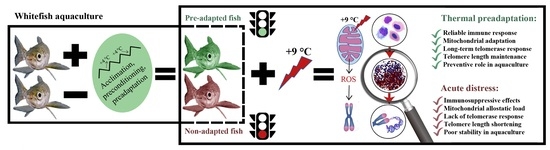
Thermal Preconditioning Alters the Stability of Hump-Snout Whitefish (Coregonus fluviatilis) and Its Hybrid Form, Showing Potential for Aquaculture
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fertilization, Incubation, and Rearing of Juveniles
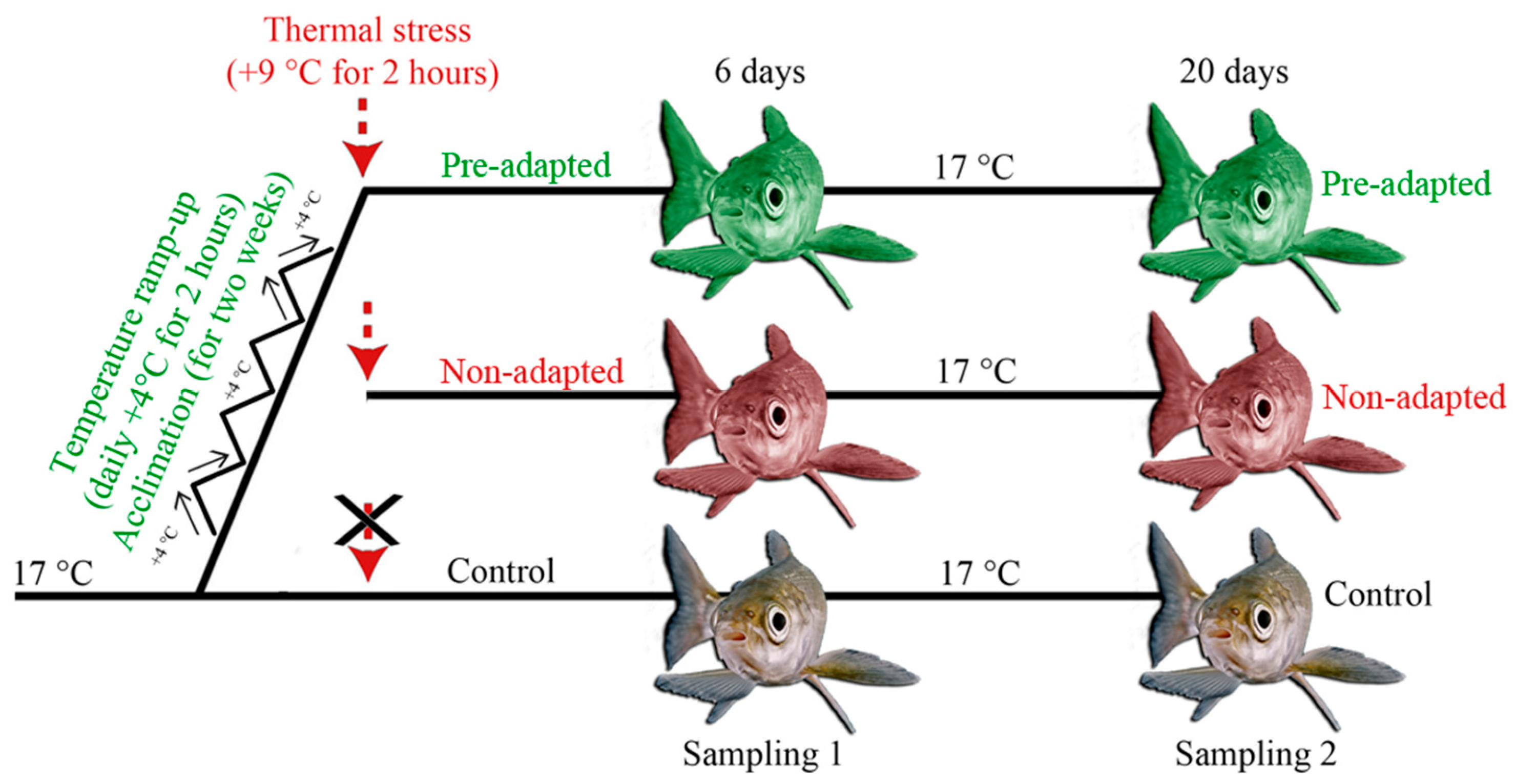
2.2. Experimental Design and Tissue Collection
2.3. Ethical Standards
2.4. Cytometric Parameters of Peripheral Blood and Hematopoietic Organs
2.5. Telomere Length and Telomerase Activity Analysis
2.6. Statistical Analysis
3. Results
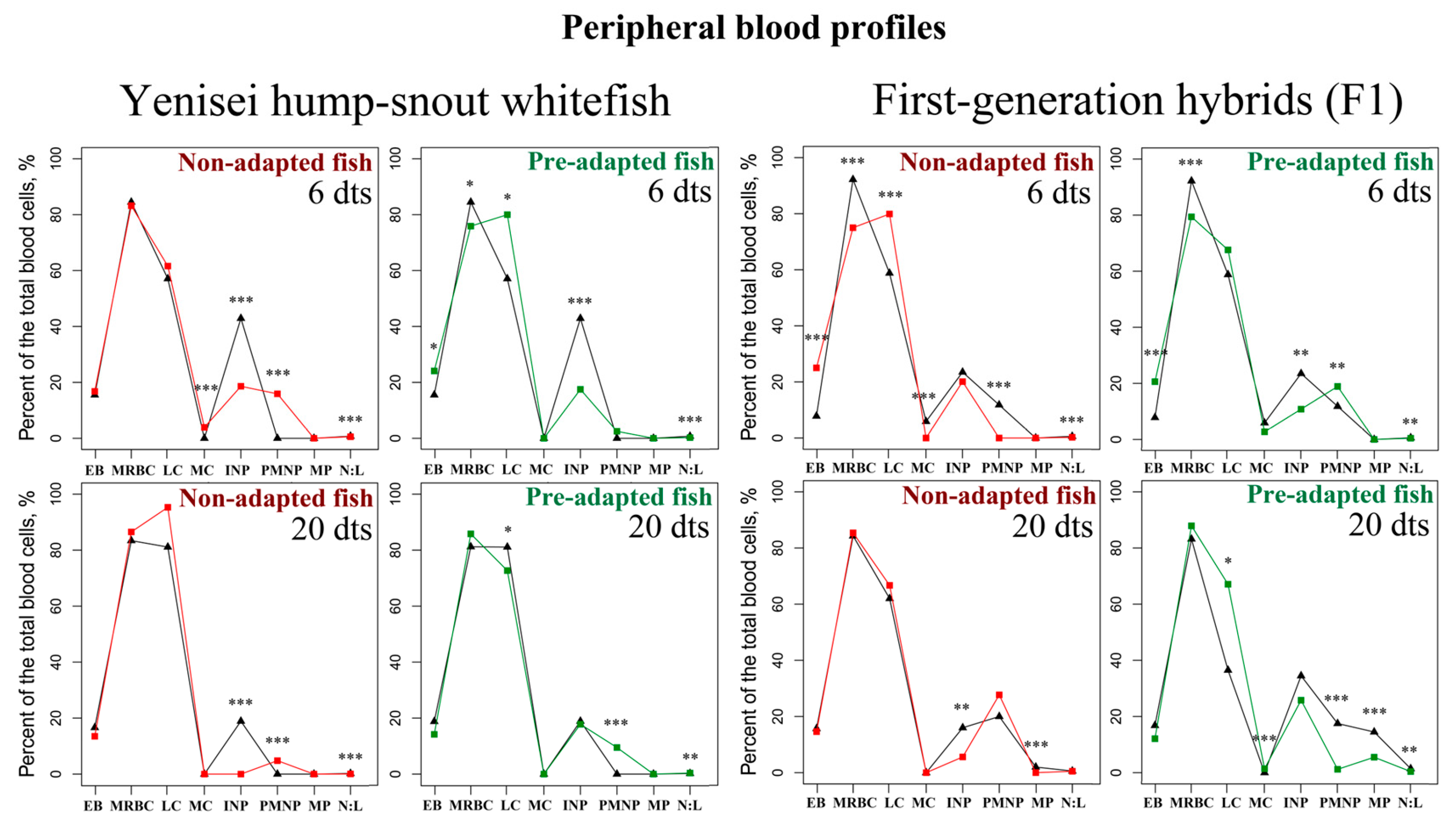
3.1. Peripheral Blood Profiles
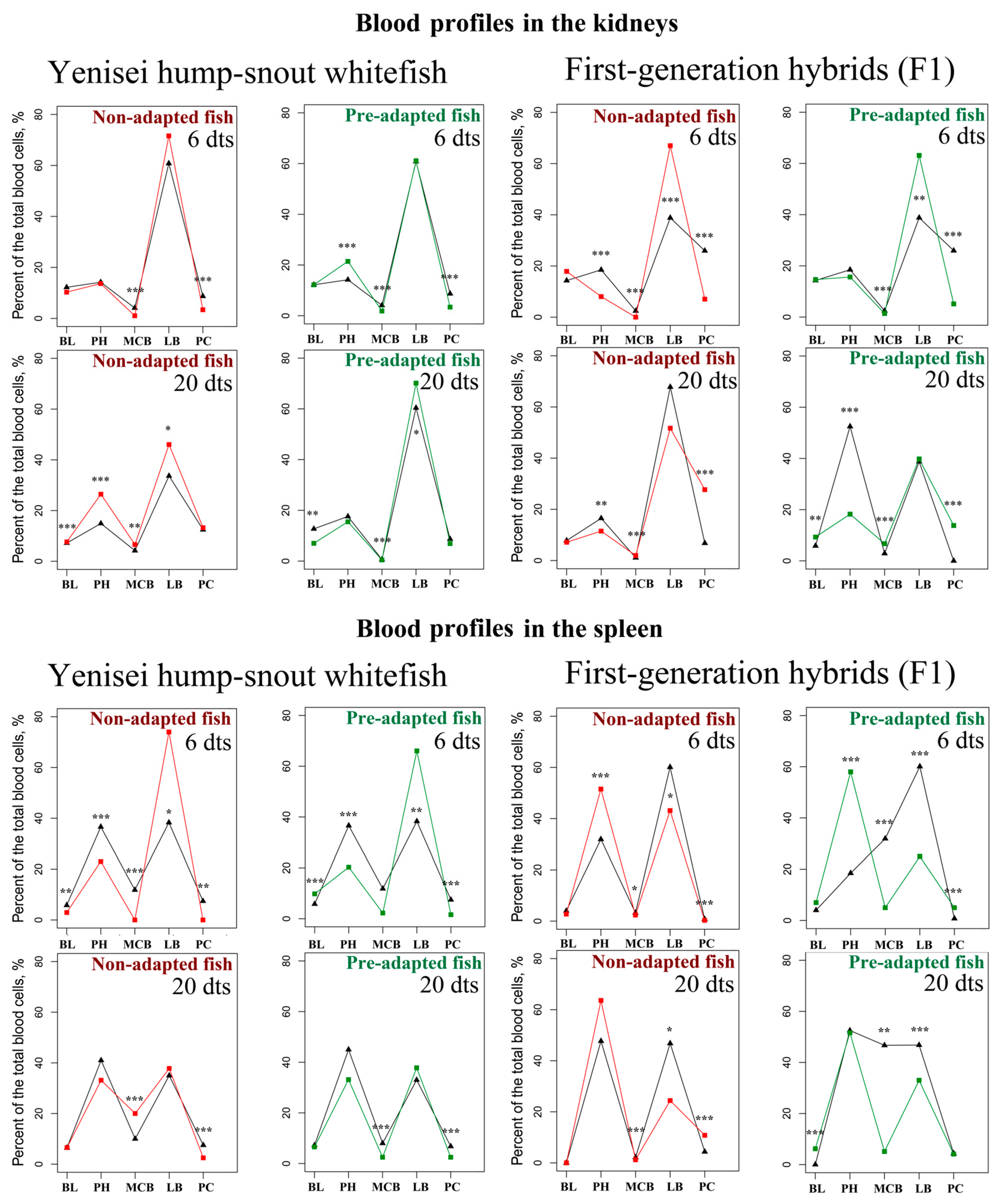
3.2. Blood Profiles in the Kidneys and Spleen
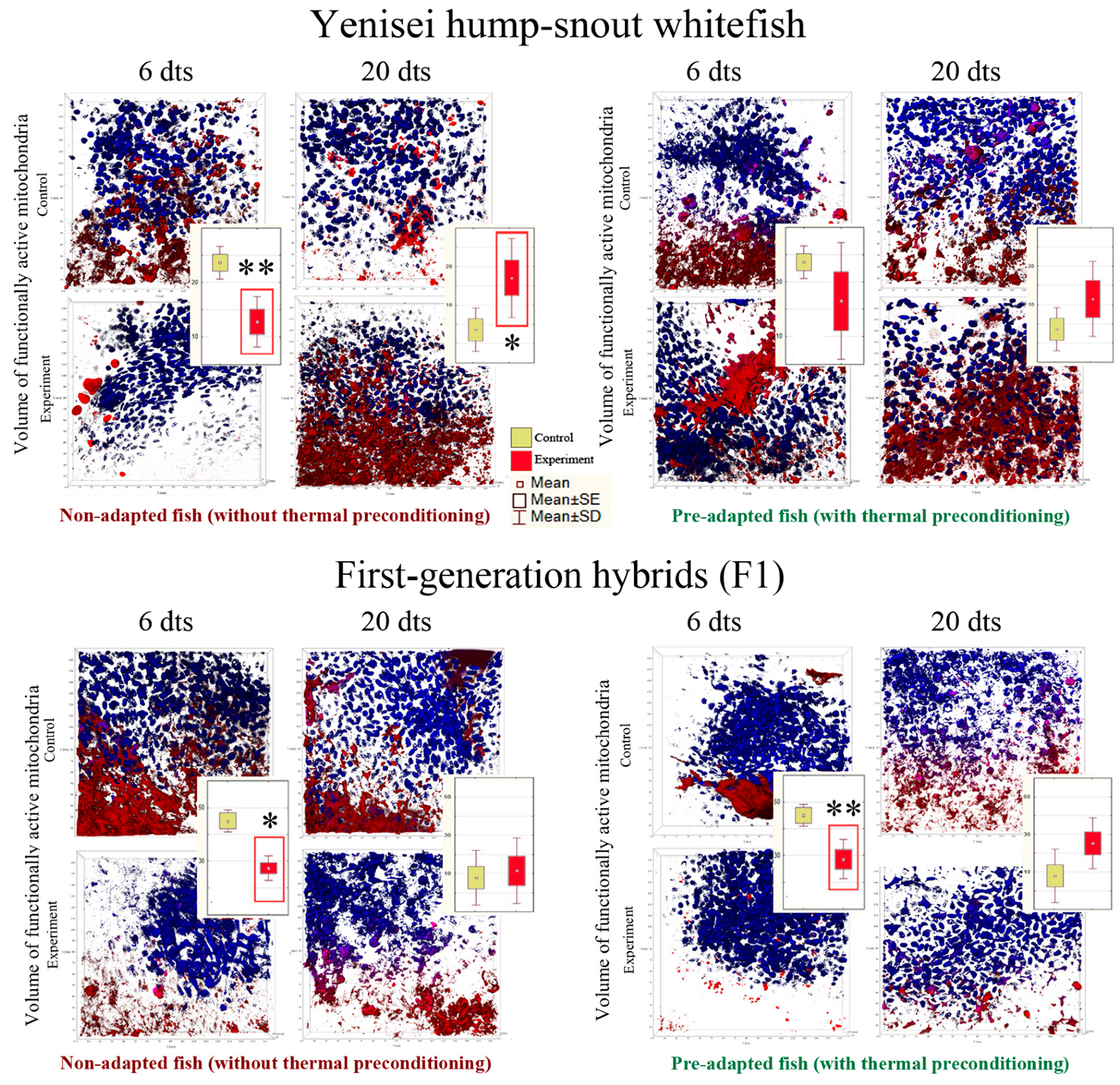
3.3. Functionally Active Mitochondria
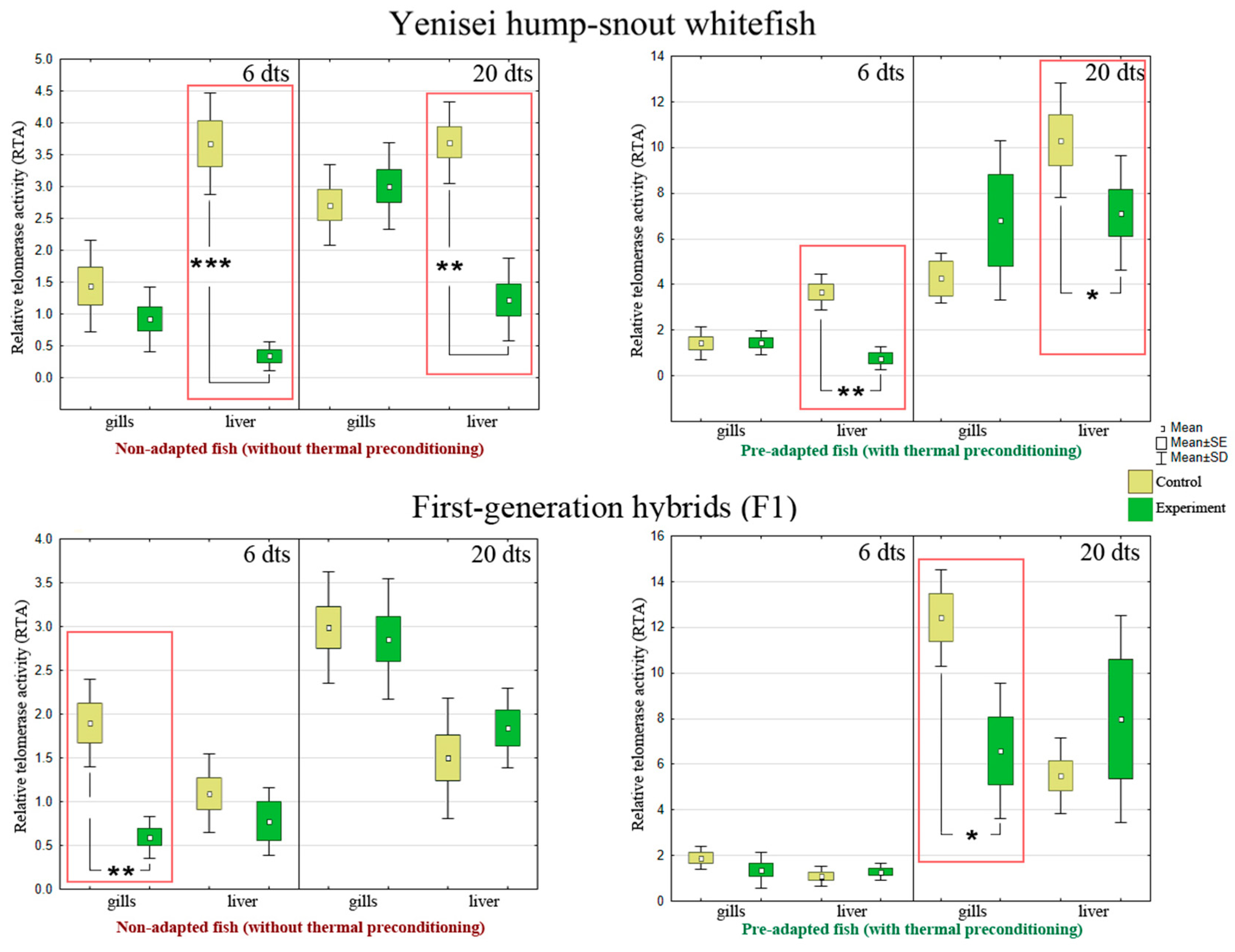
3.4. Telomerase Activity
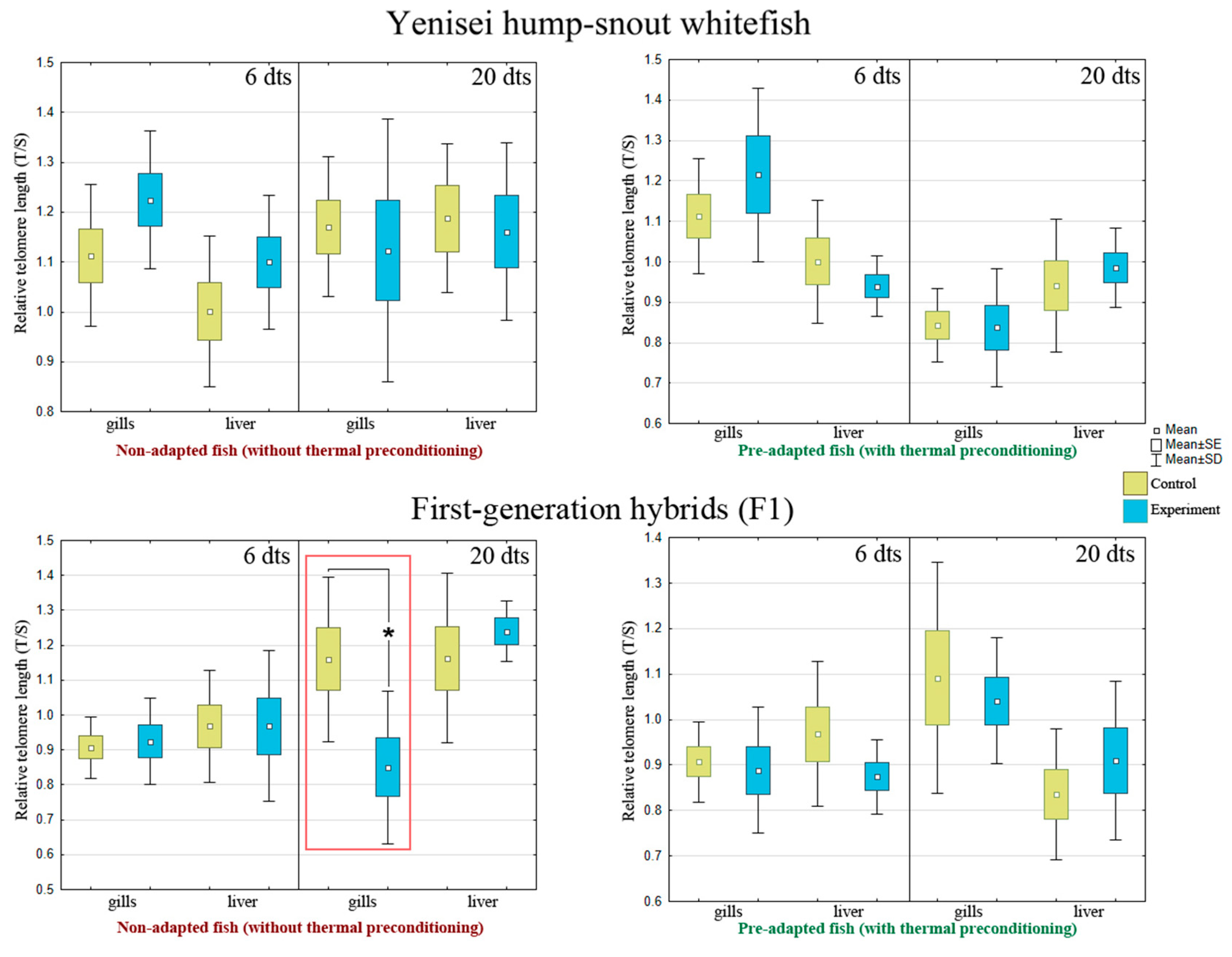
3.5. Telomere Maintenance
4. Discussion
4.1. Cellular Blood Reactions
4.2. Effects of Heat Shock on Mitochondria, Telomerase Activity, and Telomere Attrition
4.3. Relationship with Other Biomarkers and Prospects for Further Research
4.4. Understanding Whitefish Stability in the Context of their Ecology and Speciation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A
| EB 1 | MRBC 1 | LC 1 | MC 1 | INP 1 | PMNP 1 | MP 1 | N:L 1 | ||||
| Non-adapted Hump-snout whitefish | Experiment | 6d | M | 16.8 | 83.2 | 61.6 | 3.9 ***↑ | 18.6 ***↓ | 15.9 ***↑ | 0 | 0.56 ***↓ |
| SD | 1.8 | 1.8 | 8.6 | 1.2 | 3.6 | 2.3 | 0.04 | ||||
| 20d | M | 13.5 | 86.5 | 95.3 | 0 | 0 ***↓ | 4.8 ***↑ | 0 | 0.05 ***↓ | ||
| SD | 1.2 | 1.2 | 8.3 | 0.2 | 0.01 | ||||||
| Control | 6d | M | 15.5 | 84.5 | 57.1 | 0 | 42.9 | 0 | 0 | 0.75 | |
| SD | 2.3 | 2.3 | 5.8 | 1.7 | 0.02 | ||||||
| 20d | M | 16.6 | 83.4 | 81.1 | 0 | 18.9 | 0 | 0 | 0.23 | ||
| SD | 1.9 | 1.9 | 8.1 | 3.1 | 0.01 | ||||||
| Pre-adapted Hump-snout whitefish | |||||||||||
| Experiment | 6d | M | 24.1 *↑ | 75.9 *↓ | 80 *↑ | 0 | 17.5 ***↓ | 2.5 | 0 | 0.25 ***↓ | |
| SD | 23.8 | 2.8 | 7.8 | 2.4 | 0.3 | 0.01 | |||||
| 20d | M | 14.2 | 85.8 | 72.7 *↓ | 0 | 17.8 | 9.5 ***↑ | 0 | 0.38 **↑ | ||
| SD | 1.6 | 1.6 | 7.9 | 0.39 | 0.8 | 0.04 | |||||
| Control | 6d | M | 15.5 | 84.5 | 57.1 | 0 | 42.9 | 0 | 0 | 0.75 | |
| SD | 2.3 | 2.3 | 5.8 | 1.7 | 0.02 | ||||||
| 20d | M | 18.8 | 81.2 | 81.1 | 0 | 18.9 | 0 | 0 | 0.23 | ||
| SD | 1.7 | 1.7 | 3.1 | 1.1 | 0.03 | ||||||
| Non-adapted Hybrids | |||||||||||
| Experiment | 6d | M | 25 ***↑ | 75 ***↓ | 79.9 ***↑ | 0 ***↓ | 20.1 | 0 ***↓ | 0 | 0.25 ***↓ | |
| SD | 2.7 | 2.7 | 7.8 | 3.6 | 0.01 | ||||||
| 20d | M | 14.6 | 85.4 | 66.7 | 0 | 5.6 **↓ | 27.7 | 0 ***↓ | 0.5 | ||
| SD | 1.9 | 1.9 | 7.4 | 1.1 | 3.1 | 0.06 | |||||
| Control | 6d | M | 7.8 | 92.2 | 58.8 | 5,9 | 23.5 | 11.8 | 0 | 0.6 | |
| SD | 1.8 | 1.8 | 3.2 | 1.2 | 3.6 | 0.7 | 0.04 | ||||
| 20d | M | 15.7 | 84.3 | 62 | 0 | 16 | 20 | 2 | 0.58 | ||
| SD | 1.7 | 1.7 | 7.2 | 2.9 | 1.1 | 0.07 | 0.06 | ||||
| Pre-adapted Hybrids | |||||||||||
| Experiment | 6d | M | 20.6 ***↑ | 79.4 ***↓ | 67.6 | 2.7 | 10.8 **↓ | 18.9 **↑ | 0 | 0.44 **↓ | |
