Characteristics of the Intestine Extracts and Their Effect on the Crude Collagen Fibers of the Body Wall from Sea Cucumber Apostichopus japonicus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Preparation
2.3. Preparation of Extracts from Sea Cucumber Intestines
2.4. Gelatin Zymography Assay
2.5. Rheological Properties
3. Results
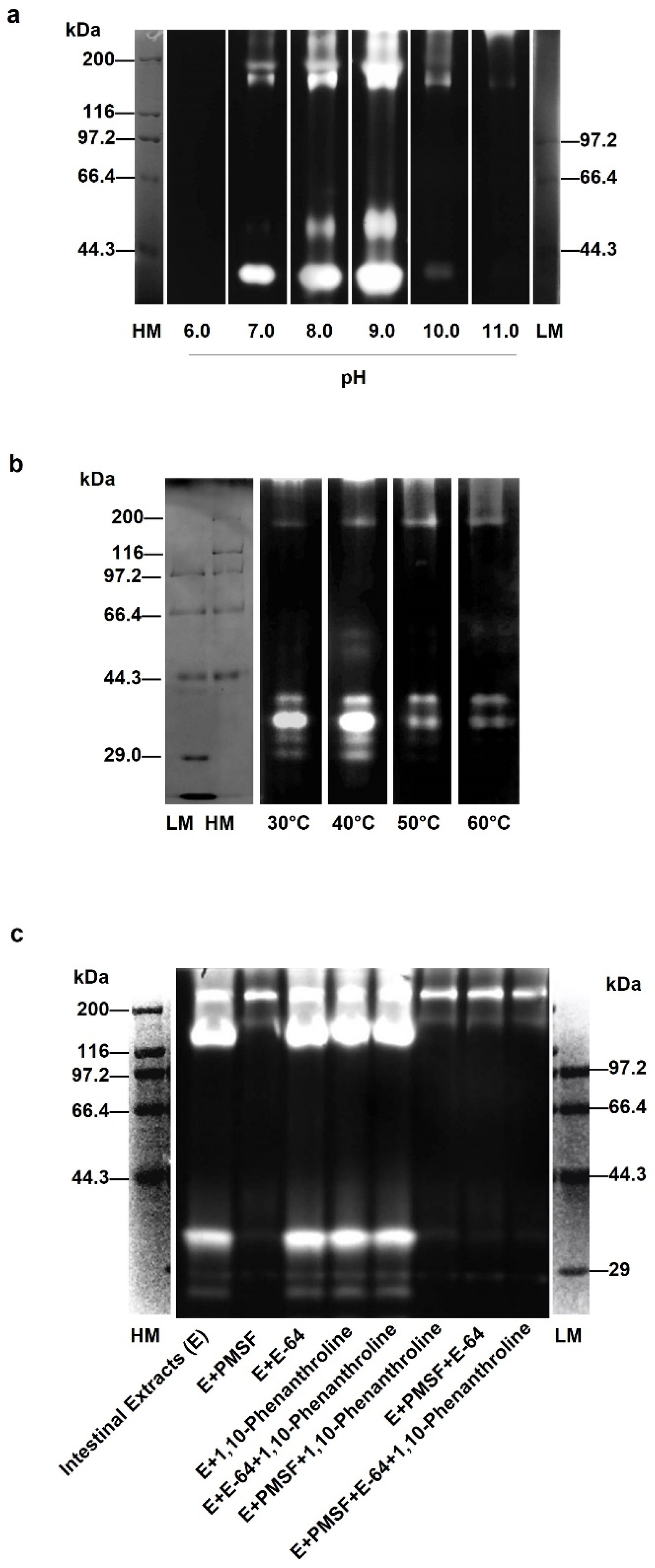
3.1. Optimal Conditions of Gelatinolytic Enzymes in Sea Cucumber Intestinal Extract
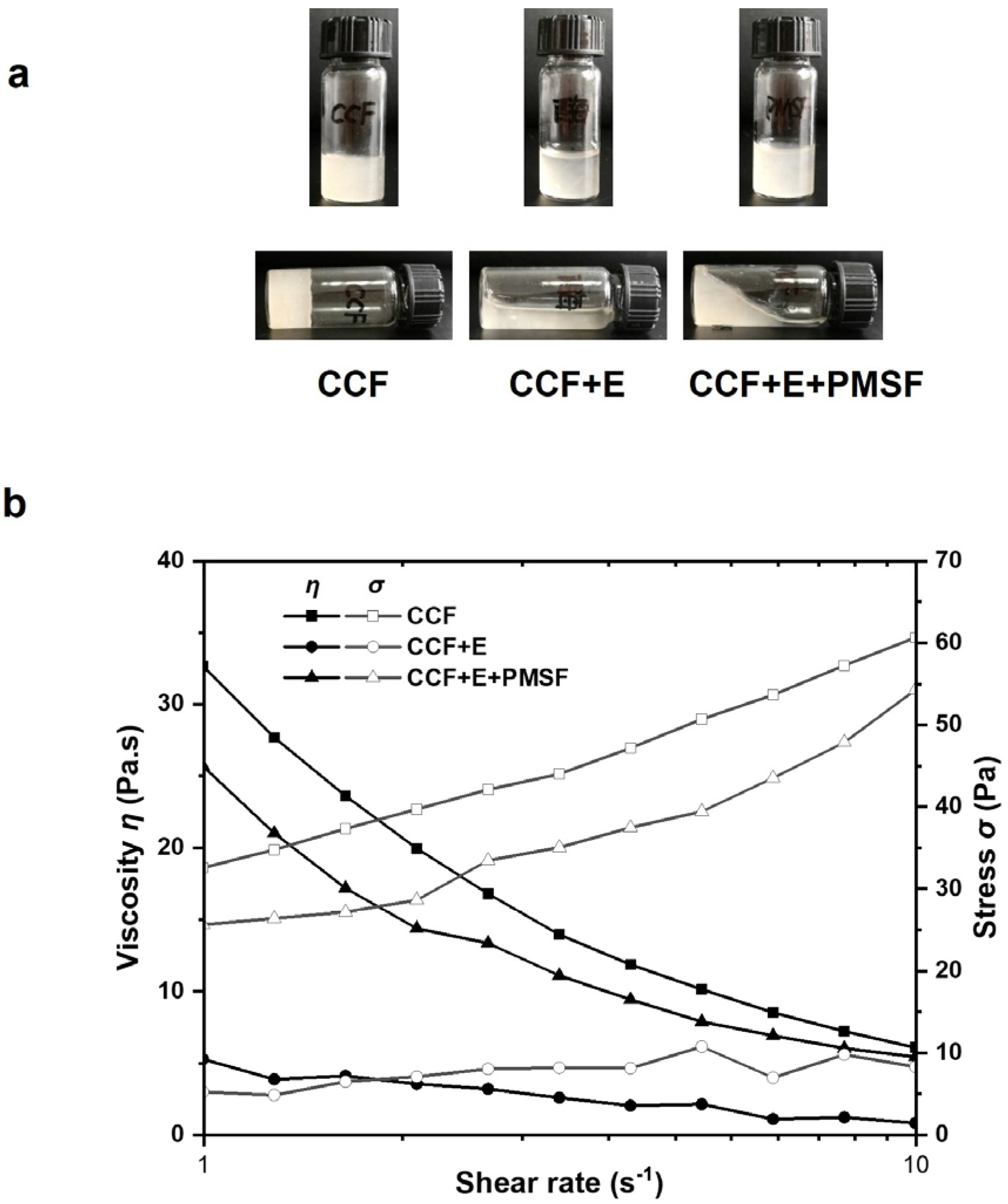
3.2. Effect of Sea Cucumber Intestinal Extract on Rheological Properties of CCF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.-X.; Zhou, D.-Y.; Ma, D.-D.; Liu, Y.-F.; Li, D.-M.; Dong, X.-P.; Tan, M.-Q.; Du, M.; Zhu, B.-W. Changes in collagenous tissue microstructures and distributions of cathepsin L in body wall of autolytic sea cucumber (Stichopus japonicus). Food Chem. 2016, 212, 341–348. [Google Scholar] [CrossRef]
- Tan, Z.F.; Ding, Y.; Tian, J.Y.; Liu, Z.Q.; Bi, J.R.; Zhou, D.Y.; Song, L.; Chen, G.B. Inhibition of ultraviolet-induced sea cucumber (Stichopus japonicus) autolysis by maintaining coelomocyte intracellular calcium homeostasis. Food Chem. 2022, 368, 130768. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zheng, J.; Zhang, Z.; Dong, X.; Zhao, L.; Tada, M. Autophagy plays a potential role in the process of sea cucumber body wall “melting” induced by UV irradiation. Wuhan Univ. J. Nat. Sci. 2008, 13, 232–238. [Google Scholar] [CrossRef]
- Chen, L.; Yao, F.; Qin, Y.; Shao, Y.; Fang, L.; Yu, X.; Wang, S.; Hou, L. The potential role of Kruppel-like factor 13 (Aj-klf13) in the intestine regeneration of sea cucumber Apostichopus japonicus. Gene 2020, 735, 144407. [Google Scholar] [CrossRef]
- Gao, F.; Yang, H.; Xu, Q.; Wang, F.; Liu, G. Effect of water temperature on digestive enzyme activity and gut mass in sea cucumber Apostichopus japonicus (Selenka), with special reference to aestivation. Chin. J. Oceanol. Limnol. 2009, 27, 714–722. [Google Scholar] [CrossRef]
