An Investigation on the Effects of Dietary Vitamin E on Juvenile Sea Urchin (Strongylocentrotus intermedius): Growth, Intestinal Microbiota, Immune Response, and Related Gene Expression
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Feeds and Feeding Procedures
2.2. Sampling
2.3. VE Content Analysis
2.4. Digestive Enzyme and Immune Enzyme Analysis
2.5. RNA Extraction and Real-Time Quantitative PCR
2.6. Microbial Diversity Analysis
2.7. Data Analysis
3. Results
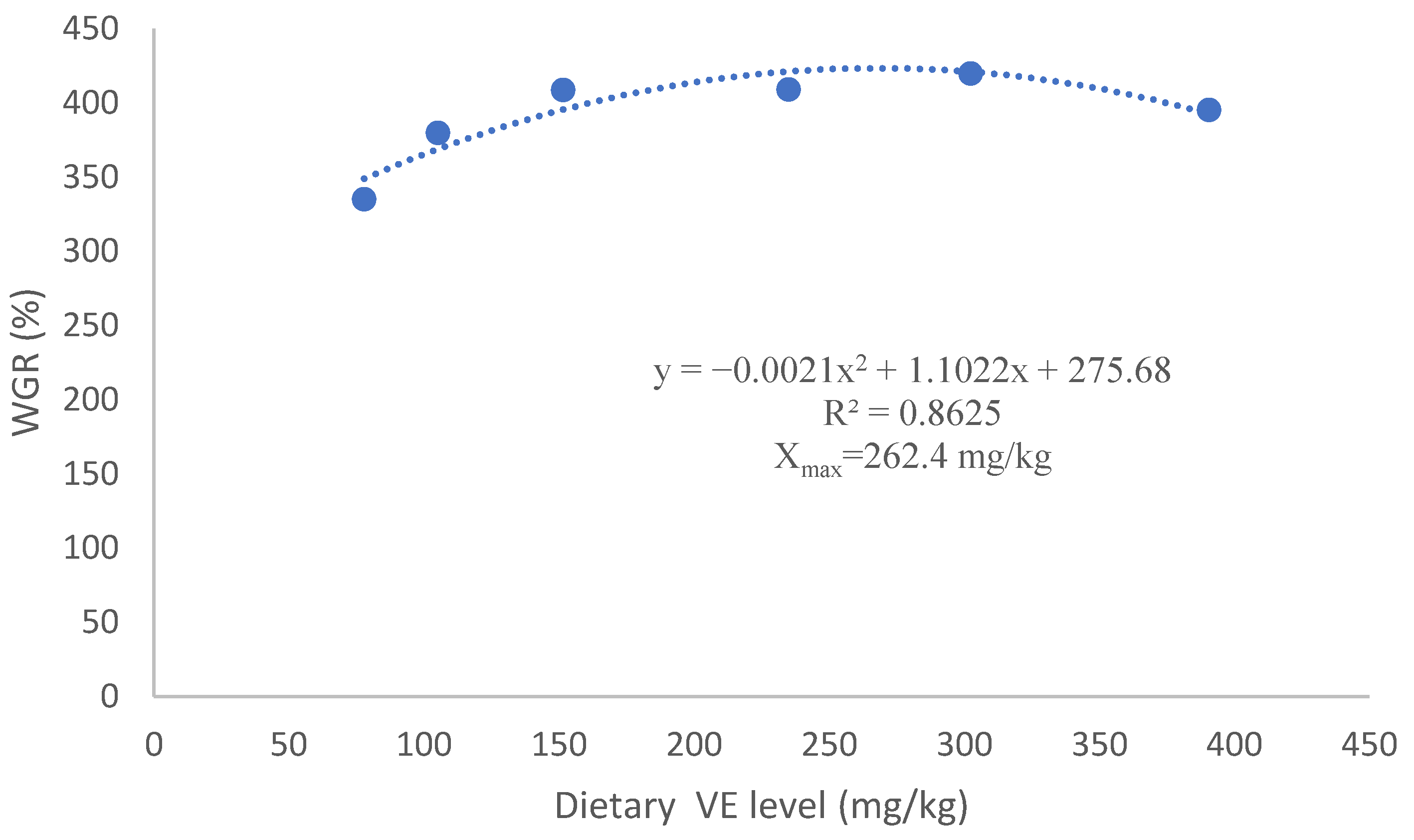
3.1. Growth Performance
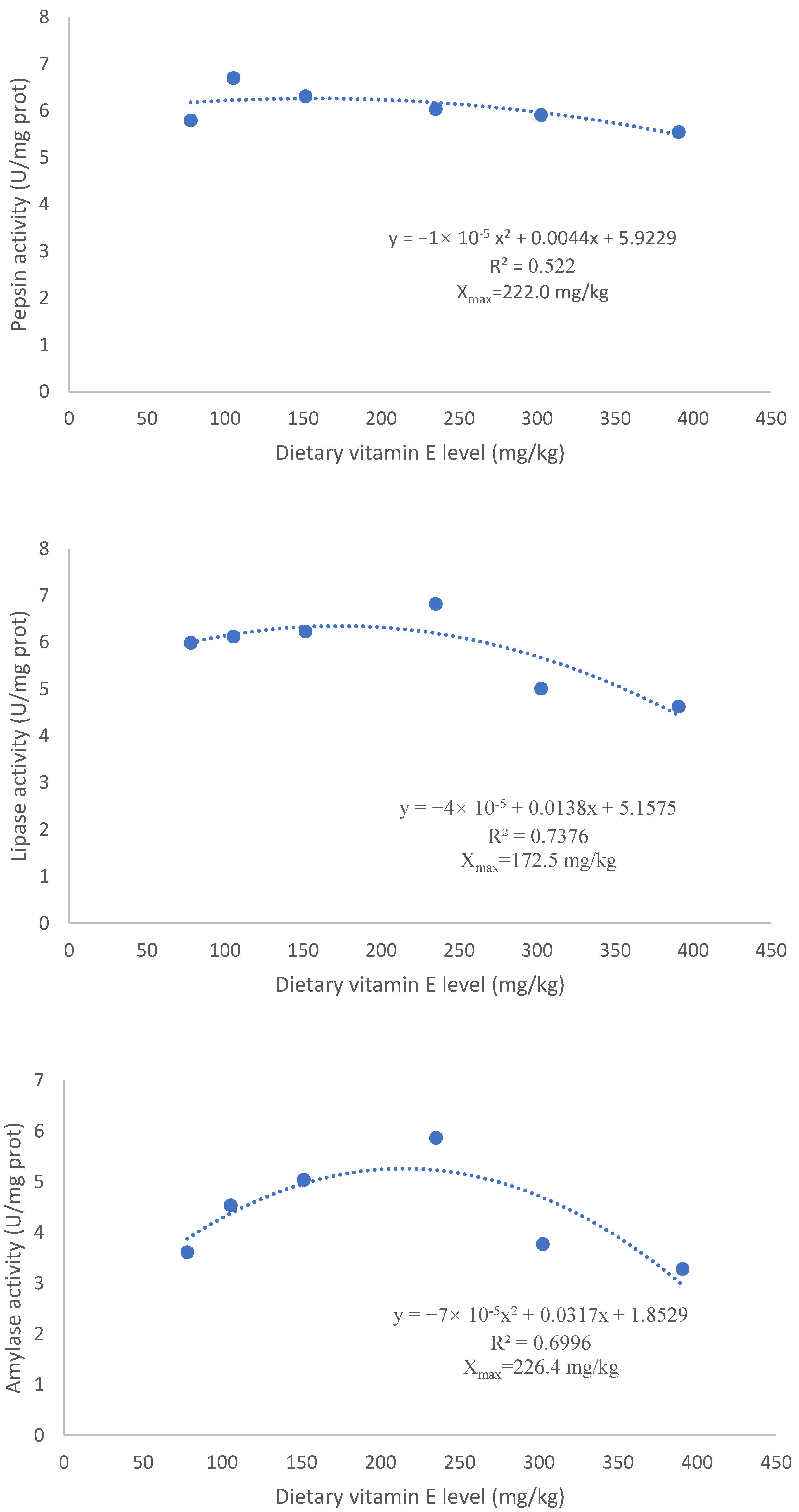
3.2. Digestive Enzyme Activities
3.3. Immune- and Antioxidation-Related Parameters
3.4. Immune-Related Gene Expression
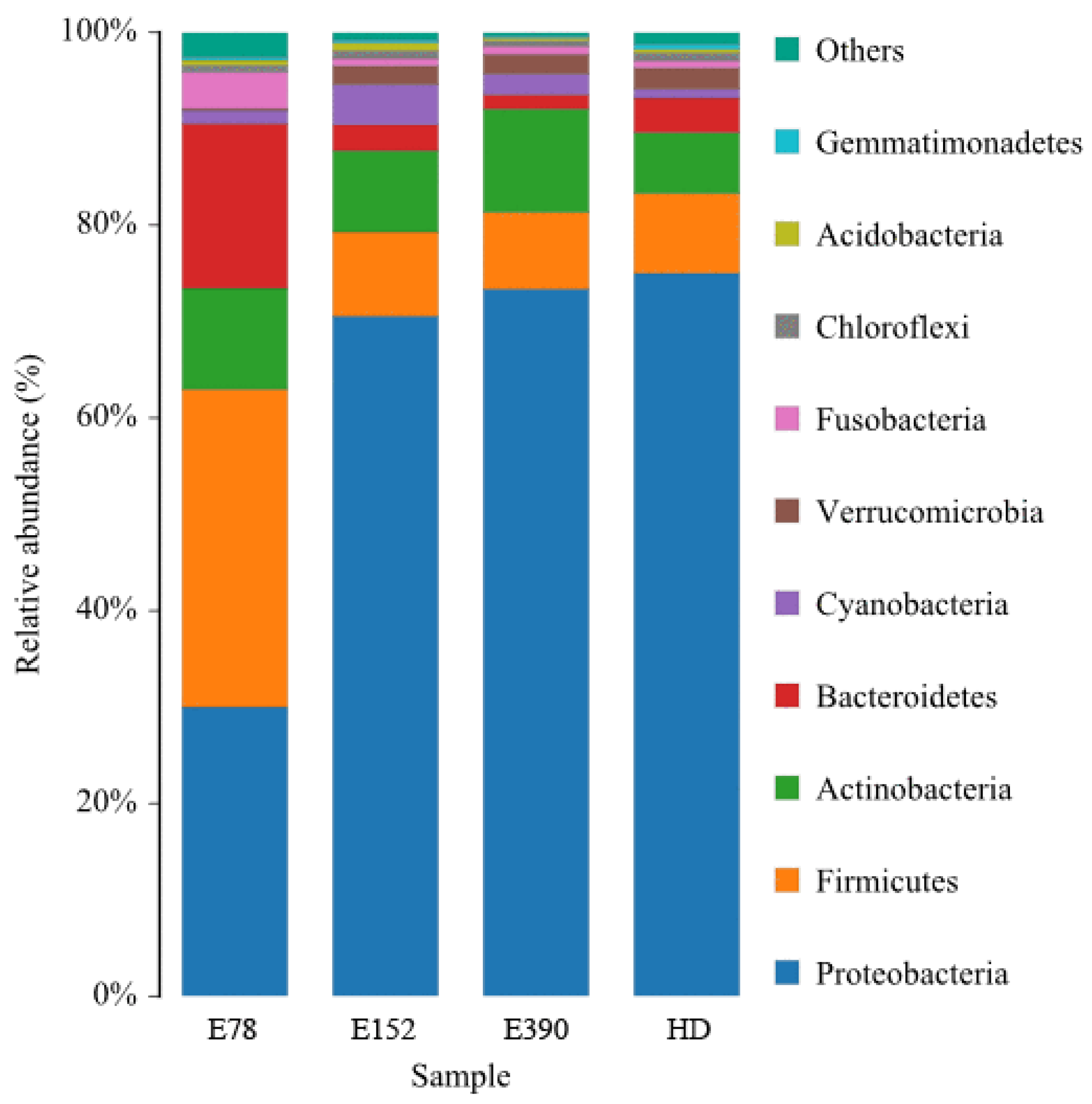
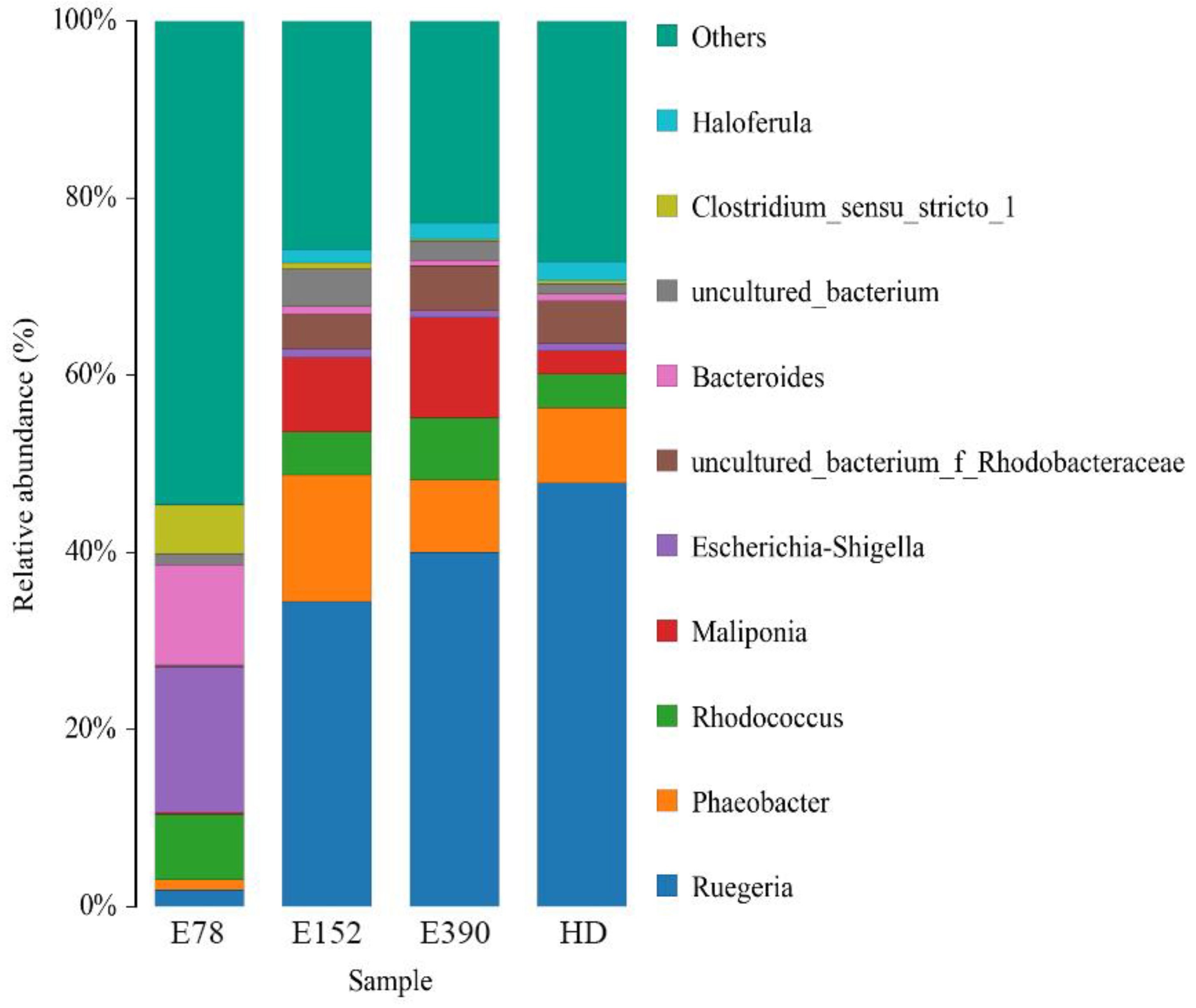
3.5. Intestinal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubilar, T.; Epherra, L.; Deias-Spreng, J. Ingestion, absorption and assimilation efficiencies, and production in the sea urchin Arbacia dufresnii fed a formulated feed. J. Shellfish Res. 2016, 35, 1083–1094. [Google Scholar] [CrossRef]
- Lourenço, S.; Valente, L.; Andrade, C. Meta-analysis on nutrition studies modulating sea urchin roe growth, colour and taste. Rev. Aquac. 2019, 11, 766–781. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, W.; Zhao, C.; Song, J. Estimates of heritabilities and genetic correlations for growth and gonad traits in the sea urchin Strongylocentrotus intermedius. Aquac. Res. 2012, 43, 271–280. [Google Scholar] [CrossRef]
- Zhan, Y.; Hu, W.; Zhang, W. The impact of CO2-driven ocean acidification on early development and calcification in the sea urchin Strongylocentrotus intermedius. Mar. Pollut. Bull. 2016, 112, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, C.; Liu, P. First report on tube feet differential pigmentation in the cultivated sea urchin Strongylocentrotus intermedius (Agassiz, 1863) and its relationship with growth performance: Tube feet differential pigmentation in sea urchins. Aquac. Res. 2010, 41, 706–708. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Leng, X. Transcriptome sequencing reveals phagocytosis as the main immune response in the pathogen-challenged sea urchin Strongylocentrotus intermedius. Fish. Shellfish Immunol. 2019, 94, 780–791. [Google Scholar] [CrossRef]
- Chang, Y.; Ding, J.; Xu, Y.; Li, D.; Zhang, W.; Li, L.; Song, J. SLAF-based high-density genetic map construction and QTL mapping for major economic traits in sea urchin Strongylocentrotus intermedius. Sci. Rep. 2018, 8, 820. [Google Scholar] [CrossRef]
- Lv, D.; Zhang, F.; Ding, J. Effects of dietary n-3 LC-PUFA on the growth performance, gonad development, fatty acid profile, transcription of related genes and intestinal microflora in adult sea urchin (Strongylocentrotus intermedius). Aquac. Res. 2020, 52, 1431–1441. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Wang, J.; Sun, Y. Requirement of vitamin E of growing sea cucumber Apostichopus japonicus Selenka. Aquac. Res. 2020, 51, 1284–1292. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, F.; Tang, L. Effects of dietary lipid sources on the growth, gonad development, nutritional and organoleptic quality, transcription of fatty acid synthesis related genes and antioxidant capacity during cold storage in adult sea urchin (Strongylocentrotus intermedius). Aquaculture 2022, 548, 737688. [Google Scholar] [CrossRef]
