Environmental Effects during Early Life-History Stages and Seed Development on Seed Functional Traits of an Australian Native Legume Species
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
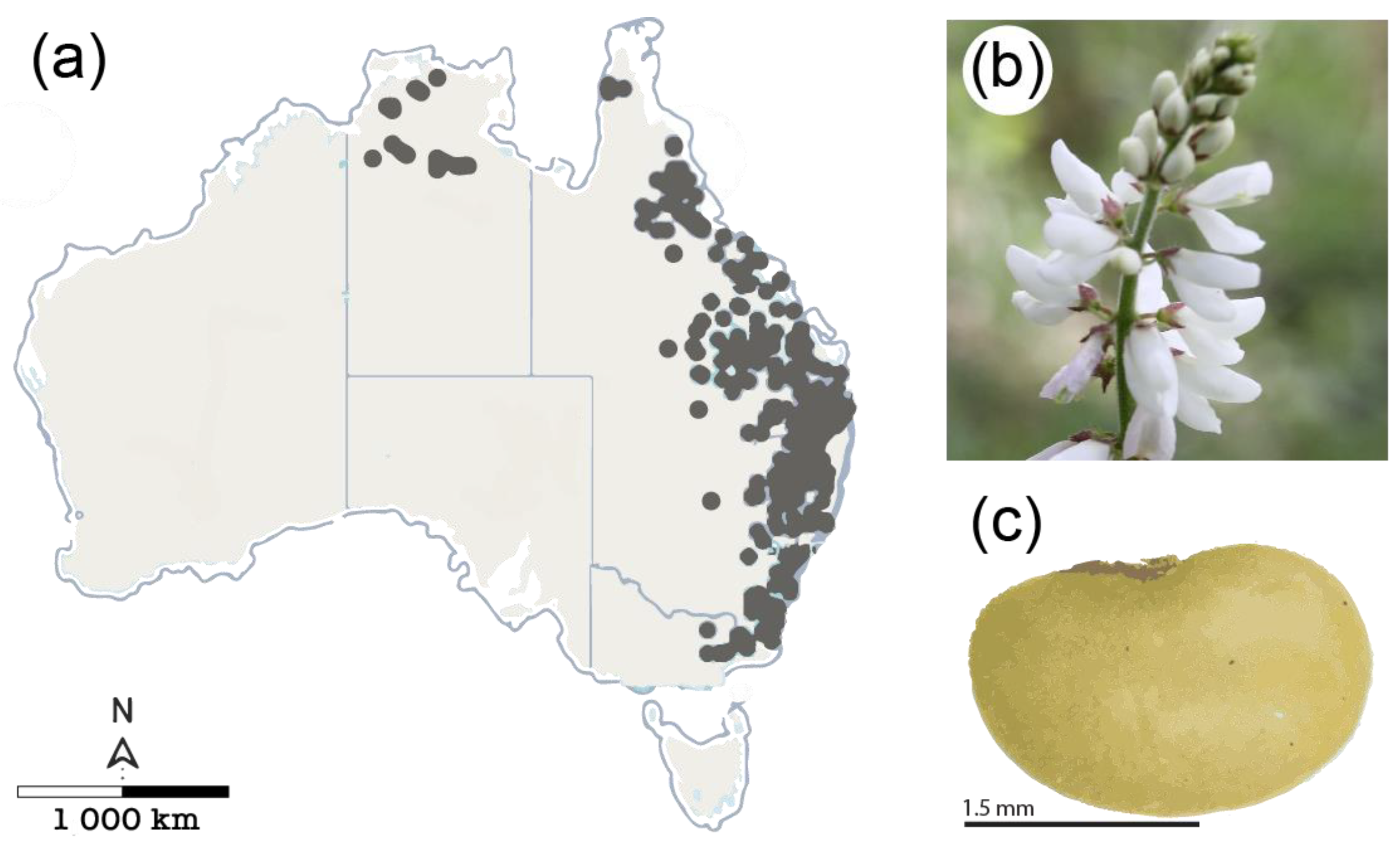
2.1. Study Species and Seed Source
2.2. Interactions between Temperature and Moisture Stress on Seed Germination (Experiment 1)
2.3. Seed and Seedling Performance following Burial, Elevated Temperature, and Reduced Soil Moisture (Experiment 2)
2.3.1. Soil Moisture
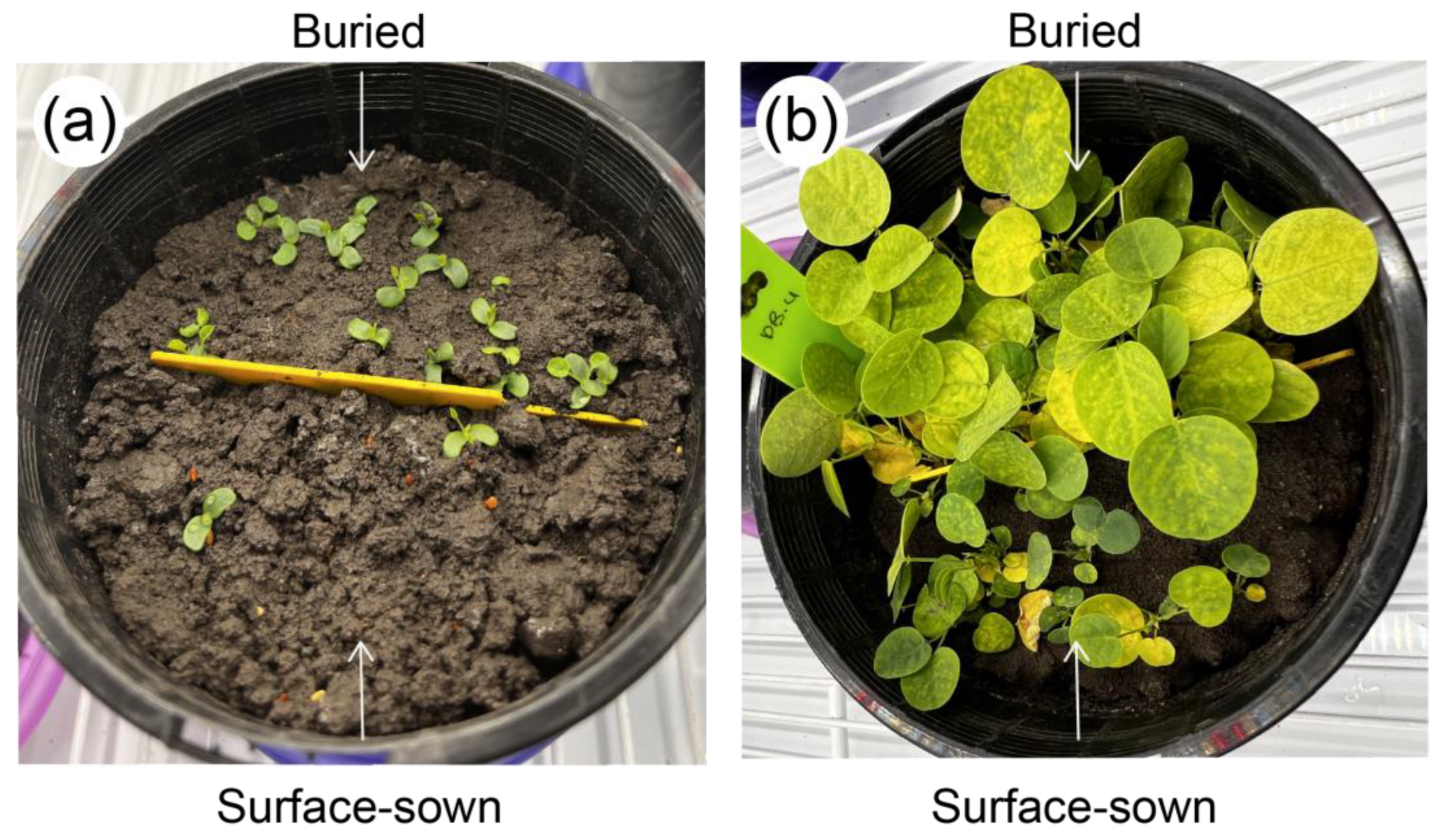
2.3.2. Seedling Emergence Trial
2.4. Effects of Post-Anthesis Maternal Soil Moisture Stress on Seed and Seedling Traits (Experiment 3)
2.4.1. Parental Seed Germination and Plant Growth
2.4.2. Soil Moisture
2.4.3. Experimental Design and Data Collection
2.4.4. Seed Harvesting
2.4.5. Germination Test of the Progeny Seeds
2.4.6. Controlled Aging Test (CAT)
2.4.7. Maternal Effects on Progeny Seedling Performance
2.5. Statistical Analysis
3. Results
3.1. Interaction between Temperature and Moisture Stress on Seed Germination (Experiment 1)
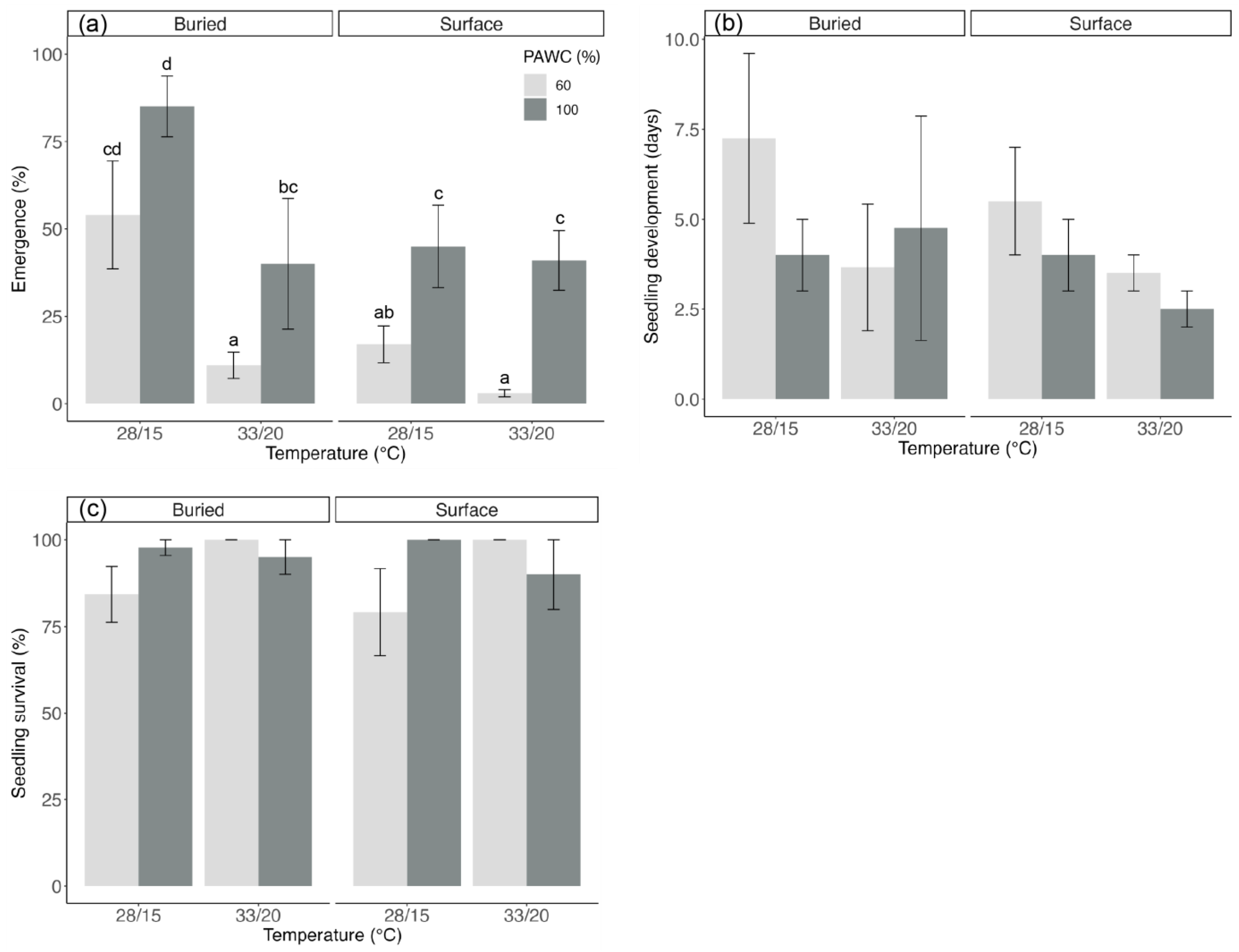
3.2. Seed and Seedling Performance following Burial, Elevated Temperature, and Reduced Soil Moisture (Experiment 2)
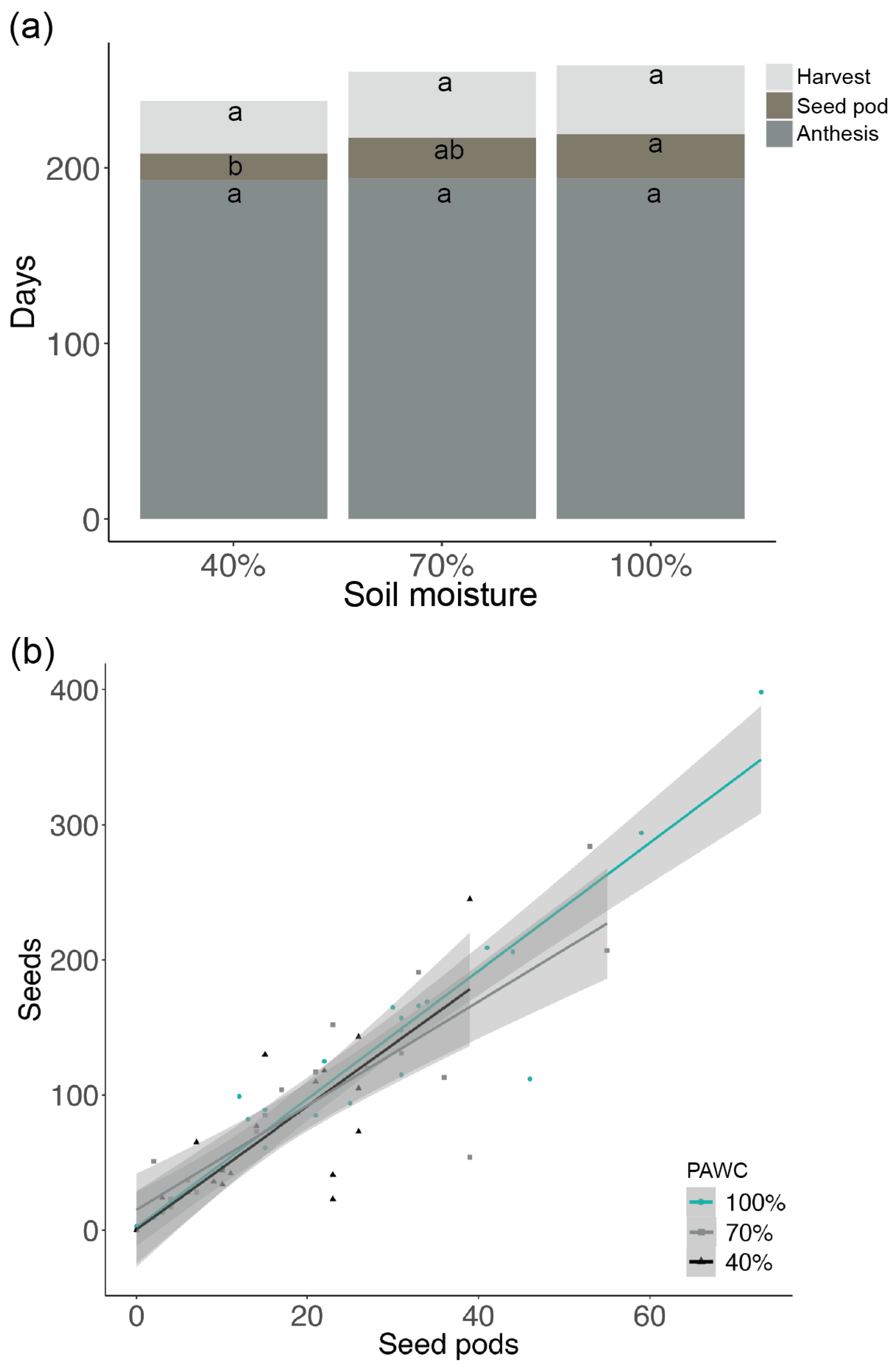
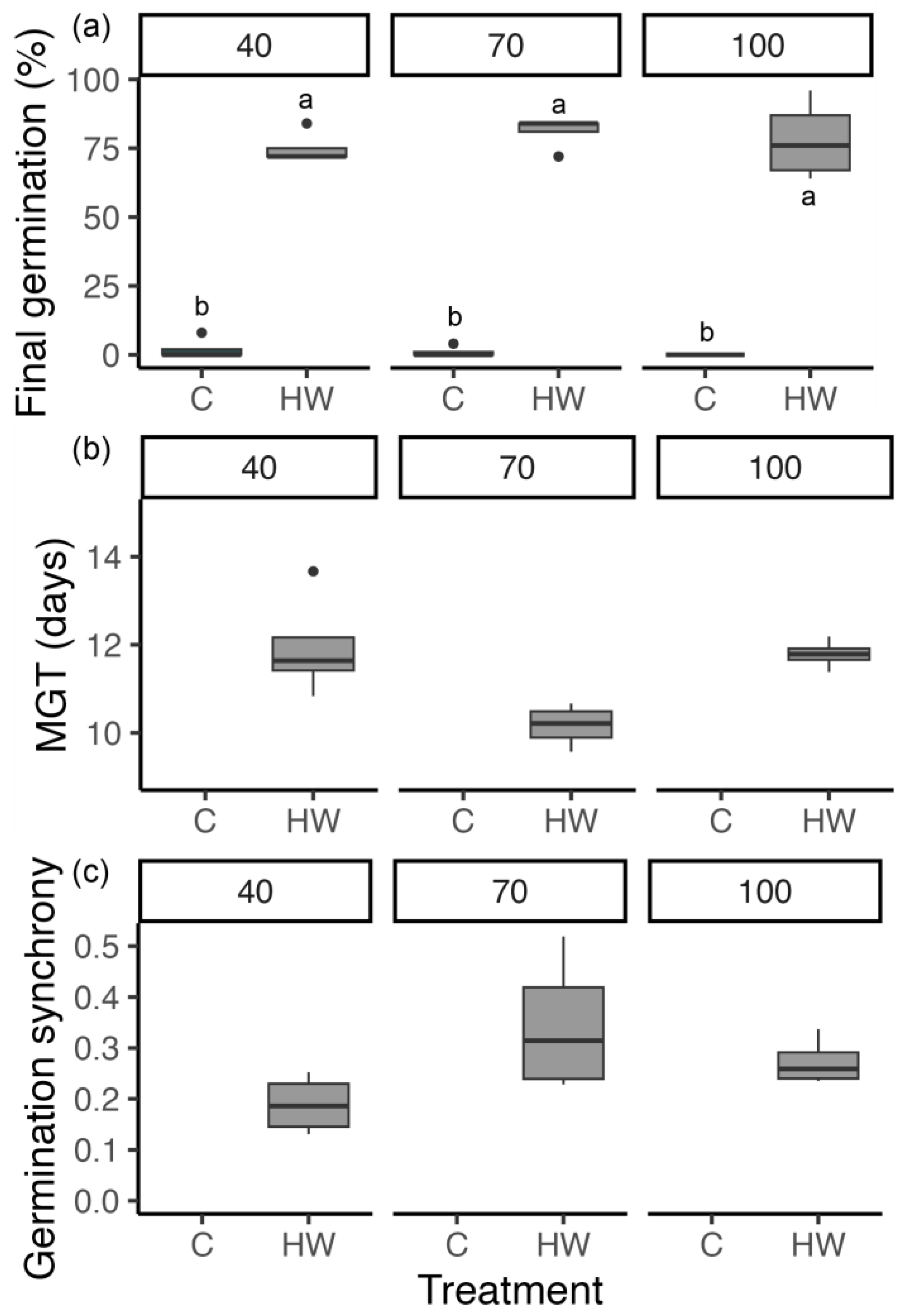
3.3. Post-Anthesis Maternal Soil Moisture Stress Effects on Plant Traits, Seed Functional Traits, and Seedling Traits (Experiment 3)
4. Discussion
4.1. Temperature and Moisture Effects on Early Plant Life History Stages
4.2. Post-Anthesis Soil Moisture Effects on Plant Life History Traits
4.3. Post-Anthesis Soil Moisture Effects on Seed and Seedling Traits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Mean Germination Time (MGT) and Germination Synchrony Calculation
