Wide-Field Calcium Imaging of Neuronal Network Dynamics In Vivo
Abstract
:Simple Summary
Abstract
1. Introduction
2. Visualizing Neural Activity with Ca2+ Sensors
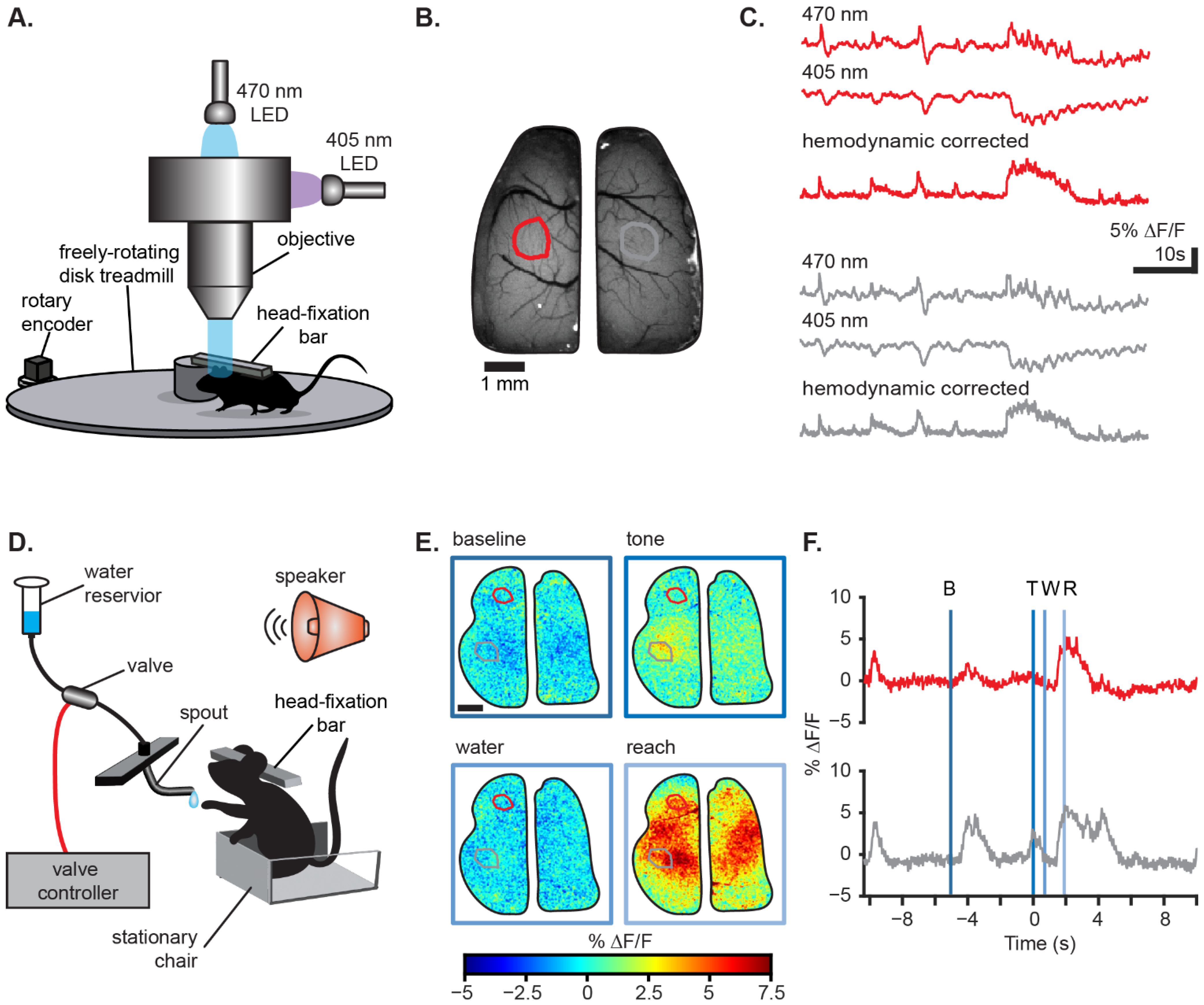
3. Monitoring Wide-Field Ca2+ Imaging Dynamics In Vivo
4. Limitations of Wide-Field Imaging
5. Analysis of Wide-Field Ca2+ Imaging Data
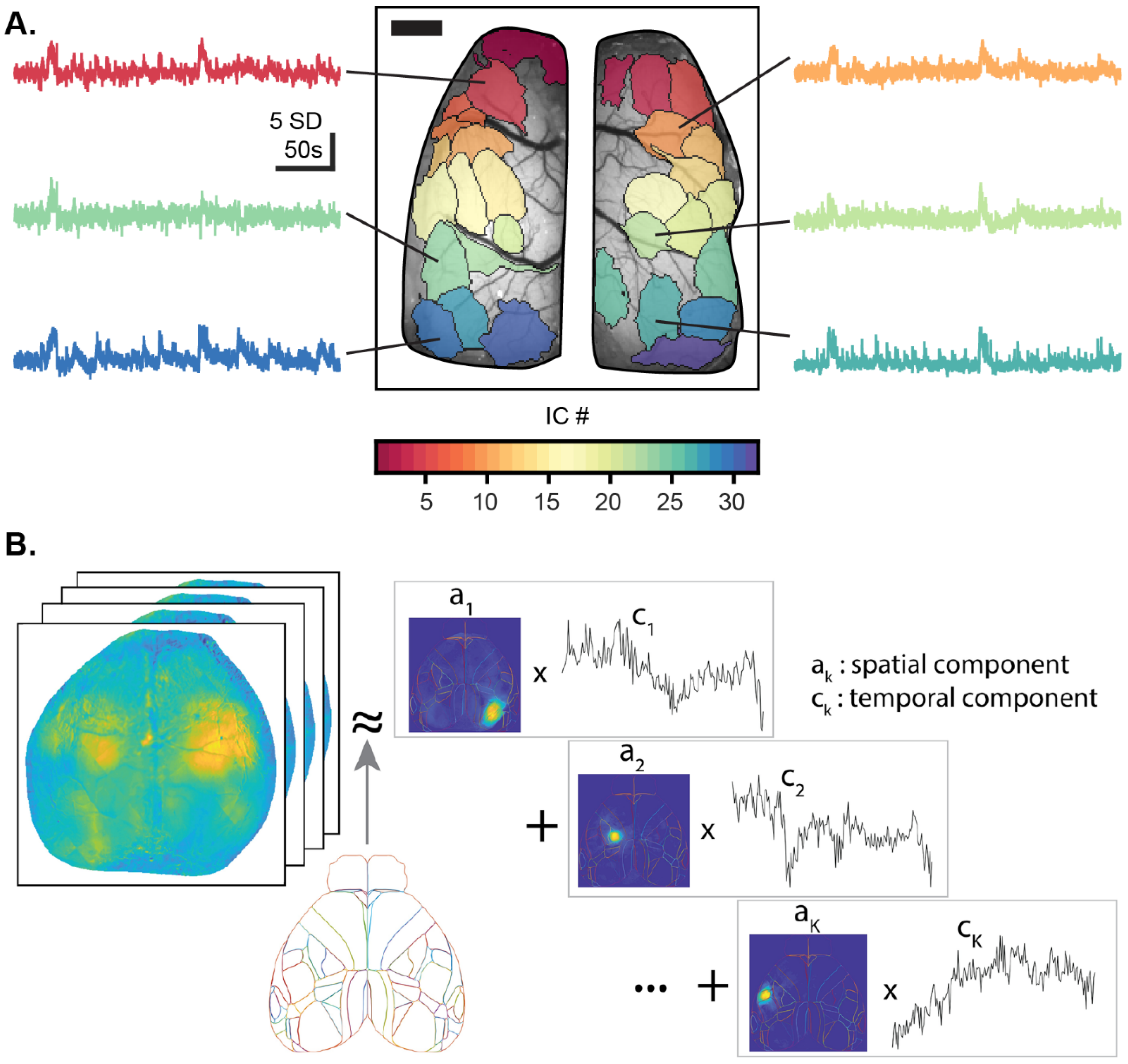
5.1. Functional Segmentation of the Imaging Field
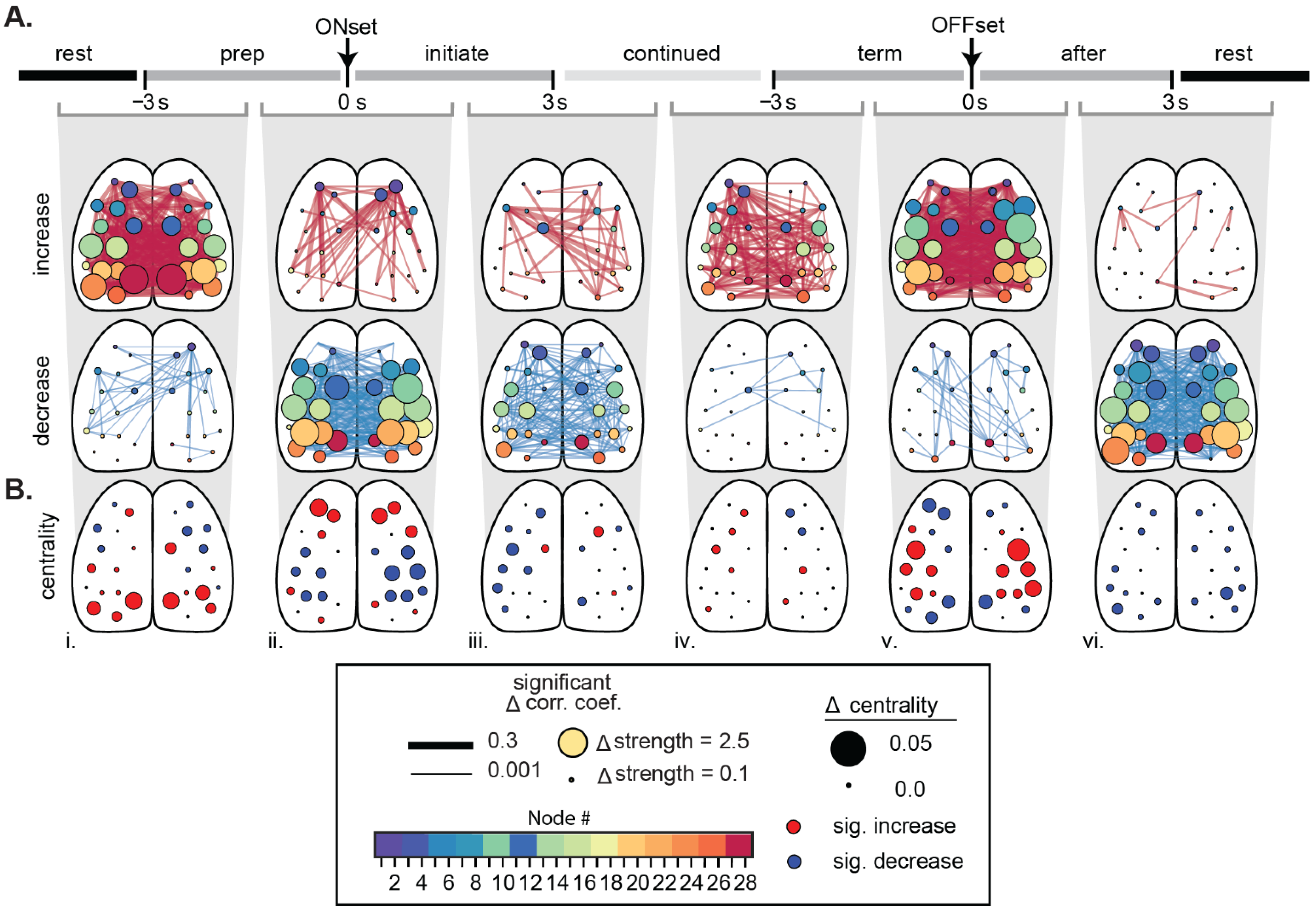
5.2. Ca2+ Imaging-Based Functional Connectivity
5.3. Analyzing the Relationship between Ca2+ and Behavior
6. Insights Gained from Wide-Field Ca2+ Imaging
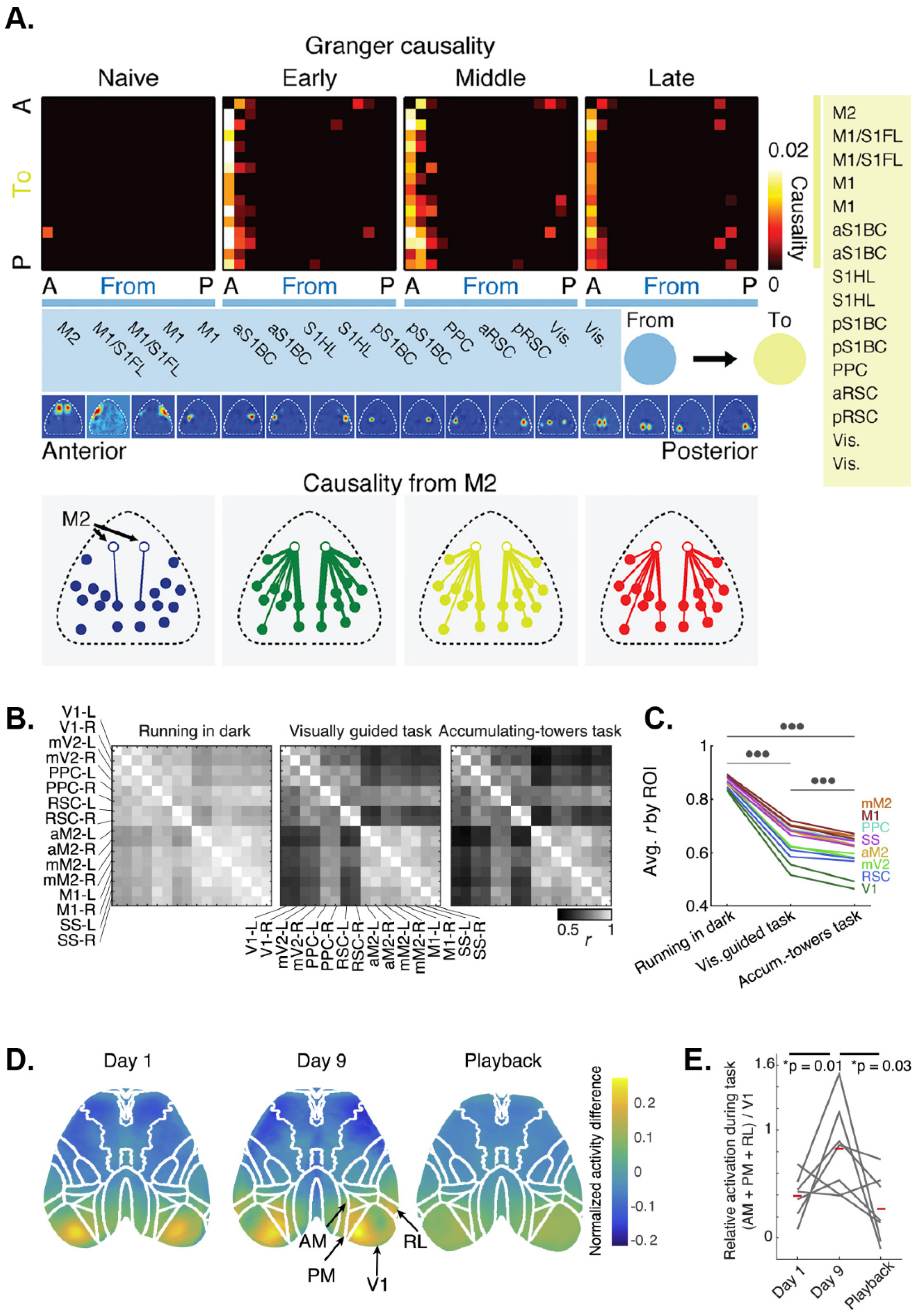
6.1. Elucidating Motor Control Using Wide-Field Ca2+ Imaging
6.2. Cortical Dynamics during Learning, Executive Functions, and Decision-Making
6.3. Cortical Activity during Visual Processing
6.4. Wide-Field Ca2+ Imaging in Neurological Disorders
7. New Developments
7.1. Voltage Sensors
7.2. Combined Recording Techniques and Multimodal Sensing
7.3. Free Range Mesoscopic Ca2+ Imaging
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buzsáki, G. Neural Syntax: Cell Assemblies, Synapsembles, and Readers. Neuron 2010, 68, 362–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, K.D. Neural signatures of cell assembly organization. Nat. Rev. Neurosci. 2005, 6, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Reid, L.; Yang, W.; Miller, J.-E.K.; Peterka, D.S.; Yuste, R. Imaging and Optically Manipulating Neuronal Ensembles. Annu. Rev. Biophys. 2017, 46, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Reid, L.; Yang, W.; Bando, Y.; Peterka, D.S.; Yuste, R. Imprinting and recalling cortical ensembles. Science 2016, 353, 691–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. From The Cover: The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avena-Koenigsberger, A.; Misic, B.; Sporns, O. Communication dynamics in complex brain networks. Nat. Rev. Neurosci. 2017, 19, 17–33. [Google Scholar] [CrossRef]
- Gilad, A.; Gallero-Salas, Y.; Groos, D.; Helmchen, F. Behavioral strategy determines frontal or posterior location of short-term memory in neocortex. Neuron 2018, 99, 814–828.e817. [Google Scholar] [CrossRef] [Green Version]
- Allen, W.E.; Kauvar, I.V.; Chen, M.Z.; Richman, E.B.; Yang, S.J.; Chan, K.; Gradinaru, V.; Deverman, B.E.; Luo, L.; Deisseroth, K. Global Representations of Goal-Directed Behavior in Distinct Cell Types of Mouse Neocortex. Neuron 2017, 94, 891–907.e896. [Google Scholar] [CrossRef]
- Ferezou, I.; Haiss, F.; Gentet, L.J.; Aronoff, R.; Weber, B.; Petersen, C.C. Spatiotemporal Dynamics of Cortical Sensorimotor Integration in Behaving Mice. Neuron 2007, 56, 907–923. [Google Scholar] [CrossRef] [Green Version]
- Dipoppa, M.; Ranson, A.; Krumin, M.; Pachitariu, M.; Carandini, M.; Harris, K.D. Vision and Locomotion Shape the Interactions between Neuron Types in Mouse Visual Cortex. Neuron 2018, 98, 602–615. [Google Scholar] [CrossRef]
- Saleem, A.B.; Ayaz, A.; Jeffery, K.J.; Harris, K.D.; Carandini, M. Integration of visual motion and locomotion in mouse visual cortex. Nat. Neurosci. 2013, 16, 1864–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.M.; Hoy, J.; Bonci, A.; Wilbrecht, L.; Stryker, M.; Niell, C.M. Identification of a Brainstem Circuit Regulating Visual Cortical State in Parallel with Locomotion. Neuron 2014, 83, 455–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, K.; Thiele, A. Cortical state and attention. Nat. Rev. Neurosci. 2011, 12, 509–523. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.J.; Vinck, M.; Reimer, J.; Batista-Brito, R.; Zagha, E.; Cadwell, C.R.; Tolias, A.S.; Cardin, J.A.; McCormick, D.A. Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron 2015, 87, 1143–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimaoka, D.; Harris, K.; Carandini, M. Effects of Arousal on Mouse Sensory Cortex Depend on Modality. Cell Rep. 2018, 25, 3230. [Google Scholar] [CrossRef]
- Blaser, R.; Heyser, C.J. Spontaneous object recognition: A promising approach to the comparative study of memory. Front. Behav. Neurosci. 2015, 9, 183. [Google Scholar] [CrossRef] [Green Version]
- Musall, S.; Kaufman, M.T.; Juavinett, A.L.; Gluf, S.; Churchland, A.K. Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci. 2019, 22, 1677–1686. [Google Scholar] [CrossRef]
- Makino, H.; Ren, C.; Liu, H.; Kim, A.N.; Kondapaneni, N.; Liu, X.; Kuzum, D.; Komiyama, T. Transformation of cortex-wide emergent properties during motor learning. Neuron 2017, 94, 880–890 e888. [Google Scholar] [CrossRef] [Green Version]
- Grinvald, A.; Omer, D.; Naaman, S.; Sharon, D. Imaging the Dynamics of Mammalian Neocortical Population Activity In-Vivo. Adv. Exp. Med. Biol. 2015, 859, 243–271. [Google Scholar] [CrossRef]
