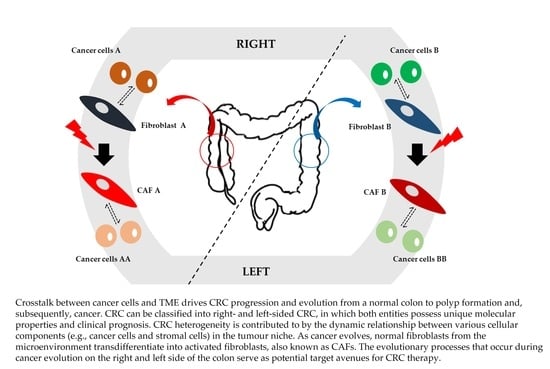
Dynamic Co-Evolution of Cancer Cells and Cancer-Associated Fibroblasts: Role in Right- and Left-Sided Colon Cancer Progression and Its Clinical Relevance
Abstract
:Simple Summary
Abstract
1. Introduction
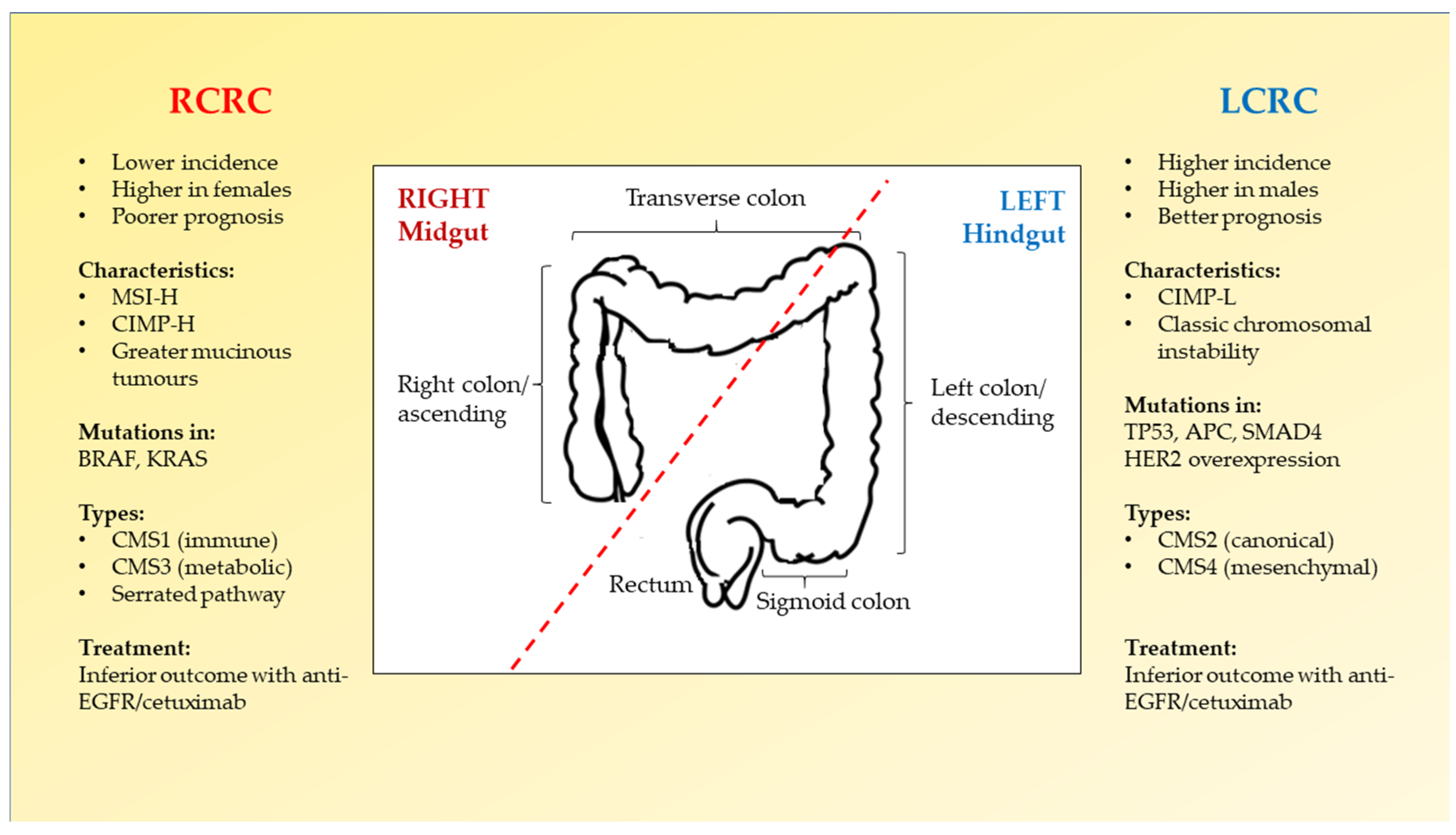
2. CRC and Sidedness
2.1. CRC
2.2. Right-Sided versus Left-Sided CRC
3. Tumour Microenvironment of CRC
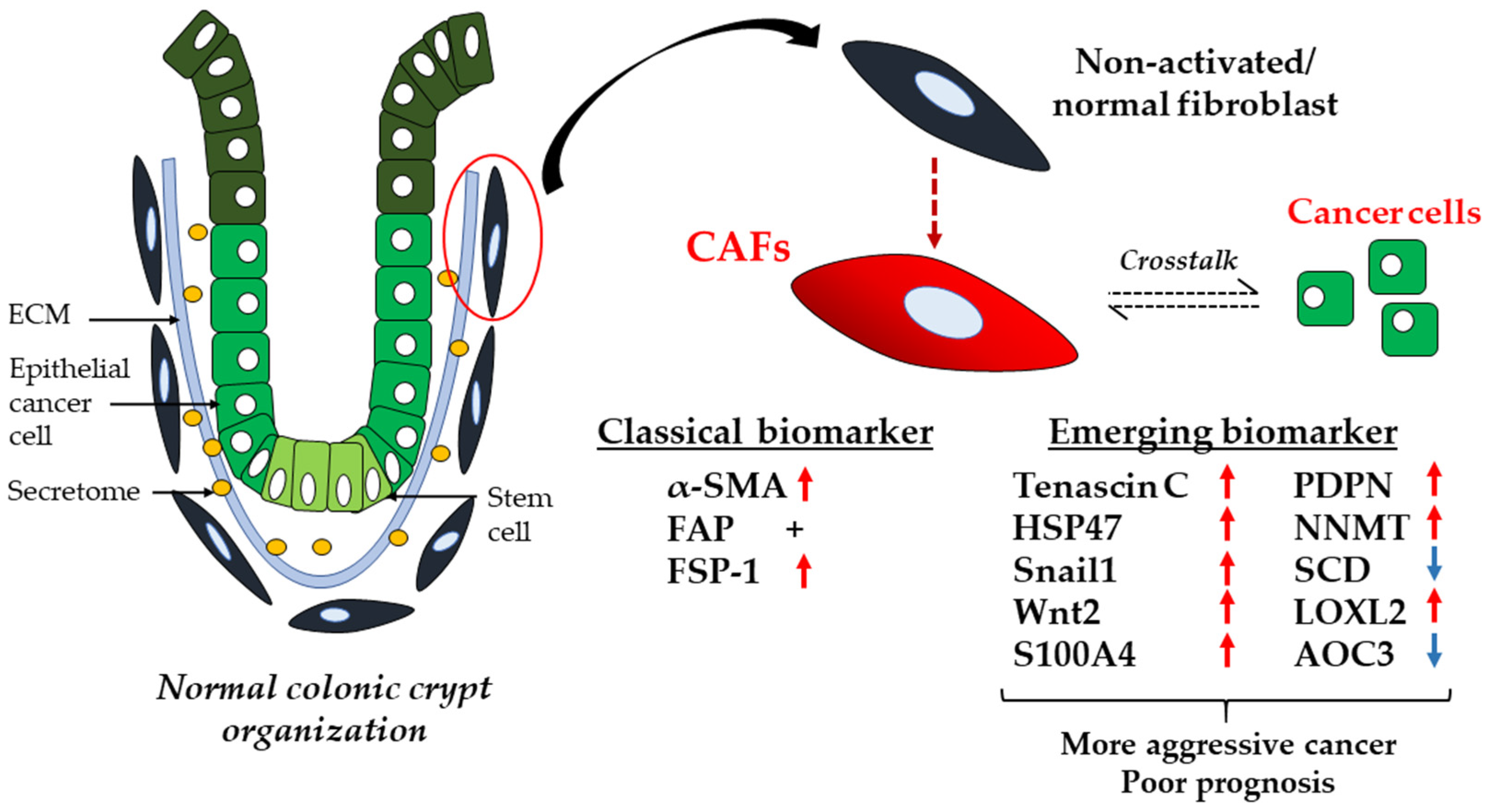
3.1. CAFs
3.2. Crosstalk between CAFs and Cancer Cells
4. Cancer Evolution
4.1. Cancer Evolution and Impact on Tumour Heterogeneity
4.2. Tumour–CAF Co-Evolution
4.3. Analyses of CRC Evolution and Heterogeneity
5. Discussion
6. Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeBleu, V.S.; Kalluri, R. A peek into cancer-associated fibroblasts: Origins, functions and translational impact. Dis. Models Mech. 2018, 11, dmm029447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calon, A.; Lonardo, E.; Berenguer-Llergo, A.; Espinet, E.; Hernando-Momblona, X.; Iglesias, M.; Sevillano, M.; Palomo-Ponce, S.; Tauriello, D.V.F.; Byrom, D.; et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015, 47, 320–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, D.W.; Adegboyega, P.A.; Di