Appendix B. Pressure Plate Method for the Determination of Plant-Available Water Content
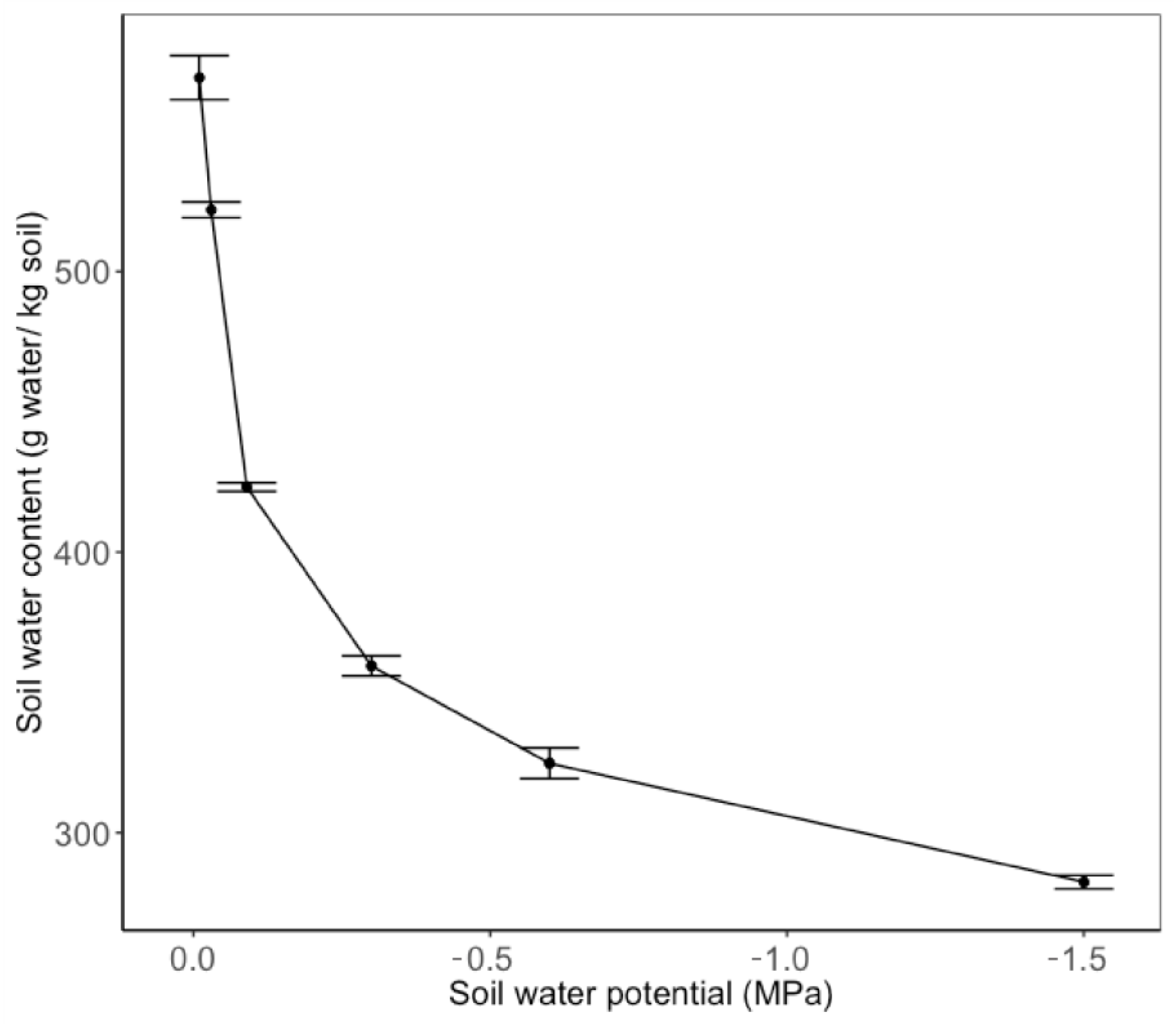
Appendix C. Temperature Regime throughout the Experiment
| Temperature (°C) | May | June | July | August | September | October | November | December |
|---|---|---|---|---|---|---|---|---|
| Maximum | 25 | 28 | 30 | 30 | 30 | 29 | 29 | 26 |
| Minimum | 12 * | 13 | 16 | 17 | 19 | 19 | 17 | 13 |
References
- Silvertown, J.; Charlesworth, D. Introduction to Plant Population Biology, 4th ed.; Blackwell Publishing: Victoria, Australia, 2009. [Google Scholar]
- Sternberg, M.; Brown, V.K.; Masters, G.J.; Clarke, I.P. Plant community dynamics in a calcareous grassland under climate change manipulations. Plant Ecol. 1999, 143, 29–37. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; García-Camacho, R.; Iriondo, J.M.; Escudero, A. Selection on flowering time in Mediterranean high-mountain plants under global warming. Evol. Ecol. 2011, 25, 777–794. [Google Scholar] [CrossRef]
- James, J.J.; Sheley, R.L.; Leger, E.A.; Adler, P.B.; Hardegree, S.P.; Gornish, E.S.; Rinella, M.J. Increased soil temperature and decreased precipitation during early life stages constrain grass seedling recruitment in cold desert restoration. J. Appl. Ecol. 2019, 56, 2609–2619. [Google Scholar] [CrossRef]
- Larson, J.E.; Funk, J.L. Regeneration: An overlooked aspect of trait-based plant community assembly models. J. Ecol. 2016, 104, 1284–1298. [Google Scholar] [CrossRef]
- Cochrane, J.A.; Hoyle, G.L.; Yates, C.J.; Wood, J.; Nicotra, A.B. Climate warming delays and decreases seedling emergence in a Mediterranean ecosystem. Oikos 2015, 124, 150–160. [Google Scholar] [CrossRef]
- Arnold, S.M. The relationship between temperature and seedling growth of two species which occur in Upper Teesdale. New Phytol. 1974, 73, 333–340. [Google Scholar] [CrossRef]