- Zuo, R.T.; Li, M.; Ding, J. Higher dietary arachidonic acid levels improved the growth performance, gonad development, nutritional value, and antioxidant enzyme activities of adult sea srchin (Strongylocentrotus intermedius). J. Ocean. Univ. China 2018, 17, 932–940. [Google Scholar] [CrossRef]
- Raposo, A.; Ferreira, S.; Ramos, R. Effect of three diets on the gametogenic development and fatty acid profile of Paracentrotus lividus (Lamarck, 1816) gonads. Aquac. Res. 2019, 50, 2023–2038. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Wang, L. Effects of dietary vitamin E levels on growth, antioxidant capacity and immune response of spotted seabass (Lateolabrax maculatus) reared at different water temperatures. Aquaculture 2023, 565, 739141. [Google Scholar] [CrossRef]
- Sau, S.K.; Paul, B.N.; Mohanta, K.N. Dietary vitamin E requirement, fish performance and carcass composition of rohu (Labeo rohita) fry. Aquaculture 2004, 240, 359–368. [Google Scholar] [CrossRef]
- Lin, Y.H.; Shiau, S.Y. Dietary vitamin E requirement of grouper, Epinephelus malabaricus, at two lipid levels, and their effects on immune responses. Aquaculture 2005, 248, 235–244. [Google Scholar] [CrossRef]
- Mehrad, B.; Sudagar, M. Dietary vitamin E requirement, fish performance and reproduction of guppy (Poecilia reticulata). Aquac. Aquar. Conserv. Legis. 2010, 3, 239–246. [Google Scholar]
- Faramarzi, M. Assessment study about effect of vitamin E (a-tocopheryl) on feeding performance, survival rate and reproductive performance of angel fish (Pterophyllum scalare). World J. Fish Mar. Sci. 2012, 4, 254–257. [Google Scholar]
- Niu, H.; Jia, Y.; Hu, P. Effect of dietary vitamin E on the growth performance and nonspecific immunity in sub-adult turbot (Scophthalmus maximus). Fish. Shellfish Immunol. 2014, 41, 501–506. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Li, X. Vitamin E requirement of sea cucumber (Apostichopus japonicus) and its’ effects on nonspecific immune responses. Aquac. Res. 2015, 46, 1628–1637. [Google Scholar] [CrossRef]
- Boglino, A.; Darias, M.J.; Estévez, A. The effect of dietary oxidized lipid levels on growth performance, antioxidant enzyme activities, intestinal lipid deposition and skeletogenesis in Senegalese sole (Solea senegalensis) larvae. Aquac. Nut. 2014, 20, 692–711. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory disease. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Yang, C.S.; Lee, H.M.; Lee, J.Y. Reactive oxygen species and p47 phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. J. Neuroinflammation 2007, 4, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, W.A.; Fakhruddin, S.; Jackson, K. Angiotensin II induces prostaglandin E2 production and oxidative stress in the renal cortex. FASEB J. 2016, 30, 1198.6. [Google Scholar] [CrossRef]
- Zhang, P.; Gan, Y.H. Prostaglandin E2 pregulated trigeminal ganglionic sodium channel 1.7 involving temporomandibular joint inflammatory pain in rats. Inflammation 2017, 40, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Sabyasachi, M.; Tanami, R.; Arunkumar, R. Characterization and identification of enzyme-producing bacteria isolated from the digestive tract of bata, Labeo bata. J. World Aouaculture Soc. 2010, 41, 369–377. [Google Scholar]
- Li, X.; Chi, Z.; Liu, Z. Phytase production by a marine yeast Kodamea ohmeri BG3. Appl. Biochem. Biotechnol. 2008, 149, 183–193. [Google Scholar] [CrossRef]
