Optimizing Moss and Lichen Transplants as Biomonitors of Airborne Anthropogenic Microfibers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
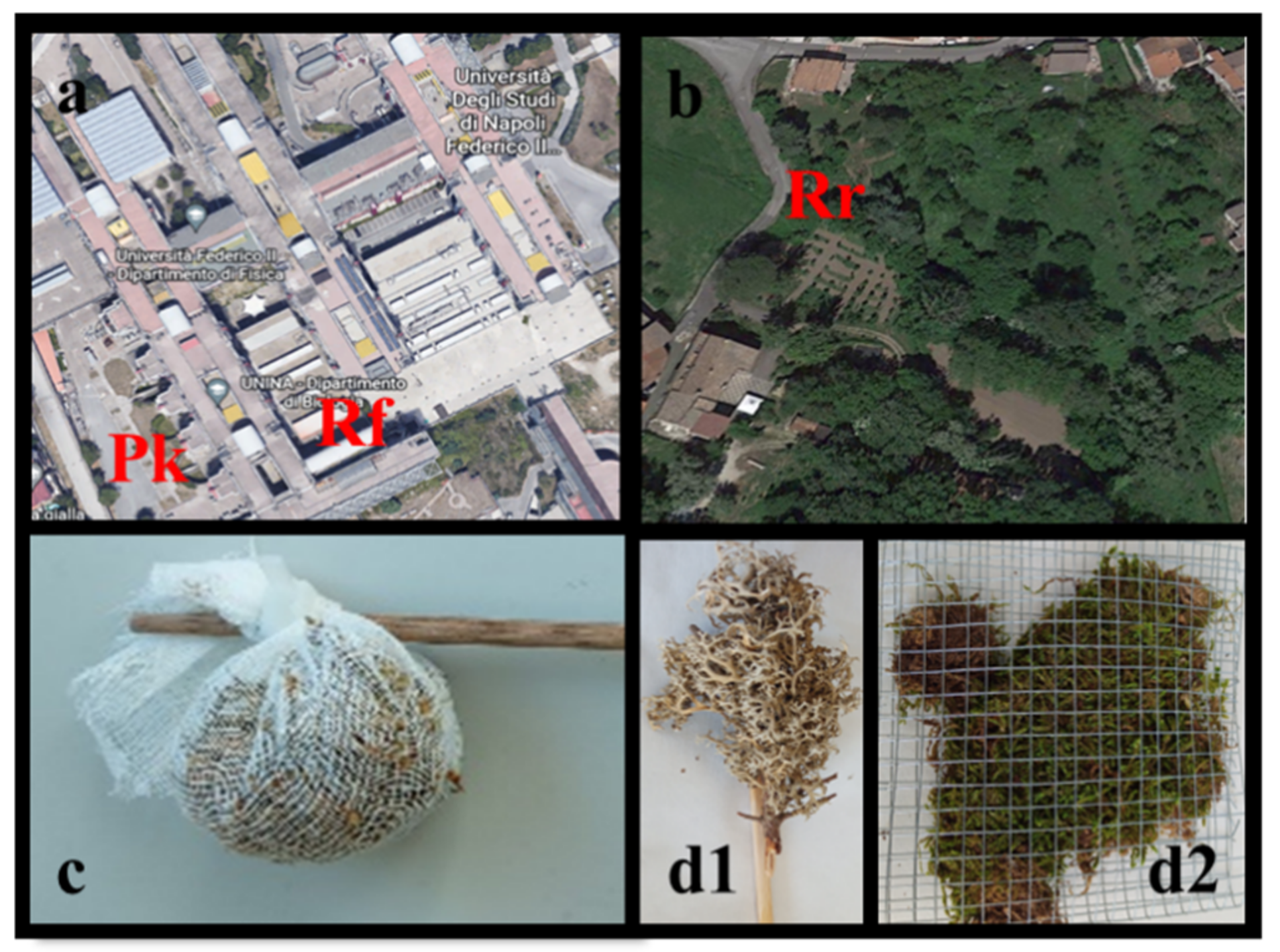
2.1. Sampling
2.2. Bag Preparation and Exposure
2.3. Anthropogenic Microfiber Extraction
2.4. Stereomicroscope Identification
2.5. Raman Microspectroscopy
2.6. Data Analysis
3. Results
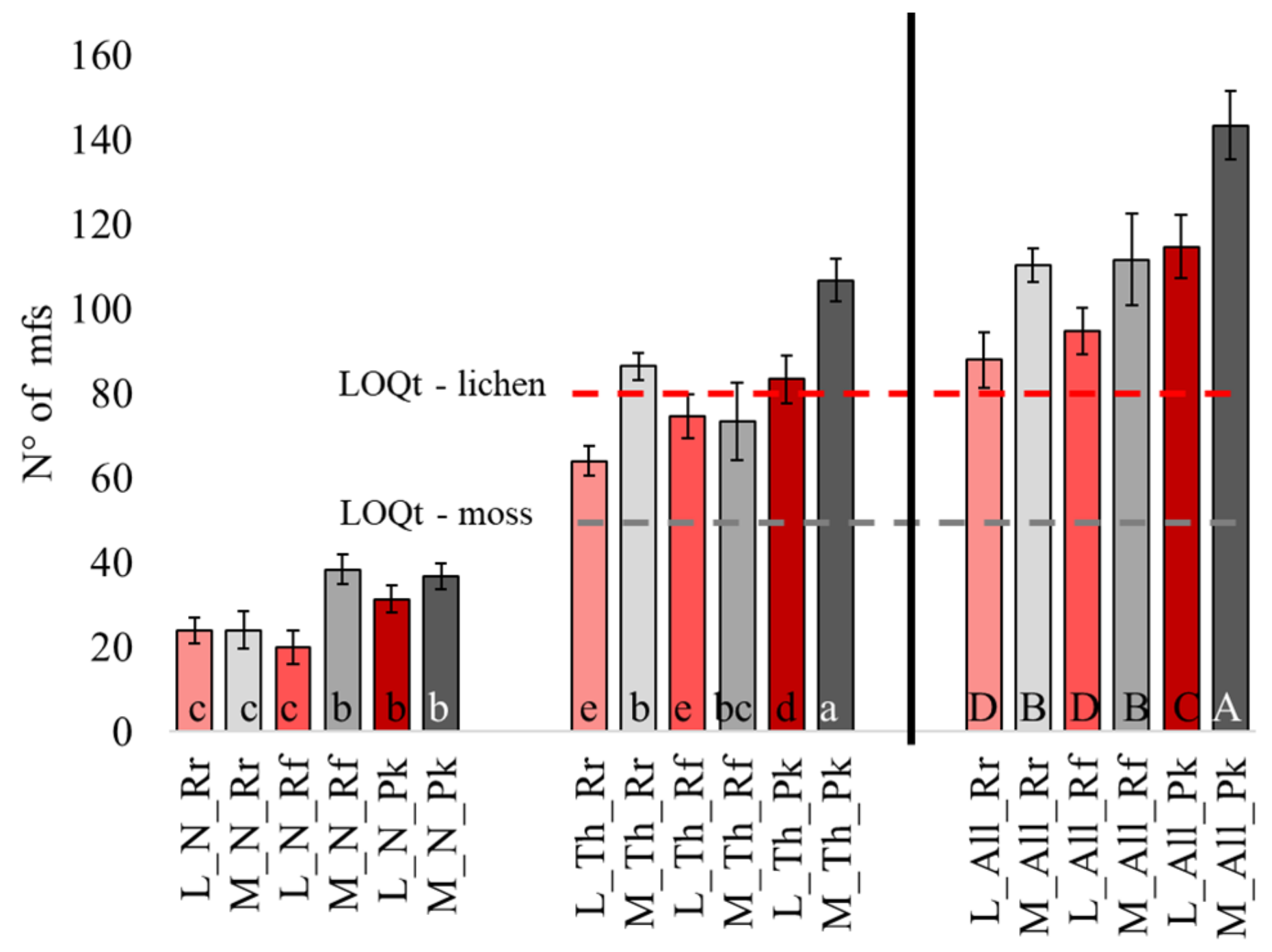
3.1. Number of Mfs in Moss and Lichen Transplants
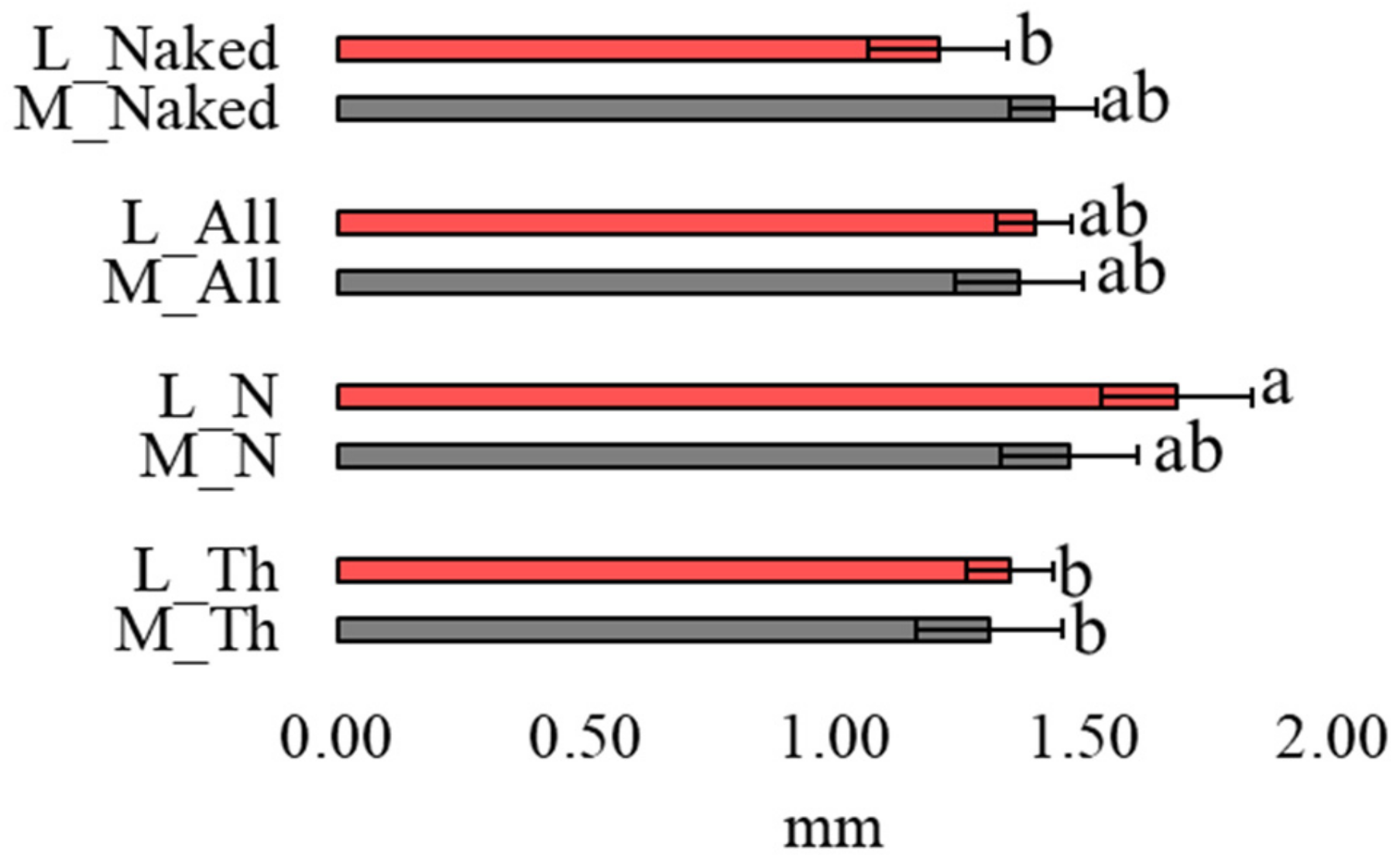
3.2. Length of Mfs in Moss and Lichen Transplants
3.3. Deposition Fluxes
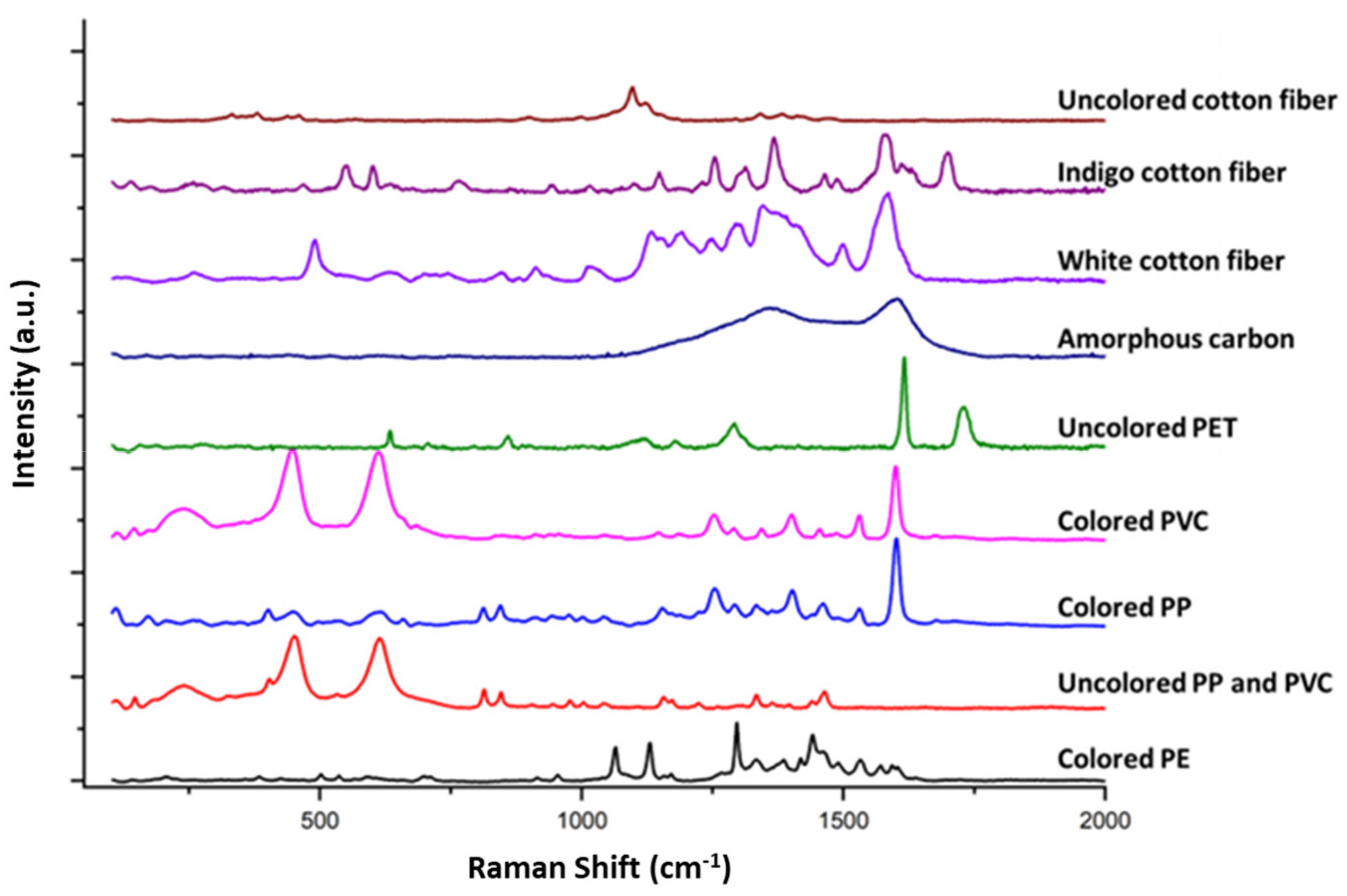
3.4. Raman Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Athey, S.N.; Erdle, L.M. Are we underestimating anthropogenic microfiber pollution? A critical review of occurrence, methods, and reporting. Environ. Toxicol. Chem. 2022, 41, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Karbalaei, S.; Maselli, V.; Walker, T.R. Micro (nano) plastics sources, fate, and effects: What we know after ten years of research. J. Hazard. Mater. Adv. 2022, 6, 100057. [Google Scholar] [CrossRef]
- Hossain, M.I.; Tuha, M.A.S.M. Biodegradable plastic production from daily household waste materials and comparison the decomposing time with synthetic polyethylene plastic. Int. J. Adv. Life Sci. Res. 2020, 3, 16–19. [Google Scholar] [CrossRef]
- Bergmann, M.; Collard, F.; Fabres, J.; Gabrielsen, G.W.; Provencher, J.F.; Rochman, C.M.; van Sebille, E.; Tekman, M.B. Plastic pollution in the Arctic. Nat. Rev. Earth Environ. 2022, 3, 323–337. [Google Scholar] [CrossRef]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro (nano) plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, J.; Guo, S.; Chen, G.; Zhu, Z. Occurrence of microplastics in air. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; Volume 100, pp. 17–31. [Google Scholar]