- Reinert, K.C.; Gao, W.; Chen, G.; Wang, X.; Peng, Y.-P.; Ebner, T.J. Cellular and Metabolic Origins of Flavoprotein Autofluorescence in the Cerebellar Cortex in vivo. Cerebellum 2011, 10, 585–599. [Google Scholar] [CrossRef]
- Ebner, T.J.; Chen, G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Prog. Neurobiol. 1995, 46, 463–506. [Google Scholar] [CrossRef]
- Ren, C.; Komiyama, T. Characterizing cortex-wide dynamics with wide-field calcium imaging. J. Neurosci. 2021, 41, 4160–4168. [Google Scholar] [CrossRef] [PubMed]
- Cardin, J.A.; Crair, M.C.; Higley, M.J. Mesoscopic Imaging: Shining a Wide Light on Large-Scale Neural Dynamics. Neuron 2020, 108, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Swanson, C.J.; Chen, J.; Li, A.; Lippert, L.G.; Boye, S.E.; Rose, K.; Sivaramakrishnan, S.; Chuong, C.-M.; Chow, R.H. The GCaMP-R Family of Genetically Encoded Ratiometric Calcium Indicators. ACS Chem. Biol. 2017, 12, 1066–1074. [Google Scholar] [CrossRef] [Green Version]
- Palazzolo, G.; Moroni, M.; Soloperto, A.; Aletti, G.; Naldi, G.; Vassalli, M.; Nieus, T.; Difato, F. Fast wide-volume functional imaging of engineered in vitro brain tissues. Sci. Rep. 2017, 7, 8499. [Google Scholar] [CrossRef] [Green Version]
- Scott, B.B.; Thiberge, S.; Guo, C.; Tervo, D.G.R.; Brody, C.D.; Karpova, A.Y.; Tank, D.W. Imaging Cortical Dynamics in GCaMP Transgenic Rats with a Head-Mounted Widefield Macroscope. Neuron 2018, 100, 1045–1058.e1045. [Google Scholar] [CrossRef] [Green Version]
- Klioutchnikov, A.; Wallace, D.J.; Frosz, M.H.; Zeltner, R.; Sawinski, J.; Pawlak, V.; Voit, K.-M.; Russell, P.S.J.; Kerr, J.N.D. Three-photon head-mounted microscope for imaging deep cortical layers in freely moving rats. Nat. Methods 2020, 17, 509–513. [Google Scholar] [CrossRef]
- Ebina, T.; Masamizu, Y.; Tanaka, Y.R.; Watakabe, A.; Hirakawa, R.; Hirayama, Y.; Hira, R.; Terada, S.-I.; Koketsu, D.; Hikosaka, K.; et al. Two-photon imaging of neuronal activity in motor cortex of marmosets during upper-limb movement tasks. Nat. Commun. 2018, 9, 1897. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.-H.; Huang, J.-F.; Chen, M.; Wen, Y.-Q.; Shen, Z.-M.; Poo, M.-M. Local homogeneity of tonotopic organization in the primary auditory cortex of marmosets. Proc. Natl. Acad. Sci. USA 2019, 116, 3239–3244. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.-H.; Huang, J.-F.; Li, J.-R.; Shen, Z.; Gong, N.; Wen, Y.-Q.; Wang, L.; Poo, M.-M. Distinct neuron populations for simple and compound calls in the primary auditory cortex of awake marmosets. Natl. Sci. Rev. 2021, 8, nwab126. [Google Scholar] [CrossRef]
- Garg, A.K.; Li, P.; Rashid, M.S.; Callaway, E.M. Color and orientation are jointly coded and spatially organized in primate primary visual cortex. Science 2019, 364, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Bollimunta, A.; Santacruz, S.R.; Eaton, R.W.; Xu, P.S.; Morrison, J.H.; Moxon, K.A.; Carmena, J.M.; Nassi, J.J. Head-mounted microendoscopic calcium imaging in dorsal premotor cortex of behaving rhesus macaque. Cell Rep. 2021, 35, 109239. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, J.; Calderón, N.C.; Tian, L.; Wabnig, S.; Prigge, M.; Tolö, J.; Gordus, A.; Orger, M.B.; Severi, K.E.; Macklin, J.J.; et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 2013, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Akerboom, J.; Chen, T.-W.; Wardill, T.; Tian, L.; Marvin, J.; Mutlu, S.; Calderón, N.C.; Esposti, F.; Borghuis, B.G.; Sun, X.R.; et al. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. J. Neurosci. 2012, 32, 13819–13840. [Google Scholar] [CrossRef] [Green Version]
- Badura, A.; Sun, X.R.; Giovannucci, A.; Lynch, L.A.; Wang, S.S.-H. Fast calcium sensor proteins for monitoring neural activity. Neurophotonics 2014, 1, 025008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grienberger, C.; Konnerth, A. Imaging Calcium in Neurons. Neuron 2012, 73, 862–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dana, H.; Sun, Y.; Mohar, B.; Hulse, B.K.; Kerlin, A.M.; Hasseman, J.P.; Tsegaye, G.; Tsang, A.; Wong, A.; Patel, R.; et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 2019, 16, 649–657. [Google Scholar] [CrossRef]
- Chen, T.-W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Nakai, J.; Ohkura, M.; Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 2001, 19, 137–141. [Google Scholar] [CrossRef]
- Tian, L.; Hires, S.A.; Mao, T.; Huber, D.; Chiappe, M.E.; Chalasani, S.H.; Petreanu, L.; Akerboom, J.; A McKinney, S.; Schreiter, E.; et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 2009, 6, 875–881. [Google Scholar] [CrossRef]
- Girven, K.S.; Sparta, D.R. Probing Deep Brain Circuitry: New Advances in in Vivo Calcium Measurement Strategies. ACS Chem. Neurosci. 2017, 8, 243–251. [Google Scholar] [CrossRef]