- Roy, T.; Mondal, S.; Ray, A.K. Phytase-producing bacteria in the digestive tracts of some freshwater fish. Aquac. Res. 2009, 40, 344–353. [Google Scholar] [CrossRef]
- Vigors, S.; John, V.D.; Kelly, A.K. The Effect of divergence in feed efficiency on the intestinal microbiota and the intestinal immune response in both unchallenged and lipopolysaccharide challenged ileal and colonic explants. PLoS ONE 2016, 11, e014814. [Google Scholar] [CrossRef]
- Attaya, A.; Wang, T.; Zou, J. Gene expression analysis of isolated salmonid GALT leucocytes in response to PAMPs and recombinant cytokines. Fish. Shellfish Immunol. 2018, 80, 426–436. [Google Scholar] [CrossRef]
- Huang, Y.W.; Ye, Y.T.; Cai, C.F. The study on damage of intestinal mucosa barrier structure with oxidized fish oil diets in Ctenopharyngodn idella. J. Fish. China 2015, 39, 1511–1520. [Google Scholar]
- Saada, H.N.; Renée, G.R.; Eltahawy, N.A. Lycopene protects the structure of the small intestine against gamma-radiation-induced oxidative stress. Phytother. Res. 2010, 24, S204–S208. [Google Scholar] [CrossRef] [PubMed]
- Heberling, C.A.; Dhurjati, P.S.; Sasser, M. Hypothesis for a systems connectivity model of autism spectrum disorder pathogenesis Links to gut bacteria, oxidative stress, and intestinal permeability. Med. Hypothesis 2013, 80, 264–270. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Yin, P. Flaxseed oil ameliorates alcoholic liver disease via anti-inflammation and modulating gut microbiota in mice. Lipids. Health Dis. 2017, 16, 44–54. [Google Scholar] [CrossRef]
- Arshadi, A.; Gharaei, A.; Mirdar Harijani, J. Effect of dietary vitamin E on reproductive performance and vitellogenin gene expression in broodstock of Litopenaeus vannamei. Iran. J. Fish. Sci. 2020, 19, 2475–2492. [Google Scholar]
- Li, M.; Zhang, F.; Ding, J. Effects of lipid sources on the growth performance, gonad development, fatty acid profile and transcription of related genes in juvenile sea urchin (Strongylocentrotus intermedius). Aquac. Nutr. 2020, 27, 28–38. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.C.; Bao, Z.M.; Dong, Y. MYP gene expressions at transcription level in different stages of gonad of sea urchin Strongylocentrotus intermedius and hybrids. Hereditas 2008, 30, 1453–1458. [Google Scholar]
- Chen, Y.D.; Liu, X.F.; Chang, Y.Q. Cloning of partial sequence and expression of NLR family in sea urchin Strongylocentrotus intermedius. J. Ocean Univ. China 2014, 29, 336–341. [Google Scholar]
- Wang, Y.N.; Yu, Z.; Liu, Y. cDNA cloning and expression analysis of the TLR gene in the shrimp and horse feces sea urchin. J. Ocean Univ. China 2014, 29, 329–335. [Google Scholar]
- Ding, J.; Chang, Y.Q.; Sun, W. Cloning and expression of immune-related genes from Strongylocentrotus intermedius. Progr. Mari. Sci. 2011, 29, 67–79. [Google Scholar]
- Bai, X.Q.; Pang, Z.G.; Zhang, W.J. Study on the expression of hsp70 and hsp90 genes in intermediate sea urchins induced by high temperature. Ocean Lake. Mar. 2015, 46, 1034–1039. [Google Scholar]
- Wang, Y.; Ding, J.; Liu, Y. Isolation of immune-relating 185/333-1 gene from sea urchin (Strongylocentrotus intermedius) and its expression analysis. J. Ocean Univ. China 2016, 15, 163–170. [Google Scholar] [CrossRef]
- Ji, N.J.; Yang, Y.F.; Ding, J. Cloning and expression analysis of the full length cDNA of the lysozyme gene from the sea urchin, Pseudomonas aeruginosa. J. Fish. Sci. China 2013, 20, 950–957. [Google Scholar]
