Effects of Transcranial Direct Current Stimulation over the Primary Motor Cortex in Improving Postural Stability in Healthy Young Adults
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
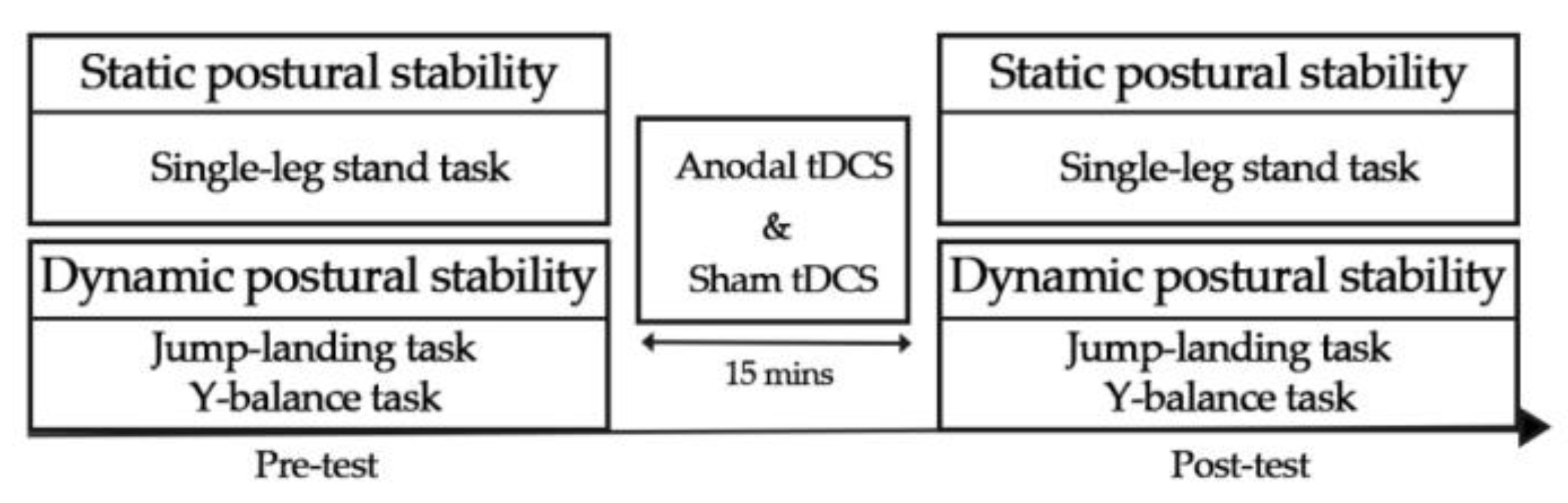
2.2. Experimental Procedure
2.3. Static Postural Stability Test
2.4. Dynamic Postural Stability Test
2.5. tDCS
2.6. Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Static Postural Stability
3.2. Dynamic Postural Stability
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wikstrom, E.A.; Tillman, M.D.; Chmielewski, T.L.; Cauraugh, J.H.; Borsa, P.A. Dynamic postural stability deficits in subjects with self-reported ankle instability. Med. Sci. Sport. Exerc. 2007, 39, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, E.A.; Tillman, M.D.; Schenker, S.M.; Borsa, P.A. Jump-landing direction influences dynamic postural stability scores. J. Sci. Med. Sport 2008, 11, 106–111. [Google Scholar] [CrossRef]
- Goldie, P.A.; Bach, T.M.; Evans, O.M. Force platform measures for evaluating postural control: Reliability and validity. Arch. Phys. Med. Rehabil. 1989, 70, 510–517. [Google Scholar] [PubMed]
- Mihara, M.; Miyai, I.; Hatakenaka, M.; Kubota, K.; Sakoda, S. Role of the prefrontal cortex in human balance control. Neuroimage 2008, 43, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Taube, W.; Mouthon, M.; Leukel, C.; Hoogewoud, H.M.; Annoni, J.M.; Keller, M. Brain activity during observation and motor imagery of different balance tasks: An fMRI study. Cortex 2015, 64, 102–114. [Google Scholar] [CrossRef]
- Dijkstra, B.W.; Bekkers, E.M.J.; Gilat, M.; de Rond, V.; Hardwick, R.M.; Nieuwboer, A. Functional neuroimaging of human postural control: A systematic review with meta-analysis. Neurosci. Biobehav. Rev. 2020, 115, 351–362. [Google Scholar] [CrossRef]
- Tse, Y.Y.; Petrofsky, J.S.; Berk, L.; Daher, N.; Lohman, E.; Laymon, M.S.; Cavalcanti, P. Postural sway and rhythmic electroencephalography analysis of cortical activation during eight balance training tasks. Med. Sci. Monit. 2013, 19, 175–186. [Google Scholar] [CrossRef]
- Paterno, M.V.; Schmitt, L.C.; Ford, K.R.; Rauh, M.J.; Myer, G.D.; Huang, B.; Hewett, T.E. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am. J. Sports Med. 2010, 38, 1968–1978. [Google Scholar] [CrossRef]
- Winter, D. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci. 2003, 15, 619–626. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Biabani, M.; Aminitehrani, M.; Zoghi, M.; Farrell, M.; Egan, G.; Jaberzadeh, S. The effects of transcranial direct current stimulation on short-interval intracortical inhibition and intracortical facilitation: A systematic review and meta-analysis. Rev. Neurosci. 2017, 29, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Kaski, D.; Quadir, S.; Patel, M.; Yousif, N.; Bronstein, A.M. Enhanced locomotor adaptation aftereffect in the “broken escalator” phenomenon using anodal tDCS. J. Neurophysiol. 2012, 107, 2493–2505. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Chugh, S. Effect of Transcranial Direct Current Stimulation on Cortico-Muscular Coherence and Standing Postural Steadiness. In Proceedings of the IASTED International Conference on Assistive Technologies, San Diego, CA, USA, 28 August–1 September 2012, pp. 7643–7646. [Google Scholar]