| SD | 2.4 | 2.3 | 8.6 | 0.9 | 2.2 | 2.3 | 0.03 | ||||
| 20d | M | 12.1 | 87.9 | 67.1 *↑ | 1.4 ***↑ | 25.8 | 1.2 ***↓ | 5.5 ***↓ | 0.4 **↓ | ||
| SD | 1.9 | 1.9 | 12.1 | 0.3 | 3.4 | 0.08 | 0.9 | 0.03 | |||
| Control | 6d | M | 7.8 | 92.2 | 58.8 | 5.9 | 23.5 | 11.8 | 0 | 0.6 | |
| SD | 1.8 | 1.8 | 3.2 | 1.2 | 3.6 | 0.7 | 0.04 | ||||
| 20d | M | 16.8 | 83.2 | 36.5 | 0 | 34.5 | 17.5 | 14.5 | 1.42 | ||
| SD | 1.6 | 1.6 | 0.73 | 2.1 | 0.9 | 0.7 | 0.32 | ||||
| E 1 | S 1 | s 1 | NCR 1 | E 1 | S (μK2)1 | s (μK2) 1 | NCR 1 | |||
| Control | Experiment | |||||||||
| Non-adapted Fish | ||||||||||
| Hump-snout whitefish | 6 d | M | 0.59 | 213.16 | 49.76 | 0.31(140) | 0.59 | 218.82 | 49.53 | 0.30 (260) |
| SD | 0.01 | 4.39 | 0.75 | 0.01 | 0.00 | 3.04 | 0.65 | 0.01 | ||
| 20 d | M | 0.61 | 277.25 | 57.93 | 0.27 (346) | 0.60 | 249.75 ***↓ | 62.80 ***↑ | 0.35 ***↑ (204) | |
| SD | 0.01 | 2.78 | 0.56 | 0.00 | 0.01 | 3.95 | 0.62 | 0.01 | ||
| Pre-adapted fish | ||||||||||
| 6 d | M | 0.59 | 213.16 | 49.76 | 0.31 (140) | 0.58 | 206.84 | 52.38 | 0.35 **↑ (160) | |
| SD | 0.01 | 4.39 | 0.75 | 0.01 | 0.01 | 3.79 | 1.10 | 0.01 | ||
| 20 d | M | 0.64 | 281.61 | 70.39 | 0.36 (156) | 0.55 ***↓ | 230.58 ***↓ | 52.08 ***↓ | 0.29 *↓ (141) | |
| SD | 0.01 | 11.49 | 5.35 | 0.03 | 0.01 | 3.09 | 0.79 | 0.01 | ||
| Hybrids | Non-adapted fish | |||||||||
| 6 d | M | 0.63 | 224.51 | 50.35 | 0.30 (165) | 0.61 | 251.13 ***↑ | 44.82 ***↓ | 0.22 ***↓ (139) | |
| SD | 0.01 | 3.88 | 0.66 | 0.01 | 0.01 | 4.84 | 1.22 | 0.01 | ||
| 20 d | M | 0.61 | 253.03 | 59.42 | 0.33 (176) | 0.58 **↓ | 245.70 | 50.13 ***↓ | 0.27 ***↓ (394) | |
| SD | 0.01 | 5.64 | 0.80 | 0.01 | 0.01 | 4.04 | 0.68 | 0.01 | ||
| Pre-adapted fish | ||||||||||
| 6 d | M | 0.63 | 224.51 | 50.35 | 0.30 (165) | 0.60 | 250.45 ***↓ | 60.21 ***↑ | 0.33 (155) | |
| SD | 0.01 | 3.88 | 0.66 | 0.01 | 0.01 | 5.72 | 1.23 | 0.01 | ||
| 20 d | M | 0.57 | 230.2 | 46.21 | 0.26 (160) | 0.59 ***↑ | 243.1 **↑ | 51.2 ***↑ | 0.28 (212) | |
| SD | 0.01 | 3.33 | 0.55 | 0.01 | 0.004 | 2.37 | 0.44 | 0.005 | ||
| BL 1 | PH 1 | MCB 1 | LB 1 | PC 1 | BL 1 | PH 1 | MCB 1 | LB 1 | PC 1 | |||
| Control | Experiment | |||||||||||
| Non-adapted Fish | ||||||||||||
| Hump-snout whitefish | 6 d | M | 12.2 | 14.2 | 4.1 | 60.8 | 8.7 | 10.3 | 13.65 | 1.05 ***↓ | 71.6 | 3.4 ***↓ |
| SD | 0.9 | 0.3 | 0.3 | 2.3 | 0.8 | 1.2 | 0.8 | 0.07 | 4.1 | 0.7 | ||
| 20 d | M | 7.2 | 14.9 | 4.2 | 33.65 | 12.45 | 7.65 ***↑ | 26.45 ***↑ | 6.6 **↑ | 46.05 *↑ | 13.25 | |
| SD | 0.8 | 0.8 | 0.3 | 2.5 | 1.9 | 0.8 | 0.8 | 0.7 | 4.2 | 0.9 | ||
| Pre-adapted fish | ||||||||||||
| 6 d | M | 12.2 | 14.2 | 4.1 | 60.8 | 8.7 | 12.2 | 21.45 ***↑ | 1.85 ***↓ | 61.1 | 3.4 ***↓ | |
| SD | 0.9 | 0.3 | 0.3 | 2.3 | 0.8 | 0.9 | 1.3 | 0.2 | 3.7 | 0.2 | ||
| 20 d | M | 12.7 | 17.6 | 0.6 | 60.4 | 8.7 | 7 **↓ | 15.5 | 0.45 ***↓ | 70.15 *↑ | 6.9 | |
| SD | 1.4 | 1.1 | 0.02 | 3.1 | 0.8 | 1.2 | 2.1 | 0.03 | 3.9 | 0.9 | ||
| Non-adapted fish | ||||||||||||
| Hybrids | 6 d | M | 14.3 | 18.5 | 2.45 | 38.8 | 25.95 | 17.9 | 8.05 ***↓ | 0 ***↓ | 67 ***↑ | 7.05 ***↓ |
