Endogenic Phenolic Compounds of Barley as Potential Biomarkers Related to Grain Mycotoxin Production and Cultivar Selection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Barley Grain Samples
2.2. Extraction and Analysis of Phenolic Compounds and Antioxidant Assay
2.3. Disease Severity and Mycotoxin Analysis
2.4. Statistical Analysis
3. Results
3.1. Assessment of Phenolic Compounds in Clean and FHB-Infected Barley Grains
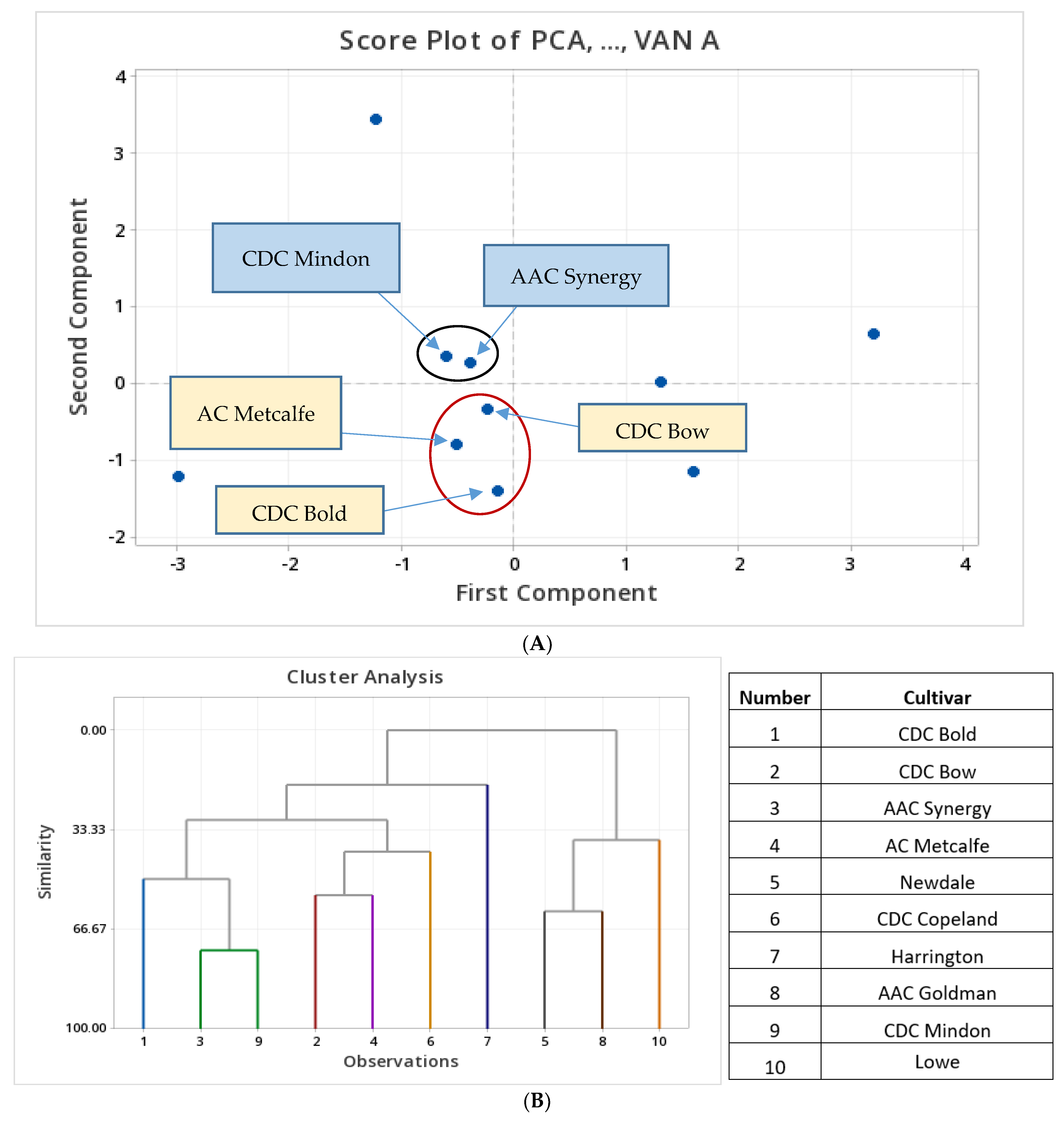
3.2. Principal Component Analysis Using Total (Free and Bound) Phenolic Contents of FHB-Infected Barley
3.3. Mycotoxin Contents of FHB-Infected Barley Cultivars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Badea, A.; Wijekoon, C. Benefits of barley grain in animal and human diets. In Cereal Grains; Goyal, A.K., Ed.; IntechOpen: London, UK, 2021; Volume 1. [Google Scholar] [CrossRef]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In Biocontrol Agents and Secondary Metabolites; Jogaiah, S., Ed.; Woodhead Publishing: Sawston, UK, 2021; ISBN 9780128229194. [Google Scholar]
- Kumaraswamy, K.G.; Bollina, V.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S.; Mamer, O.; Faubert, D. Metabolomics technology to phenotype resistance in barley against Gibberella zeae. Eur. J. Plant Pathol. 2011, 130, 29–43. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Chtioui, W.; Balmas, V.; Delogu, G.; Migheli, Q.; Oufensou, S. Bioprospecting Phenols as Inhibitors of Trichothecene-Producing Fusarium: Sustainable Approaches to the Management of Wheat Pathogens. Toxins 2022, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Singh, V. Antioxidative free and bound phenolic constituents in pericarp, germ and endosperm of Indian dent (Zea mays var. indentata) and flint (Zea mays var. indurata) maize. J. Funct. Foods. 2015, 13, 363–374. [Google Scholar]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Addit. Contam. Part A 2018, 35, 2455–2470. [Google Scholar] [CrossRef]
- Chamarthi, S.K.; Kumar, K.; Gunnaiah, R.; Kushalappa, A.C.; Dion, Y.; Choo, T.M. Identification of fusarium head blight resistance-related metabolites specific to doubled-haploid lines in barley. Eur. J. Plant Pathol. 2014, 138, 67–78. [Google Scholar] [CrossRef]
