Intraoperative Flow Cytometry for the Characterization of Gynecological Malignancies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
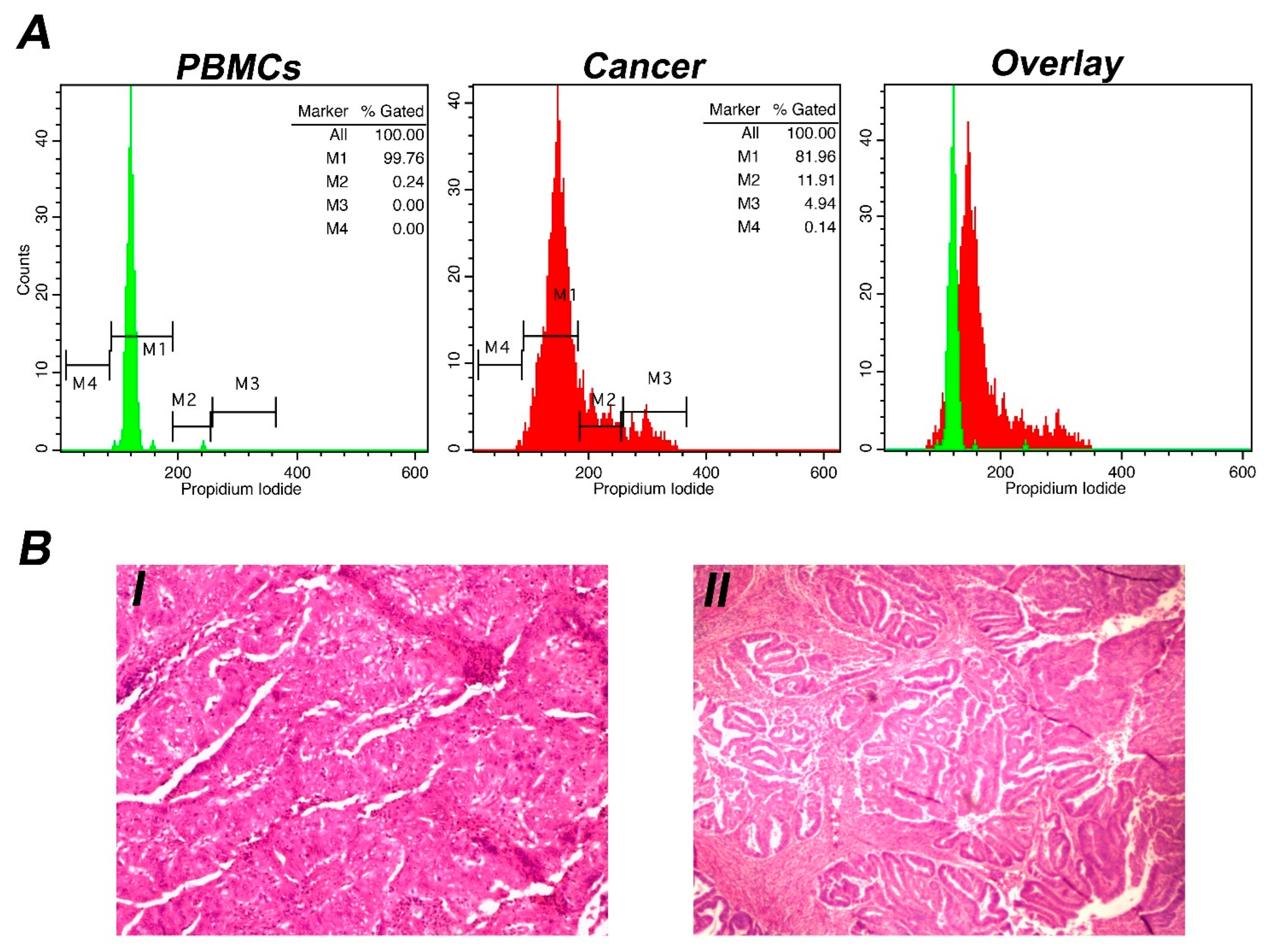
2.1. DNA Content Analysis (Ioannina Protocol)
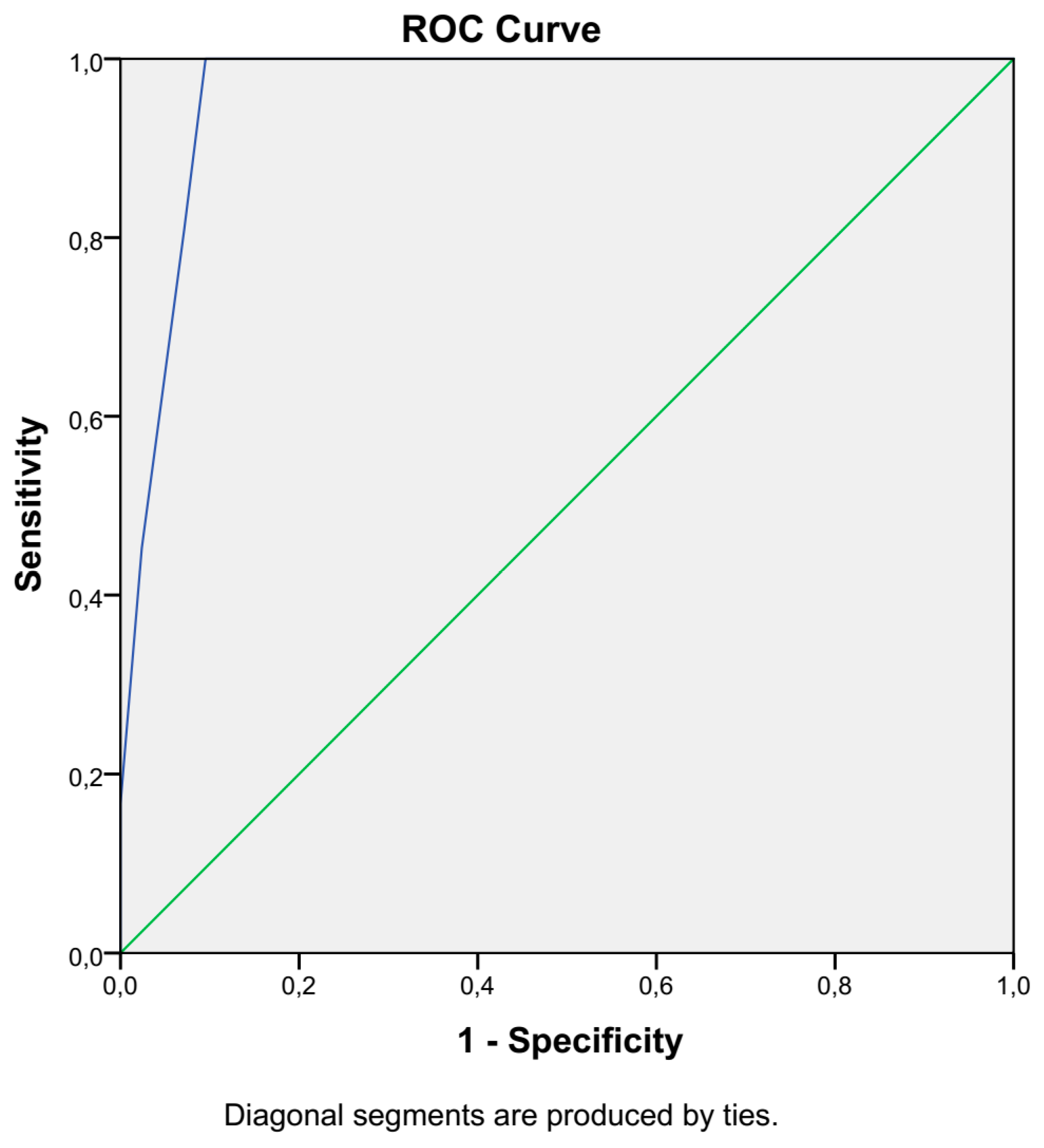
2.2. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H.K.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Stepanian, M.; Cohn, D.E. Gynecologic malignancies in adolescents. Adolesc. Med. Clin. 2004, 15, 549. [Google Scholar] [CrossRef] [PubMed]
- Lõhmussaar, K.; Boretto, M.; Clevers, H. Human-derived model systems in gynecological cancer research. Trends Cancer 2020, 6, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Colombo, N.; Herráez, A.C.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Giannone, G.; Attademo, L.; Scotto, G.; Genta, S.; Ghisoni, E.; Tuninetti, V.; Aglietta, M.; Pignata, S.; Valabrega, G. Endometrial Cancer Stem Cells: Role, Characterization and Therapeutic Implications. Cancers 2019, 11, 1820. [Google Scholar] [CrossRef]
- Ryan, A.J.; Susil, B.; Jobling, T.W.; Oehler, M.K. Endometrial cancer. Cell Tissue Res. 2005, 322, 53–61. [Google Scholar] [CrossRef]
- Felix, A.S.; Weissfeld, J.L.; Stone, R.A.; Bowser, R.; Chivukula, M.; Edwards, R.P.; Linkov, F. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control. 2010, 21, 1851–1856. [Google Scholar] [CrossRef]
- Mayr, D.; Horn, L.C.; Hiller, G.G.; Höhn, A.K.; Schmoeckel, E. Endometrial and other rare uterine sarcomas: Diagnostic aspects in the context of the 2020 WHO classification. Pathologe 2022, 43, 183–195. [Google Scholar] [CrossRef]
- Chantharasamee, J.; Wong, K.; Potivongsajarn, P.; Qorbani, A.; Motamed, N.; Brackert, S.; Cohen, J.; Chmielowski, B.; Kalbasi, A.; Rao, J.; et al. Retrospective analysis of adjuvant treatment for localized, operable uterine leiomyosarcoma. Cancer Med. 2022, 11, 2906–2912. [Google Scholar] [CrossRef]
- Ellwanger, B.; Schüler-Toprak, S.; Jochem, C.; Leitzmann, M.F.; Baurecht, H. Anthropometric factors and the risk of ovarian cancer: A systematic review and meta-analysis. Cancer Rep. 2022, e1618. [Google Scholar] [CrossRef] [PubMed]