| SD | 1.3 | 0.9 | 0.09 | 2.1 | 3 | 1.9 | 0.7 | 0 | 7.3 | 0.9 | ||
| 20 d | M | 7.8 | 16.5 | 1.1 | 67.8 | 6.8 | 7.15 | 11.5 **↓ | 1.95 ***↑ | 51.7 | 27.7 ***↑ | |
| SD | 0.9 | 0.7 | 0.05 | 3.4 | 0.3 | 1.5 | 1.7 | 0.08 | 9.2 | 6.2 | ||
| Pre-adapted fish | ||||||||||||
| 6 d | M | 14.3 | 18.5 | 2.45 | 38.8 | 25.95 | 14.65 | 15.65 | 1.4 ***↓ | 63.15 **↑ | 5.15 ***↓ | |
| SD | 1.3 | 0.9 | 0.09 | 2.1 | 3 | 2.1 | 1.2 | 0.07 | 7.1 | 0.9 | ||
| 20 d | M | 5.85 | 52.5 | 2.9 | 38.75 | 0 | 9.3 **↑ | 18.25 ***↓ | 6.7 ***↑ | 39.8 | 13.8 ***↑ | |
| SD | 0.8 | 6.8 | 0.1 | 2.3 | 0 | 0.9 | 2.4 | 0.23 | 8.1 | 0.9 | ||
| BL 1 | PH 1 | MCB 1 | LB 1 | PC 1 | BL 1 | PH 1 | MCB 1 | LB 1 | PC 1 | |||
| Control | Experiment | |||||||||||
| Non-adapted Fish | ||||||||||||
| Hump-snout whitefish | 6 d | M | 5.8 | 36.6 | 11.85 | 38.3 | 7.45 | 2.95 **↓ | 23.05 ***↓ | 0 ***↓ | 74 *↑ | 0 **↓ |
| SD | 0.9 | 2.4 | 0.9 | 3.3 | 0.8 | 0.06 | 1.3 | 0 | 11 | 0 | ||
| 20 d | M | 6.5 | 41 | 10 | 35 | 7.5 | 6.55 | 33.15 | 20 ***↑ | 37.8 | 2.5 ***↓ | |
| SD | 0.8 | 3.6 | 0.9 | 1.6 | 0.8 | 0.7 | 2.2 | 1.1 | 2.4 | 0.06 | ||
| Pre-adapted fish | ||||||||||||
| 6 d | M | 5.8 | 36.6 | 11.85 | 38.3 | 7.45 | 9.8 ***↑ | 20.3 ***↓ | 2.25 ***↓ | 66.05 **↑ | 1.6 ***↓ | |
| SD | 0.9 | 2.4 | 0.9 | 3.3 | 0.8 | 0.7 | 0.9 | 0.01 | 9.2 | 0.06 | ||
| 20 d | M | 7.2 | 45 | 8 | 33 | 6.8 | 6.55 | 33.15 | 2.5 ***↓ | 37.8 | 2.5 ***↓ | |
| SD | 1.3 | 7.2 | 0.7 | 0 | 0 | 0.7 | 1.4 | 0.02 | 7.1 | 0.06 | ||
| Non-adapted fish | ||||||||||||
| Hybrids | 6 d | M | 4 | 31.9 | 3.25 | 60.1 | 0.75 | 2.75 | 51.5 ***↑ | 2.35 *↓ | 43.1 *↓ | 0.3 ***↓ |
| SD | 0.8 | 3.1 | 0.4 | 6.9 | 0.01 | 0.03 | 4.8 | 0.08 | 3.8 | 0 | ||
| 20 d | M | 0 | 47.7 | 2.15 | 46.8 | 4.35 | 0 | 63.6 | 1.2 ***↓ | 24.4 *↓ | 10.8 ***↑ | |
| SD | 0 | 7.4 | 0.07 | 6.9 | 0.9 | 0 | 7.5 | 0.08 | 5.3 | 0 | ||
| Pre-adapted fish | ||||||||||||
| 6 d | M | 4 | 18.5 | 31.9 | 60.1 | 0.75 | 7 | 58 ***↑ | 5***↓ | 25 ***↓ | 5 ***↑ | |
| SD | 0.8 | 0.9 | 3.1 | 6.9 | 0.01 | 1.8 | 14.5 | 0.9 | 6.3 | 0.7 | ||
| 20 d | M | 0 | 52.5 | 46.7 | 46.8 | 4.35 | 6.2 ***↑ | 51.6 | 5.1 **↓ | 33 ***↓ | 4.1 | |
| SD | 0 | 6.8 | 4.7 | 5.8 | 0.12 | 1.1 | 11.8 | 0.9 | 8.2 | 0.8 | ||
| Hump-snout whitefish | Non-adapted Fish | |||||
| Gills | Liver | |||||
| Control | Experiment | Control | Experiment | |||
| 6 d | M | 1.44 | 0.92 | 3.68 | 0.34 ***↓ | |
| SD | 0.72 | 0.5 | 0.79 | 0.22 | ||
| 20 d | M | 2.71 | 3 | 3.69 | 1.22 **↓ | |
| SD | 0.63 | 0.68 | 0.64 | 0.65 | ||
| Pre-adapted fish | ||||||
| Control | Experiment | Control | Experiment | |||
| 6 d | M | 1.44 | 1.45 | 3.68 | 0.77 **↓ | |
| SD | 0.72 | 0.53 | 0.79 | 0.51 | ||
| 20 d | M | 4.27 | 6.81 | 10.32 | 7.12 *↓ | |
| SD | 1.09 | 3.5 | 2.52 | 2.5 | ||
| Hybrids | Non-adapted fish | |||||
| Gills | Liver | |||||
| Control | Experiment | Control | Experiment | |||
| 6 d | M | 1.9 | 0.59 **↓ | 1.1 | 0.78 | |
| SD | 0.50 | 0.24 | 0.45 | 0.39 | ||
| 20 d | M | 2.99 | 2.86 | 1.5 | 1.73 | |
| SD | 0.64 | 0.69 | 0.69 | 0.44 | ||
| Pre-adapted fish | ||||||
