Moss Bags as Biomonitors of Atmospheric Microplastic Deposition in Urban Environments
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Study Sites
2.2. Moss Bag Construction and Deployment
2.3. Digestion and Microplastic Extraction
2.4. Microplastic Identification
2.5. Quality Control
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibres in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Microplastics Are Contaminants of Emerging Concern in Freshwater Environments: An Overview. In Freshwater Microplastics; The Handbook of Environmental Chemistry 58; Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–23. [Google Scholar]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic rain in protected areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Roblin, B.; Ryan, M.; Vreugdenhil, A.; Aherne, J. Ambient atmospheric deposition of anthropogenic microfibers and microplastics on the western periphery of Europe (Ireland). Environ. Sci. Technol. 2020, 54, 11100–11108. [Google Scholar] [CrossRef]
- Loppi, S.; Roblin, B.; Paoli, L.; Aherne, J. Accumulation of airborne microplastics in lichens from a landfill dumping site (Italy). Sci. Rep. 2021, 11, 4564. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and evaluation of transport. Environ. Int. 2020, 156, 105411. [Google Scholar] [CrossRef]
- Welsh, B.; Aherne, J.; Paterson, A.M.; Yao, H.; McConnell, C. Atmospheric deposition of anthropogenic particles and microplastics in south-central Ontario, Canada. Sci. Total Environ. 2022, 835, 155426. [Google Scholar] [CrossRef]
- Szewc, K.; Graca, B.; Dołęga, A. Atmospheric deposition of microplastics in coastal zone: Characteristics and relationships with meteorological factors. Sci. Total Environ. 2021, 761, 143272. [Google Scholar] [CrossRef]
- Rühling, A.; Tyler, G. Sorption and retention of heavy metals in the woodland moss Hylocomium splendens (Hedw). Oikos 1970, 21, 92–97. [Google Scholar] [CrossRef]
- Berg, T.; Royset, O.; Steinnes, E. Moss (Hylocomium splendens) used as biomonitor of atmospheric trace-element deposition—Estimation of uptake efficiencies. Atmos. Environ. 1995, 29, 353–360. [Google Scholar] [CrossRef]
- Steinnes, E. Use of mosses as biomonitors of atmospheric deposition of trace elements. In Proceedings of the International Workshop on Biomonitoring of Atmospheric Pollution (with Emphasis on Trace Elements)—BioMAP, Lisbon, Portugal, 21–24 September 1997; pp. 100–107. [Google Scholar]
- Harmens, H.; Norris, D.A.; Sharps, K.; Mills, G.; Alber, R.; Aleksiayenak, Y.; Zechmeister, H.G. Heavy metal and nitrogen concentrations in mosses are declining across Europe whilst some “hotspots” remain in 2010. Environ. Pollut. 2015, 200, 93–104. [Google Scholar] [CrossRef]
- Harmens, H.; Norris, D.A.; Cooper, D.M.; Mills, G.; Steinnes, E.; Kubin, E.; Zechmeister, H.G. Nitrogen concentrations in mosses indicate the spatial distribution of atmospheric nitrogen deposition in Europe. Environ. Pollut. 2011, 159, 2852–2860. [Google Scholar] [CrossRef]
- Wilkins, K.; Aherne, J. Isothecium myosuroides and Thuidium tamariscinum mosses as bioindicators of nitrogen and heavy metal deposition in Atlantic oak woodlands. Ann. Bot. 2015, 5, 71–78. [Google Scholar]
- Olmstead, E.; Aherne, J. Are tissue concentrations of Hylocomium splendens a good predictor of nitrogen deposition? Atmos. Pollut. Res. 2019, 10, 80–87. [Google Scholar] [CrossRef]
- Berg, T.; Steinnes, E. Recent trends in atmospheric deposition of trace elements in Norway as evident from the 1995 moss survey. Sci. Total Environ. 1997, 208, 197–206. [Google Scholar] [CrossRef]
- Cowden, P.; Aherne, J.; Aherne,, J. Interspecies comparison of three moss species (Hylocomium splendens, Pleurozium schreberi and Isothecium stoloniferum) as biomonitors of trace element deposition. Environ. Monit. Assess. 2019, 191, 220. [Google Scholar] [CrossRef]
