Milk Polysialic Acid Levels Rapidly Decrease in Line with the N-Acetylneuraminic Acid Concentrations during Early Lactation in Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk and Tissue Samples
2.2. Quantification of Neu5Ac in Milk
2.3. Western Blotting
2.4. Expression Analysis
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
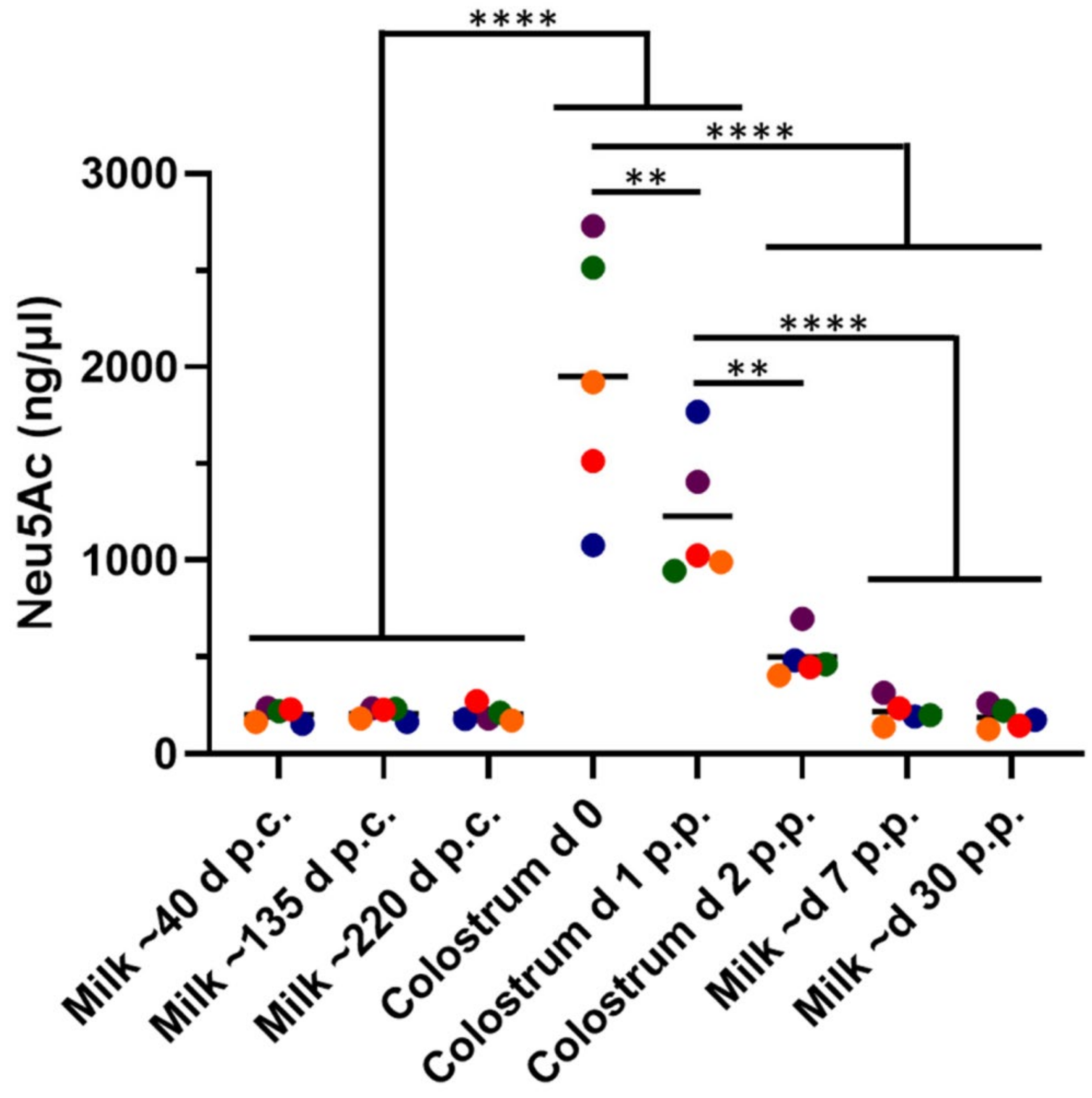
3.1. Neu5Ac Concentrations Rapidly Decrease during Early Lactation
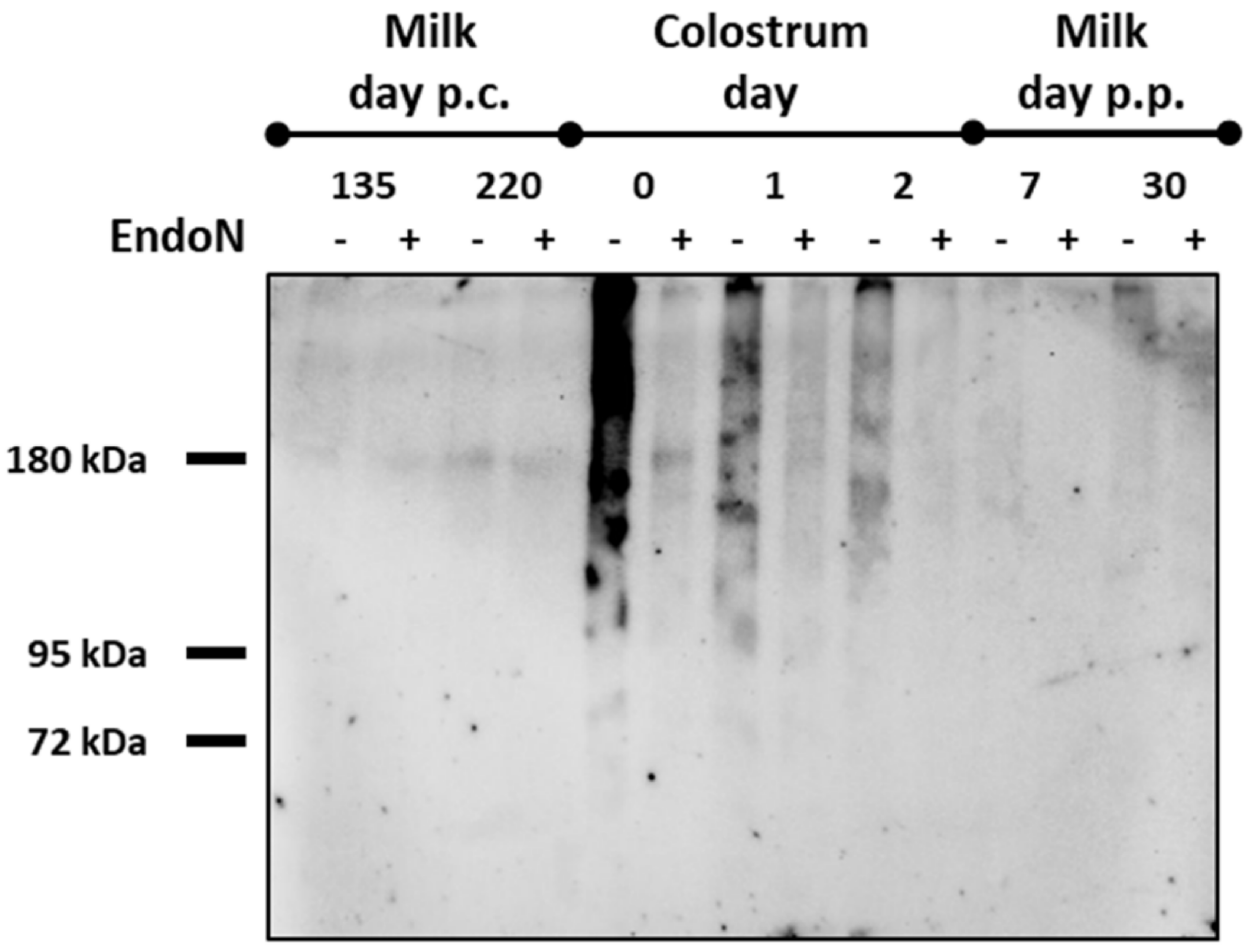
3.2. The Amount of PolySia Decreases in Line with the Neu5Ac Concentrations
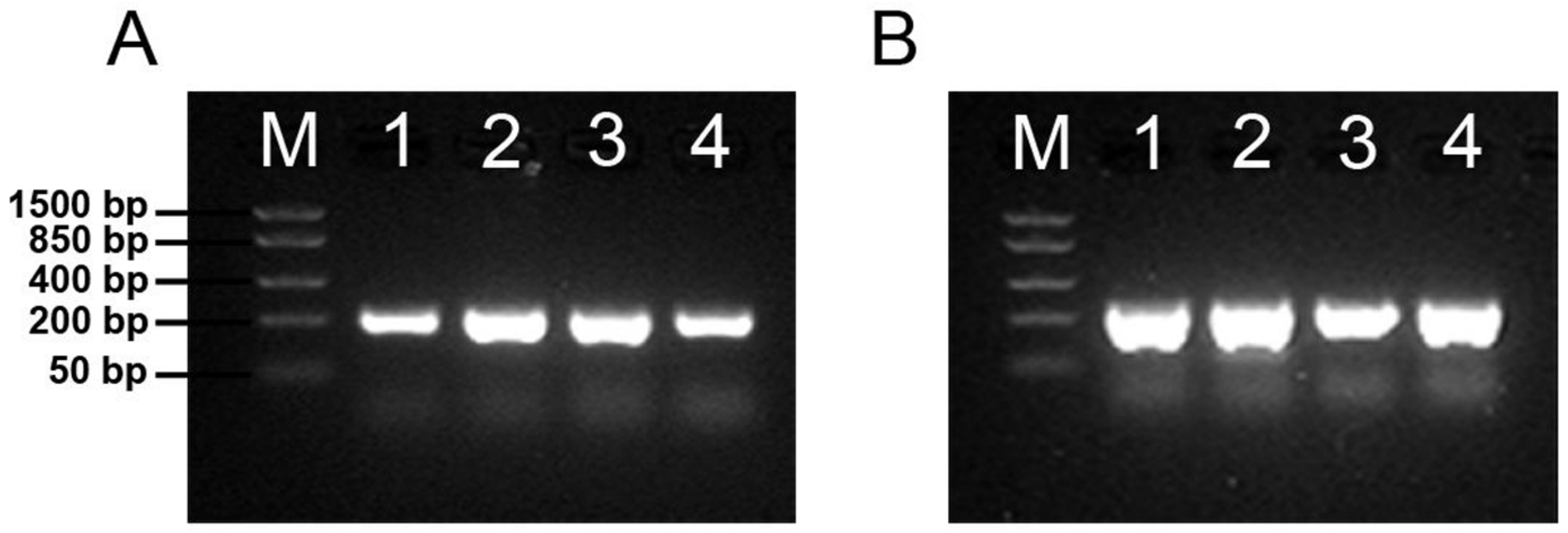
3.3. Both Polysialyltransferases Are Expressed in Udder Tissue
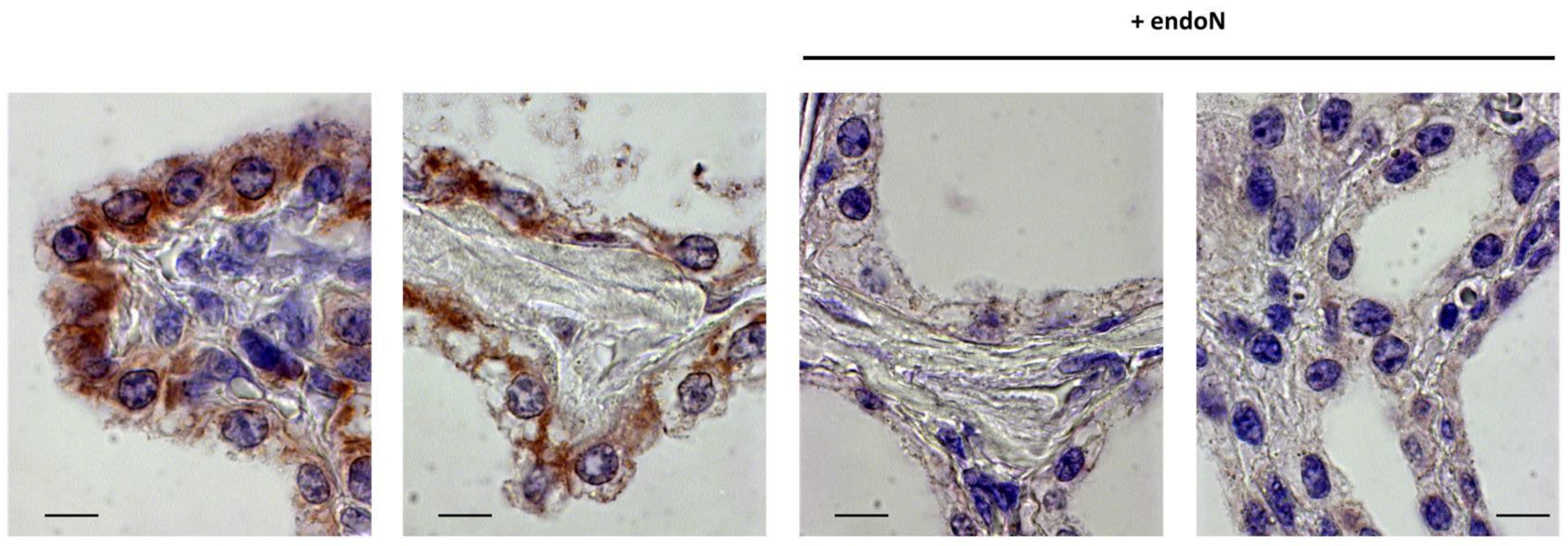
3.4. PolySia Is Located in Epithelial Cells in Udder Tissue
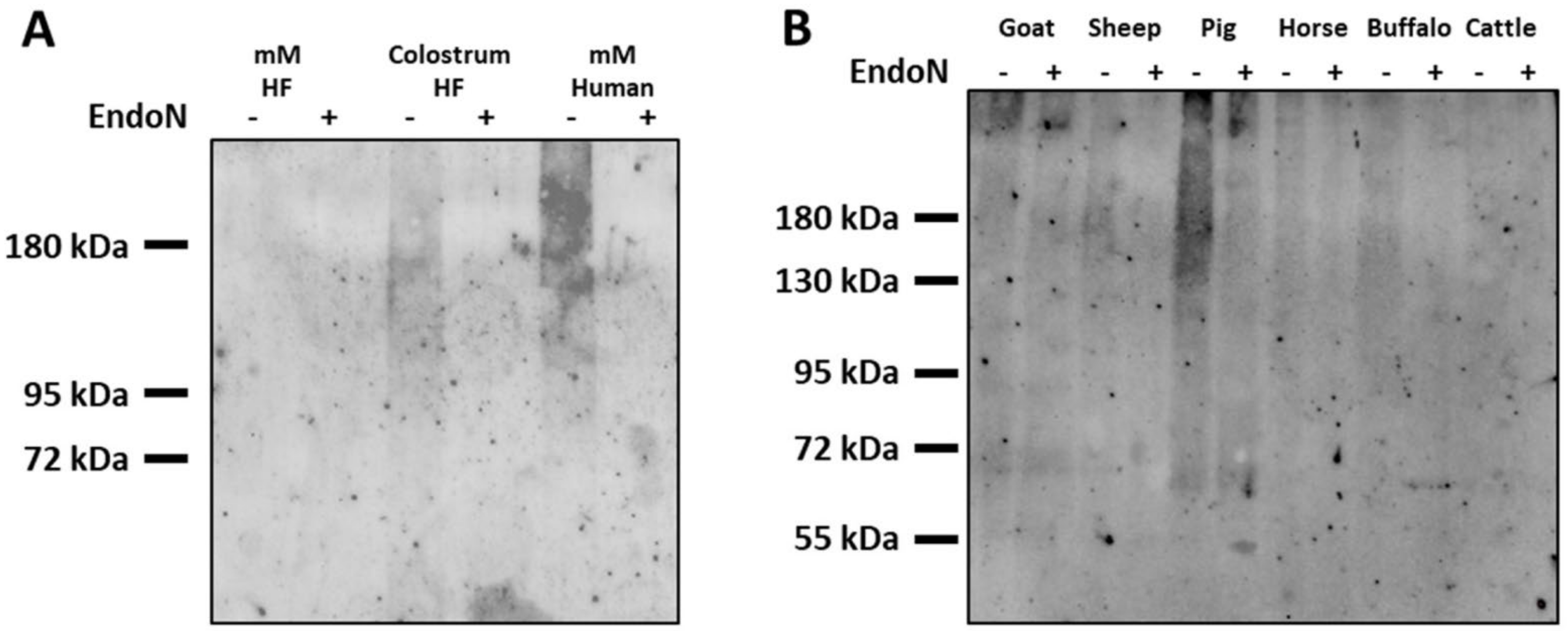
3.5. Analysis of PolySia in Milk of Different Farm Animals and Human Milk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schauer, R. Sialic acids as link to Japanese scientists. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2016, 92, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic acids: Fascinating sugars in higher animals and man. Zoology 2004, 107, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, P.; Aebi, M.; Varki, A. Evolution of Glycan Diversity. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 253–264. [Google Scholar] [CrossRef]
- Angata, T.; Varki, A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem. Rev. 2002, 102, 439–469. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef]
- Hobbs, M.; Jahan, M.; Ghorashi, S.A.; Wang, B. Current Perspective of Sialylated Milk Oligosaccharides in Mammalian Milk: Implications for Brain and Gut Health of Newborns. Foods 2021, 10, 473. [Google Scholar] [CrossRef]
- Triantis, V.; Bode, L.; van Neerven, R.J.J. Immunological Effects of Human Milk Oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef]
- Morozov, V.; Hansman, G.; Hanisch, F.-G.; Schroten, H.; Kunz, C. Human Milk Oligosaccharides as Promising Antivirals. Mol. Nutr. Food Res. 2018, 62, 1700679. [Google Scholar] [CrossRef]
