A Mouse Model of Neurodegeneration Induced by Blade Penetrating Stab Wound to the Hippocampus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
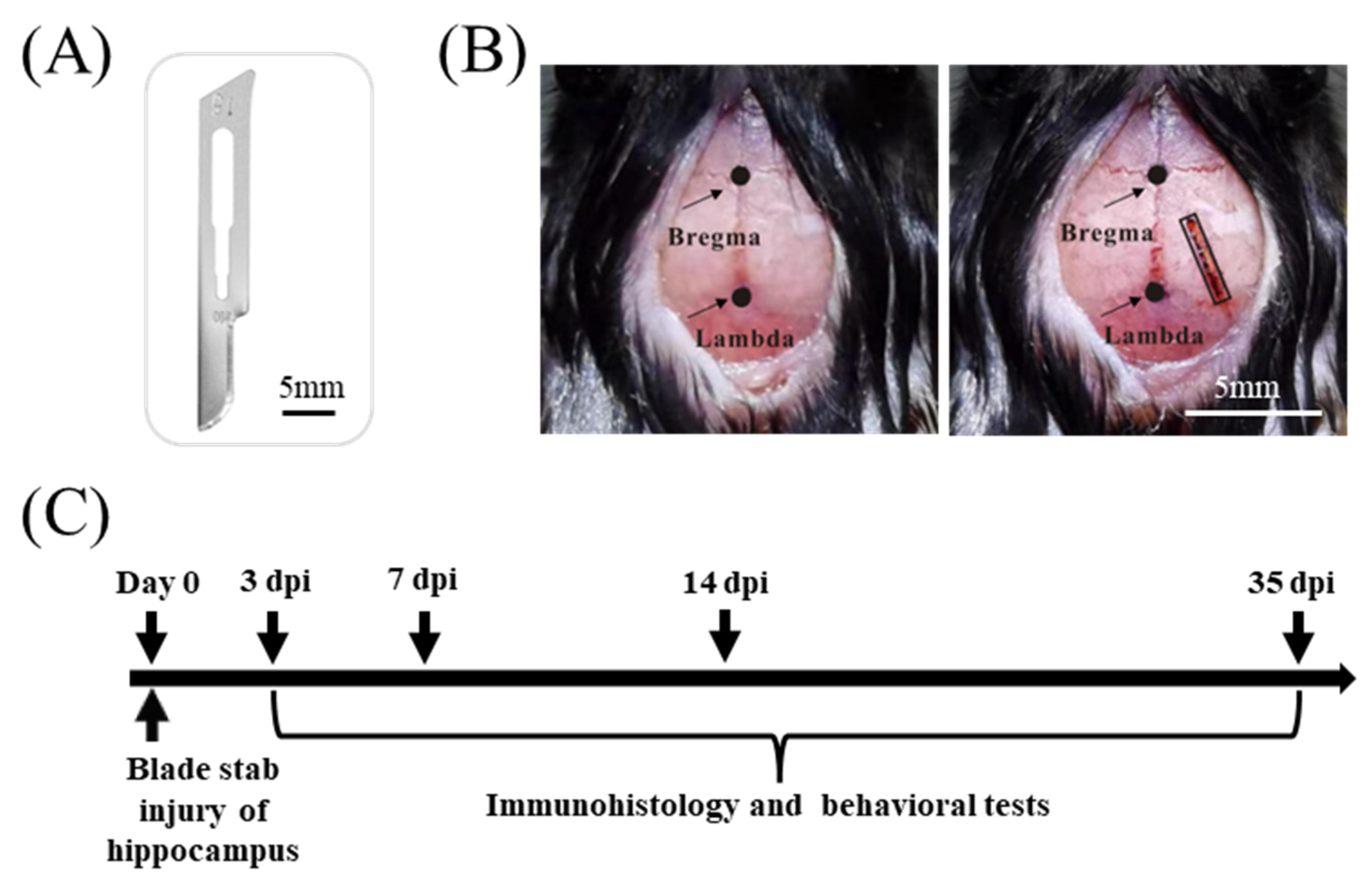
2.2. Blade Penetrating Stab Wound
2.3. Behavioral Tests
2.4. Immunofluorescence Assay
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Statistical Analysis
3. Results
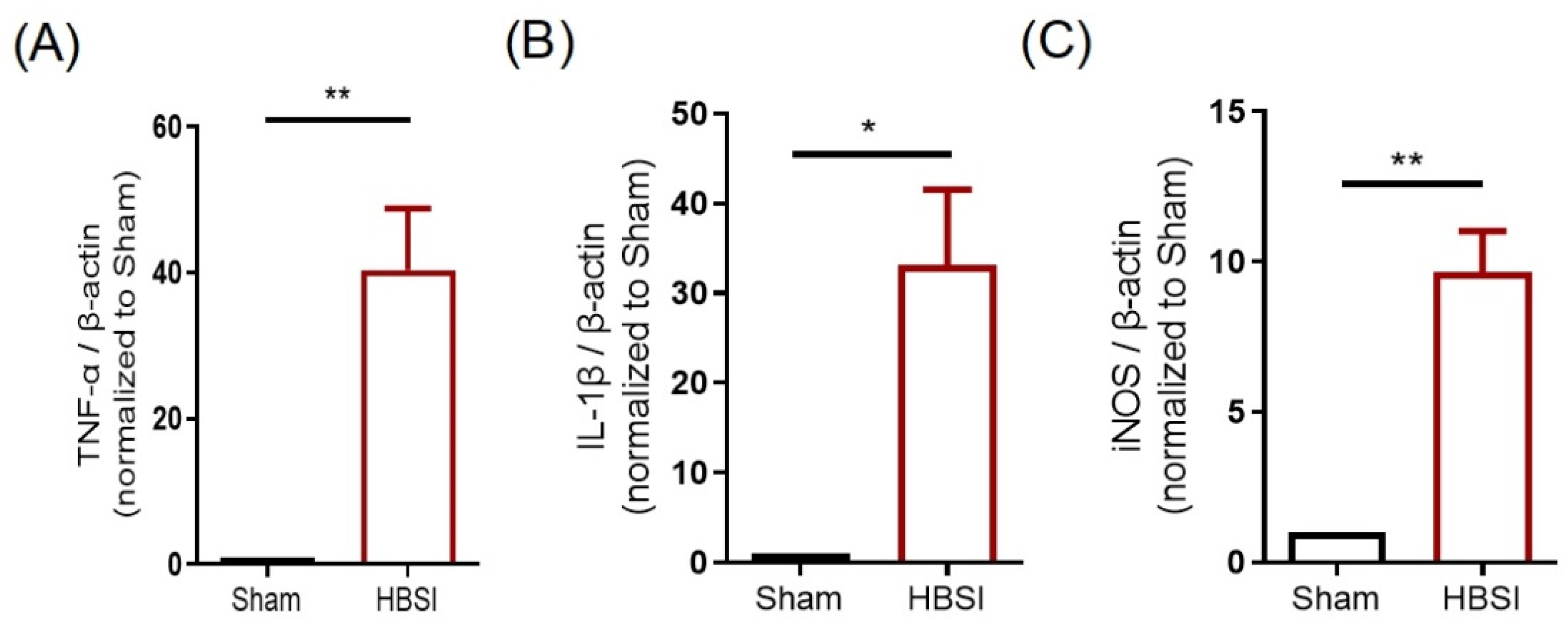
3.1. HBSI Triggers an Upregulation of Proinflammatory Cytokines in the Hippocampus
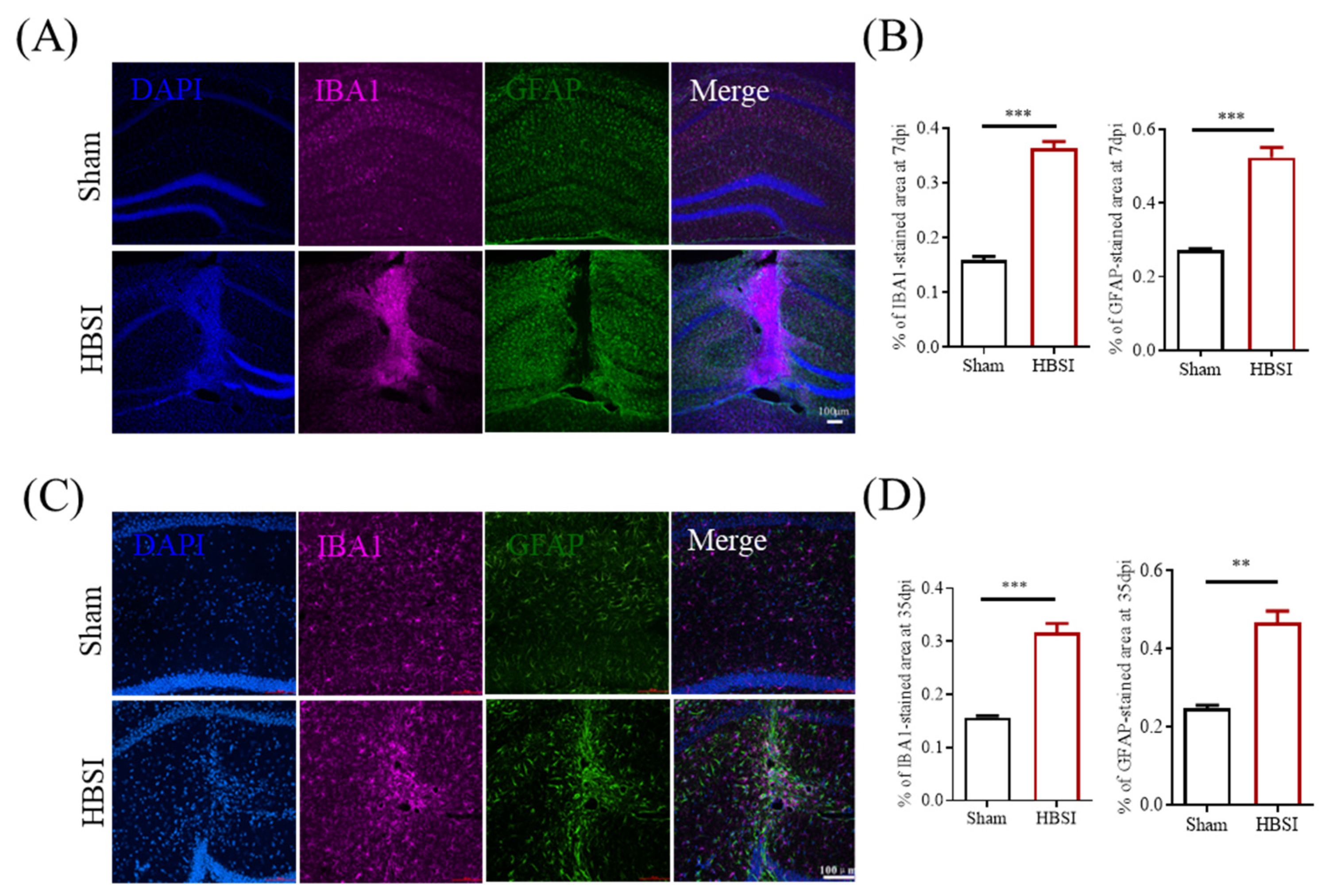
3.2. HBSI Results in Augmented Gliosis in the Hippocampus
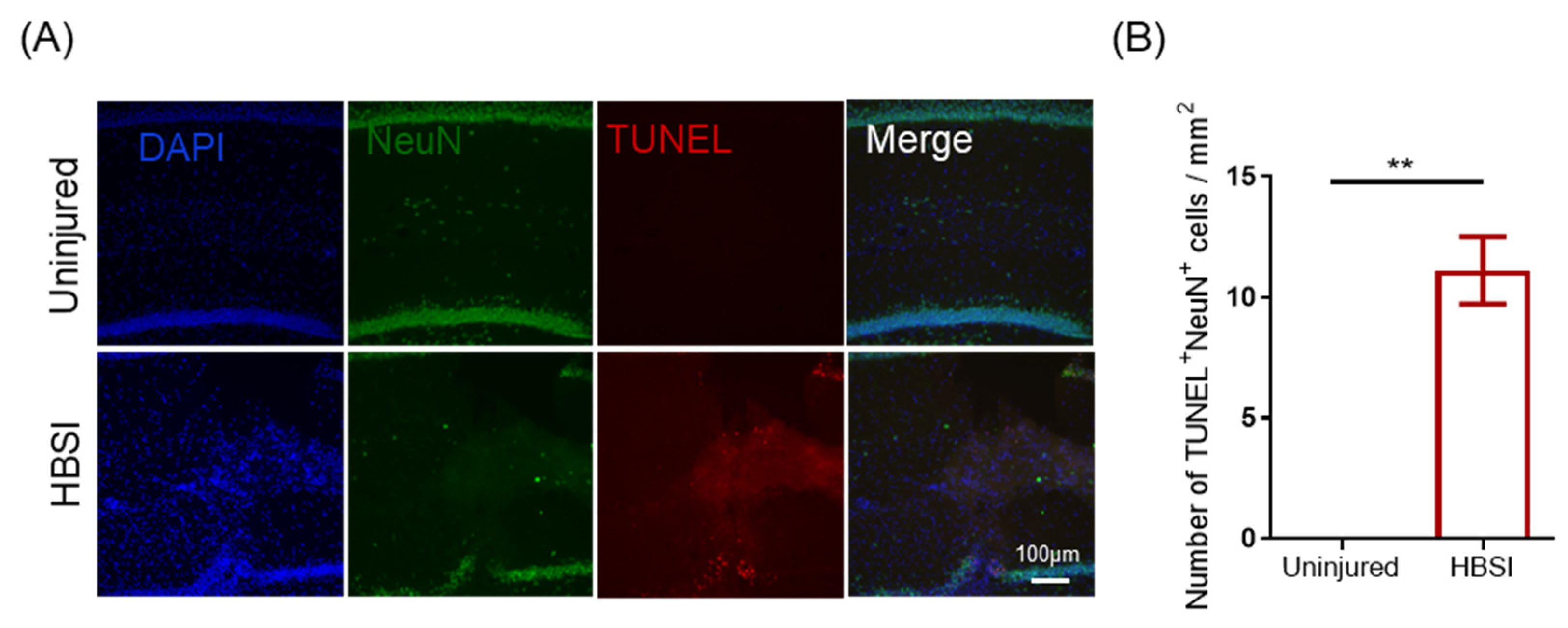
3.3. HBSI Leads to Neuronal Apoptosis in the Hippocampus
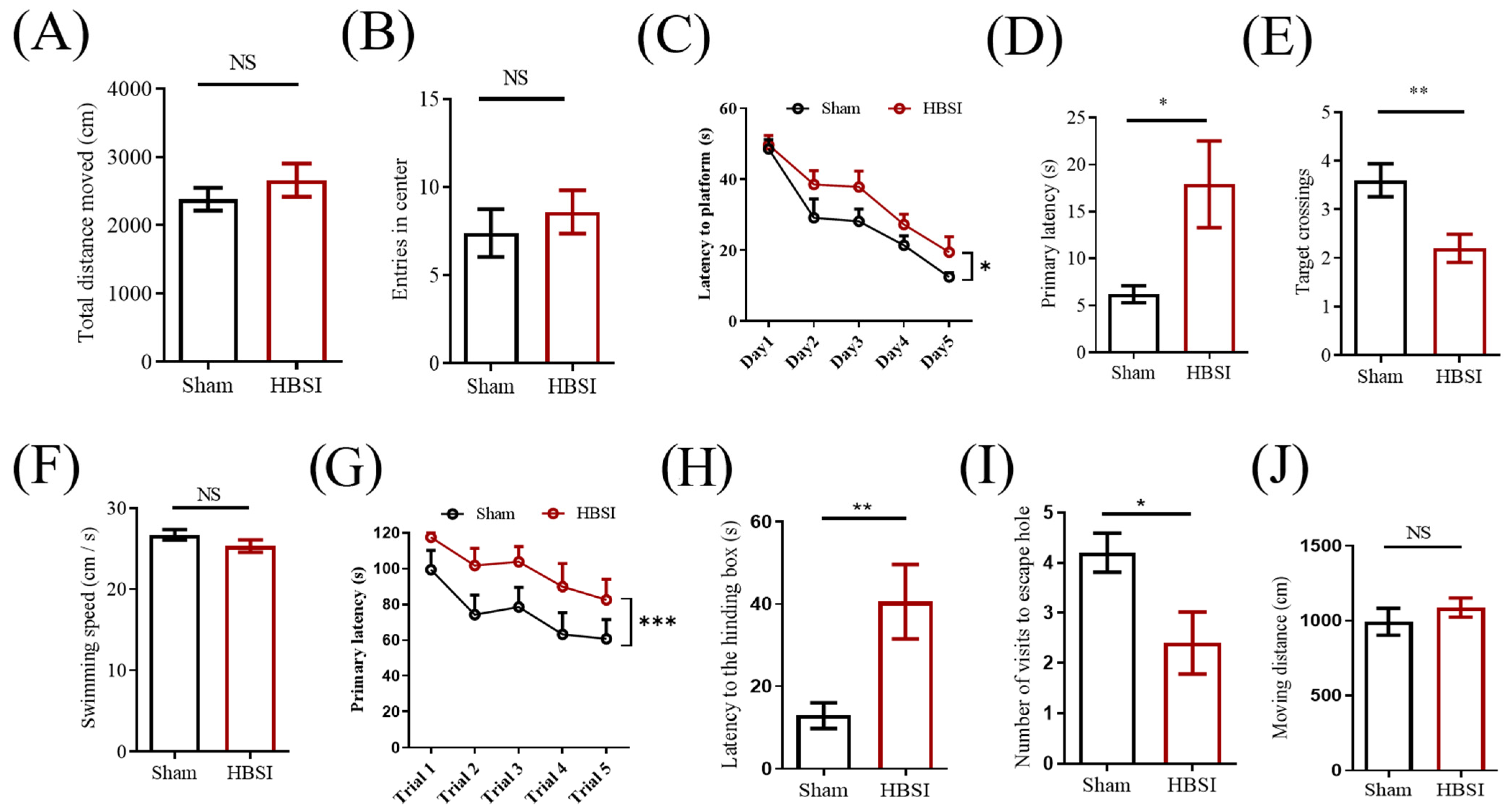
3.4. Mice with HBSI Exhibit Deficits in Learning and Memory
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pihlstrøm, L.; Wiethoff, S.; Houlden, H. Genetics of neurodegenerative diseases: An overview. Handb. Clin. Neurol. 2017, 145, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.Y.; Ho, P.W.L.; Liu, H.F.; Leung, C.T.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S.-L. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 2019, 8, 23. [Google Scholar] [CrossRef]