Mari, J.F.; Mifflin, R.C. Epithelial cells and their neighbors I. Role of intestinal myofibroblasts in development, repair, and cancer. Am. J. Physiol. Gastrointest Liver Physiol. 2005, 289, G2–G7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Ding, L.; Zhang, D.; Shi, G.; Xu, Q.; Shen, S.; Wang, Y.; Wang, T.; Hou, Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 2018, 8, 3932–3948. [Google Scholar] [CrossRef]
- Koliaraki, V.; Prados, A.; Armaka, M.; Kollias, G. The mesenchymal context in inflammation, immunity and cancer. Nature immunology 2020, 21, 974–982. [Google Scholar] [CrossRef]
- Lee, J.M.; Han, Y.D.; Cho, M.S.; Hur, H.; Min, B.S.; Lee, K.Y.; Kim, N.K. Impact of tumour sidedness on survival and recurrence patterns in colon cancer patients. Ann. Surg. Treat Res. 2019, 96, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Venook, A.P.; Niedzwiecki, D.; Innocenti, F.; Fruth, B.; Greene, C.; O’Neil, B.H.; Shaw, J.E.; Atkins, J.N.; Horvath, L.E.; Polite, B.N.; et al. Impact of primary (1°) tumour location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2016, 34 (Suppl. S15), 3504. [Google Scholar] [CrossRef]
- Loree, J.M.; Pereira, A.A.; Lam, M.; Willauer, A.N.; Raghav, K.; Dasari, A.; Morris, V.; Advani, S.; Menter, D.G.; Eng, C.; et al. Classifying colorectal cancer by tumour location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin. Cancer Res. 2018, 24, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- Nowell, P.C. The clonal evolution of tumour cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef]
- Crispo, E.; Moore, J.S.; Lee-Yaw, J.A.; Gray, S.M.; Haller, B.C. Broken barriers: Human-induced changes to gene flow and introgression in animals: An examination of the ways in which humans increase genetic exchange among populations and species and the consequences for biodiversity. BioEssays 2011, 33, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Haldar, S.; Suchanti, S.; Bhowmick, N.A. Epigenetic changes in fibroblasts drive cancer metabolism and differentiation. Endocr. Relat. Cancer 2019, 26, R673–R688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, L.R.; Campbell, P.J. Evolution of the cancer genome. Nat. Rev. Genet. 2012, 13, 795–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer (IARC). Cancer Today (Powered by GLOBOCAN 2020). Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=population (accessed on 4 March 2022).