- EFSA. Presence of microplastics and nanoplastics in food, with particular focus on seafood. Panel on contaminants in the food chain. EFSA J. 2016, 14, e04501. [Google Scholar]
- Leslie, H.; Van Velzen, J.M.; Brandsma, S.H.; Vethaak, D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Capozzi, F.; Sorrentino, M.; Di Palma, A.; Mele, F.; Arena, C.; Adamo, P.; Spagnuolo, V.; Giordano, S. Implication of vitality, seasonality and specific leaf area on PAH uptake in moss and lichen transplanted in bags. Ecol. Indic. 2020, 108, 105727. [Google Scholar] [CrossRef]
- Capozzi, F.; Carotenuto, R.; Giordano, S.; Spagnuolo, V. Evidence on the effectiveness of mosses for biomonitoring of microplastics in fresh water environment. Chemosphere 2018, 205, 1–7. [Google Scholar] [CrossRef]
- Roblin, B.; Aherne, J. Moss as a biomonitor for the atmospheric deposition of anthropogenic microfibres. Sci. Total Environ. 2020, 715, 136973. [Google Scholar] [CrossRef]
- Jafarova, M.; Grifoni, L.; Aherne, J.; Loppi, S. Comparison of Lichens and Mosses as Biomonitors of Airborne Microplastics. Atmosphere 2023, 14, 1007. [Google Scholar] [CrossRef]
- Wright, S.L.; Gouin, T.; Koelmans, A.A.; Scheuermann, L. Development of screening criteria for microplastic particles in air and atmospheric deposition: Critical review and applicability towards assessing human exposure. Microplast. Nanoplast. 2021, 1, 6. [Google Scholar] [CrossRef]
- Jafarova, M.; Contardo, T.; Aherne, J.; Loppi, S. Lichen Biomonitoring of Airborne Microplastics in Milan (N Italy). Biology 2022, 11, 1815. [Google Scholar] [CrossRef]