- Hovenden, M.J.; Newton, P.C.; Wills, K.E.; Janes, J.K.; Williams, A.L.; Vander Schoor, J.K.; Nolan, M.J. Influence of warming on soil water potential controls seedling mortality in perennial but not annual species in a temperate grassland. New Phytol. 2008, 180, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Arène, F.; Affre, L.; Doxa, A.; Saatkamp, A. Temperature but not moisture response of germination shows phylogenetic constraints while both interact with seed mass and lifespan. Seed Sci. Res. 2017, 27, 110–120. [Google Scholar] [CrossRef]
- Saatkamp, A.; Cochrane, A.; Commander, L.; Guja, L.K.; Jimenez-Alfaro, B.; Larson, J.; Nicotra, A.; Poschlod, P.; Silveira, F.A.; Cross, A.T. A research agenda for seed-trait functional ecology. New Phytol. 2019, 221, 1764–1775. [Google Scholar] [CrossRef]
- Daws, M.I.; Crabtree, L.M.; Dalling, J.W.; Mullins, C.E.; Burslem, D.F. Germination responses to water potential in neotropical pioneers suggest large-seeded species take more risks. Ann. Bot. 2008, 102, 945–951. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. What do seedlings die from and what are the implications for evolution of seed size? Oikos 2004, 106, 193–199. [Google Scholar] [CrossRef]
- Lloret, F.; Casanovas, C.; Penuelas, J. Seedling survival of Mediterranean shrubland species in relation to root: Shoot ratio, seed size and water and nitrogen use. Funct. Ecol. 1999, 13, 210–216. [Google Scholar] [CrossRef]
- Verdú, M.; Traveset, A. Early emergence enhances plant fitness: A phylogenetically controlled meta-analysis. Ecology 2005, 86, 1385–1394. [Google Scholar] [CrossRef]
- Lewandrowski, W.; Stevens, J.C.; Webber, B.L.; Dalziell, E.L.; Trudgen, M.S.; Bateman, A.M.; Erickson, T.E. Global change impacts on arid zone ecosystems: Seedling establishment processes are threatened by temperature and water stress. Ecol. Evol. 2021, 11, 8071–8084. [Google Scholar] [CrossRef]