- Dunn, A.R.; O’Connell, K.M.S.; Kaczorowski, C.C. Gene-by-environment interactions in Alzheimer’s disease and Parkinson’s disease. Neurosci. Biobehav. Rev. 2019, 103, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Izzy, S.; Chen, P.M.; Tahir, Z.; Grashow, R.; Radmanesh, F.; Cote, D.J.; Yahya, T.; Dhand, A.; Taylor, H.; Shih, S.L.; et al. Association of Traumatic Brain Injury with the Risk of Developing Chronic Cardiovascular, Endocrine, Neurological, and Psychiatric Disorders. JAMA Netw. Open 2022, 5, e229478. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sen, N. Traumatic brain injury: A risk factor for neurodegenerative diseases. Rev. Neurosci. 2016, 27, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dai, S.-K.; Shi, R.-X.; He, X.-C.; Wang, Y.-Y.; He, B.-D.; Sun, X.-W.; Du, H.-Z.; Liu, C.-M.; Teng, Z.-Q. Transcriptional profiling of microglia in the injured brain reveals distinct molecular features underlying neurodegeneration. Glia 2021, 69, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Somogyi, P.; Klausberger, T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J. Physiol. 2005, 562, 9–26. [Google Scholar] [CrossRef]
- Cohen, A.S.; Pfister, B.J.; Schwarzbach, E.; Grady, M.S.; Goforth, P.B.; Satin, L.S. Injury-induced alterations in CNS electrophysiology. Prog. Brain Res. 2007, 161, 143–169. [Google Scholar] [CrossRef]