- DoŁęgowska, S.; Migaszewski, Z.M. PAH concentrations in the moss species Hylocomium splendens (Hedw.) B.S.G. and Pleurozium schreberi (Brid.) Mitt. from the Kielce area (south-central Poland). Ecotoxicol. Environ. Saf. 2011, 74, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Holoubek, I.; Kořínek, P.; Šeda, Z.; Schneiderová, E.; Holoubková, I.; Pacl, A.; Čáslavský, J. The use of mosses and pine needles to detect persistent organic pollutants at local and regional scales. Environ. Pollut. 2000, 109, 283–292. [Google Scholar] [PubMed]
- Harmens, H.; Foan, L.; Simon, V.; Mills, G. Terrestrial mosses as biomonitors of atmospheric POPs pollution: A review. Environ. Pollut. 2013, 173, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Steinnes, E.; Njfistad, O. Use of Mosses and Lichens for Regional Mapping of 137Cs Fallout from the Chernobyl Accident. J. Environ. Radioact. 1993, 21, 65–73. [Google Scholar] [CrossRef]
- Krmar, M.; Wattanavatee, K.; Radnović, D.; Slivka, J.; Bhongsuwan, T.; Frontasyeva, M.V.; Pavlov, S.S. Airborne radionuclides in mosses collected at different latitudes. J. Environ. Radioact. 2013, 117, 45–48. [Google Scholar] [CrossRef]
- Wilkins, K.; Cathcart, H.; Hickey, P.; Hanley, O.; Vintro, L.L.; Aherne, J. Influence of climate on the spatial distribution of 210Pb, 7Be, 40K and 137Cs in moss. Pollutants 2023, 3, 102–113. [Google Scholar] [CrossRef]
- Markert, B.; Wappelhorst, O.; Weckert, V.; Herpin, U.; Siewers, U.; Friese, K.; Breulmann, G. The use of bioindicators for monitoring the heavy-metal status of the environment. J. Radioanal. Nucl. Chem. 1999, 240, 425–429. [Google Scholar] [CrossRef]
- Rühling, Å. A European survey of atmospheric heavy metal deposition in 2000–2001. Environ. Pollut. 2002, 120, 23–25. [Google Scholar] [CrossRef]
- Smodis, B.; Pginata, M.L.; Saiki, M.; Cortes, E.; Bangfa, N.; Markert, B.; Frontasyeva, M. Validation and application of plants as biomonitors of trace element atmospheric pollution—A co-ordinated effort in 14 countries. J. Atmos. Chem. 2004, 49, 3–13. [Google Scholar] [CrossRef]
- Roblin, B.; Aherne, J. Moss as a biomonitor for the atmospheric deposition of anthropogenic microfibres. Sci. Total Environ. 2020, 715, 136973. [Google Scholar] [CrossRef]
- Ares, A.; Aboal, J.R.; Carballeira, A.; Giordano, S.; Adamo, P.; Fernández, J.A. Moss bag biomonitoring: A methodological review. Sci. Total Environ. 2012, 432, 143–158. [Google Scholar] [CrossRef]
- Capozzi, F.; Di Palma, A.; Adamo, P.; Sorrentino, M.C.; Giordano, S.; Spagnuolo, V. Indoor vs. outdoor airborne element array: A novel approach using moss bags to explore possible pollution sources. Environ. Pollut. 2019, 249, 566–572. [Google Scholar] [CrossRef]
- ECCC (Environment and Climate Change Canada), 2020. Historical Climate Data. Available online: https://climate.weather.gc.ca/historical_data/search_historic_data_e.html (accessed on 31 December 2022).
- Statistics Canada, 2016. 2016 Census Profile. Available online: https://www12.statcan.gc.ca/census-recensement/index-eng.cfm (accessed on 31 December 2022).
- Peterborough City Services. 2018. Available online: https://www.peterborough.ca/en/city-services/resources/Documents/TR-Traffic-Counts-2018.pdf (accessed on 31 December 2022).
- Suchara, I.; Sucharova, J.; Hola, M.; Reimann, C.; Boyd, R.; Filzmoser, P.; Englmaier, P. The performance of moss, grass, and 1- and 2-year old spruce needles as bioindicators of contamination: A comparative study at the scale of the Czech Republic. Sci. Total Environ. 2011, 409, 2281–2297. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Giordano, S.; Aboal, J.R.; Adamo, P.; Bargagli, R.; Boquete, T.; Di Palma, A.; Real, C.; Reski, R.; Spagnuolo, V.; et al. Best options for the exposure of traditional and innovative moss bags: A systematic evaluation in three European countries. Environ. Pollut. 2016, 214, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments. NOAA Technical Memorandum NOS-OR&R-48. 2015. Available online: https://marinedebris.noaa.gov/sites/default/files/publications-files/noaa_microplastics_methods_manual.pdf (accessed on 31 December 2022).