- Röhrig, C.H.; Choi, S.S.; Baldwin, N. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Crit. Rev. Food Sci. Nutr. 2017, 57, 1017–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ze, X.; Rui, B.; Li, X.; Zeng, N.; Yuan, J.; Li, W.; Yan, J.; Li, M. Studies and Application of Sialylated Milk Components on Regulating Neonatal Gut Microbiota and Health. Front. Nutr. 2021, 8, 766606. [Google Scholar] [CrossRef]
- Spichtig, V.; Michaud, J.; Austin, S. Determination of sialic acids in milks and milk-based products. Anal. Biochem. 2010, 405, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Colley, K.J.; Kitajima, K.; Sato, C. Polysialic acid: Biosynthesis, novel functions and applications. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 498–532. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Elkashef, S.M.; Loadman, P.M.; Patterson, L.H.; Falconer, R.A. Recent advances in the analysis of polysialic acid from complex biological systems. Carbohydr. Polym. 2019, 224, 115145. [Google Scholar] [CrossRef]
- Sato, C.; Kitajima, K. Disialic, oligosialic and polysialic acids: Distribution, functions and related disease. J. Biochem. 2013, 154, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Yabe, U.; Sato, C.; Matsuda, T.; Kitajima, K. Polysialic acid in human milk. CD36 is a new member of mammalian polysialic acid-containing glycoprotein. J. Biol. Chem. 2003, 278, 13875–13880. [Google Scholar] [CrossRef]
- Kuhnle, A.; Veelken, R.; Galuska, C.E.; Saftenberger, M.; Verleih, M.; Schuppe, H.C.; Rudloff, S.; Kunz, C.; Galuska, S.P. Polysialic acid interacts with lactoferrin and supports its activity to inhibit the release of neutrophil extracellular traps. Carbohydr. Polym. 2019, 208, 32–41. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef]
- Rey-Gallardo, A.; Escribano, C.; Delgado-Martin, C.; Rodriguez-Fernandez, J.L.; Gerardy-Schahn, R.; Rutishauser, U.; Corbi, A.L.; Vega, M.A. Polysialylated neuropilin-2 enhances human dendritic cell migration through the basic C-terminal region of CCL21. Glycobiology 2010, 20, 1139–1146. [Google Scholar] [CrossRef]
