Simple Summary
Various physiological activities of organisms, including movement, feeding, reproduction, breathing, excretion, etc., all require the participation of their neuromuscular systems. Echinoderms, a phylum closely related to chordates, possess a well-differentiated but simpler muscular system, which provides a great opportunity to trace the evolutionary origins of the vertebrate muscular system. Here, we review the morphology of different musculatures and the effects of different neurotransmitters and neuropeptides involved in muscle regulation in echinoderms. In addition, we highlight the potential molecular mechanisms underpinning the action of these chemical messengers on echinoderm muscles.
Abstract
The muscular systems of echinoderms play important roles in various physiological and behavioral processes, including feeding, reproduction, movement, respiration, and excretion. Like vertebrates, echinoderm muscle systems can be subdivided into two major divisions, somatic and visceral musculature. The former usually has a myoepithelial organization, while the latter contains muscle bundles formed by the aggregation of myocytes. Neurons and their processes are also detected between these myoepithelial cells and myocytes, which are capable of releasing a variety of neurotransmitters and neuropeptides to regulate muscle activity. Although many studies have reported the pharmacological effects of these chemical messengers on various muscles of echinoderms, there has been limited research on their receptors and their signaling pathways. The muscle physiology of echinoderms is similar to that of chordates, both of which have the deuterostome mode of development. Studies of muscle regulation in echinoderms can provide new insights into the evolution of myoregulatory systems in deuterostomes.
1. Introduction
Echinoderm, originating from the Greek for “spiny skin”, is a group of marine benthic organisms. As a phylum of deuterostomate invertebrates, echinoderms form a sister clade to the chordates with hemichordates and xenoturbellids [1,2,3]. Their unique evolutionary status and close relationship to chordates, particularly when compared with other invertebrates, make them attractive as upcoming model systems. Extant echinoderms comprise five well-defined classes: Crinoidea, Asteroidea, Ophiuroidea, Holothuroidea, and Echinoidea [4]. Their biological behaviors have a great influence on the physico-chemical processes of submarine ecosystems, including maintaining and improving sediment health, the recycling of nutrients, biomass regulation, and so on [5,6]. With reference to morphology, adult echinoderms show several distinctive characteristics, such as a (usually) pentaradially symmetrical body structure, unique water vascular system, calcium carbonate endoskeleton, and mutable connective tissue [7], and, with reference to physiology, regeneration, autotomy, aestivation, and evisceration [8,9,10,11].
Echinoderms possess well-differentiated but simpler muscular systems in comparison with vertebrates, which provide a great opportunity to trace the evolutionary origins of the vertebrate muscular system [12,13]. Like vertebrates, echinoderm muscle systems can be subdivided into two major divisions, somatic and visceral musculature. However, in echinoderms, there seem to be few distinctive differences between these two muscle categories [14]. In terms of its microscopic structure, echinoderm muscle resembles more vertebrate smooth muscle than striated muscle, including body wall muscles, digestive tract muscles, appendage muscles, and arm muscles [14,15,16]. In terms of function, the muscular systems of echinoderms play important roles in various physiological and behavioral processes, including feeding, reproduction, movement, respiration, and excretion [17,18,19,20].
The muscular system of organisms and its innervation together form the neuromuscular system. There are many neurotransmitters in this system involved in regulating muscle activities such as cholines (e.g., acetylcholine, ACh), bioamines (e.g., 5-hydroxytryptamine, 5-HT), amino acids (e.g., L-glutamate), gases (e.g., nitric oxide, NO), and neuropeptides [15,21,22,23]. In vertebrates, the excitation–contraction coupling mechanism of muscles has been studied in detail, including the morphological basis of muscle fibers, action potential conduction, and intracellular Ca2+ elevation mechanism [24]. However, little research has been conducted on neuromuscular systems in marine invertebrates including echinoderms. Elphick and Melarange (2001) reviewed the neural control of muscle relaxation in echinoderms and found that it is regulated by at least two parallel neuronal signaling systems [15]. Subsequently, Hill (2001) reviewed the role of Ca2+ in muscle contraction in echinoderms, including Ca2+-dependent muscle contraction and Ca2+ storage and release sites [25]. Recently, pharmacological experiments have led to the discovery of an increasing number of myoactive neuropeptides [23,26]. However, the mechanism of muscle contraction of echinoderms has only been briefly studied at the tissue level, while the signaling pathway at the cellular and molecular levels is not well understood [27]. Here, we comprehensively review the morphology and function of different muscles in echinoderms, as well as the action characteristics and mechanisms of neurotransmitters and neuropeptides that act on these muscles.
2. Morphology and Function of Musculature in Echinoderms
Echinoderm muscles occur in many different anatomical locations, including the body wall, digestive system, reproductive system, respiratory system, and water vascular system, but they have a similar histological structure [14]. As in vertebrates, the musculature of echinoderms can be divided into visceral and somatic musculature. Visceral musculature is generally considered to have a myoepithelial organization and somatic musculature is considered to be the large muscles of the body, which consist mainly of muscle bundles containing many myocytes [14,15].
2.1. Visceral Musculature
The visceral musculature of echinoderms is the myoepithelium covering celomic surfaces of the internal organs and lining the inner surfaces of the water vascular system, including the musculature of the digestive [28], reproductive [29], and respiratory [30] systems as well as the musculature of tube feet and tentacles [31]. The myoepithelium is mainly composed of myoepithelial and peritoneal cells [14]. Typically, peritoneal cells consist of an extended apical part facing the celomic cavity and a slender basal peduncle penetrating the entire myoepithelium and adhering to the basal lamina through hemidesmosomes. The apical part has an irregularly shaped nucleus, a cilium, and microvilli, and the slender basal peduncle has bundles of intracellular filaments. Myoepithelial cells have a large number of densely packed myofilaments in the cytoplasm, which form a powerful contractile apparatus. They are connected to the basal lamina through hemidesmosomes and to other myoepithelial cells through desmosomes (Figure 1A–E). Myoepithelial cells can be arranged in different directions in different organs, forming oblique, longitudinal, or circular musculature. There are also neurons and their processes between peritoneal and myoepithelial cells, whereas there is no synaptic specialization at the neuron–muscle junction [14,31].
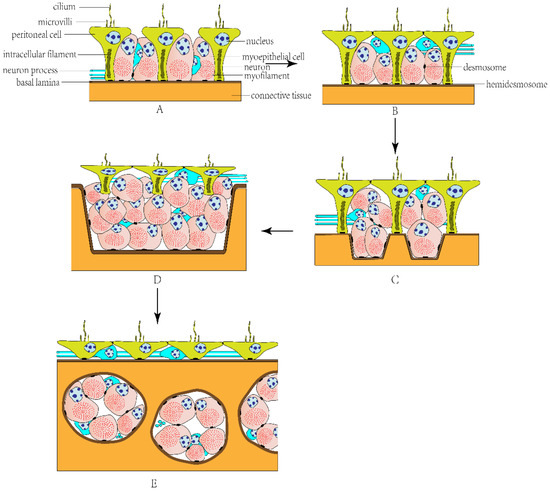
Figure 1.
The evolutionary model of echinoderm myoepithelial organization. (A) Simple myoepithelium. (B) Pseudostratified myoepithelium. (C) Bipartite pseudostratified myoepithelium. (D) Stratified myoepithelium. (E) Subepithelial musculature.
In general, the myoepithelium of the echinoderm visceral musculature is divided into the following types: (1) Simple myoepithelium (Figure 1A): a single-layered myoepithelium; most myoepithelial cells are incorporated into the adluminal surface between peritoneal cells. (2) Pseudostratified myoepithelium (Figure 1B): peritoneal cells are situated apically and myoepithelial cells are situated subapically; not all cells contribute to the adluminal surface, but all cells adhere to the common basal matrix. (3) Bipartite pseudostratified myoepithelium (Figure 1C): myoepithelial cells are either directly anchored to the basal matrix or suspended between the apical and basal surfaces of the myoepithelium. (4) Stratified myoepithelium (Figure 1D): all peritoneal cells lose contact with the basal lamina completely. (5) Subepithelial musculature (Figure 1E): myocytes are below the basal matrix and separated from the celomic lining, forming muscle bundles [31].
2.1.1. Musculature of the Water Vascular System
The tube feet and tentacles of echinoderms are body wall protuberances associated with the water vascular system, responsible for movement, adhesion, feeding, and sensation. Tube feet consist of a stem and terminal disk, with the former having a trilaminar organization consisting of an outer epidermis, a middle layer of connective tissue, and an inner celomic lining [32,33]. In all five classes of echinoderms, the celomic lining of the tube feet is a myoepithelium composed of two predominant cell types: peritoneal and myoepithelial cells. However, the morphology of the myoepithelium varies between different species from a simple myoepithelium in crinoids to a bipartite pseudostratified myoepithelium in echinoids and asteroids, with various intermediate organizations (simple or pseudostratified myoepithelium) in ophiuroids and holothurians [31,34,35,36,37]. Myoepithelial cells, consisting largely of thick and thin myofilaments, are orientated in parallel with the primary axis of tube feet and form a longitudinal muscular system, which is responsible for connecting the stem to the terminal disk [31]. The contraction of the longitudinal musculature can produce a backward pull, leading to a vacuum remaining between the basement of the tube feet and the surface of contact through the connective tissue, which facilitates adhesion [38]. The electron micrographs of myoepithelial organization in the tube feet can be seen in the figures of [31] and Figure 2.
The tentacles of holothurians can be divided into two major parts, the proximal stem and the distal branches. They are also composed of three major layers like tube feet: the epidermis, connective tissue, and celomic lining [33]. The water vascular canal of tentacles is formed by a myoepithelium where myoepithelial cells form a well-developed longitudinal muscle system within the tentacle that controls its movement. The myoepithelium of tentacles varies in different holothurians: there is a simple myoepithelium in Holothuria forskali [37] and Leptosynapta tenuis [31], and a pseudostratified myoepithelium in Leptosynapta spp. [39]. An electron micrograph of myoepithelial organization in the tentacles can be seen in the figures of [37].
2.1.2. Musculature of the Internal Organs
The histological structure of the digestive tract in echinoderms consists of three layers: an inner digestive epithelium, a middle layer of connective tissue, and an outer celomic epithelium [28,40,41,42]. The celomic epithelium is usually a pseudostratified myoepithelium, where most myoepithelial cells are orientated perpendicular to the long axis of the gut, forming the outer circular musculature, and a small portion of myoepithelial cells parallel to the long axis constitutes the inner longitudinal musculature [28,41,43]. The myoepithelial cells in the longitudinal musculature are loosely arranged and partially embedded in the underlying connective tissue. Conversely, the myoepithelial cells in the circular musculature are clustered and continuously arranged, which can produce a more powerful contraction [41,44]. The distribution and development of these two muscle layers vary in different regions of the digestive tract. Typically, the circular layer occurs all along the digestive tract, while the longitudinal layer is scarcely visible in the anterior part and better developed in the posterior part [41,42,44]. The contraction of the circular and longitudinal musculature produces intestinal peristalsis and plays a basic role in the movement of ingested food [44]. Electron micrographs of the myoepithelial organization in the digestive tract can be seen in the figures of [28,41].
