Interim Phase I Clinical Data of FT819-101, a Study of the First-Ever, Off-the-Shelf, IPSC-Derived TCR-Less CD19 CAR T-Cell Therapy for Patients with Relapsed/Refractory B-Cell Malignancies. Blood 2022, 140, 4577–4578. [Google Scholar] [CrossRef]
- Hermanson, D.L.; Kaufman, D.S. Utilizing Chimeric Antigen Receptors to Direct Natural Killer Cell Activity. Front. Immunol. 2015, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human IPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-Tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Patel, S.; Patel, M.; Musni, K.; Anderson, M.; Cooley, S.; Valamehr, B.; Chu, W. 380 Preliminary Results of an Ongoing Phase I Trial of FT500, a First-in-Class, off-the-Shelf, Induced Pluripotent Stem Cell (IPSC) Derived Natural Killer (NK) Cell Therapy in Advanced Solid Tumors. J. Immunother. Cancer 2020, 8, A405. [Google Scholar] [CrossRef]
- Gutierrez, M.; Patel, M.; Liu, F.; Szabo, P.; Valamehr, B.; Chu, Y.-W.; Beagle, B.; Chou, J.; Hong, D. 726 Phase I Results of FT516, an off-the-Shelf, IPSC-Derived NK Cell Therapy Expressing a High-Affinity, Non-Cleavable CD16 (HnCD16) Combined with Avelumab in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef]
- Goulding, J.; Hancock, B.; Blum, R.; Ge, M.; Gaidarova, S.; Rogers, P.; Mahmood, S.; Mbofung, R.; Yeh, W.-I.; Yang, B.-H.; et al. 117 FT536 Path to IND: Ubiquitous Targeting of Solid Tumors with an off-the-Shelf, First-of-Kind MICA/B-Specific CAR-INK Cellular Immunotherapy. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- Dhakal, B.; Berdeja, J.G.; Gregory, T.; Ly, T.; Bickers, C.; Zong, X.; Wong, L.; Goodridge, J.P.; Cooley, S.; Valamehr, B.; et al. Interim Phase I Clinical Data of FT576 As Monotherapy and in Combination with Daratumumab in Subjects with Relapsed/Refractory Multiple Myeloma. Blood 2022, 140, 4586–4587. [Google Scholar] [CrossRef]
- Yannoutsos, N.; Wilson, P.; Yu, W.; Chen, H.T.; Nussenzweig, A.; Petrie, H.; Nussenzweig, M.C. The Role of Recombination Activating Gene (RAG) Reinduction in Thymocyte Development In Vivo. J. Exp. Med. 2001, 194, 471–480. [Google Scholar] [CrossRef]
- Ito, T.; Kawai, Y.; Yasui, Y.; Iriguchi, S.; Minagawa, A.; Ishii, T.; Miyoshi, H.; Taketo, M.M.; Kawada, K.; Obama, K.; et al. The Therapeutic Potential of Multiclonal Tumoricidal T Cells Derived from Tumor Infiltrating Lymphocyte-Derived IPS Cells. Commun. Biol. 2021, 4, 694. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Ando, M.; Ando, J.; Ishii, M.; Sakiyama, Y.; Ohara, K.; Toyota, T.; Ohtaka, M.; Masuda, A.; Terao, Y.; et al. Sustainable Tumor-Suppressive Effect of IPSC-Derived Rejuvenated T Cells Targeting Cervical Cancers. Mol. Therapy 2020, 28, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Ando, J.; Yamazaki, S.; Ishii, M.; Sakiyama, Y.; Harada, S.; Honda, T.; Yamaguchi, T.; Nojima, M.; Ohshima, K.; et al. Long-Term Eradication of Extranodal Natural Killer/T-Cell Lymphoma, Nasal Type, by Induced Pluripotent Stem Cell-Derived Epstein-Barr Virus-Specific Rejuvenated T Cells In Vivo. Haematologica 2020, 105, 796–807. [Google Scholar] [CrossRef]