- Rutishauser, U. Polysialic acid at the cell surface: Biophysics in service of cell interactions and tissue plasticity. J. Cell. Biochem. 1998, 70, 304–312. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Zhang, G. Insights into the Role of Sialylation in Cancer Metastasis, Immunity, and Therapeutic Opportunity. Cancers 2022, 14, 5840. [Google Scholar] [CrossRef]
- Rosa, P.; Scibetta, S.; Pepe, G.; Mangino, G.; Capocci, L.; Moons, S.J.; Boltje, T.J.; Fazi, F.; Petrozza, V.; Di Pardo, A.; et al. Polysialic Acid Sustains the Hypoxia-Induced Migration and Undifferentiated State of Human Glioblastoma Cells. Int. J. Mol. Sci. 2022, 23, 9563. [Google Scholar] [CrossRef] [PubMed]
- Martersteck, C.M.; Kedersha, N.L.; Drapp, D.A.; Tsui, T.G.; Colley, K.J. Unique alpha 2, 8-polysialylated glycoproteins in breast cancer and leukemia cells. Glycobiology 1996, 6, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Otake, Y.; Nakagawa, T.; Kawano, Y.; Miyahara, R.; Li, M.; Yanagihara, K.; Inui, K.; Oyanagi, H.; Yamada, T.; et al. Prognostic significance of polysialic acid expression in resected non-small cell lung cancer. Cancer Res. 2001, 61, 1666–1670. [Google Scholar] [PubMed]
- Falconer, R.A.; Errington, R.J.; Shnyder, S.D.; Smith, P.J.; Patterson, L.H. Polysialyltransferase: A new target in metastatic cancer. Curr. Cancer Drug Targets 2012, 12, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Strubl, S.; Schubert, U.; Kuhnle, A.; Rebl, A.; Ahmadvand, N.; Fischer, S.; Preissner, K.T.; Galuska, S.P. Polysialic acid is released by human umbilical vein endothelial cells (HUVEC) in vitro. Cell Biosci. 2018, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Hane, M.; Matsuoka, S.; Ono, S.; Miyata, S.; Kitajima, K.; Sato, C. Protective effects of polysialic acid on proteolytic cleavage of FGF2 and proBDNF/BDNF. Glycobiology 2015, 25, 1112–1124. [Google Scholar] [CrossRef]