- Schoutrop, E.; Moyano-Galceran, L.; Lheureux, S.; Mattsson, J.; Lehti, K.; Dahlstrand, H.; Magalhaes, I. Molecular, cellular and systemic aspects of epithelial ovarian cancer and its tumor microenvironment. Semin. Cancer Biol. 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Burges, A.; Schmalfeldt, B. Ovarian cancer: Diagnosis and treatment. Dtsch. Arztebl. Int. 2011, 108, 635–641. [Google Scholar] [PubMed]
- Greve, B.; Kelsch, R.; Spaniol, K.; Eich, H.T.; Götte, M. Flow cytometry in cancer stem cell analysis and separation. Cytom. Part A 2012, 81, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Wang, Z.; Lu, D.; Feng, J.; Yang, D.; Chen, X.; Yan, X. CD146 is a potential marker for the diagnosis of malignancy in cervical and endometrial cancer. Oncol. Lett. 2013, 5, 1189–1194. [Google Scholar] [CrossRef]
- Corrado, G.; Laquintana, V.; Loria, R.; Carosi, M.; De Salvo, L.; Sperduti, I.; Zampa, A.; Cicchillitti, L.; Piaggio, G.; Cutillo, G.; et al. Endometrial cancer prognosis correlates with the expression of L1CAM and miR34a biomarkers. J. Exp. Clin. Cancer Res. 2018, 37, 139. [Google Scholar] [CrossRef]
- Liu, L.; Lin, J.; He, H. Identification of Potential Crucial Genes Associated With the Pathogenesis and Prognosis of Endometrial Cancer. Front. Genet. 2019, 10, 373. [Google Scholar] [CrossRef]
- D’Angelo, E.; Espinosa, I.; Ali, R.; Gilks, C.B.; van de Rijn, M.; Lee, C.-H.; Prat, J. Uterine leiomyosarcomas: Tumor size, mitotic index, and biomarkers Ki67, and Bcl-2 identify two groups with different prognosis. Gynecol. Oncol. 2011, 121, 328–333. [Google Scholar] [CrossRef]
- Salvo, G.; Odetto, D.; Perrotta, M.C.S.; Noll, F.; Perrotta, M.; Pareja, R.; Wernicke, A.; Ramirez, P.T. Measurement of tumor size in early cervical cancer: An ever-evolving paradigm. Int. J. Gynecol. Cancer 2020, 30, 1215–1223. [Google Scholar] [CrossRef]
- Shapiro, H.M. Practical Flow Cytometry; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Alexiou, G.A.; Vartholomatos, G.; Goussia, A.; Batistatou, A.; Tsamis, K.; Voulgaris, S.; Kyritsis, A.P. Fast cell cycle analysis for intraoperative characterization of brain tumor margins and malignancy. J. Clin. Neurosci. 2015, 22, 129–132. [Google Scholar] [CrossRef]