- Liotta, A.; Rösner, J.; Huchzermeyer, C.; Wojtowicz, A.; Kann, O.; Schmitz, D.; Heinemann, U.; Kovacs, R. Energy Demand of Synaptic Transmission at the Hippocampal Schaffer-Collateral Synapse. J. Cereb. Blood Flow Metab. 2012, 32, 2076–2083. [Google Scholar] [CrossRef]
- Alle, H.; Roth, A.; Geiger, J.R.P. Energy-Efficient Action Potentials in Hippocampal Mossy Fibers. Science 2009, 325, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Spalding, K.L.; Frisen, J. Adult Neurogenesis in Humans. Cold Spring Harb. Perspect. Biol. 2015, 7, a018994. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.F.; Sorrells, S.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Does Adult Neurogenesis Persist in the Human Hippocampus? Cell Stem Cell 2018, 23, 780–781. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Liu, X.-T.; Liu, C.-M.; Teng, Z.-Q. Mouse model of voluntary movement deficits induced by needlestick injuries to the primary motor cortex. J. Neurosci. Methods 2022, 365, 109380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Deng, Y.-S.; Dai, S.-K.; Mi, T.-W.; Li, R.-Y.; Liu, P.-P.; Liu, C.; He, B.-D.; He, X.-C.; Du, H.-Z.; et al. Loss of microglial EED impairs synapse density, learning, and memory. Mol. Psychiatry 2022, 27, 2999–3009. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.