- Sun, L.-M.; Zhu, B.-W.; Wu, H.-T.; Yu, L.; Zhou, D.-Y.; Dong, X.; Yang, J.-F.; Li, D.-M.; Ye, W.-X.; Murata, Y. Purification and characterization of Cathepsin B from the gut of the sea cucumber (Stichopus japonicas). Food Sci. Biotechnol. 2011, 20, 919–925. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Xue, C.-H.; Miao, B.-C.; Li, Z.-J.; Yang, W.-G.; Wang, D.-F. Study of a highly alkaline protease extracted from digestive tract of sea cucumber (Stichopus japonicus). Food Res. Int. 2005, 38, 323–329. [Google Scholar] [CrossRef]
- Zhu, B.-W.; Zhao, J.-G.; Yang, J.-F.; Mikiro, T.; Zhang, Z.-S.; Zhou, D.-Y. Purification and partial characterization of a novel β-1,3-glucanase from the gut of sea cucumber Stichopus japonicus. Process Biochem. 2008, 43, 1102–1106. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhou, D.Y.; Ma, D.D.; Liu, Z.Q.; Liu, Y.F.; Song, L.; Dong, X.P.; Li, D.M.; Zhu, B.W.; Konno, K.; et al. Effects of endogenous cysteine proteinases on structures of collagen fibres from dermis of sea cucumber (Stichopus japonicus). Food Chem. 2017, 232, 10–18. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Liu, Z.-Q.; Song, L.; Ma, Q.-R.; Zhou, D.-Y.; Zhu, B.-W.; Shahidi, F. Effects of collagenase type I on the structural features of collagen fibres from sea cucumber (Stichopus japonicus) body wall. Food Chem. 2019, 301, 125302. [Google Scholar] [CrossRef]
- Wu, H.T.; Li, D.M.; Zhu, B.W.; Sun, J.J.; Zheng, J.; Wang, F.L.; Konno, K.; Jiang, X. Proteolysis of noncollagenous proteins in sea cucumber, Stichopus japonicus, body wall: Characterisation and the effects of cysteine protease inhibitors. Food Chem. 2013, 141, 1287–1294. [Google Scholar] [CrossRef]
- Sun, L.-M.; Wang, T.-T.; Zhu, B.-W.; Niu, H.-L.; Zhang, R.; Hou, H.-M.; Zhang, G.-L.; Murata, Y. Effect of matrix metalloproteinase on autolysis of sea cucumber Stichopus japonicus. Food Sci. Biotechnol. 2013, 22, 1–3. [Google Scholar] [CrossRef]
- Wu, H.-L.; Hu, Y.-Q.; Shen, J.-D.; Cai, Q.-F.; Liu, G.-M.; Su, W.-J.; Cao, M.-J. Identification of a novel gelatinolytic metalloproteinase (GMP) in the body wall of sea cucumber (Stichopus japonicus) and its involvement in collagen degradation. Process Biochem. 2013, 48, 871–877. [Google Scholar] [CrossRef]