- Herrera, A.; Garrido-Amador, P.; Martínez, I.; Dolores Samper, M.; López-Martínez, J.; Gómez, M.; Packard, T.T. Novel methodology to isolate microplastics from vegetal-rich samples. Mar. Pollut. Bull. 2018, 129, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Löder, M.G.J.; Gerdts, G. Methodology used for the detection and identification of microplastics—A critical appraisal. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 201–227. [Google Scholar]
- MERI (Marine and Environmental Research Institute). Guide to Microplastic Identification; University of Florida: Gainesville, FL, USA, 2014; p. 13. [Google Scholar]
- Windsor, F.; Tilley, R.; Tyler, C.; Ormerod, S. Microplastic ingestion by riverine macroinvertebrates. Sci. Total Environ. 2018, 646, 68–74. [Google Scholar] [CrossRef]
- Norén, F. Small Plastic Particles in Coastal Swedish Waters; KIMO Report; KIMO: Sweden, 2007; p. 11. [Google Scholar]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, K. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Kreider, M.L.; Panko, J.M.; McAtee, B.L.; Sweet, L.I.; Finley, B.L. Physical and chemical characterization of tire-related particles: Comparison of particles generated using different methodologies. Sci. Total Environ. 2010, 408, 652–659. [Google Scholar] [CrossRef]
- Leads, R.R.; Weinstein, J.E. Occurrence of tire wear particles and other microplastics within the tributaries of the Charleston Harbor Estuary, South Carolina, USA. Mar. Pollut. Bull. 2019, 145, 569–582. [Google Scholar] [CrossRef]
- Parker, B.W.; Beckingham, B.A.; Ingram, B.C.; Ballenger, J.D.; Weinstein, J.E.; Sancho, G. Microplastic and tire wear particle occurrence in fishes from an urban estuary: Influence of feeding characteristics on exposure risk. Mar. Pollut. Bull. 2020, 160, 111539. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Jafarova, M.; Contardo, T.; Aherne, J.; Loppi, S. Lichen biomonitoring of airborne microplastics in Milan (N Italy). Biology 2022, 11, 1815. [Google Scholar] [CrossRef] [PubMed]
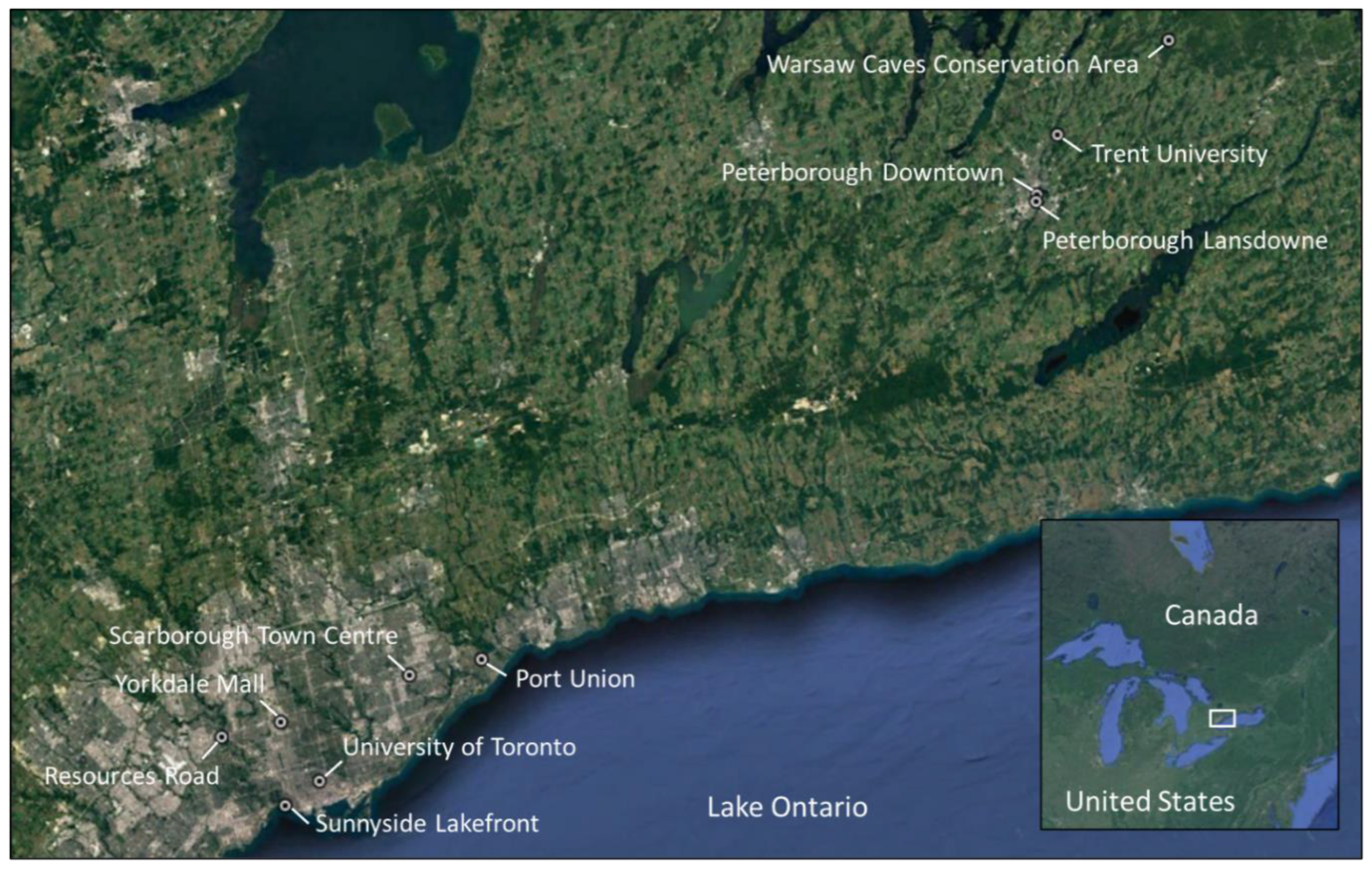


| ID | Group | Site Name | AADT | Latitude | Longitude |
|---|---|---|---|---|---|
| 1 | PTB | Peterborough Lansdowne | 24,900 | 44.288033 | –78.320410 |
| 2 | PTB | Peterborough Downtown | 10,400 | 44.295368 | –78.319133 |