- Ono, S.; Hane, M.; Kitajima, K.; Sato, C. Novel regulation of fibroblast growth factor 2 (FGF2)-mediated cell growth by polysialic acid. J. Biol. Chem. 2012, 287, 3710–3722. [Google Scholar] [CrossRef]
- Kuhnle, A.; Galuska, C.E.; Zlatina, K.; Galuska, S.P. The Bovine Antimicrobial Peptide Lactoferricin Interacts with Polysialic Acid without Loss of Its Antimicrobial Activity against Escherichia coli. Animals 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Loers, G.; Schachner, M.; Boelens, R.; Wienk, H.; Siebert, S.; Eckert, T.; Kraan, S.; Rojas-Macias, M.A.; Lutteke, T.; et al. Molecular Basis of the Receptor Interactions of Polysialic Acid (polySia), polySia Mimetics, and Sulfated Polysaccharides. ChemMedChem 2016, 11, 990–1002. [Google Scholar] [CrossRef]
- Zlatina, K.; Galuska, S.P. Polysialic Acid Modulates Only the Antimicrobial Properties of Distinct Histones. ACS Omega 2019, 4, 1601–1610. [Google Scholar] [CrossRef]
- Günther, J.; Koczan, D.; Yang, W.; Nürnberg, G.; Repsilber, D.; Schuberth, H.J.; Park, Z.; Maqbool, N.; Molenaar, A.; Seyfert, H.M. Assessment of the immune capacity of mammary epithelial cells: Comparison with mammary tissue after challenge with Escherichia coli. Vet. Res. 2009, 40, 31. [Google Scholar] [CrossRef] [PubMed]
- Petzl, W.; Zerbe, H.; Günther, J.; Yang, W.; Seyfert, H.M.; Nürnberg, G.; Schuberth, H.J. Escherichia coli, but not Staphylococcus aureus triggers an early increased expression of factors contributing to the innate immune defense in the udder of the cow. Vet. Res. 2008, 39, 18. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Yamaguchi, M.; Takemori, Y.; Furuhata, K.; Ogura, H.; Nakamura, M. Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Anal. Biochem. 1989, 179, 162–166. [Google Scholar] [CrossRef]
- Hara, S.; Takemori, Y.; Yamaguchi, M.; Nakamura, M.; Ohkura, Y. Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Anal. Biochem. 1987, 164, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zlatina, K.; Saftenberger, M.; Kuhnle, A.; Galuska, C.E.; Gartner, U.; Rebl, A.; Oster, M.; Vernunft, A.; Galuska, S.P. Polysialic Acid in Human Plasma Can Compensate the Cytotoxicity of Histones. Int. J. Mol. Sci. 2018, 19, 1679. [Google Scholar] [CrossRef] [PubMed]