- Zhao, C.C.; Yang, Y.; Wu, H.T.; Zhu, Z.M.; Tang, Y.; Yu, C.P.; Sun, N.; Lv, Q.; Han, J.R.; Li, A.T.; et al. Characterization of proteolysis in muscle tissues of sea cucumber Stichopus japonicus. Food Sci. Biotechnol. 2016, 25, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Barzkar, N.; Khan, Z.; Tamadoni Jahromi, S.; Pourmozaffar, S.; Gozari, M.; Nahavandi, R. A critical review on marine serine protease and its inhibitors: A new wave of drugs? Int. J. Biol. Macromol. 2021, 170, 674–687. [Google Scholar] [CrossRef]
- Ren, G.; Sun, H.; Li, G.; Fan, J.; Du, L.; Cui, G. Interaction mechanism of aloeemodin with trypsin: Molecular structure-affinity relationship and effect on biological activities. RSC Adv. 2020, 10, 20862–20871. [Google Scholar] [CrossRef]
- Yu, P.; Yan, C.; Yang, F.; Xu, Y.; Jiang, Q.; Xia, W. Effect of high pressure processing on the quality and endogenous enzyme activities of grass carp (Ctenopharyngodon idellus) fillets stored at 4 °C. J. Aquat. Food Prod. Technol. 2018, 27, 1093–1105. [Google Scholar] [CrossRef]
- Liu, J.Y.; Yoshida, A.; Gao, Y.L.; Shirota, K.; Shiina, Y.; Noguchi, E.; Kuwahara, K.; Osatomi, K. Purification and characterization of a sarcoplasmic serine proteinase from threadfin bream Nemipterus virgatus muscle. Food Chem. 2019, 284, 198–204. [Google Scholar] [CrossRef]
- Zhou, T.; Ding, Y.-X.; Benjakul, S.; Shui, S.-S.; Zhang, B. Characterization of endogenous enzymes in sword prawn (Parapenaeopsis hardwickii) and their effects on the quality of muscle proteins during frozen storage. LWT 2023, 177, 114563. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Proteolysis and its control using protease inhibitors in fish and fish products: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef]
- Dong, X.; Zhu, B.; Sun, L.; Zheng, J.; Jiang, D.; Zhou, D.; Wu, H.; Murata, Y. Changes of collagen in sea cucumber (Stichopus japonicas) during cooking. Food Sci. Biotechnol. 2011, 20, 1137–1141. [Google Scholar] [CrossRef]