| Control | Experiment | Control | Experiment | |||
| 6 d | M | 1.9 | 1.38 | 1.1 | 1.29 | |
| SD | 0.50 | 0.78 | 0.45 | 0.35 | ||
| 20 d | M | 12.42 | 6.58 *↓ | 5.5 | 7.98 | |
| SD | 2.11 | 2.97 | 1.65 | 4.53 | ||
| Hump-snout whitefish | Non-adapted Fish | |||||
| Gills | Liver | |||||
| Control | Experiment | Control | Experiment | |||
| 6 d | M | 1.11 | 1.22 | 1.00 | 1.10 | |
| SD | 0.14 | 0.14 | 0.15 | 0.13 | ||
| 20 d | M | 1.17 | 1.12 | 1.19 | 1.16 | |
| SD | 0.14 | 0.26 | 0.15 | 0.18 | ||
| Pre-adapted fish | ||||||
| Control | Experiment | Control | Experiment | |||
| 6 d | M | 1.11 | 1.34 | 1.00 | 0.94 | |
| SD | 0.14 | 0.27 | 0.15 | 0.08 | ||
| 20 d | M | 0.84 | 0.84 | 0.94 | 0.97 | |
| SD | 0.09 | 0.15 | 0.16 | 0.10 | ||
| Hybrids | Non-adapted fish | |||||
| Gills | Liver | |||||
| Control | Experiment | Control | Experiment | |||
| 6 d | M | 0.91 | 0.92 | 0.97 | 0.97 | |
| SD | 0.09 | 0.12 | 0.16 | 0.22 | ||
| 20 d | M | 1.16 | 0.85 | 1.16 | 1.24 | |
| SD | 0.24 | 0.22 *↓ | 0.24 | 0.09 | ||
| Pre-adapted fish | ||||||
| Control | Experiment | Control | Experiment | |||
| 6 d | M | 0.91 | 0.89 | 0.97 | 0.87 | |
| SD | 0.09 | 0.14 | 0.16 | 0.08 | ||
| 20 d | M | 1.09 | 1.04 | 0.84 | 0.85 | |
| SD | 0.25 | 1.14 | 1.14 | 0.22 | ||
References
- Simide, R.; Angelier, F.; Gaillard, S.; Stier, A. Age and Heat Stress as Determinants of Telomere Length in a Long-Lived Fish, the Siberian Sturgeon. Physiol. Biochem. Zool. 2016, 89, 441–447. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Pörtner, H.O. Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 132, 739–761. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Manzon, L.A.; Zak, M.A.; Agee, M.; Boreham, D.R.; Wilson, J.Y.; Somers, C.M.; Manzon, R.G. Thermal acclimation alters both basal heat shock protein gene expression and the heat shock response in juvenile lake whitefish (Coregonus clupeaformis). J. Therm. Biol. 2022, 104, 103185. [Google Scholar] [CrossRef] [PubMed]
- Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Houben, J.M.J.; Moonen, H.J.J.; van Schooten, F.J.; Hageman, G.J. Telomere length assessment: Biomarker of chronic oxidative stress? Free Radic. Biol. Med. 2008, 44, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, P. Organismal stress, telomeres and life histories. J. Exp. Biol. 2014, 217, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Nussey, D.H.; Baird, D.; Barrett, E.; Boner, W.; Fairlie, J.; Gemmell, N.; Hartmann, N.; Horn, T.; Haussmann, M.; Olsson, M.; et al. Measuring telomere length and telomere dynamics in evolutionary biology and ecology. Methods Ecol. Evol. 2014, 5, 299–310. [Google Scholar] [CrossRef]
- Bateson, M. Cumulative stress in research animals: Telomere attrition as a biomarker in a welfare context? Bioessays 2016, 38, 201–212. [Google Scholar] [CrossRef]
- Friesen, C.R.; Wapstra, E.; Olsson, M. Of telomeres and temperature: Measuring thermal effects on telomeres in ectothermic animals. Mol. Ecol. 2021, 31, 6069–6086. [Google Scholar] [CrossRef]