Besides the wall of the digestive tract, myoepithelial organization is also the common characteristic of the celomic epithelium in other internal organs, such as the ovaries [45], respiratory tree [42], and hemal system [31]. The ovaries of holothurians and crinoids have similar morphological detail and complexity, with both having a trilaminar structure: a germinal inner epithelium, connective tissue containing a genital hemal sinus, and an outer celomic epithelium. The outer celomic epithelium is a pseudostratified myoepithelium where myoepithelial cells form circular and longitudinal layers or only a circular layer [29,31,45,46,47]. However, in some holothurians, such as Stichopus tremulus and Mesothuria intestinalis, it is a simple myoepithelium that consists of only monociliated myoepithelial cells [48].
The ovaries of asteroids, ophiuroids, and echinoids have greater complexity, which includes an outer sac and an inner sac [47,49,50]. The organization of the inner sac is similar to and homologous to that of the ovaries of holothurians and crinoids, which consists of a germinal epithelium, connective tissue, and myoepithelium [45]. The outer sac, which is separated from the inner sac by a schizocoelic space, is also composed of three layers: an inner epithelium, a connective tissue layer, and an outer celomic epithelium. The inner epithelium is a myoepithelium in asteroids, a peritoneum in ophiuroids, and incomplete or virtually absent in echinoids [45,47,49,51]. And these muscles in ovaries mainly function to expel gametes by producing vigorous spawning contractions [46]. Electron micrographs of the myoepithelial organization in the ovaries can be seen in the figures of [29,46,49].
The respiratory trees of most holothurians are composed of an inner lining epithelium, a connective tissue layer, and an outer celomic epithelium. Myoepithelial cells in the celomic epithelium are orientated both along and perpendicular to the axis of the organ, forming a well-developed mesh of longitudinal and circular musculature. The contraction of these muscles contributes to respiratory movements together with other muscles of the body [30,42]. An electron micrograph of the myoepithelial organization in the respiratory tree can be seen in a figure in [42].
Holothuroids possess the most highly developed hemal system in echinoderms, where the wall of the hemal vessels typically has three layers: an outer epithelial layer, an intermediate circular muscle layer, and an inner connective tissue layer. The hemal vessels do not have obvious linings, and most of the lumen is occupied by connective tissue, forming a network. Epithelial and myoepithelial cells in the hemal vessel wall form a pseudostratified myoepithelium, which is anchored to a basal lamina, and most myoepithelial cells are arranged in a circular to oblique manner round the vessel. Nerve fibers are also present between the epithelial and myoepithelial cells [52,53]. An electron micrograph of a holothuroid hemal vessel can be seen in a figure in [52].
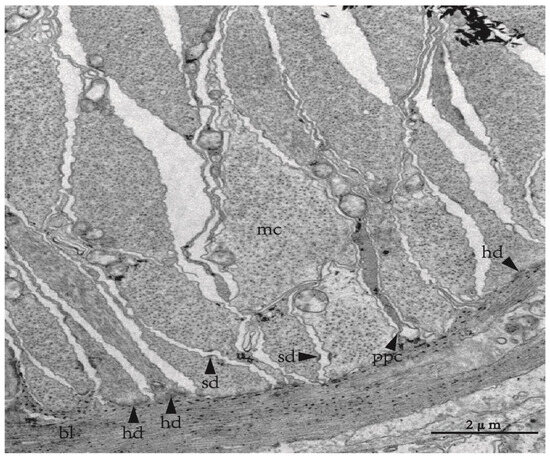
Figure 2.
The myoepithelial organization in tube foot of Apostichopus japonicus. Abbreviations: bl, basal lamina; hd, hemidesmosome; mc, myoepithelial cell; ppc, process of peritoneal cell; sd, spot desmosome.
2.2. Somatic Musculature
Most echinoderms’ somatic musculature consists of large muscles with varying organization and function, which are composed of muscle bundles containing many myocytes [14]. These include longitudinal and circular muscles of the body wall in holothurians and asteroids [54,55], pharyngeal retractor muscles in holothurians [18], Aristotle’s lantern muscles in echinoids [56], arm muscles in ophiuroids and crinoids [57,58], and spine muscles in asteroids, echinoids, and ophiuroids [59,60,61]. Most of the cytoplasm of these myocytes is occupied by myofilaments, forming a powerful contractile apparatus. There are many myocyte processes extending into the center area of each muscle bundle, called “muscle tails”, which are responsible for receiving innervation. Each muscle bundle is surrounded by a basal lamina, to which myocytes are attached by hemidesmosomes, and adjacent myocytes are connected to each other by desmosomes. There are also neurons and their processes between myocytes (Figure 3) [14,25].
2.2.1. Holothurian Somatic Musculature
- Longitudinal and circular muscles of the body wall
There are two muscle layers lining the body wall in holothurians: the inner is a circular muscle layer and the outer is a longitudinal muscle layer. Holothurians typically have five longitudinal muscles of the body wall (LMBW), which run along the longitudinal axis of the body and are connected to and innervated by the radial nerve via the mesentery. The circular muscle layer is located in the coelom lining of the body wall and interrupted by the LMBW and the radial nerve cords at each ambulacrum [21]. These two muscles work in coordination to regulate the animal’s movement. Myocytes of the LMBW have no well-developed sarcoplasmic reticulum (SR) but have many subsarcolemmal vesicles, which are considered potential storage sites for Ca2+ [25,54]. Furthermore, the LMBW has been regarded as the best model for studying excitation–contraction coupling in echinoderms due to its capacity for extreme stretch without physical damage [25]. An electron micrograph of the LMBW can be seen in a figure in [54].
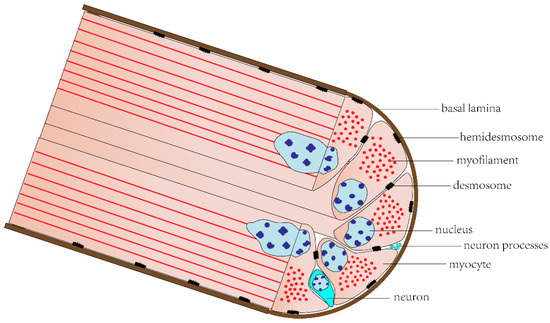
Figure 3.
Muscle bundle of echinoderm somatic musculature.
- Pharyngeal retractor muscles
Holothurians of the order Dendrochirotida have five pharyngeal retractor muscles, which are connected to the calcareous ring at their anterior end and link with the LMBW at their posterior end. These are responsible for anchoring the oral complex (pharynx and buccal tentacles) to the body wall [18,62]. Myocytes of the pharyngeal retractor muscle are joined by desmosomes and arranged in muscle bundles surrounded by a basal lamina. The organization of these muscle bundles is uniform throughout the tissue. Functionally, the contraction of the pharyngeal retractor muscle brings about the retraction of the oral complex after it has been protracted for feeding [18,63]. An electron micrograph of the pharyngeal retractor muscle can be seen in a figure in [18].
2.2.2. Echinoid Somatic Musculature
- Aristotle’s lantern muscles
Most echinoids possess a complex masticatory apparatus called Aristotle’s lantern, consisting of a muscular system and skeletal framework. The muscular system of Aristotle’s lantern is considered to be the most elaborate contractile system in echinoderms and includes seven major groups of muscles: pharyngeal levators and depressors, interpyramidal muscles, compass elevators and depressors, and lantern protractors and retractors [19,56]. Some of these muscles exhibit myoepithelial organization and others are muscle bundles [64].
Pharyngeal levators and depressors are an advanced form of bipartite pseudostratified myoepithelium, consisting of myoepithelial and peritoneal cells, where myoepithelial cells partly become immersed in the connective tissue and some peritoneal cells lose contact with the basal lamina. Interpyramidal muscles are a typical pseudostratified myoepithelium, and myoepithelial cells have well-developed myofilaments that make these muscles extremely powerful. Compass depressors and elevators function in respiratory movements and have different muscular structures. The myoepithelium on the adaxial surface of compass depressors is a bipartite pseudostratified myoepithelium. Myoepithelial cells are deeply immersed in the connective tissue and lie parallel to the longitudinal axis of the depressor. The myoepithelium on the oral surface of compass elevators is also a bipartite pseudostratified myoepithelium, but in the internal compartment, myocytes are arranged in typical muscle bundles. Protractors and retractors serve to push the lantern outwards and pull it back, respectively. The internal compartments of protractors and retractors are filled with muscle bundles surrounded by basal lamina. These muscle bundles are unevenly distributed in the connective tissue; most of them are abundant at the adaxial side of the muscle [56,64]. Electron micrographs of the lantern muscles can be seen in the figures of [56,64].
- Spine muscles
Three extant echinoderm classes (Asteroidea, Ophiuroidea, and Echinoidea) have calcified spines that are connected to the endoskeleton of the body wall through a mobile spine joint. The spine joint is the critical structure for spine movement, allowing the spine to incline and erect [59,60,61]. The spine joint of sea urchins is associated with a spine muscle and a ligament, or catch apparatus (CA), consisting of mutable collagenous tissue. The spine muscle runs longitudinally from the base of the spine to the test and surrounds the CA [60]. The CA consists mainly of collagen fibers but some smooth myocytes are also present, which run parallel to the collagen fibers, and both ends are attached to the collagen fibers [65]. The movement of the spine depends on the coordination of the spine muscle and CA, which bring about spine movement through the contraction of the spine muscle on one side, the relaxation of the spine muscle on the opposite side, and the destiffening of the whole CA [66].
2.2.3. Asteroid Somatic Musculature
- Longitudinal and circular muscles of the body wall
Each arm of asteroids has two muscle layers at the celomic side of the dorsal body wall. The superficial layer is longitudinal muscle and the inner layer is circular muscle. The longitudinal muscle is thickened along the midline of the arm to form the apical muscle, which may contribute to upward arm flexion. The circular muscle layer is separated from the longitudinal muscle layer by connective tissue, which also divides the circular muscles into transverse muscle strands that insert into the body wall ossicles. The contraction of the circular muscles can produce a widening in the ambulacral groove and arm torsion [55,67]. A photomicrograph of the longitudinal and circular muscles of the body wall in asteroids can be seen in a figure in [67].
- Spine muscles
In asteroids, the morphology of the spine and the spine joint in the crown-of-thorns starfish is well studied. There are two groups of musculature in the joint; one is the musculature in the dermis surrounding the joint, and the other is the musculature that connects the spine and the pedicel. Both musculatures are smooth and oriented along the long axis of the spine. The distribution of these muscles suggests that they permit a passive inclination of the spine and bring about its active erection [59].
2.2.4. Ophiuroid Somatic Musculature
- Arm muscles
The arms of ophiuroids consist of a series of homonomous segments. Each segment contains (1) an internal vertebral ossicle, the vertebral ossicles of adjacent segments connected by an intervertebral ligament and paired oral and aboral intervertebral muscles, and (2) four external arm plates: oral, aboral, and paired lateral plates. These intervertebral muscles are attached to special areas of the vertebral ossicle. The contraction of these intervertebral muscles causes adjacent ossicles to rotate around the intervertebral joint, which controls arm movement [57,68].