- Wang, C.; Zhan, C.L.; Cai, Q.F.; Du, C.H.; Liu, G.M.; Su, W.J.; Cao, M.J. Expression and characterization of common carp (Cyprinus carpio) matrix metalloproteinase-2 and its activity against type I collagen. J. Biotechnol. 2014, 177, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ligi, D.; Maniscalco, R.; Mannello, F. MMP-2 and MMP-9 in human peripheral blood: Optimizing gelatinase calibrator for degradome research and discovering a novel gelatinolytic enzyme. J. Proteome Res. 2020, 19, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Liu, Y.X.; Zhou, D.Y.; Liu, X.Y.; Dong, X.P.; Li, D.M.; Shahidi, F. The role of matrix metalloprotease (MMP) to the autolysis of sea cucumber (Stichopus japonicus). J. Sci. Food Agric. 2019, 99, 5752–5759. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; D’Esposito, V.; Formisano, P.; Di Carlo, A. Substrate-zymography: A still worthwhile method for gelatinases analysis in biological samples. Clin. Chem. Lab. Med. 2016, 54, 1281–1290. [Google Scholar] [CrossRef]
- Yan, L.-J.; Zhan, C.-L.; Cai, Q.-F.; Weng, L.; Du, C.-H.; Liu, G.-M.; Su, W.-J.; Cao, M.-J. Purification, Characterization, cDNA cloning and in vitro expression of a serine proteinase from the intestinal tract of sea cucumber (Stichopus japonicus) with collagen degradation activity. J. Agric. Food Chem. 2014, 62, 4769–4777. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, G.; Li, Y. Preparation and characterization of thermally stable collagens from the scales of lizardfish (Synodus macrops). Mar. Drugs 2021, 19, 597. [Google Scholar] [CrossRef]
- Xiong, X.; Xie, W.; Xie, J.; Qi, H.; Yang, X.; Li, H.; Che, H.; Song, L.; Dong, X. Protein oxidation results in textural changes in sea cucumber (Apostichopus japonicus) during tenderization. LWT-Food Sci. Technol. 2021, 144, 111231. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Li, Y.; Song, L.; Cheng, S.; Li, D.; Zhu, B.-W.; Zhou, D.; Tan, M. Combination of NMR and MRI techniques for non-invasive assessment of sea cucumber (Stichopus japonicas) tenderization during low-temperature heating process. Food Anal. Methods 2017, 10, 2207–2216. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, R.; Wang, Y. Acid-soluble and pepsin-soluble collagens from grass carp (Ctenopharyngodon idella) skin: A comparative study on physicochemical properties. Int. J. Food Sci. Tech. 2015, 50, 186–193. [Google Scholar] [CrossRef]