- Dutta, A.; Chugh, S.; Banerjee, A.; Dutta, A. Point-of-care-testing of standing posture with Wii balance board and Microsoft Kinect during transcranial direct current stimulation: A feasibility study. NeuroRehabilitation 2014, 34, 789–798. [Google Scholar] [CrossRef]
- Kaminski, E.; Steele, C.J.; Hoff, M.; Gundlach, C.; Rjosk, V.; Sehm, B.; Villringer, A.; Ragert, P. Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clin. Neurophysiol. 2016, 127, 2455–2462. [Google Scholar] [CrossRef]
- Baharlouei, H.; Sadeghi-Demneh, E.; Mehravar, M.; Manzari, P.; Jaberzadeh, S. Comparison of Transcranial Direct Current Stimulation of the Primary Motor Cortex and Cerebellum on Static Balance in Older Adults. Iran. Red Crescent Med. J. 2020, in press. [Google Scholar] [CrossRef]
- Winter, D.A.; Patla, A.E.; Rietdyk, S.; Ishac, M.G. Ankle muscle stiffness in the control of balance during quiet standing. J. Neurophysiol. 2001, 85, 2630–2633. [Google Scholar] [CrossRef]
- Bruce, A.S.; Howard, J.S.; Werkhoven, H.V.; Mcbride, J.M.; Needle, A.R. The Effects of Transcranial Direct Current Stimulation on Chronic Ankle Instability. Med. Sci. Sport. 2020, 52, 335–344. [Google Scholar] [CrossRef]
- Ma, Y.; Yin, K.; Zhuang, W.; Zhang, C.; Jiang, Y.; Huang, J.; Manor, B.; Zhou, J.; Liu, Y. Effects of Combining High-Definition Transcranial Direct Current Stimulation with Short-Foot Exercise on Chronic Ankle Instability: A Pilot Randomized and Double-Blinded Study. Brain Sci. 2020, 10, 749. [Google Scholar] [CrossRef]
- Needle, A.R.; Lepley, A.S.; Grooms, D.R. Central Nervous System Adaptation After Ligamentous Injury: A Summary of Theories, Evidence, and Clinical Interpretation. Sports Med. 2017, 47, 1271–1288. [Google Scholar] [CrossRef]
- Beck, S.; Taube, W.; Gruber, M.; Amtage, F.; Gollhofer, A.; Schubert, M. Task-specific changes in motor evoked potentials of lower limb muscles after different training interventions. Brain Res. 2007, 1179, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Pohjola, H.; Tolmunen, T.; Kotilainen, T.; Lehto, S.M. Using transcranial direct current stimulation to enhance performance in balance tasks. Clin. Neurophysiol. 2017, 128, 501–502. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, B.; Zhang, X.; Zhou, J.; Fu, W. Acute Effects of High-Definition Transcranial Direct Current Stimulation on Foot Muscle Strength, Passive Ankle Kinesthesia, and Static Balance: A Pilot Study. Brain Sci. 2020, 10, 246. [Google Scholar] [CrossRef]