- Stier, A.; Hsu, B.-Y.; Cossin-Sevrin, N.; Garcin, N.; Ruuskanen, S. From climate warming to accelerated cellular ageing: An experimental study in wild birds. BioRxiv 2021. [Google Scholar] [CrossRef]
- Oikawa, S.; Kawanishi, S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999, 453, 365–368. [Google Scholar] [CrossRef]
- Victorelli, S.; Passos, J.F. Telomeres and cell senescence—Size matters not. EbioMedicine. 2017, 21, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Ding, X.; Wang, F.; Geng, X. Telomere and its role in the aging pathways: Telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology 2019, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Angelier, F.; Weimerskirch, H.; Barbraud, C.; Chastel, O. Is telomere length a molecular marker of individual quality? Insights from a long-lived bird. Funct. Ecol. 2019, 33, 1076–1087. [Google Scholar] [CrossRef]
- Chatelain, M.; Drobniak, S.M.; Szulkin, M. The association between stressors and telomeres in non-human vertebrates: A meta-analysis. Ecol. Lett. 2020, 23, 381–398. [Google Scholar] [CrossRef]
- Axelsson, J.; Wapstra, E.; Miller, E.; Rollings, N.; Olsson, M. Contrasting seasonal patterns of telomere dynamics in response to environmental conditions in the ectothermic sand lizard, Lacerta agilis. Sci. Rep. 2020, 10, 182. [Google Scholar] [CrossRef]
- Debes, P.V.; Visse, M.; Panda, B.; Ilmonen, P.; Vasemägi, A. Is telomere length a molecular marker of past thermal stress in wild fish? Mol. Ecol. 2016, 25, 5412–5424. [Google Scholar] [CrossRef] [PubMed]
- Dupoué, A.; Rutschmann, F.; Le Galliard, G.F.; Clobert, J.; Angelier, F.; Marciau, C.; Ruault, S.; Miles, D.; Meylan, S. Shorter telomeres precede population extinction in wild lizards. Sci. Rep. 2017, 7, 16976. [Google Scholar] [CrossRef] [PubMed]
- Foley, N.M.; Petit, E.J.; Brazier, T.; Finarelli, J.A.; Hughes, G.M.; Touzalin, F.; Puechmaille, S.J.; Teeling, E.C. Drivers of longitudinal telomere dynamics in the long-lived bat species, Myotis myotis. Mol. Ecol. 2020, 29, 2963–2977. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, E.; Leone, S.; Sgura, A. Oxidative stress induces telomere dysfunction and senescence by replication fork arrest. Cells 2019, 8, 19. [Google Scholar] [CrossRef]
- Reichert, S.; Stier, A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 2017, 13, 20170463. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. N. Y. Acad. Sci. 2004, 1019, 278–284. [Google Scholar] [CrossRef]
- Monaghan, P.; Ozanne, S.E. Somatic growth and telomere dynamics in vertebrates: Relationships, mechanisms and consequences. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160446. [Google Scholar] [CrossRef]
- Passos, J.F.; Saretzki, G.; Ahmed, S.; Nelson, G.; Richter, T.; Peters, H.; Wappler, I.; Birket, M.J.; Harold, G.; Schaeuble, K.; et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007, 5, e110. [Google Scholar] [CrossRef]
- Klapper, W.; Kühne, K.; Singh, K.K.; Heidorn, K.; Parwaresch, R.; Krupp, G. Longevity of lobsters is linked to ubiquitous telomerase expression. FEBS Lett. 1998, 439, 143–146. [Google Scholar] [CrossRef]