- Nichols, P.; Malik, A.; Stockdale, M.; Colmer, T. Salt tolerance and avoidance mechanisms at germination of annual pasture legumes: Importance for adaptation to saline environments. Plant Soil 2009, 315, 241–255. [Google Scholar] [CrossRef]
- Ooi, M.K.; Auld, T.D.; Denham, A.J. Projected soil temperature increase and seed dormancy response along an altitudinal gradient: Implications for seed bank persistence under climate change. Plant Soil 2012, 353, 289–303. [Google Scholar] [CrossRef]
- Li, X.; Baskin, J.M.; Baskin, C.C. Pericarp ontogeny and anatomy in Rhus aromatica Ait. and R. glabra L.(Anacardiaceae). J. Torrey Bot. Soc. 1999, 126, 279–288. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Hudson, A.R.; Ayre, D.J.; Ooi, M.K. Physical dormancy in a changing climate. Seed Sci. Res. 2015, 25, 66–81. [Google Scholar] [CrossRef]
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2017, 68, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Jaganathan, G.K. Influence of maternal environment in developing different levels of physical dormancy and its ecological significance. Plant Ecol. 2016, 217, 71–79. [Google Scholar] [CrossRef]
- Tozer, M.G.; Ooi, M.K. Humidity-regulated dormancy onset in the Fabaceae: A conceptual model and its ecological implications for the Australian wattle Acacia saligna. Ann. Bot. 2014, 114, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Segura, F.; Vicente, M.; Franco, J.; Martinez-Sanchez, J. Effects of maternal environmental factors on physical dormancy of Astragalus nitidiflorus seeds (Fabaceae), a critically endangered species of SE Spain. Flora Morphol. Distrib. Funct. Ecol. 2015, 216, 71–76. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contributing of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Head, L.; Adams, M.; McGregor, H.V.; Toole, S. Climate change and Australia. Wiley Interdiscip. Rev. Clim. Change 2014, 5, 175–197. [Google Scholar] [CrossRef]