- Venuto, M.T.; Martorell-Ribera, J.; Bochert, R.; Harduin-Lepers, A.; Rebl, A.; Galuska, S.P. Characterization of the Polysialylation Status in Ovaries of the Salmonid Fish Coregonus maraena and the Percid Fish Sander lucioperca. Cells 2020, 9, 2391. [Google Scholar] [CrossRef]
- Sato, C.; Kitajima, K. Polysialylation and disease. Mol. Asp. Med. 2020, 79, 100892. [Google Scholar] [CrossRef]
- Martín, M.J.; Martín-Sosa, S.; García-Pardo, L.A.; Hueso, P. Distribution of Bovine Milk Sialoglycoconjugates During Lactation. J. Dairy Sci. 2001, 84, 995–1000. [Google Scholar] [CrossRef]
- Jakobsson, E.; Jokilammi, A.; Aalto, J.; Ollikka, P.; Lehtonen, J.V.; Hirvonen, H.; Finne, J. Identification of amino acid residues at the active site of endosialidase that dissociate the polysialic acid binding and cleaving activities in Escherichia coli K1 bacteriophages. Biochem. J. 2007, 405, 465–472. [Google Scholar] [CrossRef]
- Stummeyer, K.; Dickmanns, A.; Muhlenhoff, M.; Gerardy-Schahn, R.; Ficner, R. Crystal structure of the polysialic acid-degrading endosialidase of bacteriophage K1F. Nat. Struct. Mol. Biol. 2005, 12, 90–96. [Google Scholar] [CrossRef]
- Weaver, D.M.; Tyler, J.W.; VanMetre, D.C.; Hostetler, D.E.; Barrington, G.M. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 2000, 14, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- McGrath, B.A.; Fox, P.F.; McSweeney, P.L.H.; Kelly, A.L. Composition and properties of bovine colostrum: A review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar] [CrossRef]
- Varki, A. PAMPs, DAMPs and SAMPs: Host Glycans are Self-Associated Molecular Patterns, but subject to Microbial Molecular Mimicry. FASEB J. 2020, 34. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Varki, A.; Gagneux, P. Multifarious roles of sialic acids in immunity. Ann. N. Y. Acad. Sci. 2012, 1253, 16–36. [Google Scholar] [CrossRef]