- Kaminski, E.; Hoff, M.; Rjosk, V.; Steele, C.J.; Gundlach, C.; Sehm, B.; Villringer, A.; Ragert, P. Anodal Transcranial Direct Current Stimulation Does Not Facilitate Dynamic Balance Task Learning in Healthy Old Adults. Front. Hum. Neurosci. 2017, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, F.; Samaei, A.; Zoghi, M.; Hedayati, R.; Jaberzadeh, S. The effects of cerebellar transcranial direct current stimulation on static and dynamic postural stability in older individuals: A randomized double-blind sham-controlled study. Eur. J. Neurosci. 2017, 46, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Yosephi, M.H.; Ehsani, F.; Zoghi, M.; Jaberzadeh, S. Multi-session anodal tDCS enhances the effects of postural training on balance and postural stability in older adults with high fall risk: Primary motor cortex versus cerebellar stimulation. Brain Stimul. 2018, 11, 1239–1250. [Google Scholar] [CrossRef]
- Craig, C.E.; Doumas, M. Anodal Transcranial Direct Current Stimulation Shows Minimal, Measure-Specific Effects on Dynamic Postural Control in Young and Older Adults: A Double Blind, Sham-Controlled Study. PLoS ONE 2017, 12, e0170331. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Ruhe, A.; Fejer, R.; Walker, B. The test-retest reliability of centre of pressure measures in bipedal static task conditions—A systematic review of the literature. Gait Posture 2010, 32, 436–445. [Google Scholar] [CrossRef]
- Kunugi, S.; Koumura, T.; Myotsuzono, R.; Masunari, A.; Yoshida, N.; Miyakawa, S.; Mukai, N. Directions of single-leg landing affect multi-segment foot kinematics and dynamic postural stability in male collegiate soccer athletes. Gait Posture 2020, 80, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Heise, G.D. The effect of jump-landing directions on dynamic stability. J. Appl. Biomech. 2013, 29, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.; Gabbett, T.; McLellan, C.; Minahan, C. Basal Markers of Inflammation, Muscle Damage, and Performance during Five Weeks of Pre-Season Training in Elite Youth Rugby League Players. J. Athl. Enhanc. 2018, 7, 6. [Google Scholar] [CrossRef]
- Fusco, A.; Giancotti, G.F.; Fuchs, P.X.; Wagner, H.; da Silva, R.A.; Cortis, C. Y balance test: Are we doing it right? J. Sci. Med. Sport 2020, 23, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.J.; Lehr, M.E.; Fink, M.L.; Kiesel, K.B.; Plisky, P.J. Dynamic balance performance and noncontact lower extremity injury in college football players: An initial study. Sports Health 2013, 5, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, B.E. Beta-thalassemia and normal growth: Are they compatible? Eur. J. Endocrinol. 1998, 139, 143–144. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, A.F.; Volz, M.S.; Bikson, M.; Fregni, F. Electrode positioning and montage in transcranial direct current stimulation. J. Vis. Exp. 2011, 51, 2744. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef]
- Brown, C.N.; Ko, J.; Rosen, A.B.; Hsieh, K. Individuals with both perceived ankle instability and mechanical laxity demonstrate dynamic postural stability deficits. Clin. Biomech. 2015, 30, 1170–1174. [Google Scholar] [CrossRef]