- Di, W.; Heqiu, Y.; Gou, D.; Gong, P.; Ding, J.; Chang, Y.; Zuo, R. Effects of Supplementary Kelp Feeding on the Growth, Gonad Yield, and Nutritional and Organoleptic Quality of Subadult Sea Urchin (Strongylocentrotus intermedius) with Soya Lecithin Intake History. Aquac. Nutr. 2023, 16, 8894923. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, K.; Liang, X. Effects of dietary vitamin E on growth performance as well as intestinal structure and function of channel catfish (Ictalurus punctatus, Rafinesque1818). Exp. Ther. Med. 2017, 14, 5703–5710. [Google Scholar]
- Erdogan, M.; Arslan, T. Effects of vitamin E on growth and reproductive performance of pindani (Pseudotropheus socolofi Johnson, 1974). Aquaculture 2019, 509, 59–66. [Google Scholar] [CrossRef]
- Saheli, M.; Rajabi Islami, H.; Mohseni, M. Effects of dietary vitamin E on growth performance, body composition, antioxidant capacity, and some immune responses in Caspian trout (Salmo caspius). Aquac. Rep. 2021, 21, 100857. [Google Scholar] [CrossRef]
- Ahmed, S.A.A.; Ibrahim, R.E.; Farroh, K.Y. Chitosan vitamin E nanocomposite ameliorates the growth, redox, and immune status of Nile tilapia (Oreochromis niloticus) reared under different stocking densities. Aquaculture 2021, 541, 736804. [Google Scholar] [CrossRef]
- Bae, J.Y.; Park, G.H.; Yoo, K.Y. Evaluation of optimum dietary vitamin E requirements using DL-α-tocopheryl acetate in the juvenile eel, Anguilla japonica. J. Appl. Ichthyol. 2013, 29, 213–217. [Google Scholar] [CrossRef]
- Muchlisin, Z.A.; Arisa, A.A.; Muhammadar, A.A. Growth performance and feed utilization of keureling (Tor tambra) fingerlings fed a formulated diet with different doses of vitamin E (alpha-tocopherol). Arch. Polish. Fish. 2016, 24, 47–52. [Google Scholar] [CrossRef]
- Yu, L.J.; Wen, H.; Jiang, M. Effects of ferulic acid on growth performance, immunity and antioxidant status in genetically improved farmed tilapia (Oreochromis niloticus) fed oxidized fish oil. Aquac. Nut. 2020, 26, 1431–1442. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, Q.; Dong, S. Effects of supplementary selenium and vitamin E on the growth performance, antioxidant anzyme activity, and gene expression of sea ucumber Apostichopus japonicus. Biol. Trace Elem. Res. 2021, 199, 4820–4831. [Google Scholar] [CrossRef]
- Wang, W.; Ishikawa, M.; Koshio, S. Effects of dietary astaxanthin and vitamin E and their interactions on the growth performance, pigmentation, digestive enzyme activity of kuruma shrimp (Marsupenaeus japonicus). Aquac. Res. 2019, 50, 1186–1197. [Google Scholar] [CrossRef]
- Schlosser, S.C.; Lupatsch, I.; Lawrence, J.M. Protein and energy digestibility and gonad development of the European sea urchin Paracentrotus lividus (Lamarck) fed algal and prepared diets during spring and fall. Aquac. Res. 2005, 36, 972–982. [Google Scholar] [CrossRef]
- Zuo, R.; Hou, S.; Wu, F.; Song, J. Higher dietary protein increases growth performance, anti-oxidative enzymes activity and transcription of heat shock protein 70 in the juvenile sea urchin (Strongylocentrotus intermedius) under a heat stress. Aquac. Fish. 2017, 2, 18–23. [Google Scholar] [CrossRef]
- Gibbs, V.K.; Powell, M.L.; Hammer, H.S.; Jones, W.T.; Watts, S.A.; Lawrence, A.L. Dietary phospholipids affect growth and production of juvenile sea urchin Lytechinus variegatus. Aquaculture 2009, 292, 95–103. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, P.; Li, Y. Effects of dietary lipid levels on growth and gonad development of Onychostoma macrolepis broodfish. Fishes 2022, 7, 291. [Google Scholar] [CrossRef]