- Karre, S.; Kumar, A.; Yogendra, K.; Kage, U.; Kushalappa, A.; Charron, J.B. HvWRKY23 regulates flavonoid glycoside and hydroxycinnamic acid amide biosynthetic genes in barley to combat Fusarium head blight. Plant Mol. Biol. 2019, 100, 591–605. [Google Scholar] [CrossRef]
- Ponts, N.; Pinson-Gadais, L.; Boutigny, A.L.; Barreau, C.; Richard-Forget, F. Cinnamic-derived acids significantly affect Fusarium graminearum growth and in vitro synthesis of type B trichothecenes. Phytopathology 2011, 101, 929–934. [Google Scholar] [CrossRef]
- Engelharclt, G.; Koeniger, M.; Preiβ, U. Influence of wheat phenolic Acids on Fusarium Head Blight Resistance and Deoxynivalenol Accumulation. Mycotox Res. 2002, 18, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Galli, M.; Ordon, J.; Stuttmann, J.; Kogel, K.H.; Imani, J. Further analysis of barley MORC1 using a highly efficient RNA-guided Cas9 gene-editing system. Plant Biotechnol. J. 2018, 16, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, C.; Netticadan, T.; Siow, Y.L.; Sabra, A.; Yu, L.; Kodikara, C.; Badea, A. Analyses of fatty acids, proteins, ascorbic acid, bioactive phenolic compounds and antioxidant activity of Canadian barley cultivars and elite germplasm. Molecules 2022, 27, 7852. [Google Scholar] [CrossRef] [PubMed]
- Hajji, T.; Mansouri, S.; Vecino-Bello, X.; Cruz-Freire, J.M.; Rezgui, S.; Ferchichi, A. Identification and characterization of phenolic compounds extracted from barley husks by LC-MS and antioxidant activity in vitro. J. Cereal Sci. 2018, 81, 83–90. [Google Scholar] [CrossRef]
- Tucker, J.R.; Anita, L.B.; Colin, W.H.; Roger, L.; William, G.L.; Ana, B.; Dilantha, F. Genetic structure and genome-wide association study of a genomic panel of two-row, spring barley (Hordeum vulgare L.) with differential reaction to Fusarium head blight (Fusarium graminearum Schwabe) and deoxynivalenol production. Can. J. Plant Pathol. 2022, 6, 874–891. [Google Scholar] [CrossRef]