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal cancer incidence patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Grady, W.M. Genetic testing for high-risk colon cancer patients. Gastroenterology 2003, 124, 1574–1594. [Google Scholar] [CrossRef]
- Medema, J.P. Cancer stem cells: The challenges ahead. Nat. Cell Biol. 2013, 15, 338–344. [Google Scholar] [CrossRef]
- Nassar, D.; Blanpain, C. Cancer stem cells: Basic concepts and therapeutic implications. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 47–76. [Google Scholar] [CrossRef]
- De Sousa e Melo, F.; Kurtova, A.V.; Harnoss, J.M.; Kljavin, N.; Hoeck, J.D.; Hung, J.; Anderson, J.E.; Storm, E.E.; Modrusan, Z.; Koeppen, H.; et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 2017, 543, 676–680. [Google Scholar] [CrossRef]
- Shimokawa, M.; Ohta, Y.; Nishikori, S.; Matano, M.; Takano, A.; Fujii, M.; Date, S.; Sugimoto, S.; Kanai, T.; Sato, T. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature 2017, 545, 187–192. [Google Scholar] [CrossRef]
- Lee, M.S.; Menter, D.G.; Kopetz, S. Right versus left colon cancer biology: Integrating the consensus molecular subtypes. J. Natl. Compr. Cancer Netw. 2017, 15, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.D.; Tejpar, S.; Delorenzi, M.; Yan, P.; Fiocca, R.; Klingbiel, D.; Dietrich, D.; Biesmans, B.; Bodoky, G.; Barone, C.; et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: Results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J. Clin. Oncol. 2010, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Huijberts, S.; Grothey, A.; Yaeger, R.; Cuyle, P.J.; Elez, E.; Fakih, M.; Montagut, C.; Peeters, M.; Yoshino, T.; et al. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E–mutant metastatic colorectal cancer: Safety lead-in results from the Phase III BEACON colorectal cancer study. J. Clin. Oncol. 2019, 37, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopetz, S.; Guthrie, K.A.; Van Morris, K.; Lenz, H.J.; Magliocco, A.M.; Maru, D.; Yan, Y.; Lanman, R.; Manyam, G.; Hong, D.S.; et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J. Clin. Oncol. 2021, 39, 285–294. [Google Scholar] [CrossRef]
- Siravegna, G.; Mussolin, B.; Buscarino, M.; Corti, G.; Cassingena, A.; Crisafulli, G.; Ponzetti, A.; Cremolini, C.; Amatu, A.; Lauricella, C.; et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 2015, 21, 795–801, Erratum in Nat. Med. 2015, 21, 827. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.M.; Pfau, P.R.; O’Connor, E.S.; King, J.; LoConte, N.; Kennedy, G.; Smith, M.A. Mortality by stage for right- versus left-sided colon cancer: Analysis of surveillance, epidemiology, and end result—Medicare data. J. Clin. Oncol. 2011, 29, 4401–4409. [Google Scholar] [CrossRef] [Green Version]
- Zarkavelis, G.; Boussios, S.; Papadaki, A.; Katsanos, K.H.; Christodoulou, D.K.; Pentheroudakis, G. Current and future biomarkers in colorectal cancer. Ann. Gastroenterol. 2017, 30, 613–621. [Google Scholar] [CrossRef]
- Mirón Fernández, I.; Velasco, S.M.; Luque, J.D.T.; Poveda, I.G.; López, M.R.; Santoyo, J.S. Right and left colorectal cancer: Differences in post-surgical-care outcomes and survival in elderly patients. Cancers 2021, 13, 2647. [Google Scholar] [CrossRef]