- Leng, X.; Zhou, H.; Tan, Q. Integrated metabolomic and transcriptomic analyses suggest that high dietary lipid levels facilitate ovary development through the enhanced arachidonic acid metabolism, cholesterol biosynthesis and steroid hormone synthesis in Chinese sturgeon (Acipenser sinensis). Br. J. Nutr. 2019, 122, 1230–1241. [Google Scholar]
- Tao, Y.; Pan, Y.; Wang, Q. Vitamin E ameliorates impaired ovarian development, oxidative stress, and disrupted lipid metabolism in Oreochromis niloticus fed with a diet containing olive oil instead of fish oil. Antioxidants 2023, 12, 1524. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.I.; Wang, T.; Jibril Habib, Y.; Wang, J.; Fayed, W.M.; Zhang, Z. The combined effect of vitamin E, arachidonic acid, Haemtococcus pluvialis, nucleotides and yeast extract on growth and ovarian development of crayfish ( Cherax quadricarinatus) by the orthogonal array design. Aquac. Nutr. 2020, 26, 2007–2022. [Google Scholar] [CrossRef]
- Hermes-Lima, M. Oxygen in biology and biochemistry: Role of free radicals. Funct. Metab. Regul. Adapt. 2004, 30, 319–368. [Google Scholar]
- Palomero, T.; Lim, W.K.; Odom, D.T.; Sulis, M.L.; Real, P.J.; Margolin, A.; Ferrando, A.A. Notch1 directly regulates c-myc and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc. Natl. Acad. Sci. USA 2006, 103, 18261–18266. [Google Scholar] [CrossRef]
- Luschka, V.I. Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar]
- Salazar-Coria, L.; Rocha-Gomez, M.A.; Matadamas-Martinez, F.; Yepez-Mulia, L.; Vega-Lopez, A. Proteomic analysis of oxidized proteins in the brain and liver of the nile tilapia (Oreochromis niloticus) exposed to a water-accommodated fraction of maya crude oil. Ecotoxicol. Environ. Saf. 2019, 171, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Mourente, G.; Bell, J.G.; Tocher, D.R. Does dietary tocopherol level affect fatty acid metabolism in fish. Fish. Physiol. Biochem. 2007, 33, 269–280. [Google Scholar] [CrossRef]
- Wang, L.; Ma, B.; Chen, D. Effect of dietary level of vitamin E on growth performance, antioxidant ability, and resistance to Vibrio alginolyticus challenge in yellow drum Nibea albiflora. Aquaculture 2019, 507, 119–125. [Google Scholar] [CrossRef]
- Huang, Q.C.; Zhang, S.; Du, T. Effects of dietary vitamin E on growth, immunity and oxidation resistance related to the Nrf2/Keap1 signalling pathway in juvenile Sillago sihama. Anim. Feed Sci. Technol. 2020, 262, 114403. [Google Scholar] [CrossRef]
- Abd El-Gawad, E.A.; Abd El-latif, A.M.; Amin, A.A.; Abd-El-Azem, M.A. Effect of dietary fructooligosaccharide on bacterial Infection, oxidative stress and histopathological alterations in Nile tilapia (Oreochromis niloticus). Glob. Vet. 2015, 15, 339–350. [Google Scholar]
- Zuo, R.; Mai, K.; Xu, W. Dietary ALA, but not LNA, increase growth, reduce inflammatory processes, and increase anti-oxidant capacity in the marine finfish larimichthys crocea: Dietary ALA, but not LNA, increase growth, reduce inflammatory processes, and increase anti-oxidant capacity in the large yellow croaker. Lipids 2015, 50, 149–163. [Google Scholar]
- Garófolo, A.; Petrilli, A.S. Omega-3 and 6 fatty acids balance in inflammatory response in patients with cancer and cachexia. Rev. Nutr. Camp. 2006, 19, 611–621. [Google Scholar] [CrossRef]