- Fransz, D.P.; Huurnink, A.; de Boode, V.A.; Kingma, I.; van Dieen, J.H. Time to stabilization in single leg drop jump landings: An examination of calculation methods and assessment of differences in sample rate, filter settings and trial length on outcome values. Gait Posture 2015, 41, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Porta, M.; Arippa, F.; Pilloni, G.; Sorrentino, M.; Carta, M.; Mura, M.; Leban, B. Dynamic postural stability, is associated with competitive level, in youth league soccer players. Phys. Ther. Sport 2018, 35, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Sell, T.C. An examination, correlation, and comparison of static and dynamic measures of postural stability in healthy, physically active adults. Phys. Ther. Sport 2012, 13, 80–86. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Tillman, M.D.; Smith, A.N.; Borsa, P.A. A new force-plate technology measure of dynamic postural stability: The dynamic postural stability index. J. Athl. Train. 2005, 40, 305–309. [Google Scholar] [PubMed]
- Zhang, Q.; Hautier, C.A. Influence of jump-landing direction on dynamic postural stability and hamstring-to-quadriceps co-activation ratio. Res. Sports Med. 2021, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McKinley, P.; Pedotti, A. Motor strategies in landing from a jump: The role of skill in task execution. Exp. Brain Res. 1992, 90, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Arnold, B.L.; Ross, S.E. Altered Kinematics and Time to Stabilization During Drop-Jump Landings in Individuals with or Without Functional Ankle Instability. J. Athl. Train. 2016, 51, 5–15. [Google Scholar] [CrossRef]
- de Moura, M.; Hazime, F.A.; Marotti Aparicio, L.V.; Grecco, L.A.C.; Brunoni, A.R.; Hasue, R.H. Effects of transcranial direct current stimulation (tDCS) on balance improvement: A systematic review and meta-analysis. Somatosens. Mot. Res. 2019, 36, 122–135. [Google Scholar] [CrossRef]
- Herold, F.; Orlowski, K.; Bormel, S.; Muller, N.G. Cortical activation during balancing on a balance board. Hum. Mov. Sci. 2017, 51, 51–58. [Google Scholar] [CrossRef]
- Demain, A.; Westby, G.W.; Fernandez-Vidal, S.; Karachi, C.; Bonneville, F.; Do, M.C.; Delmaire, C.; Dormont, D.; Bardinet, E.; Agid, Y.; et al. High-level gait and balance disorders in the elderly: A midbrain disease? J. Neurol. 2014, 261, 196–206. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, Y.; Zhou, J.; Fried, P.J.; Wang, X.; Zhang, J.; Fang, J.; Pascual-Leone, A.; Manor, B. Direct current stimulation over the human sensorimotor cortex modulates the brain’s hemodynamic response to tactile stimulation. Eur. J. Neurosci. 2015, 42, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Lackmy, A.; Achache, V.; Bussel, B.; Katz, R. Impact of transcranial direct current stimulation on spinal network excitability in humans. J. Physiol. 2009, 587, 5653–5664. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Krishnan, C.; Kantak, S.S.; Ranganathan, R.; Nitsche, M.A. Recurrence quantification analysis of surface electromyogram supports alterations in motor unit recruitment strategies by anodal transcranial direct current stimulation. Restor. Neurol. Neurosci. 2015, 33, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Vargas, V.Z.; Baptista, A.F.; Pereira, G.O.C.; Pochini, A.C.; Ejnisman, B.; Santos, M.B.; João, S.M.A.; Hazime, F.A. Modulation of Isometric Quadriceps Strength in Soccer Players with Transcranial Direct Current Stimulation: A Crossover Study. J. Strength Cond. Res. 2018, 32, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hanson, N.J.; Wen, L.; Guo, F.; Tian, X. Transcranial Direct Current Stimulation Enhances Muscle Strength of Non-dominant Knee in Healthy Young Males. Front. Physiol. 2021, 12, 788719. [Google Scholar] [CrossRef]
- Tanaka, S.; Hanakawa, T.; Honda, M.; Watanabe, K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. J. Exp. Brain Res. 2009, 196, 459–465. [Google Scholar] [CrossRef]
- Riemann, B.L.; Lephart, S.M. The Sensorimotor System, Part II: The Role of Proprioception in Motor Control and Functional Joint Stability. J. Athl. Train. 2002, 37, 80–84. [Google Scholar] [CrossRef]
- Ross, S.E.; Guskiewicz, K.M. Examination of static and dynamic postural stability in individuals with functionally stable and unstable ankles. Clin. J. Sport Med. 2004, 14, 332–338. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Tillman, M.; Chmielewski, T.L.; Cauraugh, J.H.; Naugle, K.E.; Borsa, P.A. Dynamic postural control but not mechanical stability differs among those with and without chronic ankle instability. Scand. J. Med. Sci. Sport. 2010, 20, e137–e144. [Google Scholar] [CrossRef]
| Test | Parameters | Factor | df | F Value | p Value | ηp2 |
|---|---|---|---|---|---|---|
| Static Postural stability | non-COPSA | session | 1, 16 | 2.901 | 0.108 | 0.153 |
| time | 1, 16 | 0.261 | 0.616 | 0.016 | ||
| time × session | 1, 16 | 5.146 | 0.037 | 0.243 | ||
| dom-COPSA | session | 1, 16 | 0.186 | 0.672 | 0.012 | |
| time | 1, 16 | 0.251 | 0.623 | 0.015 | ||
| time × session | 1, 16 | 7.025 | 0.017 | 0.305 | ||
| non-COPAP | session | 1, 16 | 0.474 | 0.501 | 0.029 | |
| time | 1, 16 | 1.752 | 0.204 | 0.099 | ||
| time × session | 1, 16 | 0.016 | 0.900 | 0.001 | ||
| dom-COPAP | session | 1, 16 | 2.889 | 0.109 | 0.153 | |
| time | 1, 16 | 4.759 | 0.044 | 0.229 | ||
| time × session | 1, 16 | 3.505 | 0.080 | 0.180 | ||
| non-COPML | session | 1, 16 | 0.017 | 0.899 | 0.001 | |
| time | 1, 16 | 0.048 | 0.829 | 0.003 | ||
| time × session | 1, 16 | 0.244 | 0.628 | 0.015 | ||
| dom-COPML | session | 1, 16 | 0.129 | 0.724 | 0.008 | |
| time | 1, 16 | 1.202 | 0.289 | 0.070 | ||
| time × session | 1, 16 | 1.075 | 0.315 | 0.063 | ||
| Forward Landing task | FL-APSI | session | 1, 16 | 0.303 | 0.590 | 0.019 |
| time | 1, 16 | 27.423 | 0.0001 | 0.632 | ||
| time × session | 1, 16 | 5.013 | 0.040 | 0.239 | ||
| FL-MLSI | session | 1, 16 | 2.238 | 0.154 | 0.123 | |