- Vartholomatos, E.; Vartholomatos, G.; Alexiou, G.; Markopoulos, G. The Past, Present and Future of Flow Cytometry in Central Nervous System Malignancies. Methods Protoc. 2021, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Vartholomatos, G.; Basiari, L.; Exarchakos, G.; Kastanioudakis, I.; Komnos, I.; Michali, M.; Markopoulos, G.S.; Batistatou, A.; Papoudou-Bai, A.; Alexiou, G.A. Intraoperative flow cytometry for head and neck lesions. Assessment of malignancy and tumour-free resection margins. Oral Oncol. 2019, 99, 104344. [Google Scholar] [CrossRef] [PubMed]
- Vartholomatos, G.; Basiari, L.; Kastanioudakis, I.; Psichogios, G.; Alexiou, G.A. The Role of Intraoperative Flow Cytometry in Surgical Margins of Head and Neck Malignancies. Ear Nose Throat J. 2020, 100, 989S–990S. [Google Scholar] [CrossRef]
- Vartholomatos, G.; Harissis, H.; Andreou, M.; Tatsi, V.; Pappa, L.; Kamina, S.; Batistatou, A.; Markopoulos, G.S.; Alexiou, G.A. Rapid assessment of resection margins during breast conserving surgery using intraoperative flow cytometry. Clin. Breast Cancer 2021, 21, e602–e610. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, G.S.; Harissis, H.; Andreou, M.; Alexiou, G.A.; Vartholomatos, G. Intraoperative flow cytometry for invasive breast cancer conserving surgery: A new alternative or adjunct to cavity shaving technique? Surg. Oncol. 2022, 42, 101712. [Google Scholar] [CrossRef]
- Vartholomatos, G.; Alexiou, G.A.; Tatsi, V.; Harissis, H.; Markopoulos, G.S. Next-generation margin evaluation techniques in breast conserving surgery: A memorandum on intraoperative flow cytometry. Eur. J. Surg. Oncol. 2022; S0748-7983 (22) 00386-9. Online ahead of print. [Google Scholar]
- Markopoulos, G.S.; Glantzounis, G.K.; Goussia, A.C.; Lianos, G.D.; Karampa, A.; Alexiou, G.A.; Vartholomatos, G. Touch imprint intraoperative flow cytometry as a complementary tool for detailed assessment of resection margins and tumor biology in liver surgery for primary and metastatic liver neoplasms. Methods Protoc. 2021, 4, 66. [Google Scholar] [CrossRef]
- Markopoulos, G.S.; Goussia, A.; Bali, C.D.; Messinis, T.; Alexiou, G.; Vartholomatos, G. Resection Margins Assessment by Intraoperative Flow Cytometry in Pancreatic Cancer. Ann. Surg. Oncol. 2022, 29, 4643–4645. [Google Scholar] [CrossRef]