- Spine muscles
The spines of ophiuroids are connected to the lateral arm plate by the spine muscle and ligament. The spine muscle consists of two muscle bundles of myocytes, which originate in different areas of the lateral arm plate and converge to form a single bundle at the base of the spine. The morphology of the two muscle bundles varies widely across the class, this being smooth muscle in Ophiothrix fragilis and Amphipholis squamata and obliquely striated muscle in Ophiocomina nigra, where adjacent myofilaments are progressively staggered. The contraction of the muscle is mainly responsible for erecting the spine [61,69,70]. An electron micrograph of the spine muscle in ophiuroids can be seen in a figure in [61].
2.2.5. Crinoid Somatic Musculature
- Arm muscles
Crinoid arms consist mainly of a series of brachial ossicles that are linked at mobile joints. Adjacent brachial ossicles are connected by a pair of flexor muscles [58]. The flexor muscle bundles consist of different obliquely striated fibers (A-type and B-type fibers) and some rare smooth fibers (C-type fibers). A- and B-type fibers exhibit different myofilament arrangements and distribution. A-type fibers are present along the whole arm and form the central mass of the muscle bundle. B-type fibers only develop in the middle and proximal parts of the arm, spread more peripherally, and partly or completely surround the central mass. In addition, there are C-type fibers randomly distributed along the edge of the flexor muscle bundle or in the midzone between A- and B-type fibers. These three different muscle fibers are responsible for performing different types of arm movements, with A-type fibers performing fast movements, B-type fibers performing powerful actions, and C-type fibers related to slow movements [71,72,73]. Electron micrographs of the arm muscle in crinoids can be seen in the figures of [72].
3. Neurotransmitters That Affect Echinoderm Muscles
3.1. Cholinergic Neurotransmitters
We summarize the effects of various neurotransmitters on different muscles of echinoderms (Table 1). In the neuromuscular systems of echinoderms, the typical excitatory cholinergic neurotransmitter is acetylcholine (ACh), which induces contractile responses in various muscles in echinoderms [74]. In holothurians, Ach induces the contraction of the LMBW, intestinal muscle, cloaca, tube feet, and tentacles in different concentration ranges [75,76]. In A. japonicus, ACh leads to an initial sharp and tonic contraction of the LMBW, followed by very gradual relaxation [75]. ACh-induced intestinal contraction is observed in both longitudinal intestine strips and intestine rings, suggesting that both the longitudinal and circular muscles within the intestinal muscle layer can be activated by ACh [76]. In asteroids, ACh induces the contraction of the apical muscle, tube feet, and cardiac stomach. But the contraction of the cardiac stomach by ACh is too brief to measure the effect of relaxing agents, so in many pharmacological experiments, a high-K+ solution was used to induce a sustained contraction of the cardiac stomach [15,77,78,79]. In addition, ACh was detected in the ovaries and testes of the starfish Patiria pectinifera and induced the contraction of the gonadal wall to promote ovulation [20]. In echinoids, ACh can induce the contraction of the spine muscle, lantern retractor and protractor muscle, compass elevator muscle, tube feet, and radial muscle (echinothuriid) [80,81,82,83,84]. The subepithelial nerve plexus of the tube feet contains conspicuous amounts of ACh, which is released from nerve terminals of the subepithelial nerve plexus and diffuses through the connective tissue layer to reach the muscle layer [83]. However, ACh does not have an excitatory effect in all echinoderm muscles; for example, it cannot stimulate contraction and causes a decrease in tone of the brachial muscles in the arms of the feather star Antedon mediterranea [58].

Table 1.
The effect of classic neurotransmitters on various echinoderm muscles. LMBW, longitudinal muscle of body wall; IM, intestinal muscle; TF, tube foot; TM, tentacle muscle; CA, cloaca; AM, apical muscle; CS, cardiac stomach; EM, esophageal muscle; LRM, lantern retractor muscle; ME, myoexcitatory effect; MI, myoinhibitory effect; NE, no effect.
There are many factors that influence ACh-induced contraction, such as the external Ca2+ concentration; Mn2+, which blocks Ca2+ influx in many excitable membranes; and procaine, which inhibits Ca2+ release from the sarcoplasmic reticulum (SR) [75,95,96]. The LMBW contraction induced by ACh completely disappears in a Ca2+-free solution and the degree of contraction is significantly reduced by procaine. It can also be markedly enhanced after the mechanical response to Na+ removal, which may be due to the Na+-Ca2+ exchange mechanism that increases intracellularly stored Ca2+ by allowing Ca2+ influx and inhibiting its efflux [75]. These results suggest that the degree of ACh-induced contraction depends not only on the gradient of external Ca2+ across the plasma membrane but also the release of intracellularly stored Ca2+.
ACh acts through acetylcholine receptors (AChRs), which can be divided into nicotinic receptors (nAChRs) and muscarinic receptors (mAChRs) in deuterostomes and some protostome groups based on different pharmacological properties [91,97,98]. Pharmacological evidence suggests that nAChRs and mAChRs are co-localized in numerous echinoderm muscles and mediate different responses, respectively. When the LMBW is stimulated to contract by ACh, nAChRs are responsible for modulating muscle tone and mAChRs are responsible for initiating and enhancing rhythmicity [74,97]. Furthermore, ACh was shown to stimulate ovulation in P. pectinifera through activating mAChRs because it was inhibited by a mAChR antagonist, and a nAChR antagonist had no effect on it [20]. These results indicate that the two receptors have different signaling pathways. In fact, the nAChR is a pentameric subunit-assembled ion channel. In vertebrates, 17 nAChR subunits have been identified, and different subunit combinations show different pharmacological properties [98,99,100]. At the echinoderm neuromuscular junction, distinct subpopulations of nAChR have been identified in pre- and postsynaptic positions by using specific neuronal nAChR antagonists and muscle nAChR antagonists [74,97]. In the Strongylocentrotus purpuratus genome, at least 12 nAChR subunit-encoding genes have been identified [13]. As an ionotropic receptor, nAChR can directly gate ion channels without second messengers [101]. During the fertilization of sea urchin eggs, the activation of nAChR gated inward Na+ channels and caused depolarization by Na+ influx [100]. The mAChR is a member of the rhodophosin-like G-protein-coupled receptor (GPCR) family and acts through coupling G-proteins rather than gating ion channels directly. Therefore, the responses of mAChRs are very delayed compared with those of nAChRs [98]. Five mAChR subtypes (M1-M5) have been identified in vertebrates, with coupling to different G-proteins [98,102]. M1, M3, and M5 receptors are associated with Gq/G11 and activate intracellular Ca2+ mobilization through the phosphoinositol signaling pathway. M2 and M4 receptors are associated with Gi/Go and maintain stable intracellular Ca2+ levels by reducing protein kinase A activity [98,103,104,105,106]. Distinct mAChR subtypes are also present in echinoderm muscles, which share some pharmacological characteristics with M1-M5 receptors and may be their precursors [74,97]. In the LMBW of Sclerodactyla briareus, by using a series of M1-M5 agonists and antagonists, the presence of M1-, M3-, M5- and M2-, M4-like receptors has been identified [106]. Furthermore, pharmacological evidence suggests that the signaling pathway of mAChR activation in echinoderms is similar to that in vertebrates. For example, the LMBW contraction induced by M1, 3, and 5 receptor agonist (oxotremorine M) was blocked by LiCl treatment, an inhibitor of the phosphoinositol signaling pathway [106]. The M2/M4 antagonist methoctramine accelerated the relaxation after ACh-induced contraction, which implied a rapid decline in intracellular Ca2+ levels [97].
Although pharmacological experiments have indicated that these echinoderm AChR subtypes show similar pharmacological properties to vertebrate counterparts to some extent, due to the poor selectivity of some antagonists and agonists to echinoderm muscles and the co-expression of distinct receptor subtypes, it is difficult to accurately identify the distinct receptor subtypes and distinguish their respective functions in echinoderms [97,98]. Another approach to identify the distinct functions of AChRs is gene knockout, which has been used in mammals to describe the functional characteristics of some AChR subtypes. It would be interesting to use gene knockout or knockdown to identify the function of AChRs in echinoderms as well [107,108].
3.2. Bioamine Neurotransmitters
Many bioamine neurotransmitters, such as 5-HT, dopamine, adrenaline, and noradrenaline, have been identified in different tissues of echinoderms using biochemical and histochemical methods [21,44,109,110]. These neurotransmitters are involved in regulating a variety of physiological behaviors in echinoderms, including reproduction, ovarian maturation, embryonic development, morphogenesis, settlement, and swimming behavior [21,93,110,111,112,113]. At the same time, the effects of numerous bioamines on various echinoderm muscles have been examined, and their functional characteristics are highly variable [21,86].
In holothurians, 5-HT, dopamine, adrenaline, noradrenaline, and DOPA had no effects on the LMBW, but 5-HT had an inhibitory effect on ACh-induced contraction [21]. On the isolated cloaca preparation, 5-HT caused a contraction similar to that induced by ACh, but dopamine, adrenaline, and noradrenaline produced variable results or no effects that could be distinguished from spontaneous activities of the cloaca [85]. In asteroids, adrenaline, noradrenaline, dopamine, and histamine all had effects on tube feet. Adrenaline was five times more effective at inducing tube foot contraction than noradrenaline and ten times more effective than dopamine. However, at concentrations higher than 10−5 M, adrenaline and noradrenaline induced slow relaxation instead of contraction. This biphasic effect also occurred in skinned tube feet, suggesting that it influenced the muscle layer rather than the mechanical properties of the connective tissue. Histamine induced the relaxation of both intact and skinned tube feet. 5-HT had no effect on tube feet, even at 10−4 M [86]. In echinoids, the effects of bioamines on the lantern retractor muscle, spine muscle, tube feet, and esophageal muscle have been examined. Noradrenaline and dopamine caused a rapid relaxation of the lantern retractor muscle, and sometimes initiated long-lasting rhythmic activities [89]. Adrenaline caused relaxation and inhibited spontaneous activities and ACh-induced contraction [80]. Octopamine caused spine muscle contraction, while dopamine and noradrenaline relaxed the contraction elicited by octopamine and ACh [66]. In the larva of echinoids, 5-HT and dopamine had a strong stimulatory effect on esophageal muscle activity [109, 110]. However, no bioamines have been found to affect the tube foot muscle [88]. In crinoids, bioamines either failed to activate any responses in muscles or produced inconsistent effects [16,58].
Bioamine neurotransmitters function by activating their receptors, such as 5-HT receptors (5-HTRs), dopamine receptors, and adrenergic receptors [111,114,115]. In echinoderms, the genes encoding these receptors and the localization of some receptors have been discovered by means of genomic and immunochemical methods [13,111,114,116]. These receptors play an important role in regulating various behaviors in echinoderms. For example, dopamine receptors regulate the growth rate of the larval post-oral arms and righting behavior in S. purpuratus [115,117], and 5-HTRs regulate movement and respiratory metabolism in A. japonicus [111,114]. In mammals, the signaling pathway of these receptors has been reported in detail, including the activation of typical intracellular signaling cascades, such as G-proteins, adenylate cyclase, and phospholipase C, and altering the permeability of ion channels [118,119]. However, few studies on the signaling pathway mediated by bioamine receptors have been reported in echinoderms, especially regarding muscle regulation. Limited research indicated that 5-HT inhibited ACh-induced LMBW contraction in A. japonicus [21], and a further study found that 5-HT4/6 is highly expressed in the LMBW, which is a rhodopsin-type GPCR and mediates intracellular cAMP accumulation by coupling Gs protein [111]. However, the relationship between these receptor subtypes and the LMBW contraction process was not clear and further experiments are needed to identify the specific signaling pathway.