- Hughes, L. Climate change and Australia: Key vulnerable regions. Reg. Environ. Change 2011, 11, 189–195. [Google Scholar] [CrossRef]
- Eriksson, O.; Ehrlén, J. Seedling recruitment and population ecology. In Seedling Ecology and Evolution; Leck, M., Parker, V., Simpson, R., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 239–254. [Google Scholar]
- Australian Bureau of Meteorology. Australian Climate Variability & Change-Trend Maps. Available online: http://www.bom.gov.au/climate/change/index.shtml#tabs=Tracker&tracker=trend-maps (accessed on 30 August 2023).
- Bell, L.W.; Bennett, R.G.; Ryan, M.H.; Clarke, H. The potential of herbaceous native Australian legumes as grain crops: A review. Renew. Agric. Food Syst. 2011, 26, 72–91. [Google Scholar] [CrossRef]
- Atlas of Living Australia. Spacial Portal. Available online: https://spatial.ala.org.au/_=_ (accessed on 12 July 2023).
- Michel, B.E. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 1983, 72, 66–70. [Google Scholar] [CrossRef]
- Lozano-Isla, F.; Benites-Alfaro, O.E.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application “GerminaQuant for R”. Ecol. Res. 2019, 34, 339–346. [Google Scholar] [CrossRef]
- R Core Team: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Klute, A. Water retention: Laboratory methods. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods. Agronomy, No. 9 Part 1; Klute, A., Ed.; Soil Science Society of Amerca, American Society of Agronomy: Madison, WI, USA, 1986; Volume 5, pp. 635–662. [Google Scholar]