- Georvasili, V.K.; Markopoulos, G.S.; Batistatou, A.; Mitsis, M.; Messinis, T.; Lianos, G.D.; Alexiou, G.; Vartholomatos, G.; Bali, C.D. Detection of cancer cells and tumor margins during colorectal cancer surgery by intraoperative flow cytometry. Int. J. Surg. 2022, 104, 106717. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Vartholomatos, G.; Stefanaki, K.; Markopoulos, G.S.; Kyritsis, A.P. Intraoperative flow cytometry for diagnosis of central nervous system lesions. J. Cytol. 2019, 36, 134–135. [Google Scholar] [CrossRef]
- Andreou, M.; Vartholomatos, E.; Harissis, H.; Markopoulos, G.S.; Alexiou, G.A. Past, Present and Future of Flow Cytometry in Breast Cancer—A Systematic Review. EJIFCC 2019, 30, 423–437. [Google Scholar] [PubMed]
- Shioyama, T.; Muragaki, Y.; Maruyama, T.; Komori, T.; Iseki, H. Intraoperative flow cytometry analysis of glioma tissue for rapid determination of tumor presence and its histopathological grade. J. Neurosurg. 2013, 118, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Koriyama, S.; Nitta, M.; Shioyama, T.; Komori, T.; Maruyama, T.; Kawamata, T.; Muragaki, Y. Intraoperative Flow Cytometry Enables the Differentiation of Primary Central Nervous System Lymphoma from Glioblastoma. World Neurosurg. 2018, 112, e261–e268. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Muragaki, Y.; Shioyama, T.; Komori, T.; Maruyama, T.; Nitta, M.; Yasuda, T.; Hosono, J.; Okamoto, S.; Kawamata, T. Malignancy Index Using Intraoperative Flow Cytometry is a Valuable Prognostic Factor for Glioblastoma Treated With Radiotherapy and Concomitant Temozolomide. Neurosurgery 2018, 84, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, G.; Eguchi, S.; Anami, H.; Ishikawa, T.; Yamaguchi, K.; Nitta, M.; Muragaki, Y.; Kawamata, T. Ultrarapid Evaluation of Meningioma Malignancy by Intraoperative Flow Cytometry. World Neurosurg. 2018, 120, 320–327. [Google Scholar] [CrossRef] [PubMed]
- A Alexiou, G.; Vartholomatos, G.; Kobayashi, T.; Voulgaris, S.; Kyritsis, A.P. The emerging role of intraoperative flow cytometry in intracranial tumor surgery. Clin. Neurol. Neurosurg. 2020, 192, 105742. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Sharma, V. Surgical Oncology: An Overview. In Cancer Diagnostics and Therapeutics; Basu, S.K., Panda, C.K., Goswami, S., Eds.; Springer: Singapore, 2022; pp. 261–270. Available online: https://link.springer.com/chapter/10.1007/978-981-16-4752-9_11#citeas (accessed on 22 August 2022).