- Elmore, L.W.; Norris, M.W.; Sircar, S.; Bright, A.T.; McChesney, P.A.; Winn, R.N.; Holt, S.E. Upregulation of telomerase function during tissue regeneration. Exp. Biol. Med. 2008, 233, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Reichwald, K.; Lechel, A.; Graf, M.; Kirschner, J.; Dorn, A.; Terzibasi, E.; Wellner, J.; Platzer, M.; Rudolph, K.L.; et al. Telomeres shorten while Tert expression increases during ageing of the short-lived fish Nothobranchius furzeri. Mech. Ageing Dev. 2009, 130, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.C.; Glass, T.J.; Tolar, J.; Blazar, B.R. Expression of telomerase and telomere length are unaffected by either age or limb regeneration in Danio rerio. PLoS ONE 2009, 4, e7688. [Google Scholar] [CrossRef] [PubMed]
- Anchelin, M.; Murcia, L.; Alcaraz-Pérez, F.; García-Navarro, E.M.; Cayuela, M.L. Behaviour of telomere and telomerase during aging and regeneration in zebrafish. PLoS ONE 2011, 6, e16955. [Google Scholar] [CrossRef] [PubMed]
- McLennan, D.; Armstrong, J.D.; Stewart, D.C.; Mckelvey, S.; Boner, W.; Monaghan, P.; Metcalfe, N.B. Telomere elongation during early development is independent of environmental temperatures in Atlantic salmon. J. Exp. Biol. 2018, 4, jeb178616. [Google Scholar] [CrossRef] [PubMed]
- Rebl, A.; Verleih, M.; Nipkow, M.; Altmann, S.; Bochert, R.; Goldammer, T. Gradual and acute temperature rise induces crossing endocrine, metabolic, and immunological pathways in Maraena whitefish (Coregonus maraena). Front. Genet. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ficker, H.; Mazzucco, R.; Gassner, H.; Wanzenböck, J.; Dieckmann, U. Stocking strategies for a pre-alpine whitefish population under temperature stress. Ecol. Model. 2016, 320, 170–176. [Google Scholar] [CrossRef]
- Reed, K.M.; Izzo, L.K.; Binder, T.; Hayden, T.; Dembkowski, D.; Hansen, S.; Caroffino, D.; Vandergoot, C.H.; Krueger, C.C.; Isermann, D. Initial insights on the thermal ecology of lake whitefish in northwestern Lake Michigan. J. Great Lakes Res. 2023, 49, 757–766. [Google Scholar] [CrossRef]
- Sukhanova, L.V.; Smirnov, V.V.; Smirnova-Zalumi, N.S.; Belomestnykh, T.V.; Kirilchik, S.V. Molecular Phylogeography of Lake Baikal Coregonid Fishes. Advanc. Limnol. 2012, 63, 261–283. [Google Scholar] [CrossRef]
- Politov, D.V. Coregonids of Russia: Evolutionary genetic approach in assessment of current state of biodiversity. Fundam. Appl. Limnol. 2017, 189, 181–192. [Google Scholar] [CrossRef]
- Graham, C.F.; Boreham, D.R.; Manzon, R.G.; Wilson, J.Y.; Somers, C.M. Population structure of lake whitefish (Coregonus clupeaformis) from the Mississippian lineage in North America. FACETS 2022, 7, 853–874. [Google Scholar] [CrossRef]
- Bogdanov, B.E.; Knizhin, I.B. The Salmonid fishes of Lake Baikal and its adjacent water systems: Annotated checklist with new taxa description. Limnol. Freshw. Biol. 2022, 6, 1688–1704. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Online Database. 2023. Available online: https://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 16 October 2023).