- Bertrim, C.; Aherne, J. Moss Bags as Biomonitors of Atmospheric Microplastic Deposition in Urban Environments. Biology 2023, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Dellisanti, W.; Leung, M.M.-L.; Lam, K.W.-K.; Wang, Y.; Hu, M.; Lo, H.S.; Fang, J.K.H. A short review on the recent method development for extraction and identification of microplastics in mussels and fish, two major groups of seafood. Mar. Pollut. Bull. 2023, 186, 114221. [Google Scholar] [CrossRef]
- Vegetation, I. Heavy metals, nitrogen and pops in European mosses: 2015 survey. In Monitoring Manual; WGE: Laxenburg, Austria, 2015. [Google Scholar]
- Capozzi, F.; Giordano, S.; Aboal, J.R.; Adamo, P.; Bargagli, R.; Boquete, T.; Di Palma, A.; Real, C.; Reski, R.; Spagnuolo, V.; et al. Best options for the exposure of traditional and innovative moss bags: A systematic evaluation in three European countries. Environ. Pollut. 2016, 214, 362–373. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015. [Google Scholar]
- Herrera, A.; Garrido-Amador, P.; Martínez, I.; Samper, M.D.; López-Martínez, J.; Gómez, M.; Packard, T.T. Novel methodology to isolate microplastics from vegetal-rich samples. Mar. Pollut. Bull. 2018, 129, 61–69. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A 2014, 31, 1574–1578. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Sweden, K. Small Plastic Particles in Coastal Swedish Waters; N-Research Report Commissioned by KIMO Sweeden (Submitted to BDC); NResearch: West Caldwell, NJ, USA, 2007. [Google Scholar]
- Windsor, F.M.; Durance, I.; Horton, A.A.; Thompson, R.C.; Tyler, C.R.; Ormerod, S.J. A catchment-scale perspective of plastic pollution. Glob. Change Biol. 2019, 25, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Anger, P.M.; von der Esch, E.; Baumann, T.; Elsner, M.; Niessner, R.; Ivleva, N.P. Raman microspectroscopy as a tool for microplastic particle analysis. TrAC Trends Anal. Chem. 2018, 109, 214–226. [Google Scholar] [CrossRef]