- Terakura, S.; Yamamoto, T.N.; Gardner, R.A.; Turtle, C.J.; Jensen, M.C.; Riddell, S.R. Generation of CD19-Chimeric Antigen Receptor Modified CD8+ T Cells Derived from Virus-Specific Central Memory T Cells. Blood 2012, 119, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Bonifacius, A.; Lamottke, B.; Tischer-Zimmermann, S.; Schultze-Florey, R.; Goudeva, L.; Heuft, H.-G.; Arseniev, L.; Beier, R.; Beutel, G.; Cario, G.; et al. Patient-Tailored Adoptive Immunotherapy with EBV-Specific T Cells from Related and Unrelated Donors. J. Clin. Investig. 2023, 133, e163548. [Google Scholar] [CrossRef]
- Cobbold, M.; Khan, N.; Pourgheysari, B.; Tauro, S.; McDonald, D.; Osman, H.; Assenmacher, M.; Billingham, L.; Steward, C.; Crawley, C.; et al. Adoptive Transfer of Cytomegalovirus-Specific CTL to Stem Cell Transplant Patients after Selection by HLA-Peptide Tetramers. J. Exp. Med. 2005, 202, 379–386. [Google Scholar] [CrossRef]
- Fang, F.; Wang, W.; Chen, M.; Tian, Z.; Xiao, W. Technical Advances in NK Cell-Based Cellular Immunotherapy. Cancer Biol. Med. 2019, 16, 647–654. [Google Scholar] [CrossRef]
- Gras Navarro, A.; Björklund, A.T.; Chekenya, M. Therapeutic Potential and Challenges of Natural Killer Cells in Treatment of Solid Tumors. Front. Immunol. 2015, 6, 202. [Google Scholar] [CrossRef]
- Carlsten, M.; Childs, R.W. Genetic Manipulation of NK Cells for Cancer Immunotherapy: Techniques and Clinical Implications. Front. Immunol. 2015, 6, 266. [Google Scholar] [CrossRef]
- Afolabi, L.O.; Adeshakin, A.O.; Sani, M.M.; Bi, J.; Wan, X. Genetic Reprogramming for NK Cell Cancer Immunotherapy with CRISPR/Cas9. Immunology 2019, 158, 63–69. [Google Scholar] [CrossRef]
- Kang, S.; Gao, X.; Zhang, L.; Yang, E.; Li, Y.; Yu, L. The Advances and Challenges of NK Cell-Based Cancer Immunotherapy. Curr. Oncol. 2021, 28, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, M.; Retèl, V.; Rohaan, M.; van den Berg, J.; Haanen, J.; van Harten, W. Evaluating Different Adoption Scenarios for TIL-Therapy and the Influence on Its (Early) Cost-Effectiveness. BMC Cancer 2020, 20, 712. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.; Xiong, Y.; Hu, P.; Wu, D.; Alabanza, L.; Orentas, R.J.; Dropulic, B.; Schneider, D. Self-Driving Armored CAR-T Cells Overcome a Suppressive Milieu and Eradicate CD19+ Raji Lymphoma in Preclinical Models. Mol. Therapy 2021, 29, 2691–2706. [Google Scholar] [CrossRef]
- Heinze, D.; Park, S.; McCracken, A.; Haratianfar, M.; Lindstrom, J.; Villacorta-Martin, C.; Mithal, A.; Wang, F.; Yang, M.W.; Murphy, G.; et al. Notch Activation during Early Mesoderm Induction Modulates Emergence of the T/NK Cell Lineage from Human IPSCs. Stem Cell Rep. 2022, 17, 2610–2628. [Google Scholar] [CrossRef] [PubMed]
- Challenge Theme R&D Spotlight: Development of an End-to-End Closed IPSC Expansion Process—Cell and Gene Therapy. Available online: https://ct.catapult.org.uk/news/challenge-theme-r-d-spotlight-development-of-an-end-to-end-closed-ipsc-expansion-process (accessed on 28 October 2023).
- Pandey, P.R.; Tomney, A.; Woon, M.T.; Uth, N.; Shafighi, F.; Ngabo, I.; Vallabhaneni, H.; Levinson, Y.; Abraham, E.; Friedrich Ben-Nun, I. End-to-End Platform for Human Pluripotent Stem Cell Manufacturing. Int. J. Mol. Sci. 2019, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Kempf, H.; Hetzel, M.; Hesse, C.; Hashtchin, A.R.; Brinkert, K.; Schott, J.W.; Haake, K.; Kühnel, M.P.; Glage, S.; et al. Bioreactor-Based Mass Production of Human IPSC-Derived Macrophages Enables Immunotherapies against Bacterial Airway Infections. Nat. Commun. 2018, 9, 5088. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).