- Schauer, R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef]
- Bornhofft, K.F.; Goldammer, T.; Rebl, A.; Galuska, S.P. Siglecs: A journey through the evolution of sialic acid-binding immunoglobulin-type lectins. Dev. Comp. Immunol. 2018, 86, 219–231. [Google Scholar] [CrossRef]
- Bornhöfft, K.F.; Galuska, S.P. Glycans as Modulators for the Formation and Functional Properties of Neutrophil Extracellular Traps: Used by the Forces of Good and Evil. Front. Immunol. 2019, 10, 959. [Google Scholar] [CrossRef]
- Lizcano, A.; Secundino, I.; Dohrmann, S.; Corriden, R.; Rohena, C.; Diaz, S.; Ghosh, P.; Deng, L.; Nizet, V.; Varki, A. Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation. Blood 2017, 129, 3100–3110. [Google Scholar] [CrossRef]
- Bornhofft, K.F.; Rebl, A.; Gallagher, M.E.; Viergutz, T.; Zlatina, K.; Reid, C.; Galuska, S.P. Sialylated Cervical Mucins Inhibit the Activation of Neutrophils to Form Neutrophil Extracellular Traps in Bovine in vitro Model. Front. Immunol. 2019, 10, 2478. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Preissner, K.T. Fighting against the dark side of neutrophil extracellular traps in disease: Manoeuvres for host protection. Curr. Opin. Hematol. 2013, 20, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Knopf, J.; Naschberger, E.; Lindemann, A.; Singh, J.; Herrmann, I.; Sturzl, M.; Staats, L.; Mahajan, A.; Schauer, C.; et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 2020, 58, 102925. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Sun, H.Z.; Guan, R.W.; Shi, F.; Zhao, F.Q.; Liu, J.X. Formation of Blood Neutrophil Extracellular Traps Increases the Mastitis Risk of Dairy Cows During the Transition Period. Front. Immunol. 2022, 13, 880578. [Google Scholar] [CrossRef]