- Ruan, K.H.; Cervantes, V.; So, S.P. Engineering of a novel hybrid enzyme: An anti-inflammatory drug target with triple catalytic activities directly converting arachidonic acid into the inflammatory prostaglandin E2. Protein Eng. Des. Sel. 2009, 22, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Y.; Ma, T.; Winterthun, S. β-oxidation modulates metabolic competition between eicosapentaenoic acid and arachidonic acid regulating prostaglandin E2 synthesis in rat hepatocytes—Kupffer cells. BBA-Mol. Cell. Biol. Liplids 2010, 1801, 526–536. [Google Scholar] [CrossRef]
- Song, C.Y.; Liu, B.; Xu, P. Oxidized fish oil injury stress in Megalobrama amblycephala: Evaluated by growth, intestinal physiology, and transcriptome-based PI3K-Akt/NF-κB/TCR inflammatory signaling. Fish. Shellfish Immunol. 2018, 81, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.H.; Fang, Y.; Lin, H. Chemical characters and antioxidative properties of sulfated polysaccharides from laminaria japonica. J. Appl. Phycol. 2001, 13, 67–70. [Google Scholar] [CrossRef]
- Mei, C.H.; Zhou, S.C.; Zhu, L. Antitumor effects of laminaria extract fucoxanthin on lung cancer. Mar. Drugs 2017, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.H.; Gu, Y.; Zhang, C.X. Metabolomic approach for characterization of polyphenolic compounds in laminaria japonica, undaria pinnatifida, sargassum fusiforme and ascophyllum nodosum. Foods 2021, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Porsby, C.H.; Nielsen, K.F.; Gram, L. Phaeobacter and Ruegeria species of the Roseobacter clade colonize separate niches in a danish turbot (Scophthalmus maximus)-rearing farm and antagonize Vibrio anguillarum under different growth conditions. Appl. Environ. Microbiol. 2008, 74, 7356–7364. [Google Scholar] [CrossRef]
- Hoyles LM Cartney, A.L. What do we mean when we refer to Bacteroidetes populations in the human gastrointestinal microbiota? FEMS Microbiol. Lett. 2009, 299, 175–183. [Google Scholar] [CrossRef]
- Liu, G.; Luo, X.; Zhao, X. Gut microbiota correlates with fiber and apparent nutrients digestion in goose. Poult. Sci. 2018, 97, 3899–3909. [Google Scholar] [CrossRef]
- Nardone, G.; Compare, D.; Rocco, A. A microbiota-centric view of diseases of the upper gastrointestinal tract. Lancet Gastroenterol. Hepatol. 2017, 2, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Neef, A.; Castillejo, G. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 2014, 64, 406–417. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (%) | Experimental Feeds | |||||
|---|---|---|---|---|---|---|
| E78 | E105 | E152 | E235 | E302 | E390 | |
| Fish meal | 9.00 | 9.00 | 9.00 | 9.00 | 9.00 | 9.00 |
| Soybean meal a | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 |
| Seaweed meal | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Ruppiaceae | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Wheat bran b | 11.20 | 11.20 | 11.20 | 11.20 | 11.20 | 11.20 |
| Wheat meal c | 29.50 | 29.49 | 29.48 | 29.47 | 29.46 | 29.45 |
| Shell powder | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Gelatin | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Vitamin E acetate | 0.00 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 |