3.3. Amino Acid Neurotransmitters
The investigation of echinoderm muscle pharmacology has included many amino acid neurotransmitters, such as gamma-aminobutyric acid (GABA), glutamate, and glycine. However, only GABA has a relatively obvious role and has been extensively studied [86,88,91]. Therefore, the effects of GABA on the muscles of echinoderms are mainly discussed here. Immunocytochemical studies have indicated that GABA is mainly localized in nervous and muscular systems in echinoderms and plays an important role in the settlement of echinoderm larva [74,93,120,121,122]. Pharmacological evidence suggests that GABA has different effects on echinoderm muscles: an excitatory effect in asteroids and echinoids and an inhibitory effect in holothurians [74,86,88,120].
In asteroids and echinoids, GABA induced tube feet contraction, reaching 70-80% of that produced by ACh [86,88,92]. In holothurians, GABA was reported to cause LMBW relaxation, inhibit the rhythmicity of spontaneous contraction, reduce ACh-induced contraction, and decrease the amplitude of spontaneous cloaca contraction [85,91]. In crinoids, L-glutamate could induce rhythmic muscle contraction on the arm muscles, whereas no effect was detected for GABA and ACh, suggesting that L-glutamate may be the main excitatory neurotransmitter in crinoids [58].
GABA receptors (GABARs) can be divided into two types: GABAARs and GABABRs [74,123]. GABAARs are pentameric ligand-gated anion-selective channels and GABABRs are heterodimeric G-protein-coupled receptors [123]. In vertebrates, GABAARs mediate hyperpolarization by inducing chloride ion inflow, thereby reducing cell excitability [124], whereas in echinoderms, GABAARs mediate the excitatory responses and induced depolarization of tube feet muscle preparations by enhancing membrane permeability to Na+ [74,88,120]. Furthermore, cholinergic receptor blockers and cholinesterase inhibitors can inhibit and potentiate GABA excitatory responses in sea urchin tube feet. Therefore, Florey et al. (1975) speculated that GABA does not act directly on tube feet muscle but through the cholinergic system [88]. In vertebrates, GABABRs induce hyperpolarization by activating the G protein Gi/o, inhibit voltage-gated Ca2+ channels, or activate K+ channels [125]. As in vertebrates, GABABRs in echinoderms also mediate inhibitory responses [74,91]. However, the signaling pathway mediated by GABABRs in echinoderms remains to be studied.
3.4. Gaseous Neurotransmitters
It has been demonstrated that ACh is the major excitatory transmitter in the echinoderm neuromuscular system. However, a universal inhibitory neurotransmitter has not been identified. Neurotransmitters such as 5-HT, GABA, and dopamine all have diverse effects on echinoderm muscles. Nitric oxide (NO) is a class of gaseous neurotransmitters, which is increasingly found to play important signaling functions in many organisms [126]. In echinoderms, NO is involved in regulating reproduction, the stress response, muscle activity, and larval settlement and metamorphosis [127,128,129,130]. It has a consistent relaxation effect in many muscle preparations, including the cardiac stomach, tube feet, and apical muscle in asteroids and the pyloric sphincter in echinoids [94,127,131]. Therefore, NO may be a universal muscle relaxant in echinoderms [15].
Unlike conventional neurotransmitters that are released from vesicles and function by binding to membrane receptors, NO, as a membrane-permeant molecule, enters the target cell by free diffusion [126]. It is formed by nitric oxide synthases (NOSs) using L-arginine as a substrate [132]. In mammals, based on the different cell types, NOSs can be divided into three types: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS) [133]. In most invertebrates, however, only one NOS gene has been identified [134,135]. In asteroids, pharmacological experiments showed that L-arginine could induce cardiac stomach relaxation, suggesting the presence of NOS in this tissue [15]. In echinoids, nNOS-positive neuron-like cells were detected at the pylorus, which could produce NO to regulate the pyloric sphincter [94]. In target cells, NO activates soluble guanylyl cyclase (SGC) and causes guanosine 3′,5′-cyclic monophosphate (cGMP) accumulation, and this NO-SGC-cGMP signaling pathway is highly conserved in diverse organisms [136]. Applying the starfish cardiac stomach as a model, combined with some pharmacological inhibitors, Elphick and Melarange (2001) demonstrated the existence of the NO-SGC-cGMP pathway in an echinoderm. In this model, NO derived from the basiepithelial plexus of the starfish cardiac stomach diffuses across the connective tissue layer into the muscle cell, leading to cGMP accumulation by activating SGC [15]. In mammals, NO-induced cGMP accumulation mediates the activation of cGMP-dependent protein kinase (PKG), which then decreases IP3 production, activates sarcoplasmic/endoplasmic reticulum Ca2+ ATPase, and regulates myosin light-chain phosphatase. This pathway induces muscle relaxation by reducing the intracellular Ca2+ level and Ca2+ sensitivity [137,138]. However, the mechanism of cGMP-mediated muscle relaxation in echinoderms remains unclear and more experiments are needed to determine the universality of this pathway in echinoderm muscles.
4. Neuropeptides
Neuropeptides are an important class of signaling molecules in organisms that are widely involved in and regulate various physiological behaviors. They are derived from larger precursor proteins that are synthesized and secreted by neurons [139]. In echinoderms, a variety of neuropeptides have been predicted through genomic, transcriptomic, and proteomic analysis that have been suggested to be involved in regulating important physiological processes such as feeding, reproduction, growth, and muscle activity [22,140,141,142,143]. The first neuropeptide identified in echinoderms was a SALMFamide, which caused the relaxation of various muscles, such as the cardiac stomach, apical muscle, and tube feet in asteroids and the intestine and LMBW in holothurians [27,144,145,146]. Subsequently, an increasing number of neuropeptides in echinoderms have been investigated using pharmacological methods for their regulatory effects on muscles (Table 2). Some neuropeptides exhibit conserved muscle-regulatory function in different echinoderms, such as Pedal peptide/orcokinin-type neuropeptides, Luqin-type neuropeptides, and Calcitonin-type neuropeptides [78,79,147,148]. However, some neuropeptides, such as Vasopressin/oxytocin-type neuropeptides, exhibit the opposite effect in different echinoderms, inducing relaxation in asteroids and contraction in holothurians and echinoids [26,149]. Studies of the phylogenetic distribution of neuropeptides and their homologous receptors in different species contribute to understanding the evolutionary history of related neuropeptide families. Echinoderms, occupying an “intermediate” phylogenetic position between chordates and protostomes, are “important links” for reconstructing these evolutionary histories. For example, Somatostatin-type (SS) and allatostatin-C-type (ASTC) neuropeptides, which were identified in chordates and protostomes, respectively, both exert inhibitory effects on muscles. However, echinoderms have two SS/ASTC-type neuropeptides, SS1 and SS2, that are the orthologs of ASTC and SS, respectively, and exert completely opposite muscle regulation effects: SS-type neuropeptides cause relaxation and ASTC-type neuropeptides cause contraction [23]. This functional difference between SS/ASTC neuropeptides in echinoderms provides new insights into reconstructing the evolutionary history of this neuropeptide family.

Table 2.
The effect of neuropeptides on various echinoderm muscles. LMBW, longitudinal muscle of body wall; IM, intestine muscle; TM, tentacle muscle; AM, apical muscle; CS, cardiac stomach; EM, esophageal muscle; ME, myoexcitatory effect; MI, myoinhibitory effect; NE, no effect.
There are two noticeable problems in the muscle regulation of echinoderm neuropeptides. The first is the structure–activity relationship of neuropeptides. Some neuropeptides from the same neuropeptide family or the same precursor exhibit different potency, such as SALMFamide-2 (S2, SGPYSFNSGLTFamide) and SALMFamide-1 (S1, GFNSALMFamide) in A. rubens. Pharmacological experiments have shown that S2 has greater relaxation potency than S1, and structure–activity relationship studies of SALMFamides have suggested that the four additional N-terminal residues of S2 may be associated with neuropeptide activity because it can promote the self-association of S2 and allow S2 to form a structured conformation in aqueous solution, but S1 cannot [160]. The second is the tissue-specific effects of neuropeptides on different muscles from the same organism. In A. rubens, NGFFYamide has an inhibitory effect on apical muscle but an excitatory effect on tube feet [153]. Our research also suggested that Pedal peptide/orcokinin-type neuropeptides had a significant relaxation effect on the intestine of A. japonicus but almost no effect on LMBW [158]. So, further identification of neuropeptide receptors and clarification of the signaling pathway will help us to figure out the regulation mechanism of neuropeptides on muscle contraction/relaxation.
Typically, neuropeptides function by binding to G-protein-coupled receptors (GPCRs), causing signal transmission at the cellular level through second messengers, which later leads to physiological changes at the tissue/organism level [161]. So far, many GPCRs have been identified and cloned in echinoderms, and signaling pathways downstream of some receptors have been preliminarily explored, including Ca2+ mobilization, cAMP accumulation, and ERK1/2 phosphorylation [26,78,148]. However, the complete molecular and cellular mechanisms by which neuropeptides control muscle contraction/relaxation and the differences in regulatory mechanisms between different neuropeptides are still unknown. In schistosomes and mammals, detailed intracellular signaling cascades and membrane channel activation of neuropeptide-induced muscle contraction have been reported, which can provide guidance for related research on echinoderms [162,163].
Based on current studies of myoactive neuropeptides in echinoderms and other species, we speculated that there are three potential mechanisms mediating muscle regulation by neuropeptides in echinoderms: (1) Neuropeptides act directly on specific GPCRs located on muscle cells to control membrane channel activity through signaling cascades mediated by intracellular second messengers, thereby causing muscle contraction/relaxation. For example, Alzugaray et al. (2021) found that the allatotropin-type neuropeptide (AT) regulated muscle contraction through the AT receptor/Gq/PLC/IP3/intracellular Ca2+ signaling pathway in Hydra [164]. In addition, by using blockers of voltage-gated calcium channels (VGCCs), they found that extracellular Ca2+ influx through VGCCs also participates in muscle contraction [165]. In A. japonicus, we found that the vasopressin/oxytocin-type neuropeptide (VP/OT) has a strong contractile effect on a variety of muscle tissues, including LMBW, intestinal muscle, and tentacles. Ca2+ deprivation experiments have shown that VP/OT-induced contraction is dependent on extracellular Ca2+ influx [150]. However, the channel that mediates Ca2+ influx and the intracellular signaling pathway that activates it are still unknown. (2) Neuropeptides exert a myoexcitatory effect by directly activating peptide-gated cation channels on muscle cells. For example, Schmidt et al. (2018) found a peptide-gated ion channel in the marine annelid Platynereis dumerilii that is activated by myoinhibitory peptides (MIPs) and belongs to the degenerin/epithelial Na+ channel (DEG/ENaC) family [166]. A previous study showed that MIPs can function by activating a specific GPCR [166,167]. These results suggest that neuropeptides may have a dual signaling system in some organisms, transmitting distinct signaling through both ionotropic and metabotropic receptors [166]. However, no peptide-gated ion channels have been identified in echinoderms; the neuropeptides reported so far all function by activating metabotropic GPCRs. (3) Neuropeptides control muscle contraction/relaxation by activating other neurons that secrete other neurochemicals. In Caenorhabditis elegans, neuropeptide-like protein 40 induced GABAergic neurons to release γ-GABA by binding with a specific receptor located on GABAergic neurons to control the rhythmic contraction of intestinal muscle [168]. In echinoderms, Odekunle et al. (2019) were the first to report the anatomical expression patterns of the VP/OT-type neuropeptide and its receptor in the starfish A. rubens using double-labelling fluorescence immunohistochemistry. Based on the expression of the receptor in the basiepithelial neural plexus, they speculated that VP/OT-type neuropeptides may indirectly regulate muscle relaxation by participating in other neural processes [26]. Elphick and Melarange (2001) also reported the relationship between SALMFamides and NO, which both induced cardiac stomach relaxation in starfish. However, SALMFamides do not function through NO, and they belong to different neural signaling systems [15]. So far, in echinoderms, no conclusive evidence has been provided that shows neuropeptides can mediate muscle contraction/relaxation through other neurons.