- Chen, Q.; Cichon, J.; Wang, W.; Qiu, L.; Lee, S.-J.R.; Campbell, N.R.; DeStefino, N.; Goard, M.J.; Fu, Z.; Yasuda, R.; et al. Imaging Neural Activity Using Thy1-GCaMP Transgenic Mice. Neuron 2012, 76, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Zariwala, H.A.; Borghuis, B.G.; Hoogland, T.M.; Madisen, L.; Tian, L.; De Zeeuw, C.I.; Zeng, H.; Looger, L.L.; Svoboda, K.; Chen, T.W. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci. 2012, 32, 3131–3141. [Google Scholar] [CrossRef] [Green Version]
- Madisen, L.; Garner, A.R.; Shimaoka, D.; Chuong, A.S.; Klapoetke, N.C.; Linda, L.; Van Der Bourg, A.; Niino, Y.; Egolf, L.; Monetti, C.; et al. Transgenic Mice for Intersectional Targeting of Neural Sensors and Effectors with High Specificity and Performance. Neuron 2015, 85, 942–958. [Google Scholar] [CrossRef] [Green Version]
- Nicola, F.D.C.; Hua, I.; Levine, A.J. Intersectional genetic tools to study skilled reaching in mice. Exp. Neurol. 2021, 347, 113879. [Google Scholar] [CrossRef]
- de Vries, S.E.J.; Lecoq, J.A.; Buice, M.A.; Groblewski, P.A.; Ocker, G.K.; Oliver, M.; Feng, D.; Cain, N.; Ledochowitsch, P.; Millman, D.; et al. A large-scale standardized physiological survey reveals functional organization of the mouse visual cortex. Nat. Neurosci. 2019, 23, 138–151. [Google Scholar] [CrossRef]
- Kauvar, I.V.; Machado, T.A.; Yuen, E.; Kochalka, J.; Choi, M.; Allen, W.E.; Wetzstein, G.; Deisseroth, K. Cortical Observation by Synchronous Multifocal Optical Sampling Reveals Widespread Population Encoding of Actions. Neuron 2020, 107, 351–367.e19. [Google Scholar] [CrossRef]
- Couto, J.; Musall, S.; Sun, X.R.; Khanal, A.; Gluf, S.; Saxena, S.; Kinsella, I.; Abe, T.; Cunningham, J.P.; Paninski, L.; et al. Chronic, cortex-wide imaging of specific cell populations during behavior An integrative approach for analyzing hundreds of neurons in task performing mice using wide-field calcium imaging. Nat. Protoc. 2021, 16, 3241–3263. [Google Scholar] [CrossRef]
- Ohkura, M.; Sasaki, T.; Sadakari, J.; Gengyo-Ando, K.; Kagawa-Nagamura, Y.; Kobayashi, C.; Ikegaya, Y.; Nakai, J. Genetically Encoded Green Fluorescent Ca2+ Indicators with Improved Detectability for Neuronal Ca2+ Signals. PLoS ONE 2012, 7, e51286. [Google Scholar] [CrossRef] [Green Version]
- Dana, H.; Mohar, B.; Sun, Y.; Narayan, S.; Gordus, A.; Hasseman, J.P.; Tsegaye, G.; Holt, G.T.; Hu, A.; Walpita, D.; et al. Sensitive red protein calcium indicators for imaging neural activity. eLife 2016, 5, 12727. [Google Scholar] [CrossRef]
- Berlin, S.; Carroll, E.C.; Newman, Z.L.; O Okada, H.; Quinn, C.M.; Kallman, B.; Rockwell, N.C.; Martin, S.S.; Lagarias, J.C.; Isacoff, E.Y. Photoactivatable genetically encoded calcium indicators for targeted neuronal imaging. Nat. Methods 2015, 12, 852–858. [Google Scholar] [CrossRef] [Green Version]
- Hussein, W.; Berlin, S. Red Photoactivatable Genetic Optical-Indicators. Front. Cell. Neurosci. 2020, 14, 113. [Google Scholar] [CrossRef]
- Chan, K.Y.; Jang, M.J.; Yoo, B.B.; Greenbaum, A.; Ravi, N.; Wu, W.-L.; Sanchez-Guardado, L.; Lois, C.; Mazmanian, S.K.; E Deverman, B.; et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017, 20, 1172–1179. [Google Scholar] [CrossRef]
- Deverman, B.E.; Pravdo, P.L.; Simpson, B.P.; Kumar, S.R.; Chan, K.Y.; Banerjee, A.; Wu, W.-L.; Yang, B.; Huber, N.; Pasca, S.; et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016, 34, 204–209. [Google Scholar] [CrossRef]
- Cramer, S.W.; Carter, R.E.; Aronson, J.D.; Kodandaramaiah, S.B.; Ebner, T.J.; Chen, C.C. Through the looking glass: A review of cranial window technology for optical access to the brain. J. Neurosci. Methods 2021, 354, 109100. [Google Scholar] [CrossRef]
- Silasi, G.; Boyd, J.D.; LeDue, J.; Murphy, T.H. Improved methods for chronic light-based motor mapping in mice: Automated movement tracking with accelerometers, and chronic EEG recording in a bilateral thin-skull preparation. Front. Neural Circuits 2013, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.-J.; Yu, T.-T.; Zhang, C.; Li, Z.; Luo, Q.-M.; Xu, T.-H.; Zhu, D. Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution. Light. Sci. Appl. 2017, 7, 17153. [Google Scholar] [CrossRef] [Green Version]
- Vanni, M.P.; Murphy, T.H. Mesoscale transcranial spontaneous activity mapping in GCaMP3 transgenic mice reveals extensive reciprocal connections between areas of somatomotor cortex. J. Neurosci. 2014, 34, 15931–15946. [Google Scholar] [CrossRef] [Green Version]
- Silasi, G.; Xiao, D.; Vanni, M.P.; Chen, A.C.; Murphy, T.H. Intact skull chronic windows for mesoscopic wide-field imaging in awake mice. J. Neurosci. Methods 2016, 267, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, L.; Carter, R.E.; Rynes, M.L.; Dominguez, J.; Chen, G.; Naik, A.; Hu, J.; Sagar, A.K.; Haltom, L.; Mossazghi, N.; et al. Cortex-wide neural interfacing via transparent polymer skulls. Nat. Commun. 2019, 10, 1500. [Google Scholar] [CrossRef]