- Bochkarev, N.A.; Zuykova, E.I.; Politov, D.V. Taxonomic status and origin of some ecological forms of whitefish Coregonus lavaretus (L.) from water bodies of Siberia. Russ. J. Genet. 2017, 53, 875–884. [Google Scholar] [CrossRef]
- Sukhanova, L.V.; Zhuravlyov, O.I.; Smirnov, V.V.; Smirnova-Zalumi, N.S.; Glyzina, O.J.; Kirilchik, S.V. Prospects for abundance recovery of Baikal lacustrine-riverine whitefish and its application. Fish. Farming Fish. 2011, 10, 26–28. [Google Scholar]
- Bochkarev, N.A.; Zuykova, E.I.; Solovyev, M.M. Hybridization between different lineages of pidschian-like whitefishes (Coregonus lavaretus pidschian, coregonidae) in water bodies of Southern Siberia. Ecol. Genet. 2017, 15, 31–43. [Google Scholar] [CrossRef]
- Savchenko, A.P.; Lopatin, V.N.; Zyryanov, A.N.; Smirnov, M.N.; Vyshegorodsev, A.A. Appendix to the Red Book of Krasnoyarsk Territory. Animals; Publishing House of KSU: Krasnoyarsk, Russia, 2004; 147p. [Google Scholar]
- Skryabin, A.G. Biology of Baikal Whitefish; Science: Moscow, Russia, 1969; 125p. [Google Scholar]
- Sessions, K.J.; Whitehouse, L.M.; Manzon, L.A.; Boreham, D.R.; Somers, C.M.; Wilson, J.Y.; Manzon, R.G. The heat shock response shows plasticity in embryonic lake whitefish (Coregonus clupeaformis) exposed to repeated thermal stress. J. Therm. Biol. 2021, 100, 103036. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.H.; Joncs, B.R.; Syrctlc, R.F. Temperature requircments for growth and survival of larval ciscos (Coregonus urtedii). J. Fish. Res. Board. Curi. 1971, 28, 924–927. [Google Scholar] [CrossRef]
- Golovanov, V.K. Temperature Criteria of the Life Activity of Freshwater Fish; Poligraf-Plyus: Moscow, Russia, 2013; 300p. [Google Scholar]
- Semenchenko, S.M.; Smeshlivaya, N.V. Resistance of larves and fry of Coregonus tugun and Stenodus leucichthys nelma to effects of high temperatures. Artif. Reprod. Aquat. Biol. Resour. 2022, 2, 235–239. [Google Scholar]
- Edsall, T.; Rottiers, D.V. Temperature tolerance of young-of-the-year lake whitefish, Coregonus clupeaformis. Women’s Stud. Q. 1976, 33, 177–180. [Google Scholar] [CrossRef]
- Stewart, T.R.; Vinson, M.R.; Stockwell, J.D. Effects of warming winter embryo incubation temperatures on larval cisco (Coregonus artedi) survival, growth, and critical thermal maximum. J. Great Lakes Res. 2022, 48, 1042–1049. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Anthropogenic temperature fluctuations and their effect on aquaculture: A comprehensive review. Aquac. Fish. 2022, 7, 223–243. [Google Scholar] [CrossRef]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef]
- Tang, S.; Liang, J.; Xiang, C.; Xiao, Y.; Wang, X.; Wu, J.; Li, G.; Cheke, R.A. A general model of hormesis in biological systems and its application to pest management. J. R. Soc. Interface 2019, 16, 20190468. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Calabrese, E.J. A global environmental health perspective and optimisation of stress. Sci. Total Environ. 2020, 20, 135263. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Whitehouse, L.; Danzmann, R.; Dixon, B. Effects of juvenile thermal preconditioning on the heat-shock, immune, and stress responses of rainbow trout upon a secondary thermal challenge. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 280, 111413. [Google Scholar] [CrossRef]
- Wedemeyer, G.A.; Barton, B.A.; McLeay, D.J. Stress and Acclimation. In Methods for Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 451–489. [Google Scholar]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Chernyaev, Z.A. Reproduction of Whitefish. In Ecological and Physiological Features of Reproduction and Development; Partnership of Scientific Publications KMK: Moscow, Russia, 2017; p. 329. [Google Scholar]
- Sapozhnikova, Y.P.; Koroleva, A.G.; Yakhnenko, V.M.; Tyagun, M.L.; Glyzina, O.Y.; Coffin, A.B.; Makarov, M.M.; Shagun, A.N.; Kulikov, V.A.; Gasarov, P.V.; et al. Molecular and cellular responses to long-term sound exposure in peled (Coregonus peled). J. Acoust. Soc. Am. 2020, 148, 895. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.P.; Koroleva, A.G.; Yakhnenko, V.M.; Khanaev, I.V.; Glyzina, O.Y.; Avezova, T.N.; Volkova, A.A.; Mushinskaya, A.V.; Tyagun, M.L.; Shagun, A.N.; et al. Sex associated effects of noise pollution in stone sculpin (Paracottus knerii) as a model object in the context of human-induced rapid environmental change. Biology 2021, 10, 1063. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, R.; Likens, G. Limnological Analysis; Springer: New York, NY, USA, 2000; pp. 69–80. [Google Scholar]
- Klimenkov, I.V.; Sudakov, N.P.; Pastukhov, M.V.; Kositsyn, N.S. The phenomenon of compensatory cell proliferation in olfactory epithelium in fish caused by prolonged exposure to natural odorants. Sci. Rep. 2020, 10, 8908. [Google Scholar] [CrossRef] [PubMed]
- Green, N.G.; Thomas, B.J. Numerical determination of the effective moments of non spherical particles. J. Phys. D Appl. Phys. 2007, 40, 78–85. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.R.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Voropaeva, E.; Maksimov, V.; Malyutina, S.; Bobak, M.; Voevoda, M. Effects of DNA quality on the measurement of telomere length. Mol. Biol. 2015, 49, 508–512. [Google Scholar] [CrossRef]
- Cawthon, R. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Yip, B.W.; Mok, H.O.; Peterson, D.R.; Wan, M.T.; Taniguchi, Y.; Ge, W.; Au, D.W. Sex-dependent telomere shortening, telomerase activity and oxidative damage in marine medaka Oryzias melastigma during aging. Mar. Pollut. Bull. 2017, 124, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Kim, N.; Wu, F. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res. 1997, 25, 2595–2597. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ellsaesser, C.F.; Clem, L.W. Hematological and immunological changes in channel catfish stressed by handling and transport. J. Fish. Biol. 2006, 28, 511–521. [Google Scholar] [CrossRef]
- Harris, J.; Bird, D.J. Modulation of the fish immune system by hormones. Vet. Immunol. Immunopathol. 2000, 77, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Kordinas, V.; Ioannidis, A.; Chatzipanagiotou, S. The Telomere/Telomerase System in Chronic Inflammatory Diseases. Cause or Effect? Genes 2016, 7, 60. [Google Scholar] [CrossRef]
- Pulsford, A.L.; Lemaire-Gony, S.; Tomlinson, M.; Collingwood, N.; Glynn, P.J. Effects of acute stress on the immune system of the dab, Limanda limanda. Comp. Biochem. Physiol. Part. C Pharmacol. Toxicol. Endocrinol. 1994, 109, 129–139. [Google Scholar] [CrossRef]
- Tang, L.; Cai, N.; Zhou, Y.; Liu, Y.; Hu, J.; Li, Y.; Yi, S.; Song, W.; Kang, L.; He, H. Acute stress induces an inflammation dominated by innate immunity represented by neutrophils in mice. Front. Immunol. 2022, 13, 1014296. [Google Scholar] [CrossRef] [PubMed]
- Thrall, M.A. Hematology of amphibians, Veterinary Hematology and Clinical Chemistry: Text and Clinical Case Presentations; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; 618p. [Google Scholar]
- Campbell, T.W. Clinical pathology. In Reptile Medicine and Surgery; Mader, D.R., Ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1996; pp. 248–257. [Google Scholar]
- Gordon, A.S. Some aspects of hormonal influences upon the leukocytes. Ann. N. Y. Acad. Sci. 1955, 59, 907–927. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Systematic Review. Psychosom. Med. 2018, 80, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.J.; Schulte, P.M. Mechanisms and costs of mitochondrial thermal acclimation in a eurythermal killifish (Fundulus heteroclitus). J. Exp. Biol. 2015, 218 Pt 11, 1621–1631. [Google Scholar] [CrossRef]
- Chung, D.J.; Schulte, P.M. Mitochondria and the thermal limits of ectotherms. J. Exp. Biol. 2020, 223 Pt 20, jeb227801. [Google Scholar] [CrossRef] [PubMed]