5. Conclusions
The musculature of echinoderms includes the myoepithelium and muscle bundles. The myoepithelium is composed mainly of myoepithelial and peritoneal cells and can be divided into different types according to the relative position of these cells, including the simple myoepithelium, pseudostratified myoepithelium, bipartite pseudostratified myoepithelium, and stratified myoepithelium. Muscle bundles are formed by the aggregation of several myocytes surrounded by basal lamina, most of which consist of smooth muscle fibers, but obliquely striated muscle fibers are also present in ophiuroids and crinoids. Pharmacological experiments have demonstrated the effects of many classic neurotransmitters on echinoderm muscles, of which ACh and NO are considered to be the main excitatory and inhibitory neurotransmitters in echinoderms, respectively. However, other classic neurotransmitters, such as 5-HT, dopamine, adrenaline, noradrenaline, GABA, and L-glutamate, have variable effects on different muscles. In recent years, an increasing number of neuropeptides have also been identified to have muscle-regulating effects in different echinoderm species. Neurotransmitters and neuropeptides usually function through specific receptors, and although many receptors have been identified in echinoderms, the complete downstream signaling pathways of these receptors are unknown. Studies on how these neurotransmitters induce muscle contraction/relaxation will help to understand the potential mechanisms of muscle regulation. Echinoderms have a deuterostome mode of development, which results in their muscle physiology having more in common with that of vertebrates than that of other invertebrates. Therefore, studies on the regulatory mechanisms of echinoderm muscle systems will provide new insights into the evolution of regulatory systems in deuterostome muscle.
Author Contributions
H.L. and M.C. jointly designed the structure, wrote, and edited this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (grant numbers 31972767 and 42276103).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bromham, L.D.; Degnan, B.M. Hemichordates and deuterostome evolution: Robust molecular phylogenetic support for a hemichordate + echinoderm clade. Evol. Dev. 1999, 1, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bourlat, S.J.; Juliusdottir, T.; Lowe, C.J.; Freeman, R.; Aronowicz, J.; Kirschner, M.; Lander, E.S.; Thorndyke, M.; Nakano, H.; Kohn, A.B.; et al. Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature 2006, 444, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.W.; Hejnol, A.; Matus, D.Q.; Pang, K.; Browne, W.E.; Smith, S.A.; Seaver, E.; Rouse, G.W.; Obst, M.; Edgecombe, G.D.; et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 2008, 452, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, C.T.; Miyake, T.; Rast, J.P. Echinoderms. Curr. Biol. 2005, 15, R944–R946. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Ecology of Paracentrotus lividus. Dev. Aquacult. Fish. Sci. 2001, 32, 177–216. [Google Scholar] [CrossRef]
- Purcell, S.W.; Conand, C.; Uthicke, S.; Byrne, M. Ecological roles of exploited sea cucumbers. Oceanogr. Mar. Biol. 2017, 54, 367–386. [Google Scholar] [CrossRef]
- Kalinin, V.I. Echinoderms Metabolites: Structure, Functions, and Biomedical Perspectives. Mar. drugs 2021, 19, 125. [Google Scholar] [CrossRef]
- Kondo, M.; Akasaka, K. Regeneration in crinoids. Dev. Growth Differ. 2010, 52, 57–68. [Google Scholar] [CrossRef]
- Li, Q.; Ren, Y.; Liang, C.; Qiao, G.; Wang, Y.; Ye, S.; Li, R. Regeneration of coelomocytes after evisceration in the sea cucumber, Apostichopus japonicus. Fish. Shellfish. Immunol. 2018, 76, 266–271. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Candia Carnevali, M.D. Morphological and Physiological Aspects of Mutable Collagenous Tissue at the Autotomy Plane of the Starfish Asterias rubens L. (Echinodermata, Asteroidea): An Echinoderm Paradigm. Mar. drugs 2023, 21, 138. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, H.; Wang, L.; Zhou, Y.; Zhang, T.; Liu, Y. Effects of aestivation on the energy budget of sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Acta Ecol. Sinica. 2007, 27, 3155–3161. [Google Scholar] [CrossRef]
- Dolmatov, I.Y.; Ginanova, T.T. Muscle regeneration in holothurians. Microsc. Res. Technol. 2001, 55, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Sea Urchin Genome Sequencing Consortium; Sodergren, E.; Weinstock, G.M.; Davidson, E.H.; Cameron, R.A.; Gibbs, R.A.; Angerer, R.C.; Angerer, L.M.; Arnone, M.I.; Burgess, D.R.; et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [Google Scholar] [CrossRef] [PubMed]
- García-Arrarás, J.E.; Dolmatov, I.Y. Echinoderms: Potential model systems for studies on muscle regeneration. Curr. Pharm. Des. 2010, 16, 942–955. [Google Scholar] [CrossRef]
- Elphick, M.R.; Melarange, R. Neural control of muscle relaxation in echinoderms. J. Exp. Biol. 2001, 204, 875–885. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Barbaglio, A.; Maclaren, W.M.; Carnevali, M.D. Physiological and immunocytochemical evidence that glutamatergic neurotransmission is involved in the activation of arm autotomy in the featherstar Antedon mediterranea (Echinodermata: Crinoidea). J. Exp. Biol. 2010, 213, 2104–2115. [Google Scholar] [CrossRef]
- McCurley, R.S.; Kier, W.M. The Functional Morphology of Starfish Tube Feet: The Role of a Crossed-Fiber Helical Array in Movement. Biol. Bull. 1995, 188, 197–209. [Google Scholar] [CrossRef]
- Byrne, M. The morphology of autotomy structures in the sea cucumber Eupentacta quinquesemita before and during evisceration. J. Exp. Biol. 2001, 204, 849–863. [Google Scholar] [CrossRef]
- Ziegler, A.; Schröder, L.; Ogurreck, M.; Faber, C.; Stach, T. Evolution of a novel muscle design in sea urchins (Echinodermata: Echinoidea). PLoS ONE 2012, 7, e37520. [Google Scholar] [CrossRef]
- Mita, M.; Osugi, T.; Takahashi, T.; Watanabe, T.; Satake, H. Mechanism of gamete shedding in starfish: Involvement of acetylcholine in extracellular Ca2+-dependent contraction of gonadal walls. Gen. Comp. Endocrinol. 2020, 290, 113401. [Google Scholar] [CrossRef]
- Inoue, M.; Tamori, M.; Motokawa, T. Innervation of holothurian body wall muscle: Inhibitory effects and localization of 5-HT. Zool. Sci. 2002, 19, 1217–1222. [Google Scholar] [CrossRef]
- Zandawala, M.; Moghul, I.; Yañez Guerra, L.A.; Delroisse, J.; Abylkassimova, N.; Hugall, A.F.; O’Hara, T.D.; Elphick, M.R. Discovery of novel representatives of bilaterian neuropeptide families and reconstruction of neuropeptide precursor evolution in ophiuroid echinoderms. Open Biol. 2017, 7, 170129. [Google Scholar] [CrossRef]
- Zhang, Y.; Yañez-Guerra, L.A.; Tinoco, A.B.; Escudero Castelán, N.; Egertová, M.; Elphick, M.R. Somatostatin-type and allatostatin-C-type neuropeptides are paralogous and have opposing myoregulatory roles in an echinoderm. Proc. Natl. Acad. Sci. USA 2022, 119, e2113589119. [Google Scholar] [CrossRef]
- Calderón, J.C.; Bolaños, P.; Caputo, C. The excitation-contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef]
- Hill, R.B. Role of Ca2+ in excitation-contraction coupling in echinoderm muscle: Comparison with role in other tissues. J. Exp. Biol. 2001, 204, 897–908. [Google Scholar] [CrossRef]
- Odekunle, E.A.; Semmens, D.C.; Martynyuk, N.; Tinoco, A.B.; Garewal, A.K.; Patel, R.R.; Blowes, L.M.; Zandawala, M.; Delroisse, J.; Slade, S.E.; et al. Ancient role of vasopressin/oxytocin-type neuropeptides as regulators of feeding revealed in an echinoderm. BMC Biol. 2019, 17, 60. [Google Scholar] [CrossRef]
- Melarange, R.; Elphick, M.R. Comparative analysis of nitric oxide and SALMFamide neuropeptides as general muscle relaxants in starfish. J. Exp. Biol. 2003, 20, 893–899. [Google Scholar] [CrossRef][Green Version]
- Mashanov, V.S.; Frolova, L.T.; Dolmatov, I.Y. Structure of the digestive tube in the holothurian Eupentacta fraudatrix (holothuroidea: Dendrochirota). Russ. J. Mar. Biol. 2004, 30, 314–322. [Google Scholar] [CrossRef]
- Holland, N.D. The fine structure of the ovary of the feather star Nemaster rubiginosa (Echinodermata: Crinoidea). Tissue Cell 1971, 3, 161–175. [Google Scholar] [CrossRef]
- Spirina, I.S.; Dolmatov, I.Y. Morphology of the respiratory trees in the holothurians Apostichopus japonicus and Cucumaria japonica. Russ. J. Mar. Biol. 2001, 27, 367–375. [Google Scholar] [CrossRef]
- Rieger, R.M.; Lombardi, J. Ultrastructure of celomic lining in echinoderm podia: Significance for concepts in the evolution of muscle and peritoneal cells. Zoomorphology 1987, 107, 191–208. [Google Scholar] [CrossRef]
- Cavey, M.J. Organization of the celomic lining and a juxtaposed nerve plexus in the suckered tube feet of Parastichopus californicus (Echinodermata: Holothuroida). J. Morphol. 2006, 267, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Balzac, C.A.; Abreu-Arbelo, J.E.; García-Arrarás, J.E. Neuroanatomy of the tube feet and tentacles in Holothuria glaberrima (Holothuroidea, Echinodermata). Zoomorphology 2010, 129, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Florey, E.; Cahill, M.A. Ultrastructure of sea urchin tube feet. Evidence for connective tissue involvement in motor control. Cell Tissue Res. 1977, 177, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.L.; Rieger, R.M. Rudimentary cilia in muscle cells of annelids and echinoderms. Cell Tissue Res. 1980, 213, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.L.; Cavey, M.J. Ultrastructure of the celomic lining in the podium of the starfish Stylasterias forreri. Cell Tissue Res. 1981, 218, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Bouland, C.; Massin, C.; Jangoux, M. The fine structure of buccal tentacles of Holothuria forskali (Echinodermta, Holothuroidea). Zoomorphology 1982, 101, 133–149. [Google Scholar] [CrossRef]