| time | 1, 16 | 0.073 | 0.790 | 0.005 | ||
| time × session | 1, 16 | 0.003 | 0.956 | 0.0001 | ||
| FL-VSI | session | 1, 16 | 1.878 | 0.189 | 0.105 | |
| time | 1, 16 | 3.739 | 0.071 | 0.189 | ||
| time × session | 1, 16 | 0.085 | 0.774 | 0.005 | ||
| FL-DPSI | session | 1, 16 | 2.563 | 0.129 | 0.138 | |
| time | 1, 16 | 3.673 | 0.073 | 0.187 | ||
| time × session | 1, 16 | 2.617 | 0.125 | 0.141 | ||
| FL-TTS | session | 1, 16 | 0.245 | 0.627 | 0.015 | |
| time | 1, 16 | 6.145 | 0.025 | 0.277 | ||
| time × session | 1, 16 | 0.280 | 0.604 | 0.017 | ||
| Anterior lateral landing task | LL-APSI | session | 1, 16 | 1.616 | 0.222 | 0.092 |
| time | 1, 16 | 6.089 | 0.025 | 0.276 | ||
| time × session | 1, 16 | 0.002 | 0.963 | 0.0001 | ||
| LL-MLSI | session | 1, 16 | 0.454 | 0.510 | 0.028 | |
| time | 1, 16 | 0.958 | 0.342 | 0.057 | ||
| time × session | 1, 16 | 1.506 | 0.237 | 0.086 | ||
| LL-VSI | session | 1, 16 | 0.706 | 0.413 | 0.042 | |
| time | 1, 16 | 2.245 | 0.154 | 0.123 | ||
| time × session | 1, 16 | 0.319 | 0.580 | 0.020 | ||
| LL-DPSI | session | 1, 16 | 1.140 | 0.302 | 0.067 | |
| time | 1, 16 | 3.347 | 0.086 | 0.173 | ||
| time × session | 1, 16 | 0.042 | 0.841 | 0.003 | ||
| LL-TTS | session | 1, 16 | 1.419 | 0.251 | 0.081 | |
| time | 1, 16 | 7.976 | 0.012 | 0.333 | ||
| time × session | 1, 16 | 9.698 | 0.007 | 0.377 | ||
| Anterior medial landing task | ML-APSI | session | 1, 16 | 0.083 | 0.777 | 0.005 |
| time | 1, 16 | 0.731 | 0.405 | 0.044 | ||
| time × session | 1, 16 | 1.980 | 0.178 | 0.110 | ||
| ML-MLSI | session | 1, 16 | 2.954 | 0.105 | 0.156 | |
| time | 1, 16 | 0.008 | 0.932 | 0.0001 | ||
| time × session | 1, 16 | 1.213 | 0.287 | 0.070 | ||
| ML-VSI | session | 1, 16 | 2.284 | 0.150 | 0.125 | |
| time | 1, 16 | 4.290 | 0.055 | 0.211 | ||
| time × session | 1, 16 | 1.868 | 0.191 | 0.105 | ||
| ML-DPSI | session | 1, 16 | 2.631 | 0.124 | 0.141 | |
| time | 1, 16 | 0.002 | 0.968 | 0.0001 | ||
| time × session | 1, 16 | 2.897 | 0.108 | 0.153 | ||
| ML-TTS | session | 1, 16 | 0.302 | 0.590 | 0.019 | |
| time | 1, 16 | 8.086 | 0.012 | 0.336 | ||
| time × session | 1, 16 | 6.524 | 0.021 | 0.290 | ||
| Y-balance test | dom-YBTCS | session | 1, 16 | 0.190 | 0.669 | 0.012 |
| time | 1, 16 | 10.159 | 0.006 | 0.388 | ||
| time × session | 1, 16 | 1.337 | 0.264 | 0.077 | ||
| non-YBTCS | session | 1, 16 | 18.198 | 0.001 | 0.532 | |
| time | 1, 16 | 148.075 | 0.0001 | 0.902 | ||
| time × session | 1, 16 | 71.372 | 0.0001 | 0.817 |
| Test | Parameter | Anodal Stimulation | Sham Stimulation | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | p | ||
| Static postural stability | non-COPSA | 1387.084 ± 351.054 | 1300.327 ± 333.207* | 1462.003 ± 422.593 | 1506.350 ± 383.414 | 0.015 |
| dom-COPSA | 1453.194 ± 365.467 | 1283.514 ± 314.143 | 1275.224 ± 343.593 | 1396.662 ± 401.482 | 0.231 | |
| non-COPAP | 53.287 ± 7.837 | 52.389 ± 7.866 | 51.384 ± 8.598 | 50.339 ± 10.442 | 0.524 | |