-M.; Tan, W.; Wang, X.; Li, Y.; Bai, B.; Li, Y.; Zhang, S.-F.; Yan, H.-L.; Chen, Z.-L.; et al. Partial loss of psychiatric risk gene Mir137 in mice causes repetitive behavior and impairs sociability and learning via increased Pde10a. Nat. Neurosci. 2018, 21, 1689–1703. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Liu, C.; Wang, Y.-Y.; Deng, Y.-S.; He, X.-C.; Du, H.-Z.; Liu, C.-M.; Teng, Z.-Q. SerpinA3N deficiency deteriorates impairments of learning and memory in mice following hippocampal stab injury. Cell Death Discov. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Corps, K.N.; Roth, T.; McGavern, D.B. Inflammation and Neuroprotection in Traumatic Brain Injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, R.S.B.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef]
- Diaz-Arrastia, R.; Wang, K.; Papa, L.; Sorani, M.D.; Yue, J.; Puccio, A.M.; McMahon, P.J.; Inoue, T.; Yuh, E.L.; Lingsma, H.F.; et al. Acute Biomarkers of Traumatic Brain Injury: Relationship between Plasma Levels of Ubiquitin C-Terminal Hydrolase-L1 and Glial Fibrillary Acidic Protein. J. Neurotrauma 2014, 31, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ontiveros, D.G.; Tajiri, N.; Acosta, S.; Giunta, B.; Tan, J.; Borlongan, C.V. Microglia Activation as a Biomarker for Traumatic Brain Injury. Front. Neurol. 