- Smith, J.E. The structure and function of the tube feet in certain echinoderms. J. Mar. Biol. Assoc. UK 1937, 22, 345–357. [Google Scholar] [CrossRef]
- Mckenzie, J.D. Ultrastructure of the tentacles of the apodous holothurian Leptosynapta spp. (holothurioidea: Echinodermata) with special reference to the epidermis and surface coats. Cell Tissue Res. 1988, 251, 387–397. [Google Scholar] [CrossRef]
- Martinez, A.; Lopez, J.; Villaro, A.C.; Sesma, P. Choanocyte-like cells in the digestive system of the starfish Marthasterias glacialis (Echinodermata). J. Morphol. 1991, 208, 215–225. [Google Scholar] [CrossRef]
- Deridder, C.; Jangoux, M. The digestive tract of the spatangoid echinoid Echinocardium cordatum (echinodermata): Morphofunctional study. Acta Zool. 1993, 74, 337–351. [Google Scholar] [CrossRef]
- Kamenev, Y.O.; Dolmatov, I.Y.; Frolova, L.T.; Khang, N.A. The morphology of the digestive tract and respiratory organs of the holothurian Cladolabes schmeltzii (Holothuroidea, Dendrochirotida). Tissue Cell 2013, 45, 126–139. [Google Scholar] [CrossRef] [PubMed]
- García-Arrarás, J.E.; Rojas-Soto, M.; Jiménez, L.B.; Díaz-Miranda, L. The enteric nervous system of echinoderms: Unexpected complexity revealed by neurochemical analysis. J. Exp. Biol. 2001, 204, 865–873. [Google Scholar] [CrossRef]
- Féral, J.P.; Massin, C. Digestive systems: Holothurioidea. In Echinoderm Nutrition; Balkema: Rotterdam, The Netherlands, 1982; pp. 191–212. [Google Scholar]
- Smiley, S. A review of echinoderm oogenesis. J. Electron. Microsc. Technol. 1990, 16, 93–114. [Google Scholar] [CrossRef]
- Smiley, S.; Cloney, R.A. Ovulation and the fine structure of the stichopus californicus (Echinodermata: Holothuroidea) fecund ovarian tubules. Biol. Bull. 1985, 169, 342–364. [Google Scholar] [CrossRef] [PubMed]
- Atwood, D.G. Ultrastructure of the gonadal wall of the sea cucumber, Leptosynapta clarki (Echinodermata: Holothuroidea). Z. Zellforsch. Mikrosk. Anat. 1973, 141, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Nerrevang, D.A.; Wingstrand, K.G. On the occurrence and structure of choanocyte-like cells in some echinoderms. Acta Zool. 1970, 51, 249–270. [Google Scholar] [CrossRef]
- Beijnink, F.B.; Walker, C.W.; Voogt, P.A. An ultrastructural study of relationships between the ovarian haemal system, follicle cells, and primary oocytes in the sea star, Asterias rubens. Implications for oocyte nutrition. Cell Tissue Res. 1984, 238, 339–347. [Google Scholar] [CrossRef]
- Byrne, M. Ultrastructure of the ovary and oogenesis in the ovoviviparous ophiuroid Ophiolepis paucispina (echinodermata). Biol. Bull. 1989, 176, 79–95. [Google Scholar] [CrossRef]
- Davis, H.S. The Gonad Walls of Echinodermata: A Comparative Study Based on Electron Microscopy. Master’s Thesis, California University, San Diego, CA, USA, 1971. [Google Scholar]
- Jensen, H. Ultrastructure of the dorsal hemal vessel in the sea-cucumber Parastichopus tremulus (Echinodermata: Holothuroidea). Cell Tissue Res. 1975, 160, 355–369. [Google Scholar] [CrossRef]
- Prosser, C.L.; Judson, C.L. Pharmacology of haemal vessels of Stichopus californicus. Biol. Bull. 1952, 102, 249–251. [Google Scholar] [CrossRef]
- Suzuki, S. Physiological and cytochemical studies on activator calcium in contraction by smooth muscle of a sea cucumber, Isostichopus badionotus. Cell Tissue Res. 1982, 222, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Blowes, L.M.; Egertová, M.; Liu, Y.; Davis, G.R.; Terrill, N.J.; Gupta, H.S.; Elphick, M.R. Body wall structure in the starfish Asterias rubens. J. Anat. 2017, 231, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Dolmatov, I.Y.; Mashanov, V.S.; Zueva, O.R. Derivation of muscles of the Aristotle’s lantern from celomic epithelia. Cell Tissue Res. 2007, 327, 371–384. [Google Scholar] [CrossRef] [PubMed]
- LeClair, E.E. Arm joint articulations in the ophiuran brittlestars (Echinodermata: Ophiuroidea): A morphometric analysis of ontogenetic, serial, and interspecific variation. J. Zool. 1996, 240, 245–275. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Barbaglio, A.; Carnevali, M.D. The elusive role of L-glutamate as an echinoderm neurotransmitter: Evidence for its involvement in the control of crinoid arm muscles. Zoology 2013, 116, 1–8. [Google Scholar] [CrossRef]
- Motokawa, T. Morphology of spines and spine joint in the crown-of-thorns starfish Acanthaster planci (echinodermata, asteroida). Zoomorphology. 1986, 106, 247–253. [Google Scholar] [CrossRef]
- Motokawa, T.; Fuchigami, Y. Coordination between catch connective tissue and muscles through nerves in the spine joint of the sea urchin Diadema setosum. J. Exp. Biol. 2015, 218, 703–710. [Google Scholar] [CrossRef]
- Wilkie, I.C. Functional Morphology of the Arm Spine Joint and Adjacent Structures of the Brittlestar Ophiocomina nigra (Echinodermata: Ophiuroidea). PLoS ONE 2016, 11, e0167533. [Google Scholar] [CrossRef][Green Version]
- Hyman, L.H. The Invertebrates: Echinodermata, the Coelomate Bilateria; McGraw-Hill: New York, NY, USA, 1955; Volume 4. [Google Scholar]
- Pople, W.; Ewer, D.W. Studies on the myoneural physiology of echinodermata. i. the pharyngeal retractor muscle of cucumaria. J. Exp. Biol. 1954, 31, 114–126. [Google Scholar] [CrossRef]
- Stauber, M. The lantern of Aristotle: Organization of its coelom and origin of its muscles (Echinodermata, Echinoida). Zoomorphology. 1993, 113, 137–151. [Google Scholar] [CrossRef]
- Hidaka, M.; Takahashi, K. Fine structure and mechanical properties of the catch apparatus of the sea-urchin spine, a collagenous connective tissue with muscle-like holding capacity. J. Exp. Biol. 1983, 103, 1–14. [Google Scholar] [CrossRef]
- Shingyoji, C.; Yamaguchi, M. Effects of acetylcholine, octopamine, atp, dopamine, and electrical stimulation on the spine muscle of the sea urchin, Anthocidaris crassispina. Comp. Biochem. Physiol. Part. C Pharmacol. Toxicol. Endocrinol. 1995, 111, 23–32. [Google Scholar] [CrossRef]
- O’Neill, P. Structure and mechanics of starfish body wall. J. Exp. Biol. 1989, 147, 53–89. [Google Scholar] [CrossRef] [PubMed]
- Saita, A.; Candia Carnevali, M.D.; Canonaco, M. Muscle system organization in the Echinoderms. I. Intervertebral muscles of Ophioderma longicaudum (Ophiuroidea). J. Submicrosc. Cytol. 1982, 14, 291–304. [Google Scholar]
- Stauber, M.; Märkel, K. Comparative morphology of muscle-skeleton attachments in the Echinodermata. Zoomorphology 1988, 108, 137–148. [Google Scholar] [CrossRef]
- Byrne, M. Ophiuroidea. In Microscopic Anatomy of Invertebrates; Frederick, W.H., Fu-Shiang, C., Eds.; Wiley-Liss: Hoboken, NJ, USA, 1996; Volume 14, pp. 247–343. [Google Scholar]
- Carnevali, M.D.C.; Saita, A. Muscle system organization in the echinoderms: II. Microscopic anatomy and functional significance of the muscle-ligament-skeleton system in the arm of the comatulids (Antedon mediterranea). J. Morphol. 1985, 185, 59–74. [Google Scholar] [CrossRef]
- Carnevali, M.D.C.; Saita, A. Muscle system organization in the echinoderms: III. Fine structure of the contractile apparatus of the arm flexor muscles of the comatulids (Antedon mediterranea). J. Morphol. 1985, 185, 75–87. [Google Scholar] [CrossRef]
- Candia Carnevali, M.D.; Saita, A.; Fedrigo, A. An unusual Z-system in the obliquely striated muscles of crinoids: Three-dimensional structure and computer simulations. J. Muscle Res. Cell Motil. 1986, 7, 568–578. [Google Scholar] [CrossRef]
- Devlin, C.L. The pharmacology of gamma-aminobutyric acid and acetylcholine receptors at the echinoderm neuromuscular junction. J. Exp. Biol. 2001, 204, 887–896. [Google Scholar] [CrossRef]
- Suzuki, S.; Sugi, H. Physiological and ultrastructural studies on the longitudinal retractor muscle of a sea cucumber Stichopus japonicus. II. Intracellular localization and translocation of activator calcium during mechanical activity. J. Exp. Biol. 1982, 97, 113–119. [Google Scholar] [CrossRef] [PubMed]
- García-Arrarás, J.E.; Torres-Avillán, I.; Ortíz-Miranda, S. Cells in the intestinal system of holothurians (Echinodermata) express cholecystokinin-like immunoreactivity. Gen. Comp. Endocrinol. 1991, 83, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, E.J.; Go, H.J.; Oh, H.Y.; Lin, M.; Elphick, M.R.; Park, N.G. Identification of a novel starfish neuropeptide that acts as a muscle relaxant. J. Neurochem. 2016, 137, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Egertová, M.; Zampronio, C.G.; Jones, A.M.; Elphick, M.R. Pedal peptide/orcokinin-type neuropeptide signaling in a deuterostome: The anatomy and pharmacology of starfish myorelaxant peptide in Asterias rubens. J. Comp. Neurol. 2017, 525, 3890–3917. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Kim, C.H.; Go, H.J.; Egertová, M.; Zampronio, C.G.; Jones, A.M.; Park, N.G.; Elphick, M.R. Biochemical, Anatomical, and Pharmacological Characterization of Calcitonin-Type Neuropeptides in Starfish: Discovery of an Ancient Role as Muscle Relaxants. Front. Neurosci. 2018, 12, 382. [Google Scholar] [CrossRef]
- Boltt, R.E.; Ewer, D.W. Studies on the myoneural physiology of Echinodermata. V. The lantern retractor muscle of Parechinus: Responses to drugs. J. Exp. Biol. 1963, 40, 727–733. [Google Scholar] [CrossRef]
- Mendes, E.G.; Abbud, L.; Lopez, A.A. Pharmacological studies on the invertebrate non-striated muscles. I. The response to drugs. Comp. Gen. Pharmacol. 1970, 1, 11–22. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Amemiya, S. Studies on the radial muscle of an echinothuriid sea-urchin, Asthenosoma-I. Mechanical responses to electrical stimulation and drugs. Comp. Biochem. Physiol. C. Comp. Pharmacol. 1977, 57, 69–73. [Google Scholar] [CrossRef]