- Chakraborty, I.; Banik, S.; Biswas, R.; Yamamoto, T.; Noothalapati, H.; Mazumder, N. Raman spectroscopy for microplastic detection in water sources: A systematic review. Int. J. Environ. Sci. Technol. 2022, 20, 10435–10448. [Google Scholar] [CrossRef]
- Vergara, A.; Vitagliano, L.; Merlino, A.; Sica, F.; Marino, K.; Verde, C.; di Prisco, G.; Mazzarella, L. An order-disorder transition plays a role in switching off the Root effect in fish hemoglobins. J. Biol. Chem. 2010, 285, 32568–32575. [Google Scholar]
- Couto, J.; Aboal, J.; Fernandez, J.; Carballeira, A. A new method for testing the sensitivity of active biomonitoring: An example of its application to a terrestrial moss. Chemosphere 2004, 57, 303–308. [Google Scholar] [CrossRef]
- Ares, A.; Aboal, J.; Carballeira, A.; Fernández, J. Do moss bags containing devitalized Sphagnum denticulatum reflect heavy metal concentrations in bulk deposition? Ecol. Indic. 2015, 50, 90–98. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Zapata, F.; Ortega-Ojeda, F.E.; García-Ruiz, C. Forensic examination of textile fibres using Raman imaging and multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120695. [Google Scholar] [CrossRef]
- Karapanayiotis, T.; Villar, S.E.J.; Bowen, R.D.; Edwards, H.G. Raman spectroscopic and structural studies of indigo and its four 6, 6′-dihalogeno analogues. Analyst 2004, 129, 613–618. [Google Scholar] [CrossRef]