- West, S.L.; Aronson, J.D.; Popa, L.S.; Feller, K.D.; E Carter, R.; Chiesl, W.M.; Gerhart, M.L.; Shekhar, A.C.; Ghanbari, L.; Kodandaramaiah, S.B.; et al. Wide-Field Calcium Imaging of Dynamic Cortical Networks during Locomotion. Cereb. Cortex 2021, 32, 2668–2687. [Google Scholar] [CrossRef]
- Kim, T.H.; Zhang, Y.; Lecoq, J.; Jung, J.C.; Li, J.; Zeng, H.; Niell, C.M.; Schnitzer, M.J. Long-Term Optical Access to an Estimated One Million Neurons in the Live Mouse Cortex. Cell Rep. 2016, 17, 3385–3394. [Google Scholar] [CrossRef]
- Dombeck, D.; Khabbaz, A.N.; Collman, F.; Adelman, T.L.; Tank, D.W. Imaging Large-Scale Neural Activity with Cellular Resolution in Awake, Mobile Mice. Neuron 2007, 56, 43–57. [Google Scholar] [CrossRef] [Green Version]
- Andermann, M.L.; Kerlin, A.M.; Reid, C. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front. Cell. Neurosci. 2010, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Shaik, M.A.; Kim, S.H.; Kozberg, M.G.; Thibodeaux, D.N.; Zhao, H.T.; Yu, H.; Hillman, E.M. Wide-field optical mapping of neural activity and brain haemodynamics: Considerations and novel approaches. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150360. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, E.A.; Steinmetz, N.A.; Peters, A.J.; Carandini, M.; Harris, K.D. Cortical State Fluctuations during Sensory Decision Making. Curr. Biol. 2020, 30, 4944–4955.e7. [Google Scholar] [CrossRef]
- Valley, M.T.; Moore, M.G.; Zhuang, J.; Mesa, N.; Castelli, D.; Sullivan, D.; Reimers, M.; Waters, J.; Hayashi, Y.; Yawata, S.; et al. Separation of hemodynamic signals from GCaMP fluorescence measured with wide-field imaging In vivo calcium imaging from dentate granule cells with wide-field fluorescence microscopy. J. Neurophysiol. 2020, 123, 356–366. [Google Scholar] [CrossRef]
- Wekselblatt, J.B.; Flister, E.D.; Piscopo, D.M.; Niell, C.M. Large-scale imaging of cortical dynamics during sensory perception and behavior. J. Neurophysiol. 2016, 115, 2852–2866. [Google Scholar] [CrossRef] [Green Version]
- Xiao, D.; Vanni, M.P.; Mitelut, C.C.; Chan, A.W.; LeDue, J.M.; Xie, Y.; Chen, A.C.; Swindale, N.V.; Murphy, T.H. Mapping cortical mesoscopic networks of single spiking cortical or sub-cortical neurons. eLife 2017, 6, 19976. [Google Scholar] [CrossRef]
- MacDowell, C.J.; Buschman, T.J. Low-Dimensional Spatiotemporal Dynamics Underlie Cortex-wide Neural Activity. Curr. Biol. 2020, 30, 2665–2680.e8. [Google Scholar] [CrossRef]
- Waters, J. Sources of widefield fluorescence from the brain. eLife 2020, 9, e59841. [Google Scholar] [CrossRef] [PubMed]
- Bethge, P.; Carta, S.; Lorenzo, D.A.; Egolf, L.; Goniotaki, D.; Madisen, L.; Voigt, F.; Chen, J.L.; Schneider, B.; Ohkura, M.; et al. An R-CaMP1.07 reporter mouse for cell-type-specific expression of a sensitive red fluorescent calcium indicator. PLoS ONE 2017, 12, e0179460. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, M.B.; Orger, M.B.; Robson, D.N.; Li, J.M.; Keller, P.J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 2013, 10, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Quirin, S.; Vladimirov, N.; Yang, C.-T.; Peterka, D.S.; Yuste, R.; Ahrens, M.B. Calcium imaging of neural circuits with extended depth-of-field light-sheet microscopy. Opt. Lett. 2016, 41, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Homma, R.; Baker, B.J.; Jin, L.; Garaschuk, O.; Konnerth, A.; Cohen, L.B.; Bleau, C.X.; Canepari, M.; Djurisic, M.; Zecevic, D. Wide-Field and Two-Photon Imaging of Brain Activity with Voltage and Calcium-Sensitive Dyes. Dyn. Brain Imaging 2009, 489, 43–79. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Do, Q.B.; Antic, S.D.; Knöpfel, T. Transgenic Strategies for Sparse but Strong Expression of Genetically Encoded Voltage and Calcium Indicators. Int. J. Mol. Sci. 2017, 18, 1461. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, P.D.; Navabi, Z.S.; Carter, R.E.; Fausner, S.M.L.; Ghanbari, L.; Ebner, T.J.; Swisher, S.L.; Kodandaramaiah, S.B. Polymer Skulls With Integrated Transparent Electrode Arrays for Cortex-Wide Opto-Electrophysiological Recordings. Adv. Healthc. Mater. 2022, 11, 2200626. [Google Scholar] [CrossRef]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef]
- Jercog, P.; Rogerson, T.; Schnitzer, M.J. Large-Scale Fluorescence Calcium-Imaging Methods for Studies of Long-Term Memory in Behaving Mammals. Cold Spring Harb. Perspect. Biol. 2016, 8, a021824. [Google Scholar] [CrossRef] [Green Version]
- Hoang, H.; Sato, M.-A.; Shinomoto, S.; Tsutsumi, S.; Hashizume, M.; Ishikawa, T.; Kano, M.; Ikegaya, Y.; Kitamura, K.; Kawato, M.; et al. Improved hyperacuity estimation of spike timing from calcium imaging. Sci. Rep. 2020, 10, 17844. [Google Scholar] [CrossRef]