- Sinha, R.C.; Savard, M.E. Comparison of immunoassay and gas chromatography methods for the detection of the mycotoxin deoxynivalenol in grain samples. Can. J. Plant Pathol. 1996, 18, 233–236. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Fernandes, J.M.C.; Bergstrom, G.C. Influence of Growth Stage on Fusarium Head Blight and Deoxynivalenol Production in Wheat. J. Phytopathol. 2007, 155, 577–581. [Google Scholar] [CrossRef]
- Legge, W.G.; Badea, A.; Tucker, J.R.; Fetch, T.G., Jr.; Banik, M.; Haber, S.; Menzies, J.G.; Tekauz, A.; Turkington, T.K.; Martin, R.A.; et al. AAC Goldman barley. Can. J. Plant Sci. 2018, 98, 1203–1211. [Google Scholar] [CrossRef]
- Juskiw, P.E.; Oatway, L.; Oro, M.; Nyachiro, J.M.; Anbessa, Y.; Xi, K.; Turkington, T.K.; Lohr, S.; Bowness, J.; Capettini, F. Registration of ‘Lowe’, a Two-Rowed Malting Barley with Enhanced Resistance to Fusarium Head Blight. J. Plant Regist. 2019, 13, 301–310. [Google Scholar] [CrossRef]
- Siranidou, E.; Kang, Z.; Buchenauer, H. Studies on symptom development, phenolic compounds and morphological defence responses in wheat cultivars differing in resistance to Fusarium Head Blight. J. Phytopathol. 2002, 150, 200–208. [Google Scholar] [CrossRef]
- Kulik, T.; Stuper-Szablewska, K.; Bilska, K.; Buśko, M.; Ostrowska-Kołodziejczak, A.; Załuski, D.; Perkowski, J. Trans-cinnamic and chlorogenic acids affect the secondary metabolic profiles and ergosterol biosynthesis by Fusarium culmorum and F. graminearum sensu stricto. Toxins 2017, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in Phenylpropanoid and Trichothecene Production by Fusarium culmorum and F. graminearum Sensu Stricto via Exposure to Flavonoids. Toxins 2018, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of Fungicides and Factors Affecting Their Fate and Removal Efficacy: A Review. Sustainability. 2022, 14, 4056. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Over-looked Pesticide Class? Environ Sci Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Carbon footprint for Canadian barley. 2017. Available online: http://metrics.sustainablecrops.ca/home/datacollection/3 (accessed on 25 September 2023).