2013, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.E. Mechanisms of Traumatic Brain Injury: Biomechanical, Structural and Cellular Considerations. Crit. Care Nurs. Q. 2000, 23, 1–13. [Google Scholar] [CrossRef]
- Ma, X.; Aravind, A.; Pfister, B.J.; Chandra, N.; Haorah, J. Animal Models of Traumatic Brain Injury and Assessment of Injury Severity. Mol. Neurobiol. 2019, 56, 5332–5345. [Google Scholar] [CrossRef]
- Cernak, I. Animal Models of Head Trauma. NeuroRx 2005, 2, 410–422. [Google Scholar] [CrossRef]
- Gaetz, M. The neurophysiology of brain injury. Clin. Neurophysiol. 2004, 115, 4–18. [Google Scholar] [CrossRef]
- Hay, J.; Johnson, V.E.; Smith, D.H.; Stewart, W. Chronic Traumatic Encephalopathy: The Neuropathological Legacy of Traumatic Brain Injury. Annu. Rev. Pathol. 2016, 11, 21–45. [Google Scholar] [CrossRef]
- Marklund, N.; Bakshi, A.; Castelbuono, D.; Conte, V.; McIntosh, T. Evaluation of Pharmacological Treatment Strategies in Traumatic Brain Injury. Curr. Pharm. Des. 2006, 12, 1645–1680. [Google Scholar] [CrossRef]
- Jawabri, K.H.; Sharma, S. Physiology, Cerebral Cortex Functions. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef]