- Grewe, B.; Langer, D.; Kasper, H.; Kampa, B.; Helmchen, F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nat. Methods 2010, 7, 399–405. [Google Scholar] [CrossRef]
- Göbel, W.; Helmchen, F. In Vivo Calcium Imaging of Neural Network Function. Physiology 2007, 22, 358–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibau, E.; Ludl, A.-A.; Rudiger, S.; Orlandi, J.G.; Soriano, J. Neuronal Spatial Arrangement Shapes Effective Connectivity Traits of in vitro Cortical Networks. IEEE Trans. Netw. Sci. Eng. 2018, 7, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Deneux, T.; Kaszas, A.; Szalay, G.; Katona, G.; Lakner, T.; Grinvald, A.; Rózsa, B.; Vanzetta, I. Accurate spike estimation from noisy calcium signals for ultrafast three-dimensional imaging of large neuronal populations in vivo. Nat. Commun. 2016, 7, 12190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barson, D.; Hamodi, A.S.; Shen, X.; Lur, G.; Constable, R.T.; Cardin, J.A.; Crair, M.C.; Higley, M.J. Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits. Nat. Methods 2019, 17, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Yoshida, T.; Matsui, T.; Ohki, K. Wide-field Ca2+ imaging reveals visually evoked activity in the retrosplenial area. Front. Mol. Neurosci. 2015, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busche, M.A.; Kekuš, M.; Adelsberger, H.; Noda, T.; Förstl, H.; Nelken, I.; Konnerth, A. Rescue of long-range circuit dysfunction in Alzheimer’s disease models. Nat. Neurosci. 2015, 18, 1623–1630. [Google Scholar] [CrossRef]
- Montagni, E.; Resta, F.; Conti, E.; Scaglione, A.; Pasquini, M.; Micera, S.; Mascaro, A.L.A.; Pavone, F.S. Wide-field imaging of cortical neuronal activity with red-shifted functional indicators during motor task execution. J. Phys. D Appl. Phys. 2018, 52, 074001. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, S.-L.; Li, Y.; Royall, J.; Feng, D.; Lesnar, P.; Graddis, N.; Naeemi, M.; Facer, B.; Ho, A.; et al. The Allen Mouse Brain Common Coordinate Framework: A 3D Reference Atlas. Cell 2020, 181, 936–953.e20. [Google Scholar] [CrossRef]
- Vanni, M.P.; Chan, A.W.; Balbi, M.; Silasi, G.; Murphy, T.H. Mesoscale Mapping of Mouse Cortex Reveals Frequency-Dependent Cycling between Distinct Macroscale Functional Modules. J. Neurosci. 2017, 37, 7513–7533. [Google Scholar] [CrossRef]
- Mohajerani, M.H.; Chan, A.W.; Mohsenvand, M.; LeDue, J.; Liu, R.; A McVea, D.; Boyd, J.D.; Wang, Y.T.; Reimers, M.; Murphy, T.H. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat. Neurosci. 2013, 16, 1426–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, B.R.; Bauer, A.Q.; Snyder, A.Z.; Schlaggar, B.L.; Lee, J.-M.; Culver, J.P. Imaging of Functional Connectivity in the Mouse Brain. PLoS ONE 2011, 6, e16322. [Google Scholar] [CrossRef] [Green Version]
- Nagayama, M.; Aritake, T.; Hino, H.; Kanda, T.; Miyazaki, T.; Yanagisawa, M.; Akaho, S.; Murata, N. Detecting cell assemblies by NMF-based clustering from calcium imaging data. Neural. Netw. 2022, 149, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Song, M.; Paik, S.-B.; Jung, M.W. Spatial organization of functional clusters representing reward and movement information in the striatal direct and indirect pathways. Proc. Natl. Acad. Sci. USA 2020, 117, 27004–27015. [Google Scholar] [CrossRef]
- Pinto, L.; Rajan, K.; DePasquale, B.; Thiberge, S.Y.; Tank, D.W.; Brody, C.D. Task-Dependent Changes in the Large-Scale Dynamics and Necessity of Cortical Regions. Neuron 2019, 104, 810–824.e9. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Matsuzaki, M. Neuronal representations of reward-predicting cues and outcome history with movement in the frontal cortex. Cell Rep. 2021, 34, 108704. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.J.; Fabre, J.M.J.; Steinmetz, N.A.; Harris, K.D.; Carandini, M. Striatal activity topographically reflects cortical activity. Nature 2021, 591, 420–425. [Google Scholar] [CrossRef]
- Brown, G.D.; Yamada, S.; Sejnowski, T.J. Independent component analysis at the neural cocktail party. Trends Neurosci. 2001, 24, 54–63. [Google Scholar] [CrossRef]
- Stone, J.V. Independent component analysis: An introduction. Trends Cogn. Sci. 2002, 6, 59–64. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Adali, T. Unmixing fMRI with independent component analysis. IEEE Eng. Med. Biol. Mag. 2006, 25, 79–90. [Google Scholar] [CrossRef]
- Sahonero-Alvarez, G.; Calderon, H. A comparison of SOBI, FastICA, JADE and Infomax algorithms. In Proceedings of the Proceedings of the 8th International Multi-Conference on Complexity, Informatics and Cybernetics, Orlando, FL, USA, 21 March 2017; pp. 17–22. [Google Scholar]
- Belouchrani, A.; Abed-Meraim, K.; Cardoso, J.-F.; Moulines, E. A blind source separation technique using second-order statistics. IEEE Trans. Signal Process. 1997, 45, 434–444. [Google Scholar] [CrossRef] [Green Version]