- Was-Gubala, J.; Machnowski, W. Application of Raman spectroscopy for differentiation among cotton and viscose fibers dyed with several dye classes. Spectrosc. Lett. 2014, 47, 527–535. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.; Ragas, A.M. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Loppi, S.; Roblin, B.; Paoli, L.; Aherne, J. Accumulation of airborne microplastics in lichens from a landfill dumping site (Italy). Sci. Rep. 2021, 11, 4564. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Sorrentino, M.C.; Cascone, E.; Iuliano, M.; De Tommaso, G.; Granata, A.; Giordano, S.; Spagnuolo, V. Biomonitoring of airborne microplastic deposition in semi-natural and rural sites using the moss hypnum cupressiforme. Plants 2023, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Adamo, P.; Spagnuolo, V.; Tretiach, M.; Bargagli, R. Accumulation of airborne trace elements in mosses, lichens and synthetic materials exposed at urban monitoring stations: Towards a harmonisation of the moss-bag technique. Chemosphere 2013, 90, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Chen, G.; Bao, J.; Song, M.; Li, Y.; Luo, C. Interactions between microplastics and organic compounds in aquatic environments: A mini review. Sci. Total Environ. 2020, 736, 139472. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef]
- Meeßen, J.; Ott, S. Recognition mechanisms during the pre-contact state of lichens: I. Mycobiont-photobiont interactions of the mycobiont of Fulgensia bracteata. Symbiosis 2013, 59, 121–130. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total Environ. 2019, 666, 377–389. [Google Scholar] [CrossRef]


| Sample | Site | n. | mean | s.d. | Tukey’s |
|---|---|---|---|---|---|
| L_All | Rr | 3 | 1.50 | 0.03 | a * |
| L_All | Pk | 3 | 1.42 | 0.07 | ab |
| L_All | Rf | 3 | 1.40 | 0.08 | ab |
| M_All | Rr | 3 | 1.26 | 0.06 | b |
| M_All | Pk | 3 | 1.08 | 0.04 | c |
| M_All | Rf | 3 | 1.37 | 0.02 | ab |