- Florey, E.; Cahill, M.A. Cholinergic motor control of sea urchin tube feet: Evidence for chemical transmission without synapses. J. Exp. Biol. 1980, 88, 281–292. [Google Scholar] [CrossRef]
- McKew, M.; Wilkie, I.C. Organisation and pharmacology of the compass elevator muscles of the sea-urchin Echinus esculentus L. In Echinoderm Research; Candia Carnevali, M.D., Bonasoro, F., Eds.; Balkema: Rotterdam, The Netherlands, 1999; pp. 103–108. [Google Scholar]
- Hill, R.B. Effects of Some Postulated Neurohumors on Rhythmicity of the Isolated Cloaca of a Holothurian. Physiol. Zool. 1970, 43, 109–123. [Google Scholar] [CrossRef]
- Protas, L.L.; Muske, G.A. The effects of some transmitter substances of the tube foot muscles of the starfish, Asterias amurensis (Lütken). Gen. Pharmacol. 1980, 11, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, T. Pharmacological control of muscular activity in the sea urchin larva--IV. Effects of monoamines and adenosine. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1991, 98, 307–315. [Google Scholar] [CrossRef]
- Florey, E.; Cahill, M.A.; Rathmayer, M. Excitatory actions of GABA and of acetyl-choline in sea urchin tube feet. Comp. Biochem. Physiol. C Comp. Pharmacol. 1975, 51, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Pentreath, V.W.; Cobb, J.L. Neurobiology of echinodermata. Biol. Rev. Cambridge Philos. Soc. 1972, 47, 363–392. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, T.; Lundgren, B.; Treufeldt, R. Serotonin and contractile activity in the echinopluteus. A study of the cellular basis of larval behaviour. Exp. Cell Res. 1972, 72, 115–139. [Google Scholar] [CrossRef]
- Devlin, C.L.; Schlosser, W. Gamma-aminobutyric acid modulation of acetylcholine-induced contractions of a smooth muscle from an echinoderm (Sclerodactyla briareus). Invertebr. Neurosci. 1999, 4, 1–8. [Google Scholar] [CrossRef]
- Florey, E.; McLennan, H. The effects of factor I and of gamma-aminobutyric acid on smooth muscle preparations. J. Physiol. 1959, 145, 66–76. [Google Scholar] [CrossRef]
- Nontunha, N.; Tinikul, R.; Chaichotranunt, S.; Poomtong, T.; Sobhon, P.; Tinikul, Y. The presence and distribution of gamma-aminobutyric acid and dopamine during the developmental stages of the sea cucumber, Holothuria scabra, with emphasis on settlement organs. Cell Tissue Res. 2023, 391, 457–483. [Google Scholar] [CrossRef]
- Yaguchi, J.; Yaguchi, S. Evolution of nitric oxide regulation of gut function. Proc. Natl. Acad. Sci. USA 2019, 116, 5607–5612. [Google Scholar] [CrossRef]
- Hagiwara, S.; Nakajima, S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J. Gen. Physiol. 1966, 49, 793–806. [Google Scholar] [CrossRef]
- Weber, A.; Herz, R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J. Gen. Physiol. 1968, 52, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Devlin, C.L.; Schlosser, W.; Belz, D.T.; Kodiak, K.; Nash, R.F.; Zitomer, N. Pharmacological identification of acetylcholine receptor subtypes in echinoderm smooth muscle (Sclerodactyla briareus). Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2000, 125, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A. Acetylcholine. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S120–S126. [Google Scholar] [CrossRef]
- Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005, 346, 967–989. [Google Scholar] [CrossRef]
- Aluigi, M.G.; Diaspro, A.; Ramoino, P.; Russo, P.; Falugi, C. The sea urchin, Paracentrotus lividus, as a model to investigate the onset of molecules immunologically related to the α-7 subunit of nicotinic receptors during embryonic and larval development. Curr. Drug Targets 2012, 13, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Venter, J.C. The structure and evolution of adrenergic and muscarinic cholinergic receptors. J. Cardiovasc. Pharmacol. 1987, 10 (Suppl. S12), S69–S73. [Google Scholar] [CrossRef]
- Bonner, T.I.; Buckley, N.J.; Young, A.C.; Brann, M.R. Identification of a family of muscarinic acetylcholine receptor genes. Science 1987, 237, 527–532. [Google Scholar] [CrossRef]
- Eglen, R.M.; Hegde, S.S.; Watson, N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996, 48, 531–565. [Google Scholar]
- Ehlert, F.J.; Ostrom, R.S.; Sawyer, G.W. Subtypes of the muscarinic receptor in smooth muscle. Life Sci. 1997, 61, 1729–1740. [Google Scholar] [CrossRef]
- Caulfield, M.P.; Birdsall, N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar]
- Devlin, C.L.; Amole, W.; Anderson, S.; Shea, K. Muscarinic acetylcholine receptor compounds alter net Ca2+ flux and contractility in an invertebrate smooth muscle. Invert. Neurosci. 2003, 5, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Erausquin, M.; Marubio, L.M.; Klink, R.; Changeux, J.P. Nicotinic receptor function: New perspectives from knockout mice. Trends Pharmacol. Sci. 2000, 21, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Wess, J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 423–450. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, M.D.C.; Bonasoro, F.; Invernizzi, R.; Lucca, E.; Welsch, U.; Thorndyke, M.C. Tissue distribution of monoamine neurotransmitters in normal and regenerating arms of the feather star Antedon mediterranea. Cell Tissue Res. 1996, 285, 341–352. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Wang, C.; Tian, H.; Wang, W.; Ru, S. Effects of monocrotophos pesticide on cholinergic and dopaminergic neurotransmitter systems during early development in the sea urchin Hemicentrotus pulcherrimus. Toxicol. Appl. Pharmacol. 2017, 328, 46–53. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, J.W.; Han, T.; Huang, D.X.; Zhao, Z.H.; Feng, J.Q.; Zhou, N.M.; Xie, H.Q.; Wang, T.M. Identification and characterization of a novel 5-hydroxytryptamine receptor in the sea cucumber Apostichopus japonicus (Selenka). J. Exp. Zool. Part A 2021, 335, 367–380. [Google Scholar] [CrossRef]
- Chaiyamoon, A.; Tinikul, R.; Chaichotranunt, S.; Poomthong, T.; Suphamungmee, W.; Sobhon, P.; Tinikul, Y. Distribution and dynamic expression of serotonin and dopamine in the nervous system and ovary of Holothuria scabra during ovarian maturation. J. Comp. Physiol. A 2018, 204, 391–407. [Google Scholar] [CrossRef]
- Kalachev, A.V.; Tankovich, A.E. The dopamine effect on sea urchin larvae depends on their age. Dev. Growth Differ. 2023, 65, 120–131. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Z.; Zhou, N.; Sun, L.; Lv, Z.; Wu, C. Identification and functional characterisation of 5-HT4 receptor in sea cucumber Apostichopus japonicus (Selenka). Sci. Rep. 2017, 7, 40247. [Google Scholar] [CrossRef]
- McDonald, M.; Griffin, N.P.; Howell, E.; Li, D.; Harnew-Spradley, S.; Rodriguez, P.; Lancaster, A.; Umutoni, F.; Besh, J.; Shelley, C. Effects of neurotransmitter receptor antagonists on sea urchin righting behavior and tube foot motility. J. Exp. Biol. 2022, 225, jeb243076. [Google Scholar] [CrossRef]
- Katow, H.; Suyemitsu, T.; Ooka, S.; Yaguchi, J.; Jin-Nai, T.; Kuwahara, I.; Katow, T.; Yaguchi, S.; Abe, H. Development of a dopaminergic system in sea urchin embryos and larvae. J. Exp. Biol. 2010, 213, 2808–2819. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.K.; Sewell, M.A.; Angerer, R.C.; Angerer, L.M. Rapid adaptation to food availability by a dopamine-mediated morphogenetic response. Nat. Commun. 2011, 2, 592. [Google Scholar] [CrossRef] [PubMed]
- Girault, J.A.; Greengard, P. The neurobiology of dopamine signaling. Arch. Neurol. 2004, 61, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Barnes, N.M. Central 5-HT receptors and their function; present and future. Neuropharmacology 2020, 177, 108155. [Google Scholar] [CrossRef] [PubMed]
- Katow, H.; Yoshida, H.; Kiyomoto, M. Initial report of γ-aminobutyric acidergic locomotion regulatory system and its 3-mercaptopropionic acid-sensitivity in metamorphic juvenile of sea urchin, Hemicentrotus pulcherrimus. Sci. Rep. 2020, 10, 778. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Yu, H.; Kong, L. The effect of chemical cues on the settlement of sea cucumber (Apostichopus japonicus) larvae. J. Ocean. Univ. China. 2014, 13, 321–330. [Google Scholar] [CrossRef]
- Nontunha, N.; Chaiyamoon, A.; Chaichotranunt, S.; Tinikul, R.; Tinikul, Y. Neurotransmitters induce larval settlement and juvenile growth of the sea cucumber, Holothuria scabra. Aquaculture 2021, 535, 736427. [Google Scholar] [CrossRef]
- Söderhielm, P.C.; Klein, A.B.; Bomholtz, S.H.; Jensen, A.A. Profiling of GABAA and GABAB receptor expression in the myometrium of the human uterus. Life Sci. 2018, 214, 145–152. [Google Scholar] [CrossRef]
- Jorgensen, E.M. GABA. In Worm book: The online review of C. elegans biology; WormBook: Online, 2005; pp. 1–13. [Google Scholar] [CrossRef]
- Campbell, V.; Berrow, N.; Dolphin, A.C. GABAB receptor modulation of Ca2+ currents in rat sensory neurones by the G protein G(o): Antisense oligonucleotide studies. J. Physiol. 1993, 470, 1–11. [Google Scholar] [CrossRef]
- Jacklet, J.W. Nitric oxide signaling in invertebrates. Invertebr. Neurosci. 1997, 3, 1–14. [Google Scholar] [CrossRef]