- Iftikar, F.I.; Hickey, A.J. Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS ONE 2013, 8, e64120. [Google Scholar] [CrossRef]
- Roussel, D.; Voituron, Y. Mitochondrial costs of being hot: Effects of acute thermal change on liver bioenergetics in toads (Bufo bufo). Front. Physiol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Pichaud, N.; Ekström, A.; Breton, S.; Sundström, F.; Rowinski, P.; Blier, P.U.; Sandblom, E. Adjustments of cardiac mitochondrial phenotype in a warmer thermal habitat is associated with oxidative stress in European perch, Perca fluviatilis. Sci. Rep. 2020, 10, 17697. [Google Scholar] [CrossRef] [PubMed]
- Hazel, J.R. Thermal adaptation in biological-membranes: Is homeoviscous adaptation the explanation. Annu. Rev. Physiol. 1995, 57, 19–42. [Google Scholar] [CrossRef]
- Liang, S.; Li, W.; Zhang, Y.; Tang, X.; He, J.; Bai, Y.; Li, D.; Wang, Y.; Chen, Q. Seasonal variation of metabolism in lizard Phrynocephalus vlangalii at high altitude. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2017, 203, 341–347. [Google Scholar] [CrossRef]
- Dahlhoff, E.; Somero, G.N. Effects of temperature on mitochondria from abalone (genus Haliotis): Adaptive plasticity and its limits. J. Exp. Biol. 1993, 185, 151–168. [Google Scholar] [CrossRef]
- O’Brien, J.; Dahlhoff, E.; Somero, G.N. Thermal resistance of mitochondrial respiration: Hydrophobic interactions of membrane proteins may limit thermal resistance. Physiol. Zool. 1991, 64, 1509–1526. [Google Scholar] [CrossRef]
- Martin-Aragon, S.; Villar, A.; Benedi, J. Age-dependent effects of esculetin on mood-related behavior and cognition from stressed mice are associated with restoring brain antioxidant status. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, C.F.; Reus, G.Z.; Ignacio, Z.M.; Abelaira, H.M.; Titus, S.E.; de Carvalho, P.; Arent, C.O.; Dos Santos, M.A.; Matias, B.I.; Martins, M.M.; et al. Enriched flavonoid fraction from Cecropia pachystachya Trécul leaves exerts antidepressant-like behavior and protects brain against oxidative stress in rats subjected to chronic mild stress. Neurotox. Res. 2016, 29, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef]
- Haendeler, J.; Dröse, S.; Büchner, N.; Jakob, S.; Altschmied, J.; Goy, C.; Spyridopoulos, I.; Zeiher, A.M.; Brandt, U.; Dimmeler, S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Reyes, A.; Green, P.; Caron, M.J.; Bonini, M.G.; Gordon, D.M.; Holt, I.J.; Santos, J.H. Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res. 2012, 40, 712–725. [Google Scholar] [CrossRef]
- Qian, W.; Kumar, N.; Roginskaya, V.; Fouquerel, E.; Opresko, P.L.; Shiva, S.; Watkins, S.C.; Kolodieznyi, D.; Bruchez, M.P.; Van Houten, B. Chemoptogenetic damage to mitochondria causes rapid telomere dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 18435–18444. [Google Scholar] [CrossRef] [PubMed]
- Saretzki, G. Telomerase, mitochondria and oxidative stress. Exp. Gerontol. 2009, 44, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, G. Peroxyl radicals: Inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free. Radic. Biol. Med. 2006, 41, 362–387. [Google Scholar] [CrossRef]
- Rollings, N.; Miller, E.; Olsson, M. Telomeric attrition with age and temperature in Eastern mosquitofish (Gambusia holbrooki). Naturwissenschaften 2014, 101, 241–244. [Google Scholar] [CrossRef]
- McLennan, D.; Armstrong, J.D.; Stewart, D.C.; Mckelvey, S.; Boner, W.; Monaghan, P.; Metcalfe, N.B. Interactions between parental traits, environmental harshness and growth rate in determining telomere length in wild juvenile salmon. Mol. Ecol. 2016, 25, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Noguera, J.C.; Velando, A. Carry-over effects of early thermal conditions on somatic and germline oxidative damages are mediated by compensatory growth in sticklebacks. J. Anim. Ecol. 2019, 88, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, N.B.; Olsson, M. How telomere dynamics are influenced by the balance between mitochondrial efficiency, ROS production and DNA damage. Mol. Ecol. 2021, 31, 6040–6052. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Lin, J.; Dhabhar, F.S.; Wolkowitz, O.M.; Puterman, E.; Karan, L.; Blackburn, E.H. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav. Immun. 2010, 24, 531–539. [Google Scholar] [CrossRef]
- Scott, G.R.; Johnston, I.A. Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proc. Natl. Acad. Sci. USA 2012, 109, 14247–14252. [Google Scholar] [CrossRef]
- Stewart, T.R.; Mäkinen, M.; Goulon, C.; Guillard, J.; Marjomäki, T.J.; Lasne, E.; Karjalainen, J.; Stockwell, J.D. Influence of warming temperatures on coregonine embryogenesis within and among species. Hydrobiologia 2021, 848, 4363–4385. [Google Scholar] [CrossRef]
- Lindström, J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999, 14, 343–348. [Google Scholar] [CrossRef]
- Bernatchez, L.; Renaut, S.; Whiteley, A.R.; Derome, N.; Jeukens, J.; Landry, L.; Lu, G.; Nolte, A.W.; Ostbye, K.; Rogers, S.M.; et al. On the origin of species: Insights from the ecological genomics of lake whitefish. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1783–1800. [Google Scholar] [CrossRef]
- De-Kayne, R.; Selz, O.M.; Marques, D.A.; Frei, D.; Seehausen, O.; Feulner, P. Genomic architecture of adaptive radiation and hybridization in Alpine whitefish. Nat. Commun. 2022, 13, 4479. [Google Scholar] [CrossRef]
- Brauer, C.J.; Sandoval-Castillo, J.; Gates, K.; Hammer, M.P.; Unmack, P.J.; Bernatchez, L.; Beheregaray, L.B. Natural hybridization reduces vulnerability to climate change. Nat. Clim. Chang. 2023, 13, 282–289. [Google Scholar] [CrossRef]

| Average Length, TL, cm | Weight, g | Number of Fish | Number of Samples | ||
|---|---|---|---|---|---|
| Hump-snout whitefish (control and experiment) | 6 d | 6.31 ± 0.43 | 3.80 ± 0.48 | 28 | 224 |
| 20 d | 7.57 ± 1.70 | 5.83 ± 1.60 | 28 | 224 | |
| Hybrids (control and experiment) | 6 d | 5.94 ± 0.75 | 2.32 ± 0.94 | 28 | 224 |
| 20 d | 6.60 ± 0.62 | 3.47 ± 0.92 | 28 | 224 | |
| Aquariums | Dissolved Oxygen, mg/L | Saturation O2, % | CO2, mg/L |
|---|---|---|---|
| Control, 17 °C | 9.9 | 107 | 2.4 |
| Training, 21 °C | 8.6 | 105 | 2.6 |
| Experimental, 26 °C | 7.8 | 102 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapozhnikova, Y.P.; Koroleva, A.G.; Yakhnenko, V.M.; Volkova, A.A.; Avezova, T.N.; Glyzina, O.Y.; Sakirko, M.V.; Tolstikova, L.I.; Sukhanova, L.V. Thermal Preconditioning Alters the Stability of Hump-Snout Whitefish (Coregonus fluviatilis) and Its Hybrid Form, Showing Potential for Aquaculture. Biology 2023, 12, 1348. https://doi.org/10.3390/biology12101348
Sapozhnikova YP, Koroleva AG, Yakhnenko VM, Volkova AA, Avezova TN, Glyzina OY, Sakirko MV, Tolstikova LI, Sukhanova LV. Thermal Preconditioning Alters the Stability of Hump-Snout Whitefish (Coregonus fluviatilis) and Its Hybrid Form, Showing Potential for Aquaculture. Biology. 2023; 12(10):1348. https://doi.org/10.3390/biology12101348
Chicago/Turabian StyleSapozhnikova, Yulia P., Anastasia G. Koroleva, Vera M. Yakhnenko, Aleksandra A. Volkova, Tatyana N. Avezova, Olga Yu. Glyzina, Mariya V. Sakirko, Lyubov I. Tolstikova, and Lyubov V. Sukhanova. 2023. "Thermal Preconditioning Alters the Stability of Hump-Snout Whitefish (Coregonus fluviatilis) and Its Hybrid Form, Showing Potential for Aquaculture" Biology 12, no. 10: 1348. https://doi.org/10.3390/biology12101348
APA StyleSapozhnikova, Y. P., Koroleva, A. G., Yakhnenko, V. M., Volkova, A. A., Avezova, T. N., Glyzina, O. Y., Sakirko, M. V., Tolstikova, L. I., & Sukhanova, L. V. (2023). Thermal Preconditioning Alters the Stability of Hump-Snout Whitefish (Coregonus fluviatilis) and Its Hybrid Form, Showing Potential for Aquaculture. Biology, 12(10), 1348. https://doi.org/10.3390/biology12101348