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell. Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Fröhling, S.; Döhner, H. Chromosomal abnormalities in cancer. N. Engl. J. Med. 2008, 359, 722–734. [Google Scholar] [CrossRef]
- Danielsen, H.E.; Pradhan, M.; Novelli, M. Revisiting tumour aneuploidy—The place of ploidy assessment in the molecular era. Nat. Rev. Clin. Oncol. 2016, 13, 291–304. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- AAmin, A.R.; Karpowicz, P.A.; Carey, T.E.; Arbiser, J.; Nahta, R.; Chen, Z.G.; Dong, J.-T.; Kucuk, O.; Khan, G.N.; Huang, G.S.; et al. Evasion of anti-growth signaling: A key step in tumorigenesis and potential target for treatment and prophylaxis by natural compounds. Semin. Cancer Biol. 2015, 35, S55–S77. [Google Scholar] [CrossRef]
- Geels, Y.; Pijnenborg, J.; Erp, S.V.D.B.-V.; Snijders, M.; Bulten, J.; Massuger, L. Absolute depth of myometrial invasion in endometrial cancer is superior to the currently used cut-off value of 50%. Gynecol. Oncol. 2013, 129, 285–291. [Google Scholar] [CrossRef]
- Vartholomatos, E.; Mantziou, S.; Alexiou, G.A.; Lazari, D.; Sioka, C.; Kyritsis, A.; Markopoulos, G.S. An NF-kappaB- and Therapy-Related Regulatory Network in Glioma: A Potential Mechanism of Action for Natural Antiglioma Agents. Biomedicines 2022, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Vartholomatos, E.; Alexiou, G.A.; Markopoulos, G.S.; Lazari, D.; Tsiftsoglou, O.; Chousidis, I.; Leonardos, I.; Kyritsis, A.P. Deglucohellebrin: A Potent Agent for Glioblastoma Treatment. Anti-Cancer Agents Med. Chem. 2020, 20, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Demirel, D.; Akyürek, N.; Ramzy, I. Diagnostic and prognostic significance of image cytometric DNA ploidy measurement in cytological samples of cervical squamous intraepithelial lesions. Cytopathology 2013, 24, 105–112. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiadi, Z.; Mantziou, S.; Akrivis, C.; Paschopoulos, M.; Balasi, E.; Lianos, G.D.; Alexiou, G.A.; Mitsis, M.; Vartholomatos, G.; Markopoulos, G.S. Intraoperative Flow Cytometry for the Characterization of Gynecological Malignancies. Biology 2022, 11, 1339. https://doi.org/10.3390/biology11091339
Anastasiadi Z, Mantziou S, Akrivis C, Paschopoulos M, Balasi E, Lianos GD, Alexiou GA, Mitsis M, Vartholomatos G, Markopoulos GS. Intraoperative Flow Cytometry for the Characterization of Gynecological Malignancies. Biology. 2022; 11(9):1339. https://doi.org/10.3390/biology11091339
Chicago/Turabian StyleAnastasiadi, Zoi, Stefania Mantziou, Christos Akrivis, Minas Paschopoulos, Eufemia Balasi, Georgios D. Lianos, George A. Alexiou, Michail Mitsis, George Vartholomatos, and Georgios S. Markopoulos. 2022. "Intraoperative Flow Cytometry for the Characterization of Gynecological Malignancies" Biology 11, no. 9: 1339. https://doi.org/10.3390/biology11091339
APA StyleAnastasiadi, Z., Mantziou, S., Akrivis, C., Paschopoulos, M., Balasi, E., Lianos, G. D., Alexiou, G. A., Mitsis, M., Vartholomatos, G., & Markopoulos, G. S. (2022). Intraoperative Flow Cytometry for the Characterization of Gynecological Malignancies. Biology, 11(9), 1339. https://doi.org/10.3390/biology11091339