- Molgedey, L.; Schuster, H.G. Separation of a mixture of independent signals using time delayed correlations. Phys. Rev. Lett. 1994, 72, 3634–3637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calhoun, V.; Adali, T.; Pearlson, G.; Pekar, J. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum. Brain Mapp. 2001, 13, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calhoun, V.D.; de Lacy, N. Ten key observations on the analysis of resting-state functional MR imaging data using independent component analysis. Neuroimaging Clin. N. Am. 2017, 27, 561–579. [Google Scholar] [CrossRef]
- Saxena, S.; Kinsella, I.; Musall, S.; Kim, S.H.; Meszaros, J.; Thibodeaux, D.N.; Kim, C.; Cunningham, J.; Hillman, E.M.C.; Churchland, A.; et al. Localized semi-nonnegative matrix factorization (LocaNMF) of widefield calcium imaging data. PLoS Comput. Biol. 2020, 16, e1007791. [Google Scholar] [CrossRef] [Green Version]
- Mirzal, A. NMF versus ICA for blind source separation. Adv. Data Anal. Classif. 2017, 11, 25–48. [Google Scholar] [CrossRef]
- Yang, W.; Miller, J.-E.K.; Carrillo-Reid, L.; Pnevmatikakis, E.; Paninski, L.; Yuste, R.; Peterka, D.S. Simultaneous Multi-plane Imaging of Neural Circuits. Neuron 2016, 89, 269–284. [Google Scholar] [CrossRef] [Green Version]
- Quarta, E.; Scaglione, A.; Lucchesi, J.; Sacconi, L.; Mascaro, A.L.A.; Pavone, F.S. Distributed and Localized Dynamics Emerge in the Mouse Neocortex during Reach-to-Grasp Behavior. J. Neurosci. 2021, 42, 777–788. [Google Scholar] [CrossRef]
- Chapter 3—Connectivity Matrices and Brain Graphs. In Fundamentals of Brain Network Analysis; Fornito, A.; Zalesky, A.; Bullmore, E.T. (Eds.) Academic Press: Cambridge, MA, USA, 2016; pp. 89–113. [Google Scholar]
- Fornito, A.; Zalesky, A.; Bullmore, E.T. Network scaling effects in graph analytic studies of human resting-state fMRI data. Front. Syst. Neurosci. 2010, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Bullmore, E.T.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Mijalkov, M.; Kakaei, E.; Pereira, J.B.; Westman, E.; Volpe, G.; Initiative, F.T.A.D.N. BRAPH: A graph theory software for the analysis of brain connectivity. PLoS ONE 2017, 12, e0178798. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.; Sporns, O.; MacLean, J.N. Network Analysis of Murine Cortical Dynamics Implicates Untuned Neurons in Visual Stimulus Coding. Cell Rep. 2020, 31, 107483. [Google Scholar] [CrossRef] [PubMed]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Salkoff, D.B.; Zagha, E.; McCarthy, E.; A McCormick, D. Movement and Performance Explain Widespread Cortical Activity in a Visual Detection Task. Cereb. Cortex 2019, 30, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Galiñanes, G.L.; Bonardi, C.; Huber, D. Directional reaching for water as a cortex-dependent behavioral framework for mice. Cell Rep. 2018, 22, 2767–2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapin, J.K.; Woodward, D.J. Somatic sensory transmission to the cortex during movement: Phasic modulation over the locomotor step cycle. Exp. Neurol. 1982, 78, 670–684. [Google Scholar] [CrossRef]
- Favorov, O.V.; Nilaweera, W.U.; Miasnikov, A.A.; Beloozerova, I.N. Activity of Somatosensory-Responsive Neurons in High Subdivisions of SI Cortex during Locomotion. J. Neurosci. 2015, 35, 7763–7776. [Google Scholar] [CrossRef] [Green Version]
- Ayaz, A.; Stäuble, A.; Hamada, M.; Wulf, M.-A.; Saleem, A.B.; Helmchen, F. Layer-specific integration of locomotion and sensory information in mouse barrel cortex. Nat. Commun. 2019, 10, 2585. [Google Scholar] [CrossRef] [Green Version]
- Dadarlat, M.C.; Stryker, M.P. Locomotion Enhances Neural Encoding of Visual Stimuli in Mouse V1. J. Neurosci. 2017, 37, 3764–3775. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Higley, M.J. Layer 5 Circuits in V1 Differentially Control Visuomotor Behavior. Neuron 2019, 105, 346–354.e5. [Google Scholar] [CrossRef]
- Schneider, D.M.; Mooney, R. How Movement Modulates Hearing. Annu. Rev. Neurosci. 2018, 41, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.M. Reflections of action in sensory cortex. Curr. Opin. Neurobiol. 2020, 64, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Clancy, K.B.; Mrsic-Flogel, T.D. The sensory representation of causally controlled objects. Neuron 2020, 109, 677–689.e4. [Google Scholar] [CrossRef] [PubMed]
- Zatka-Haas, P.; A Steinmetz, N.; Carandini, M.; Harris, K.D. Sensory coding and the causal impact of mouse cortex in a visual decision. eLife 2021, 10, e63163. [Google Scholar] [CrossRef]
- Orsolic, I.; Rio, M.; Mrsic-Flogel, T.D.; Znamenskiy, P. Mesoscale cortical dynamics reflect the interaction of sensory evidence and temporal expectation during perceptual decision-making. Neuron 2021, 109, 1861–1875.e10. [Google Scholar] [CrossRef]