| Mineral premix d | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Calcium propionate | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Betaine | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Glycine | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Fish oil | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Soybean lecithin | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Approximate analysis | ||||||
| Crude lipid | 12.24 | 12.22 | 12.22 | 12.21 | 12.20 | 12.23 |
| Crude protein | 25.79 | 25.84 | 25.87 | 25.84 | 25.85 | 25.82 |
| Vitamin E (mg/kg) | 77.86 | 105.27 | 151.55 | 235.01 | 302.43 | 390.67 |
| Gene | Sequence (5′–3′) | Reference |
|---|---|---|
| 18S | F: GTTCGAAGGCGATCAGATAC R: CTGTCAATCCTCACTGTGTC | Zhou et al. [37]. |
| COX-2 | F: GAGGTGGATAACCGATTGA R: AGCATTGCCCATAGAACAG | MH516324 |
| NLR-6 | F: GTTCAGGGAGAGGCAGG R: CATGGGCGAGTGGTCAC | Chen et al. [38] |
| TLR | F: TCAAATGGAGCCCGTATGTAGAG R: CTAATGTCCCCTGCTCTGCCA | Wang et al. [39] |
| GPX | F: CGAGTTTGAGAAGCGTGGTG R: GGATCAGCTATGATTGGGTATGG | Ding et al. [40] |
| GST | F: CTCGGAGATTCGCTCACCA R: GCTGGCTGGAGAAATGAACAA | Ding et al. [41] |
| HSP70 | F: ACACTCATCTCGGAGGAG R: CTTTCTTATGCTTTCGCTTGA | Bai et al. [41] |
| 185/333-1 | F: GCTCTTGCTATCTCGGCTCAC R: AAGCGACCTTGTCCTCTCTCTCT | Wang et al. [42] |
| LYZ | F: GAGACGGTACAGGGCTACA R: CGGGCAAAATCCTCACAAG | Ji et al. [43] |
| AIF-1 | F: TCGAACGTGCAAGGTGGCAAG R: CGTCATTGTCATCGAGGTCTCCAC | MH516330 |
| TNF-α | F: GCTGTAACGGCGTTCGTCTCC R: TGGTGTACTTGTGCTGGTTGTTGG | MH516331 |
| Index | Dietary Treatments | ||||||
|---|---|---|---|---|---|---|---|
| E78 | E105 | E152 | E235 | E302 | E390 | HD | |
| Wi (g) | 3.66 ± 0.09 | 3.66 ± 0.25 | 3.97 ± 0.18 | 3.56 ± 0.15 | 3.90 ± 0.60 | 3.53 ± 0.09 | 3.98 ± 0.27 |
| Wf (g) | 19.83 ± 0.80 b | 20.86 ± 1.35 ab | 21.57 ± 1.22 ab | 20.10 ± 1.27 ab | 21.78 ± 1.21 ab | 22.18 ± 1.10 ab | 24.17 ± 1.87 a |
| WGR (%) | 335.1 ± 26.7 b | 380.1 ± 38.6 ab | 408.8 ± 38.0 ab | 409.1 ± 35.7 ab | 419.9 ± 34.0 ab | 395.6 ± 14.6 ab | 475.1 ± 54.9 a |
| GM (g) | 2.97 ± 0.11 ab | 3.17 ± 0.37 a | 3.28 ± 0.20 a | 3.02 ± 0.25 ab | 3.26 ± 0.36 a | 2.64 ± 0.72 ab | 2.24 ± 0.25 b |
| GSI (%) | 15.21 ± 0.36 a | 14.42 ± 0.90 a | 14.61 ± 0.61 a | 15.36 ± 0.37 a | 15.83 ± 0.56 a | 14.41 ± 0.26 a | 8.67 ± 0.56 b |
| DM (g) | 1.15 ± 0.08 b | 1.39 ± 0.12 b | 1.21 ± 0.13 b | 1.11 ± 0.12 b | 1.32 ± 0.17 b | 1.12 ± 0.09 b | 1.81 ± 0.22 a |
| DTI (%) | 5.80 ± 0.34 bc | 6.70 ± 0.18 a | 6.31 ± 0.33 bc | 6.04 ± 0.44 bc | 5.91 ± 0.30 bc | 5.55 ± 0.71 c | 7.62 ± 0.44 a |
| Index | Dietary Treatments | ||||||
|---|---|---|---|---|---|---|---|
| E78 | E105 | E152 | E235 | E302 | E390 | HD | |
| Pepsin (U/mg prot) | 6.42 ± 0.33 a | 6.75 ± 0.20 a | 5.90 ± 0.21 a | 7.00 ± 0.33 a | 7.42 ± 0.91 a | 3.91 ± 0.55 b | 7.13 ± 0.57 a |
| Lipase (U/g prot) | 5.99 ± 0.34 abc | 6.12 ± 0.19 abc | 6.23 ± 0.37 ab | 6.82 ± 0.20 a | 5.01 ± 0.44 cd | 4.63 ± 0.64 d | 5.58 ± 0.25 bcd |
| Amylase (U/mg prot) | 3.61 ± 0.50 bc | 4.54 ± 0.97 ab | 5.04 ± 0.59 ab | 5.87 ± 0.52 a | 3.77 ± 0.49 bc | 3.28 ± 0.23 bc | 2.72 ± 0.60 c |
| Cellulase (U/mg prot) | 18.83 ± 2.78 ab | 23.31 ± 0.48 a | 15.74 ± 0.96 bc | 15.79 ± 2.46 bc | 15.23 ± 1.84 bc | 11.35 ± 1.33 c | 22.13 ± 2.47 a |
| Index | Dietary Treatments | ||||||
|---|---|---|---|---|---|---|---|
| E78 | E105 | E152 | E235 | E302 | E390 | HD | |
| LYZ (U/mL) | 168.54 ± 29.00 a | 129.59 ± 16.93 a | 112.35 ± 1.98 ab | 163.29 ± 23.94 a | 154.49 ± 9.05 a | 155.06 ± 31.19 a | 57.98 ± 7.96 c |