- Fisher, E.M.C.; Bannerman, D.M. Mouse models of neurodegeneration: Know your question, know your mouse. Sci. Transl. Med. 2019, 11, eaaq1818. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-Y.; Cho, H.; Lee, L.P. Human mini-brain models. Nat. Biomed. Eng. 2021, 5, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Oyefeso, F.A.; Muotri, A.R.; Wilson, C.G.; Pecaut, M.J. Brain organoids: A promising model to assess oxidative stress-induced central nervous system damage. Dev. Neurobiol. 2021, 81, 653–670. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.-D.; Liu, C.-M.; Teng, Z.-Q. A Mouse Model of Neurodegeneration Induced by Blade Penetrating Stab Wound to the Hippocampus. Biology 2022, 11, 1365. https://doi.org/10.3390/biology11091365
He B-D, Liu C-M, Teng Z-Q. A Mouse Model of Neurodegeneration Induced by Blade Penetrating Stab Wound to the Hippocampus. Biology. 2022; 11(9):1365. https://doi.org/10.3390/biology11091365
Chicago/Turabian StyleHe, Bao-Dong, Chang-Mei Liu, and Zhao-Qian Teng. 2022. "A Mouse Model of Neurodegeneration Induced by Blade Penetrating Stab Wound to the Hippocampus" Biology 11, no. 9: 1365. https://doi.org/10.3390/biology11091365
APA StyleHe, B.-D., Liu, C.-M., & Teng, Z.-Q. (2022). A Mouse Model of Neurodegeneration Induced by Blade Penetrating Stab Wound to the Hippocampus. Biology, 11(9), 1365. https://doi.org/10.3390/biology11091365