- Pisanu, S.; Cubeddu, T.; Pagnozzi, D.; Rocca, S.; Cacciotto, C.; Alberti, A.; Marogna, G.; Uzzau, S.; Addis, M.F. Neutrophil extracellular traps in sheep mastitis. Vet. Res. 2015, 46, 59. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Wang, Y.; Wang, C.; Liu, X.; Han, Z.; Fu, Y.; Yang, Z. Effects of Neutrophil Extracellular Traps on Bovine Mammary Epithelial Cells in vitro. Front. Immunol. 2019, 10, 1003. [Google Scholar] [CrossRef]
- Wall, S.K.; Gross, J.J.; Kessler, E.C.; Villez, K.; Bruckmaier, R.M. Blood-derived proteins in milk at start of lactation: Indicators of active or passive transfer. J. Dairy Sci. 2015, 98, 7748–7756. [Google Scholar] [CrossRef]
- Hoeksema, M.; van Eijk, M.; Haagsman, H.P.; Hartshorn, K.L. Histones as mediators of host defense, inflammation and thrombosis. Future Microbiol. 2016, 11, 441–453. [Google Scholar] [CrossRef]
- Galuska, S.P.; Galuska, C.E.; Tharmalingam, T.; Zlatina, K.; Prem, G.; Husejnov, F.C.O.; Rudd, P.M.; Vann, W.F.; Reid, C.; Vionnet, J.; et al. In vitro generation of polysialylated cervical mucins by bacterial polysialyltransferases to counteract cytotoxicity of extracellular histones. FEBS J. 2017, 284, 1688–1699. [Google Scholar] [CrossRef]
- Mishra, B.; von der Ohe, M.; Schulze, C.; Bian, S.; Makhina, T.; Loers, G.; Kleene, R.; Schachner, M. Functional role of the interaction between polysialic acid and extracellular histone H1. J. Neurosci. 2010, 30, 12400–12413. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Kamiya, M.; Urano, Y.; Nishi, H.; Herter, J.M.; Mayadas, T.; Hirohama, D.; Suzuki, K.; Kawakami, H.; Tanaka, M.; et al. Lactoferrin Suppresses Neutrophil Extracellular Traps Release in Inflammation. EBioMedicine 2016, 10, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Thiesler, H.; Beimdiek, J.; Hildebrandt, H. Polysialic acid and Siglec-E orchestrate negative feedback regulation of microglia activation. Cell. Mol. Life Sci. 2020, 78, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, S.; Buettner, F.F.; Erben, L.; Mathews, M.; Neumann, H.; Muhlenhoff, M.; Hildebrandt, H. Polysialylation and lipopolysaccharide-induced shedding of E-selectin ligand-1 and neuropilin-2 by microglia and THP-1 macrophages. Glia 2016, 64, 1314–1330. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, S.; Muhlenhoff, M.; Stangel, M.; Hildebrandt, H. Polysialic acid on SynCAM 1 in NG2 cells and on neuropilin-2 in microglia is confined to intracellular pools that are rapidly depleted upon stimulation. Glia 2015, 63, 1240–1255. [Google Scholar] [CrossRef]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Frangsmyr, L.; Frank, M.; Blaum, B.S.; Chai, W.; Podgorski, I.I.; Harrach, B.; Benko, M.; et al. Polysialic acid is a cellular receptor for human adenovirus 52. Proc. Natl. Acad. Sci. USA 2018, 115, E4264–E4273. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinterseher, J.; Günther, J.; Zlatina, K.; Isernhagen, L.; Viergutz, T.; Wirthgen, E.; Hoeflich, A.; Vernunft, A.; Galuska, S.P. Milk Polysialic Acid Levels Rapidly Decrease in Line with the N-Acetylneuraminic Acid Concentrations during Early Lactation in Dairy Cows. Biology 2023, 12, 5. https://doi.org/10.3390/biology12010005
Hinterseher J, Günther J, Zlatina K, Isernhagen L, Viergutz T, Wirthgen E, Hoeflich A, Vernunft A, Galuska SP. Milk Polysialic Acid Levels Rapidly Decrease in Line with the N-Acetylneuraminic Acid Concentrations during Early Lactation in Dairy Cows. Biology. 2023; 12(1):5. https://doi.org/10.3390/biology12010005
Chicago/Turabian StyleHinterseher, Julia, Juliane Günther, Kristina Zlatina, Lisa Isernhagen, Torsten Viergutz, Elisa Wirthgen, Andreas Hoeflich, Andreas Vernunft, and Sebastian Peter Galuska. 2023. "Milk Polysialic Acid Levels Rapidly Decrease in Line with the N-Acetylneuraminic Acid Concentrations during Early Lactation in Dairy Cows" Biology 12, no. 1: 5. https://doi.org/10.3390/biology12010005
APA StyleHinterseher, J., Günther, J., Zlatina, K., Isernhagen, L., Viergutz, T., Wirthgen, E., Hoeflich, A., Vernunft, A., & Galuska, S. P. (2023). Milk Polysialic Acid Levels Rapidly Decrease in Line with the N-Acetylneuraminic Acid Concentrations during Early Lactation in Dairy Cows. Biology, 12(1), 5. https://doi.org/10.3390/biology12010005