- Hay, F.; Klin, J.; Probert, R. Can a post-harvest ripening treatment extend the longevity of Rhododendron L. seeds? Sci. Hortic. 2006, 111, 80–83. [Google Scholar] [CrossRef]
- Probert, R.J.; Daws, M.I.; Hay, F.R. Ecological correlates of ex situ seed longevity: A comparative study on 195 species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.R.; Vieira, D.L. Direct seeding of 16 Brazilian savanna trees: Responses to seed burial, mulching and an invasive grass. Appl. Veg. Sci. 2017, 20, 410–421. [Google Scholar] [CrossRef]
- Long, R.L.; Panetta, F.D.; Steadman, K.J.; Probert, R.; Bekker, R.M.; Brooks, S.; Adkins, S.W. Seed persistence in the field may be predicted by laboratory-controlled aging. Weed Sci. 2008, 56, 523–528. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Tribulato, A.; Patanè, C. Effects of drought stress on seed germination of ornamental sunflowers. Acta Physiol. Plant. 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Notarnicola, R.F.; Nicotra, A.B.; Kruuk, L.E.; Arnold, P.A. Effects of warming temperatures on germination responses and trade-offs between seed traits in an alpine plant. J. Ecol. 2023, 111, 62–76. [Google Scholar] [CrossRef]
- Delachiave, M.; De Pinho, S. Scarification, temperature and light in germination of Senna occidentalis seed (Caesalpinaceae). Seed Sci. Technol. 2003, 31, 225–230. [Google Scholar] [CrossRef]
- Cochrane, A. Modelling seed germination response to temperature in Eucalyptus L’Her.(Myrtaceae) species in the context of global warming. Seed Sci. Res. 2017, 27, 99–109. [Google Scholar] [CrossRef]
- CSIRO and Bureau of Meteorology. State of the Climate; Commonwealth Scientific and Industrial Research Organization/Australian Bureau of Meteorology: Melbourne, Australia, 2020. [Google Scholar]