- Nishio, N.; Tsukano, H.; Hishida, R.; Abe, M.; Nakai, J.; Kawamura, M.; Aiba, A.; Sakimura, K.; Shibuki, K. Higher visual responses in the temporal cortex of mice. Sci. Rep. 2018, 8, 11136. [Google Scholar] [CrossRef] [Green Version]
- Sit, K.K.; Goard, M.J. Distributed and retinotopically asymmetric processing of coherent motion in mouse visual cortex. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Cramer, S.W.; Popa, L.S.; Carter, R.E.; Chen, G.; Ebner, T.J. Abnormal excitability and episodic low-frequency oscillations in the cerebral cortex of the tottering mouse. J. Neurosci. 2015, 35, 5664–5679. [Google Scholar] [CrossRef] [Green Version]
- Cramer, J.V.; Gesierich, B.; Roth, S.; Dichgans, M.; Düring, M.; Liesz, A. In vivo widefield calcium imaging of the mouse cortex for analysis of network connectivity in health and brain disease. NeuroImage 2019, 199, 570–584. [Google Scholar] [CrossRef]
- Balbi, M.; Vanni, M.P.; Vega, M.J.; Silasi, G.; Sekino, Y.; Boyd, J.D.; LeDue, J.M.; Murphy, T.H. Longitudinal monitoring of mesoscopic cortical activity in a mouse model of microinfarcts reveals dissociations with behavioral and motor function. J. Cereb. Blood Flow Metab. 2018, 39, 1486–1500. [Google Scholar] [CrossRef]
- Busche, M.A.; Konnerth, A. Impairments of neural circuit function in Alzheimer’s disease. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doostdar, N.; Airey, J.; Radulescu, C.I.; Melgosa-Ecenarro, L.; Zabouri, N.; Pavlidi, P.; Kopanitsa, M.; Saito, T.; Saido, T.; Barnes, S.J. Multi-scale network imaging in a mouse model of amyloidosis. Cell Calcium 2021, 95, 102365. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.F.; Wykes, R.C.; Kullmann, D.M.; Carandini, M. Focal cortical seizures start as standing waves and propagate respecting homotopic connectivity. Nat. Commun. 2017, 8, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Huang, C.; Li, J.Z.; Grewe, B.F.; Zhang, Y.; Eismann, S.; Schnitzer, M.J. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 2015, 350, 1361–1366. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Han, Z.; Platisa, J.; Wooltorton, J.; Cohen, L.B.; Pieribone, V.A. Single Action Potentials and Subthreshold Electrical Events Imaged in Neurons with a Fluorescent Protein Voltage Probe. Neuron 2012, 75, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberland, S.; Yang, H.H.; Pan, M.M.; Evans, S.W.; Guan, S.; Chavarha, M.; Yang, Y.; Salesse, C.; Wu, H.; Wu, J.C.; et al. Fast two-photon imaging of subcellular voltage dynamics in neuronal tissue with genetically encoded indicators. eLife 2017, 6, e25690. [Google Scholar] [CrossRef]
- Platisa, J.; Vasan, G.; Yang, A.; Pieribone, V.A. Directed Evolution of Key Residues in Fluorescent Protein Inverses the Polarity of Voltage Sensitivity in the Genetically Encoded Indicator ArcLight. ACS Chem. Neurosci. 2017, 8, 513–523. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Wang, Y.; Liu, Z.; Gou, Y.; Jaeger, D.; St-Pierre, F. Detecting rapid pan-cortical voltage dynamics in vivo with a brighter and faster voltage indicator. bioRxiv 2022, 2022.2008.2029.505018. [Google Scholar] [CrossRef]
- Kannan, M.; Vasan, G.; Pieribone, V.A. Optimizing Strategies for Developing Genetically Encoded Voltage Indicators. Front. Cell. Neurosci. 2019, 13, 53. [Google Scholar] [CrossRef]
- Kannan, M.; Vasan, G.; Huang, C.; Haziza, S.; Li, J.Z.; Inan, H.; Schnitzer, M.J.; Pieribone, V.A. Fast, in vivo voltage imaging using a red fluorescent indicator. Nat. Methods 2018, 15, 1108–1116. [Google Scholar] [CrossRef]
- Kannan, M.; Vasan, G.; Haziza, S.; Huang, C.; Chrapkiewicz, R.; Luo, J.; Cardin, J.A.; Schnitzer, M.J.; Pieribone, V.A. Dual polarity voltage imaging reveals subthreshold dynamics and concurrent spiking patterns of multiple neuron-types. bioRxiv 2021, 2021.2010.2013.463730. [Google Scholar] [CrossRef]
- Clancy, K.B.; Orsolic, I.; Mrsic-Flogel, T.D. Locomotion-dependent remapping of distributed cortical networks. Nat. Neurosci. 2019, 22, 778–786. [Google Scholar] [CrossRef]
- Terada, S.-I.; Kobayashi, K.; Ohkura, M.; Nakai, J.; Matsuzaki, M. Super-wide-field two-photon imaging with a micro-optical device moving in post-objective space. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, K.; Oisi, Y.; Suzuki, T.; Ikeda, M.; Ito, Y.; Ito, T.; Uwamori, H.; Kobayashi, K.; Kobayashi, M.; Odagawa, M.; et al. Fast, cell-resolution, contiguous-wide two-photon imaging to reveal functional network architectures across multi-modal cortical areas. Neuron 2021, 109, 1810–1824.e9. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-H.; Stirman, J.N.; Yu, Y.; Hira, R.; Smith, S.L. Diesel2p mesoscope with dual independent scan engines for flexible capture of dynamics in distributed neural circuitry. Nat. Commun. 2021, 12, 6639. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Liang, Y.; Meng, G.; Zhou, P.; Svoboda, K.; Paninski, L.; Ji, N. Rapid mesoscale volumetric imaging of neural activity with synaptic resolution. Nat. Methods 2020, 17, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Lake, E.M.R.; Ge, X.; Shen, X.; Herman, P.; Hyder, F.; Cardin, J.A.; Higley, M.J.; Scheinost, D.; Papademetris, X.; Crair, M.C.; et al. Simultaneous cortex-wide fluorescence Ca2+ imaging and whole-brain fMRI. Nat. Methods 2020, 17, 1262–1271. [Google Scholar] [CrossRef]