| M_Pre-expo | M_Rr | M_Pk | M_Rf | M_Rf_Naked | L_Pre-expo | L_Rr | L_Pk | L_Rf | L_Rf_Naked | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0 < mfs < 1 | 72 | 118 | 197 | 108 | 117 | 141 | 77 | 108 | 95 | 112 |
| B | 1 ≤ mfs < 2 | 83 | 158 | 191 | 181 | 109 | 103 | 117 | 165 | 123 | 110 |
| C | 2 ≤ mfs < 3 | 15 | 46 | 38 | 50 | 76 | 39 | 54 | 51 | 51 | 29 |
| D | 3 ≤ mfs < 4 | 0 | 7 | 3 | 8 | 12 | 8 | 11 | 6 | 10 | 11 |
| E | 4 ≤ mfs ≤ 5 | 0 | 2 | 0 | 2 | 2 | 2 | 5 | 3 | 4 | 0 |
| b | a | a | a | a | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Friedman ANOVA | Chi Sqr. (N = 5, df = 4) = 10; p = 0.04 | Chi Sqr. (N = 5, df = 4) = 2.5; p = 0.65 | |||||||||
| Biomonitor | Site | Test Portion | Mean mfs m2 d−1 | s.d. |
|---|---|---|---|---|
| L | Rr | Th | −15.6 | 3.3 |
| L | Pk | Th | 2.1 | 5.2 |
| L | Rf | Th | −5.8 | 4.7 |
| L | Rf | Naked | 9.5 | 7.4 |
| M | Rr | Th | 5.9 | 0.6 |
| M | Pk | Th | 9.5 | 0.9 |
| M | Rf | Th | 3.6 | 1.6 |
| M | Rf | Naked | 9.3 | 2.3 |
| Class | Number of Samples | Raman Shift (cm−1) | Raman Assignment | Reference |
|---|---|---|---|---|
| Microplastics | 3 | 1065, 1131, 1171, 1297, 1334, 1386, 1418, 1441, 2724, 2849, 2883 | Colored PE | Anger et al., (2018) [26] |
| Microplastics | 3 | 404, 452, 532, 615, 814, 846, 944, 977, 1003, 1042, 1157, 1225, 1334, 1440, 1464, 2725, 2845, 2888, 2909, 2929, 2957 | Uncolored PP and PVC | Chakraborty et al., (2022) [27] |
| Microplastics | 4 | 446, 617, 812, 844, 944, 976, 1000, 1040, 1154, 1253, 1334, 1402, 1461, 1530, 1601, 2843, 2887, 2908, 2927, 2956 | Colored PP | Anger et al., (2018) [26] |
| Microplastics | 1 | 447, 611, 1145, 1184, 1254, 1291, 1343, 1401, 1455, 1530, 1600 | Colored PVC | Chakraborty et al., (2022) [27] |
| Microplastics | 2 | 634, 859, 1118, 1178, 1292, 1617, 1731, 2962, 3082 | Uncolored PET | Anger et al., (2018) [26] |