| Cultivar | Clean | Infected | % Change | Significance |
|---|---|---|---|---|
| CDC Bold | 140.5 ± 17 | 304.5 ± 38 | 117 | 0.0024 ** |
| CDC Bow | 228.3 ± 53 | 307.9 ± 80 | 34.9 | ns |
| AAC Synergy | 213.7 ± 22 | 206.5 ± 22 | –3.4 | ns |
| AC Metcalfe | 201.5 ± 23 | 238.1 ± 9.0 | 18.2 | ns |
| Newdale | 262.2 ± 45 | 260.5 ± 42 | –0.6 | ns |
| CDC Copeland | 235.9 ± 34 | 151.9 ± 31 | –35.6 | 0.0337 * |
| Harrington | 208.8 ± 27 | 176.8 ± 43 | –15.3 | ns |
| AAC Goldman | 245.1 ± 54 | 262.6 ± 21 | 7.1 | ns |
| CDC Mindon | 265.3 ± 35 | 207.7 ± 50 | –21.7 | ns |
| Lowe | 330.5 ± 96 | 324.7 ± 67 | –1.8 | ns |
| Cultivar | Clean | Infected | % Change | Significance |
|---|---|---|---|---|
| CDC Bold | 191.3 ± 47 | 317.4 ± 54 | 65.9 | 0.03775 * |
| CDC Bow | 292.6 ± 42 | 292.8 ± 60 | 0.1 | ns |
| AAC Synergy | 382.2 ± 14 | 292.5 ± 60 | –23.5 | ns |
| AC Metcalfe | 337.8 ± 15 | 299.9 ± 52 | –11.2 | ns |
| Newdale | 359.0 ± 48 | 288.3 ± 49 | –19.7 | ns |
| CDC Copeland | 342.4 ± 4.2 | 227.7 ± 28 | –33.5 | 0.00218 ** |
| Harrington | 468.7 ± 101 | 412.4 ± 85 | –12.0 | ns |
| AAC Goldman | 377.3 ± 33 | 321.5 ± 16 | –14.8 | ns |
| CDC Mindon | 375.3 ± 51.5 | 304.0 ± 53.5 | −19.0 | ns |
| Lowe | 367.7 ± 25.5 | 416.7 ± 73.5 | 13.3 | ns |
| PCA | CAF | SIN | FA | CAT | 4HBA | VAN A | IsoFA | DON | |
|---|---|---|---|---|---|---|---|---|---|
| PCA | −0.036 | 0.323 | 0.314 | 0.091 | 0.54 | 0.712 | 0.096 | 0.318 | |
| CAF | −0.227 | −0.23 | −0.062 | 0.116 | 0.244 | 0.136 | −0.053 | ||
| SIN | 0.288 | 0.019 | 0.534 | 0.206 | 0.451 | −0.091 | |||
| FA | −0.313 | −0.041 | 0.006 | 0.745 * | −0.228 | ||||
| CAT | −0.093 | −0.096 | −0.459 | 0.813 ** | |||||
| 4HBA | 0.792 ** | 0.05 | −0.216 | ||||||
| VAN A | 0.042 | 0.048 | |||||||
| IsoFA | −0.493 | ||||||||
| DON |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badea, A.; Tucker, J.R.; Sabra, A.; Netticadan, T.; Blackwell, B.; Yu, L.; Kodikara, C.; Wijekoon, C. Endogenic Phenolic Compounds of Barley as Potential Biomarkers Related to Grain Mycotoxin Production and Cultivar Selection. Biology 2023, 12, 1306. https://doi.org/10.3390/biology12101306
Badea A, Tucker JR, Sabra A, Netticadan T, Blackwell B, Yu L, Kodikara C, Wijekoon C. Endogenic Phenolic Compounds of Barley as Potential Biomarkers Related to Grain Mycotoxin Production and Cultivar Selection. Biology. 2023; 12(10):1306. https://doi.org/10.3390/biology12101306
Chicago/Turabian StyleBadea, Ana, James R. Tucker, Ali Sabra, Thomas Netticadan, Barbara Blackwell, Liping Yu, Chamali Kodikara, and Champa Wijekoon. 2023. "Endogenic Phenolic Compounds of Barley as Potential Biomarkers Related to Grain Mycotoxin Production and Cultivar Selection" Biology 12, no. 10: 1306. https://doi.org/10.3390/biology12101306
APA StyleBadea, A., Tucker, J. R., Sabra, A., Netticadan, T., Blackwell, B., Yu, L., Kodikara, C., & Wijekoon, C. (2023). Endogenic Phenolic Compounds of Barley as Potential Biomarkers Related to Grain Mycotoxin Production and Cultivar Selection. Biology, 12(10), 1306. https://doi.org/10.3390/biology12101306