- Esmaeili, V.; Tamura, K.; Muscinelli, S.P.; Modirshanechi, A.; Boscaglia, M.; Lee, A.B.; Oryshchuk, A.; Foustoukos, G.; Liu, Y.; Crochet, S. Rapid suppression and sustained activation of distinct cortical regions for a delayed sensory-triggered motor response. Neuron 2021, 109, 2183–2201.e2189. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Boyd, J.D.; Bolaños, F.; Vanni, M.P.; Silasi, G.; Haupt, D.; LeDue, J.M. High-throughput automated home-cage mesoscopic functional imaging of mouse cortex. Nat. Commun. 2016, 7, 11611. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Lu, Y.; Zhao, R.; Liu, X.; De-Eknamkul, C.; Ren, C.; Mehrsa, A.; Komiyama, T.; Kuzum, D. Evaluation of Durability of Transparent Graphene Electrodes Fabricated on Different Flexible Substrates for Chronic In Vivo Experiments. IEEE Trans. Biomed. Eng. 2020, 67, 3203–3210. [Google Scholar] [CrossRef]
- Park, D.-W.; Schendel, A.A.; Mikael, S.; Brodnick, S.K.; Richner, T.J.; Ness, J.P.; Hayat, M.R.; Atry, F.; Frye, S.T.; Pashaie, R.; et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 5258. [Google Scholar] [CrossRef] [Green Version]
- Qiang, Y.; Artoni, P.; Seo, K.J.; Culaclii, S.; Hogan, V.; Zhao, X.; Zhong, Y.; Han, X.; Wang, P.-M.; Lo, Y.-K.; et al. Transparent arrays of bilayer-nanomesh microelectrodes for simultaneous electrophysiology and two-photon imaging in the brain. Sci. Adv. 2018, 4, eaat0626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donahue, M.J.; Kaszas, A.; Turi, G.F.; Rózsa, B.; Slézia, A.; Vanzetta, I.; Katona, G.; Bernard, C.; Malliaras, G.G.; Williamson, A. Multimodal Characterization of Neural Networks Using Highly Transparent Electrode Arrays. Eneuro 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.K.; Burns, L.D.; Cocker, E.D.; Nimmerjahn, A.; Ziv, Y.; El Gamal, A.; Schnitzer, M.J. Miniaturized integration of a fluorescence microscope. Nat. Methods 2011, 8, 871–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evoigts, J.; Siegle, J.H.; Pritchett, D.L.; Moore, C.I. The flexDrive: An ultra-light implant for optical control and highly parallel chronic recording of neuronal ensembles in freely moving mice. Front. Syst. Neurosci. 2013, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Rynes, M.L.; Surinach, D.A.; Linn, S.; Laroque, M.; Rajendran, V.; Dominguez, J.; Hadjistamoulou, O.; Navabi, Z.S.; Ghanbari, L.; Johnson, G.W.; et al. Miniaturized head-mounted microscope for whole-cortex mesoscale imaging in freely behaving mice. Nat. Methods 2021, 18, 417–425. [Google Scholar] [CrossRef]
- Rynes, M.L.; Ghanbari, L.; Schulman, D.S.; Linn, S.; Laroque, M.; Dominguez, J.; Navabi, Z.S.; Sherman, P.; Kodandaramaiah, S.B.; Surinach, D.A.; et al. Assembly and operation of an open-source, computer numerical controlled (CNC) robot for performing cranial microsurgical procedures Miniaturized head-mounted microscope for whole-cortex mesoscale imaging in freely behaving mice. Nat. Protoc. 2020, 15, 1992–2023. [Google Scholar] [CrossRef]
- Juneau, J.; Duret, G.; Chu, J.P.; Rodriguez, A.V.; Morozov, S.; Aharoni, D.; Robinson, J.T.; St-Pierre, F.; Kemere, C. MiniFAST: A sensitive and fast miniaturized microscope for in vivo neural recording. bioRxiv 2020, 2020.2011.2003.367466. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nietz, A.K.; Popa, L.S.; Streng, M.L.; Carter, R.E.; Kodandaramaiah, S.B.; Ebner, T.J. Wide-Field Calcium Imaging of Neuronal Network Dynamics In Vivo. Biology 2022, 11, 1601. https://doi.org/10.3390/biology11111601
Nietz AK, Popa LS, Streng ML, Carter RE, Kodandaramaiah SB, Ebner TJ. Wide-Field Calcium Imaging of Neuronal Network Dynamics In Vivo. Biology. 2022; 11(11):1601. https://doi.org/10.3390/biology11111601
Chicago/Turabian StyleNietz, Angela K., Laurentiu S. Popa, Martha L. Streng, Russell E. Carter, Suhasa B. Kodandaramaiah, and Timothy J. Ebner. 2022. "Wide-Field Calcium Imaging of Neuronal Network Dynamics In Vivo" Biology 11, no. 11: 1601. https://doi.org/10.3390/biology11111601
APA StyleNietz, A. K., Popa, L. S., Streng, M. L., Carter, R. E., Kodandaramaiah, S. B., & Ebner, T. J. (2022). Wide-Field Calcium Imaging of Neuronal Network Dynamics In Vivo. Biology, 11(11), 1601. https://doi.org/10.3390/biology11111601