- Pearson, T.R.H.; Burslem, D.F.R.P.; Mullins, C.E.; Dalling, J.W. Germination ecology of neotropical pioneers: Interacting effects of environmental conditions and seed size. Ecology 2002, 83, 2798–2807. [Google Scholar] [CrossRef]
- Wang, T.-T.; Chu, G.-M.; Jiang, P.; Niu, P.-X.; Wang, M. Effects of sand burial and seed size on seed germination, seedling emergence and seedling biomass of Anabasis aphylla. Pak. J. Bot. 2017, 49, 391–396. [Google Scholar]
- Bell, D.T.; Rokich, D.P.; McChesney, C.J.; Plummer, J.A. Effects of temperature, light and gibberellic acid on the germination of seeds of 43 species native to Western Australia. J. Veg. Sci. 1995, 6, 797–806. [Google Scholar] [CrossRef]
- Gugger, S.; Kesselring, H.; Stöcklin, J.; Hamann, E. Lower plasticity exhibited by high-versus mid-elevation species in their phenological responses to manipulated temperature and drought. Ann. Bot. 2015, 116, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.J. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol. 2011, 190, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Baskin, J.M.; Baskin, C.C.; Yang, X.; Liu, G.; Ye, X.; Huang, Z.; Cornelissen, J.H. Great granny still ruling from the grave: Phenotypical response of plant performance and seed functional traits to salt stress affects multiple generations of a halophyte. J. Ecol. 2022, 110, 117–128. [Google Scholar] [CrossRef]
- Everingham, S.E.; Offord, C.A.; Sabot, M.E.; Moles, A.T. Time-traveling seeds reveal that plant regeneration and growth traits are responding to climate change. Ecology 2021, 102, e03272. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.; Ding, J. Phenotypic plasticity in resource allocation to sexual trait of alligatorweed in wetland and terrestrial habitats. Sci. Total Environ. 2021, 757, 143819. [Google Scholar] [CrossRef]