- Elphick, M.R.; Melarange, R. Nitric Oxide Function in an Echinoderm. Biol. Bull. 1998, 194, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.D.; Brandhorst, B.P. Development of nitric oxide synthase-defined neurons in the sea urchin larval ciliary band and evidence for a chemosensory function during metamorphosis. Dev. Dyn. 2007, 236, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Mohri, T.; Sokabe, M.; Kyozuka, K. Nitric oxide (NO) increase at fertilization in sea urchin eggs upregulates fertilization envelope hardening. Dev. Biol. 2008, 322, 251–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romano, G.; Costantini, M.; Buttino, I.; Ianora, A.; Palumbo, A. Nitric oxide mediates the stress response induced by diatom aldehydes in the sea urchin Paracentrotus lividus. PLoS ONE 2011, 6, e25980. [Google Scholar] [CrossRef]
- Billack, B.; Laskin, J.D.; Heck, P.T.; Troll, W.; Gallo, M.A.; Heck, D.E. Alterations in cholinergic signaling modulate contraction of isolated sea urchin tube feet: Potential role of nitric oxide. Biol. Bull. 1998, 195, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Bogdan, C. The multiplex function of nitric oxide in (auto)immunity. J. Exp. Med. 1998, 187, 1361–1365. [Google Scholar] [CrossRef]
- Regulski, M.; Tully, T. Molecular and biochemical characterization of dNOS: A Drosophila Ca2+/calmodulin-dependent nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1995, 92, 9072–9076. [Google Scholar] [CrossRef]
- Chen, T.; Wong, N.K.; Jiang, X.; Luo, X.; Zhang, L.; Yang, D.; Ren, C.; Hu, C. Nitric oxide as an antimicrobial molecule against Vibrio harveyi infection in the hepatopancreas of Pacific white shrimp, Litopenaeus vannamei. Fish. Shellfish. Immunol. 2015, 42, 114–120. [Google Scholar] [CrossRef]
- Bicker, G. Pharmacological approaches to nitric oxide signalling during neural development of locusts and other model insects. Arch. Insect Biochem. Physiol. 2007, 64, 43–58. [Google Scholar] [CrossRef]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the .NO/cGMP signaling pathway. Biochim. Biophys. Acta 1999, 1411, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Petkov, G.V.; Boev, K.K. The role of sarcoplasmic reticulum and sarcoplasmic reticulum Ca2+-ATPase in the smooth muscle tone of the cat gastric fundus. Pflugers Arch. 1996, 431, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; Civelli, O.; Herbert, E. Polyprotein gene expression: Generation of diversity of neuroendocrine peptides. Annu. Rev. Biochem. 1984, 53, 665–715. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.L.; Elphick, M.R. The neuropeptide transcriptome of a model echinoderm, the sea urchin Strongylocentrotus purpuratus. Gen. Comp. Endocrinol. 2012, 179, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Semmens, D.C.; Mirabeau, O.; Moghul, I.; Pancholi, M.R.; Wurm, Y.; Elphick, M.R. Transcriptomic identification of starfish neuropeptide precursors yields new insights into neuropeptide evolution. Open Biol. 2016, 6, 150224. [Google Scholar] [CrossRef]
- Chen, M.; Talarovicova, A.; Zheng, Y.; Storey, K.B.; Elphick, M.R. Neuropeptide precursors and neuropeptides in the sea cucumber Apostichopus japonicus: A genomic, transcriptomic and proteomic analysis. Sci. Rep. 2019, 9, 8829. [Google Scholar] [CrossRef]
- Aleotti, A.; Wilkie, I.C.; Yañez-Guerra, L.A.; Gattoni, G.; Rahman, T.A.; Wademan, R.F.; Ahmad, Z.; Ivanova, D.A.; Semmens, D.C.; Delroisse, J.; et al. Discovery and functional characterization of neuropeptides in crinoid echinoderms. Front. Neurosci. 2022, 16, 1006594. [Google Scholar] [CrossRef]
- Elphick, M.R.; Price, D.A.; Lee, T.D.; Thorndyke, M.C. The SALMFamides: A new family of neuropeptides isolated from an echinoderm. Proc. Biol. Sci. 1991, 243, 121–127. [Google Scholar] [CrossRef]
- Díaz-Miranda, L.; García-Arrarás, J.E. Pharmacological action of the heptapeptide GFSKLYFamide in the muscle of the sea cucumber Holothuria glaberrima (Echinodermata). Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1995, 110, 171–176. [Google Scholar] [CrossRef]
- Melarange, R.; Potton, D.J.; Thorndyke, M.C.; Elphick, M.R. SALMFamide neuropeptides cause relaxation and eversion of the cardiac stomach in starfish. Proc. R. Soc. London. 1999, 266, 1785–1789. [Google Scholar] [CrossRef]
- Yañez-Guerra, L.A.; Delroisse, J.; Barreiro-Iglesias, A.; Slade, S.E.; Scrivens, J.H.; Elphick, M.R. Discovery and functional characterisation of a luqin-type neuropeptide signalling system in a deuterostome. Sci. Rep. 2018, 8, 7220. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Y.; Cong, X.; Liu, H.; Storey, K.B.; Chen, M. Molecular and functional characterization of the luqin-type neuropeptide signaling system in the sea cucumber Apostichopus japonicus. Peptides 2022, 155, 170839. [Google Scholar] [CrossRef]
- Elphick, M.R.; Rowe, M.L. NGFFFamide and echinotocin: Structurally unrelated myoactive neuropeptides derived from neurophysin-containing precursors in sea urchins. J. Exp. Biol. 2009, 212, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Zheng, Y.Q.; Liu, H.C.; Chen, M.Y. A Putative Role of Vasopressin/Oxytocin-Type Neuropeptide in Osmoregulation and Feeding Inhibition of Apostichopus japonicus. Int. J. Mol. Sci. 2023; accepted. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M.; Iwakoshi, E.; Muneoka, Y.; Minakata, H.; Nomoto, K. Isolation and characterization of bioactive peptides from the sea cucumber, Stichopus japonicus. In Peptide Science—Present and Future; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 419–420. [Google Scholar] [CrossRef]
- Inoue, M.; Birenheide, R.; Koizumi, O.; Kobayakawa, Y.; Muneoka, Y.; Motokawa, T. Localization of the neuropeptide NGIWYamide in the holothurian nervous system and its effects on muscular contraction. Proc. R. Soc. B 1999, 266, 993–1000. [Google Scholar] [CrossRef]
- Tinoco, A.B.; Semmens, D.C.; Patching, E.C.; Gunner, E.F.; Egertová, M.; Elphick, M.R. Characterization of NGFFYamide Signaling in Starfish Reveals Roles in Regulation of Feeding Behavior and Locomotory Systems. Front. Endocrinol. 2018, 9, 507. [Google Scholar] [CrossRef]
- Semmens, D.C.; Dane, R.E.; Pancholi, M.R.; Slade, S.E.; Scrivens, J.H.; Elphick, M.R. Discovery of a novel neurophysin-associated neuropeptide that triggers cardiac stomach contraction and retraction in starfish. J. Exp. Biol. 2013, 216, 4047–4053. [Google Scholar] [CrossRef]
- Zhang, Y.; Yañez Guerra, L.A.; Egertová, M.; Zampronio, C.G.; Jones, A.M.; Elphick, M.R. Molecular and functional characterization of somatostatin-type signalling in a deuterostome invertebrate. Open Biol. 2020, 10, 200172. [Google Scholar] [CrossRef]
- Tinoco, A.B.; Barreiro-Iglesias, A.; Yañez Guerra, L.A.; Delroisse, J.; Zhang, Y.; Gunner, E.F.; Zampronio, C.G.; Jones, A.M.; Egertová, M.; Elphick, M.R. Ancient role of sulfakinin/cholecystokinin-type signalling in inhibitory regulation of feeding processes revealed in an echinoderm. eLife 2021, 10, e65667. [Google Scholar] [CrossRef]
- Tian, S.; Egertová, M.; Elphick, M.R. Functional Characterization of Paralogous Gonadotropin-Releasing Hormone-Type and Corazonin-Type Neuropeptides in an Echinoderm. Front. Endocrinol. 2017, 8, 259. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Liu, H.C.; Cong, X.; Storey, K.B.; Chen, M.Y. A potential feeding regulation strategy during aestivation: Relaxation of intestine mediated by peptide/orcokinin-type neuropeptide in sea cucumber Apostichopus japonicus. Aquaculture 2023, accepted. [Google Scholar] [CrossRef]
- Lin, M.; Egertová, M.; Zampronio, C.G.; Jones, A.M.; Elphick, M.R. Functional characterization of a second pedal peptide/orcokinin-type neuropeptide signaling system in the starfish Asterias rubens. J. Comp. Neurol. 2018, 526, 858–876. [Google Scholar] [CrossRef]
- Otara, C.B.; Jones, C.E.; Younan, N.D.; Viles, J.H.; Elphick, M.R. Structural analysis of the starfish SALMFamide neuropeptides S1 and S2: The N-terminal region of S2 facilitates self-association. Biochim. Biophys. Acta 2014, 1844, 358–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fredriksson, R.; Lagerström, M.C.; Lundin, L.G.; Schiöth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef] [PubMed]
- Novozhilova, E.; Kimber, M.J.; Qian, H.; McVeigh, P.; Robertson, A.P.; Zamanian, M.; Maule, A.G.; Day, T.A. FMRFamide-like peptides (FLPs) enhance voltage-gated calcium currents to elicit muscle contraction in the human parasite Schistosoma mansoni. PLoS Neglected Trop. Dis. 2010, 4, e790. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Butler, A.; Stewart, R.; Gonzalez-Cota, A.L.; Lybaert, P.; Amazu, C.; Reinl, E.L.; Wakle-Prabagaran, M.; Salkoff, L.; England, S.K.; et al. Oxytocin can regulate myometrial smooth muscle excitability by inhibiting the Na+ -activated K+ channel, Slo2.1. J. Physiol. 2019, 597, 137–149. [Google Scholar] [CrossRef]
- Alzugaray, M.E.; Gavazzi, M.V.; Ronderos, J.R. G protein-coupled receptor signal transduction and Ca2+ signaling pathways of the allatotropin/orexin system in Hydra. Gen. Comp. Endocrinol. 2021, 300, 113637. [Google Scholar] [CrossRef]
- Alzugaray, M.E.; Ronderos, J.R. Allatoregulatory-like systems and changes in cytosolic Ca2+ modulate feeding behavior in Hydra. Gen. Comp. Endocrinol. 2018, 258, 70–78. [Google Scholar] [CrossRef]
- Schmidt, A.; Bauknecht, P.; Williams, E.A.; Augustinowski, K.; Gründer, S.; Jékely, G. Dual signaling of Wamide myoinhibitory peptides through a peptide-gated channel and a GPCR in Platynereis. FASEB J. 2018, 32, 5338–5349. [Google Scholar] [CrossRef]
- Conzelmann, M.; Williams, E.A.; Tunaru, S.; Randel, N.; Shahidi, R.; Asadulina, A.; Berger, J.; Offermanns, S.; Jékely, G. Conserved MIP receptor-ligand pair regulates Platynereis larval settlement. Proc. Natl. Acad. Sci. USA 2013, 110, 8224–8229. [Google Scholar] [CrossRef]
- Wang, H.; Girskis, K.; Janssen, T.; Chan, J.P.; Dasgupta, K.; Knowles, J.A.; Schoofs, L.; Sieburth, D. Neuropeptide secreted from a pacemaker activates neurons to control a rhythmic behavior. Curr. Biol. 2013, 23, 746–754. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).