| AKP (U/100 mL) | 1.27 ± 0.24 a | 0.81 ± 0.12 bc | 0.64 ± 0.12 bc | 0.99 ± 0.11 ab | 0.65 ± 0.31 bc | 0.66 ± 0.08 bc | 0.41 ± 0.03 c |
| ACP (U/100 mL) | 3.06 ± 0.36 a | 1.52 ± 0.18 bc | 1.07 ± 0.02 bc | 2.29 ± 0.13 ab | 1.96 ± 0.44 ab | 1.99 ± 0.62 ab | 0.64 ± 0.05 d |
| SOD (U/mL) | 57.27 ± 2.81 a | 47.96 ± 2.50 b | 47.46 ± 2.16 b | 53.85 ± 1.79 ab | 48.27 ± 1.11 b | 48.40 ± 2.11 b | 53.45 ± 0.50 ab |
| CAT (U/mL) | 0.80 ± 0.03 a | 0.51 ± 0.08 bc | 0.36 ± 0.00 d | 0.39 ± 0.05 cd | 0.62 ± 0.03 b | 0.55 ± 0.02 b | 0.63 ± 0.05 ab |
| GST (U/mL) | 12.70 ± 0.28 a | 8.61 ± 1.72 b | 3.12 ± 0.18 c | 7.33 ± 0.64 b | 7.64 ± 0.37 b | 8.85 ± 0.58 b | 11.92 ± 0.38 a |
| GPX (U/mL) | 24.74 ± 1.47 a | 19.71 ± 1.23 b | 14.89 ± 1.15 c | 21.60 ± 0.76 ab | 21.71 ± 0.39 ab | 22.79 ± 0.58 ab | 23.79 ± 0.52 a |
| MDA (nmol/mL) | 0.64 ± 0.09 b | 0.47 ± 0.02 bc | 0.61 ± 0.04 b | 0.90 ± 0.06 a | 0.53 ± 0.03 b | 0.55 ± 0.03 b | 0.33 ± 0.07 c |
| Phylum | Dietary Treatments | |||
|---|---|---|---|---|
| E78 | E152 | E390 | HD | |
| Proteobacteria | 0.31 ± 0.01 b | 0.71 ± 0.03 a | 0.71 ± 0.05 a | 0.75 ± 0.01 a |
| Firmicutes | 0.32 ± 0.01 a | 0.08 ± 0.02 b | 0.07 ± 0.00 b | 0.09 ± 0.02 b |
| Actinobacteria | 0.10 ± 0.03 ab | 0.08 ± 0.02 ab | 0.12 ± 0.03 a | 0.06 ± 0.00 b |
| Bacteroidetes | 0.18 ± 0.01 a | 0.03 ± 0.01 b | 0.02 ± 0.00 b | 0.04 ± 0.00 b |
| Firmicutes/Bacteroidetes | 1.88 ± 0.18 b | 3.37 ± 0.20 ab | 4.14 ± 0.81 a | 2.48 ± 0.51 ab |
| Genus | Dietary Treatments | |||
|---|---|---|---|---|
| E78 | E152 | E390 | HD | |
| Ruegeria | 0.02 ± 0.00 c | 0.35 ± 0.03 b | 0.42 ± 0.01 a | 0.47 ± 0.01 a |
| Phaeobacter | 0.01 ± 0.00 c | 0.14 ± 0.03 a | 0.08 ± 0.01 b | 0.09 ± 0.01 b |
| Rhodococcus | 0.07 ± 0.01 a | 0.05 ± 0.00 b | 0.07 ± 0.00 a | 0.04 ± 0.00 b |
| Maliponia | 0.00 ± 0.00 b | 0.08 ± 0.02 a | 0.12 ± 0.01 a | 0.02 ± 0.01 b |
| Escherichia-Shigella | 0.17 ± 0.01 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| Bacteroides | 0.12 ± 0.01 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| Clostridium sensu stricto1 | 0.06 ± 0.00 a | 0.01 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Gou, D.; Gong, P.; Di, W.; Wang, L.; Ding, J.; Chang, Y.; Zuo, R. An Investigation on the Effects of Dietary Vitamin E on Juvenile Sea Urchin (Strongylocentrotus intermedius): Growth, Intestinal Microbiota, Immune Response, and Related Gene Expression. Biology 2023, 12, 1523. https://doi.org/10.3390/biology12121523
Li M, Gou D, Gong P, Di W, Wang L, Ding J, Chang Y, Zuo R. An Investigation on the Effects of Dietary Vitamin E on Juvenile Sea Urchin (Strongylocentrotus intermedius): Growth, Intestinal Microbiota, Immune Response, and Related Gene Expression. Biology. 2023; 12(12):1523. https://doi.org/10.3390/biology12121523
Chicago/Turabian StyleLi, Min, Dan Gou, Panke Gong, Weixiao Di, Lina Wang, Jun Ding, Yaqing Chang, and Rantao Zuo. 2023. "An Investigation on the Effects of Dietary Vitamin E on Juvenile Sea Urchin (Strongylocentrotus intermedius): Growth, Intestinal Microbiota, Immune Response, and Related Gene Expression" Biology 12, no. 12: 1523. https://doi.org/10.3390/biology12121523
APA StyleLi, M., Gou, D., Gong, P., Di, W., Wang, L., Ding, J., Chang, Y., & Zuo, R. (2023). An Investigation on the Effects of Dietary Vitamin E on Juvenile Sea Urchin (Strongylocentrotus intermedius): Growth, Intestinal Microbiota, Immune Response, and Related Gene Expression. Biology, 12(12), 1523. https://doi.org/10.3390/biology12121523