- Abedin, M.Z.; Karim, A.A.; Latiff, A.A.; Gan, C.-Y.; Che Ghazali, F.; Zzaman, W.; Hossain, M.M.; Ahmed, F.; Absar, N.; Sarker, M.Z.I. Physicochemical and biochemical properties of pepsin-solubilized collagen isolated from the integument of sea cucumber (Stichopus vastus). J. Food Process Preserv. 2014, 38, 2027–2036. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Tuo, F.Y.; Song, L.; Liu, Y.X.; Dong, X.P.; Li, D.M.; Zhou, D.Y.; Shahidi, F. Action of trypsin on structural changes of collagen fibres from sea cucumber (Stichopus japonicus). Food Chem. 2018, 256, 113–118. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.-Q.; Zhang, Z.-Y.; Nie, B.; Du, Y.-N.; Tang, Y.; Wu, H.-T. Characteristics of the Intestine Extracts and Their Effect on the Crude Collagen Fibers of the Body Wall from Sea Cucumber Apostichopus japonicus. Biology 2023, 12, 705. https://doi.org/10.3390/biology12050705
Xu S-Q, Zhang Z-Y, Nie B, Du Y-N, Tang Y, Wu H-T. Characteristics of the Intestine Extracts and Their Effect on the Crude Collagen Fibers of the Body Wall from Sea Cucumber Apostichopus japonicus. Biology. 2023; 12(5):705. https://doi.org/10.3390/biology12050705
Chicago/Turabian StyleXu, Shi-Qi, Zheng-Yu Zhang, Bin Nie, Yi-Nan Du, Yue Tang, and Hai-Tao Wu. 2023. "Characteristics of the Intestine Extracts and Their Effect on the Crude Collagen Fibers of the Body Wall from Sea Cucumber Apostichopus japonicus" Biology 12, no. 5: 705. https://doi.org/10.3390/biology12050705
APA StyleXu, S.-Q., Zhang, Z.-Y., Nie, B., Du, Y.-N., Tang, Y., & Wu, H.-T. (2023). Characteristics of the Intestine Extracts and Their Effect on the Crude Collagen Fibers of the Body Wall from Sea Cucumber Apostichopus japonicus. Biology, 12(5), 705. https://doi.org/10.3390/biology12050705