- Dornbos, D., Jr.; Mullen, R. Influence of stress during soybean seed fill on seed weight, germination, and seedling growth rate. Can. J. Plant Sci. 1991, 71, 373–383. [Google Scholar] [CrossRef]
- Chamorro, D.; Parra, A.; Moreno, J. Reproductive output, seed anatomy and germination under water stress in the seeder Cistus ladanifer subjected to experimental drought. Environ. Exp. Bot. 2016, 123, 59–67. [Google Scholar] [CrossRef]
- Boyko, A.; Blevins, T.; Yao, Y.; Golubov, A.; Bilichak, A.; Ilnytskyy, Y.; Hollander, J.; Meins, F., Jr.; Kovalchuk, I. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS ONE 2010, 5, e9514. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beveridge, F.C.; Williams, A.; Cave, R.; Kalaipandian, S.; Haque, M.M.; Adkins, S.W. Environmental Effects during Early Life-History Stages and Seed Development on Seed Functional Traits of an Australian Native Legume Species. Biology 2024, 13, 148. https://doi.org/10.3390/biology13030148
Beveridge FC, Williams A, Cave R, Kalaipandian S, Haque MM, Adkins SW. Environmental Effects during Early Life-History Stages and Seed Development on Seed Functional Traits of an Australian Native Legume Species. Biology. 2024; 13(3):148. https://doi.org/10.3390/biology13030148
Chicago/Turabian StyleBeveridge, Fernanda C., Alwyn Williams, Robyn Cave, Sundaravelpandian Kalaipandian, Mirza M. Haque, and Steve W. Adkins. 2024. "Environmental Effects during Early Life-History Stages and Seed Development on Seed Functional Traits of an Australian Native Legume Species" Biology 13, no. 3: 148. https://doi.org/10.3390/biology13030148
APA StyleBeveridge, F. C., Williams, A., Cave, R., Kalaipandian, S., Haque, M. M., & Adkins, S. W. (2024). Environmental Effects during Early Life-History Stages and Seed Development on Seed Functional Traits of an Australian Native Legume Species. Biology, 13(3), 148. https://doi.org/10.3390/biology13030148