| 3 | PTB | Trent University | <200 | 44.359686 | –78.287522 |
| 4 | GTA | Port Union | 233,900 | 43.796374 | –79.154208 |
| 5 | GTA | Scarborough Town Centre | 315,900 | 43.779017 | –79.262005 |
| 6 | GTA | Yorkdale Mall | 397,000 | 43.727960 | –79.454669 |
| 7 | GTA | Resources Road | 442,900 | 43.711166 | –79.543352 |
| 8 | TOR | Sunnyside Lakefront | 77,000 § | 43.637672 | –79.447855 |
| 9 | TOR | University of Toronto St. George | 17,800 $ | 43.664392 | –79.396623 |
| 10 | CON | Warsaw Caves Conservation Area | 130 | 44.460905 | –78.117738 |
| ID | Study Site | Count | RPD | %Fibre | mp g−1 |
|---|---|---|---|---|---|
| 1 | Peterborough Lansdowne | 5.5 $ | 18 | 55 | 2.8 |
| 2 | Peterborough Downtown | 8.0 | 25 | 69 | 5.7 |
| 3 | Trent University | 5.0 $ | 80 | 60 | 2.5 |
| 4 | Port Union | 15.0 | 13 | 50 | 12.9 |
| 5 | Scarborough Town Centre | 8.5 | 12 | 71 | 5.8 |
| 6 | Yorkdale Mall | 11.5 | 9 | 30 | 9.2 |
| 7 | Resources Road | 17.0 | 59 | 47 | 15.0 |
| 8 | Sunnyside Lakefront | 10.5 | 29 | 43 | 8.1 |
| 9 | University of Toronto | 12.0 | 17 | 25 | 9.5 |
| 10 | Warsaw Caves § | 2.8 | 47 | 93 |
| Group | Microplastic Count | Fibre % | Volume mm3 g−1 | Concentration mp g−1 (45 Days) | Deposition mp m−2 Day−1 | Deposition mf m−2 Day−1 |
|---|---|---|---|---|---|---|
| CON | 2.8 | 93 | 0.002 | |||
| PTB | 6.2 | 62 | 0.007 | 3.7 | 21 | 13 |
| TOR | 11.3 | 33 | 0.019 | 8.8 | 50 | 17 |
| GTA | 13.0 | 48 | 0.064 | 10.7 | 60 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertrim, C.; Aherne, J. Moss Bags as Biomonitors of Atmospheric Microplastic Deposition in Urban Environments. Biology 2023, 12, 149. https://doi.org/10.3390/biology12020149
Bertrim C, Aherne J. Moss Bags as Biomonitors of Atmospheric Microplastic Deposition in Urban Environments. Biology. 2023; 12(2):149. https://doi.org/10.3390/biology12020149
Chicago/Turabian StyleBertrim, Carter, and Julian Aherne. 2023. "Moss Bags as Biomonitors of Atmospheric Microplastic Deposition in Urban Environments" Biology 12, no. 2: 149. https://doi.org/10.3390/biology12020149
APA StyleBertrim, C., & Aherne, J. (2023). Moss Bags as Biomonitors of Atmospheric Microplastic Deposition in Urban Environments. Biology, 12(2), 149. https://doi.org/10.3390/biology12020149