| dom-COPAP | 53.016 ± 5.089 | 50.437 ± 5.913 * | 48.881 ± 5.530 | 48.660 ± 5.254 | 0.322 | |
| non-COPML | 52.373 ± 12.182 | 52.120 ± 10.282 | 51.593 ± 7.547 | 52.219 ± 7.682 | 0.967 | |
| dom-COPML | 52.349 ± 11.661 | 49.352 ± 10.868 | 49.930 ± 7.730 | 50.206 ± 8.750 | 0.746 | |
| Forward landing task | FL-APSI | 0.031 ± 0.005 | 0.027 ± 0.005 * | 0.029 ± 0.004 | 0.028 ± 0.005 | 0.480 |
| FL-MLSI | 0.102 ± 0.009 | 0.102 ± 0.007 | 0.100 ± 0.006 | 0.100 ± 0.07 | 0.252 | |
| FL-VSI | 0.304 ± 0.031 | 0.297 ± 0.028 | 0.296 ± 0.032 | 0.291 ± 0.028 | 0.297 | |
| FL-DPSI | 0.323 ± 0.030 | 0.314 ± 0.029 | 0.311 ± 0.028 | 0.309 ± 0.028 | 0.354 | |
| FL-TTS | 1.313 ± 0.929 | 1.052 ± 0.671 | 1.329 ± 0.692 | 1.199 ± 0.720 | 0.174 | |
| Anterior lateral landing task | LL-APSI | 0.078 ± 0.009 | 0.076 ± 0.010 | 0.076 ± 0.010 | 0.073 ± 0.009 | 0.226 |
| LL-MLSI | 0.097 ± 0.008 | 0.096 ± 0.009 | 0.095 ± 0.009 | 0.095 ± 0.007 | 0.839 | |
| LL-VSI | 0.293 ± 0.033 | 0.290 ± 0.027 | 0.288 ± 0.040 | 0.281 ± 0.029 | 0.251 | |
| LL-DPSI | 0.321 ± 0.033 | 0.315 ± 0.029 | 0.311 ± 0.037 | 0.306 ± 0.028 | 0.281 | |
| LL-TTS | 1.558 ± 0.604 | 1.268 ± 0.405* | 1.553 ± 0.555 | 1.595 ± 0.699 | 0.044 | |
| Anterior medial landing task | ML-APSI | 0.087 ± 0.011 | 0.085 ± 0.010 | 0.086 ± 0.009 | 0.086 ± 0.010 | 0.433 |
| ML-MLSI | 0.094 ± 0.008 | 0.095 ± 0.010 | 0.092 ± 0.009 | 0.090 ± 0.006 | 0.032 | |
| ML-VSI | 0.310 ± 0.038 | 0.300 ± 0.031 | 0.293 ± 0.029 | 0.292 ± 0.029 | 0.298 | |
| ML-DPSI | 0.335 ± 0.037 | 0.325 ± 0.031 | 0.314 ± 0.028 | 0.319 ± 0.029 | 0.336 | |
| ML-TTS | 1.640 ± 0.643 | 1.154 ± 0.434 * | 1.491 ± 0.604 | 1.448 ± 0.795 | 0.055 | |
| Y-balance test | non-YBTCS | 101.693 ± 11.665 | 118.786 ± 13.464 * | 102.400 ± 8.257 | 104.481 ± 9.266 * | 0.0001 |
| dom-YBTCS | 100.954 ± 11.195 | 104.342 ± 10.311 * | 102.679 ± 7.853 | 103.832 ± 9.083 | 0.630 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Nitsche, M.A.; Yi, L.; Kong, Z.; Qi, F. Effects of Transcranial Direct Current Stimulation over the Primary Motor Cortex in Improving Postural Stability in Healthy Young Adults. Biology 2022, 11, 1370. https://doi.org/10.3390/biology11091370
Hou J, Nitsche MA, Yi L, Kong Z, Qi F. Effects of Transcranial Direct Current Stimulation over the Primary Motor Cortex in Improving Postural Stability in Healthy Young Adults. Biology. 2022; 11(9):1370. https://doi.org/10.3390/biology11091370
Chicago/Turabian StyleHou, Jinqian, Michael A. Nitsche, Longyan Yi, Zhaowei Kong, and Fengxue Qi. 2022. "Effects of Transcranial Direct Current Stimulation over the Primary Motor Cortex in Improving Postural Stability in Healthy Young Adults" Biology 11, no. 9: 1370. https://doi.org/10.3390/biology11091370
APA StyleHou, J., Nitsche, M. A., Yi, L., Kong, Z., & Qi, F. (2022). Effects of Transcranial Direct Current Stimulation over the Primary Motor Cortex in Improving Postural Stability in Healthy Young Adults. Biology, 11(9), 1370. https://doi.org/10.3390/biology11091370