| Other | 12 | 1367, 1601 | Amorphous carbon | Li et al., (2023) [31] |
| Cellulose based fibers | 24 | 491, 1016, 1134, 1149, 1192, 1249, 1294, 1347, 1375, 1409, 1500, 1586 | Grey/white dyed cotton fiber | Zapata et al., (2022) [32] |
| Cellulose based fibers | 16 | 258, 468, 553, 601, 766, 945, 1016, 1099, 1149, 1254, 1313, 1368, 1464, 1576, 1611, 1700 | Indigo dyed cotton fiber | Karapanayiotis et al., (2004) [33] |
| Cellulose based fibers | 2 | 332, 381, 437, 461, 897, 1000, 1098, 1121, 1149, 1342, 1381, 1413 | Uncolored cotton fiber | Was-Gubala and Machnowski, (2014) [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, F.; Sorrentino, M.C.; Granata, A.; Vergara, A.; Alberico, M.; Rossi, M.; Spagnuolo, V.; Giordano, S. Optimizing Moss and Lichen Transplants as Biomonitors of Airborne Anthropogenic Microfibers. Biology 2023, 12, 1278. https://doi.org/10.3390/biology12101278
Capozzi F, Sorrentino MC, Granata A, Vergara A, Alberico M, Rossi M, Spagnuolo V, Giordano S. Optimizing Moss and Lichen Transplants as Biomonitors of Airborne Anthropogenic Microfibers. Biology. 2023; 12(10):1278. https://doi.org/10.3390/biology12101278
Chicago/Turabian StyleCapozzi, Fiore, Maria Cristina Sorrentino, Angelo Granata, Alessandro Vergara, Miriam Alberico, Manuela Rossi, Valeria Spagnuolo, and Simonetta Giordano. 2023. "Optimizing Moss and Lichen Transplants as Biomonitors of Airborne Anthropogenic Microfibers" Biology 12, no. 10: 1278. https://doi.org/10.3390/biology12101278
APA StyleCapozzi, F., Sorrentino, M. C., Granata, A., Vergara, A., Alberico, M., Rossi, M., Spagnuolo, V., & Giordano, S. (2023). Optimizing Moss and Lichen Transplants as Biomonitors of Airborne Anthropogenic Microfibers. Biology, 12(10), 1278. https://doi.org/10.3390/biology12101278