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.P.; Tabernero, J.; et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yang, Y.; Li, X.; Huang, M.; Xu, F.; Ge, W.; Zhang, S.; Zheng, S. Multi-omics approach reveals distinct differences in left- and right-sided colon cancer. Mol. Cancer Res. 2018, 16, 476–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warschkow, R.; Sulz, M.C.; Marti, L.; Tarantino, I.; Schmied, B.M.; Cerny, T.; Güller, U. Better survival in right-sided versus left-sided stage I–III colon cancer patients. BMC Cancer 2016, 16, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moritani, K.; Hasegawa, H.; Okabayashi, K.; Ishii, Y.; Endo, T.; Kitagawa, Y. Difference in the recurrence rate between right- and left-sided colon cancer: A 17-year experience at a single institution. Surg. Today 2013, 44, 1685–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, H.; Zaanan, A.; Sinicrope, F.A. Microsatellite instability testing and its role in the management of colorectal cancer. Curr. Treat. Options Oncol. 2015, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Garcia, E.; Argiles, G.; Elez, E.; Tabernero, J. BRAF mutant colorectal cancer: Prognosis, treatment, and new perspectives. Ann. Oncol. 2017, 28, 2648–2657. [Google Scholar] [CrossRef]
- Sveen, A.; Kopetz, S.; Lothe, R.A. Biomarker-guided therapy for colorectal cancer: Strength in complexity. Nat. Rev. Clin. Oncol. 2019, 17, 11–32. [Google Scholar] [CrossRef]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S.; Renz, P.; Wegner, R.E.; Finley, G.G.; Raj, M.S.; Monga, D.K.; McCormick, J.; Kirichenko, A.V. Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer: A National Cancer Database (NCDB) analysis. Ann. Surg. 2020, 271, 716–723. [Google Scholar] [CrossRef]
- Arakawa, K.; Hata, K.; Kawai, K.; Tanaka, T.; Nishikawa, T.; Sasaki, K.; Shuno, Y.; Kaneko, M.; Hiyoshi, M.; Emoto, S.; et al. Predictors for high microsatellite instability in patients with colorectal cancer fulfilling the revised Bethesda Guidelines. Anticancer Res. 2018, 38, 4871–4876. [Google Scholar] [CrossRef]
- Puccini, A.; Marshall, J.L.; Salem, M.E. Molecular variances between right- and left-sided colon cancers. Curr. Color. Cancer Rep. 2018, 14, 152–158. [Google Scholar] [CrossRef]
- Xing, F.; Saidou, J.; Watabe, K. Cancer associated fibroblasts (CAFs) in tumour microenvironment. Front. Biosci. 2010, 15, 166–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wever, O.; Demetter, P.; Mareel, M.; Bracke, M. Stromal myofibroblasts are drivers of invasive cancer growth. Int. J. Cancer 2008, 123, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Ishii, G.; Ochiai, A.; Neri, S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumour microenvironment. Adv. Drug Deliv. Rev. 2016, 99 Pt B, 186–196. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, H.; Zhao, H.; Wu, Z.; Long, Y.; Zhang, J.; Yan, X.; You, Z.; Zhou, L.; Xia, T.; et al. Matrix-transmitted paratensile signaling enables myofibroblast–fibroblast cross talk in fibrosis expansion. Proc. Natl. Acad. Sci. USA 2020, 117, 10832–10838. [Google Scholar] [CrossRef]
- Arina, A.; Idel, C.; Hyjek, E.M.; Alegre, M.L.; Wang, Y.; Bindokas, V.P.; Weichselbaum, R.R.; Schreiber, H. Tumour-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl. Acad. Sci. USA 2016, 113, 7551–7556. [Google Scholar] [CrossRef] [Green Version]
- Conti, J.; Thomas, G. The role of tumour stroma in colorectal cancer invasion and metastasis. Cancers 2011, 3, 2160–2168. [Google Scholar] [CrossRef] [Green Version]
- Cirri, P.; Chiarugi, P. Cancer-associated-fibroblasts and tumour cells: A diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2011, 31, 195–208. [Google Scholar] [CrossRef]
- Wu, J.; Liang, C.; Chen, M.; Su, W. Association between tumour-stroma ratio and prognosis in solid tumour patients: A systematic review and meta-analysis. Oncotarget 2016, 7, 68954–68965. [Google Scholar] [CrossRef] [Green Version]
- NBSP; Tommelein, J.; Verset, L.; Boterberg, T.; Demetter, P.; Bracke, M.; De Wever, O. Cancer-Associated Fibroblasts Connect Metastasis-Promoting Communication in Colorectal Cancer. Front. Oncol. 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2019, 146, 895–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikberg, M.L.; Edin, S.; Lundberg, I.V.; Van Guelpen, B.; Dahlin, A.M.; Rutegård, J.; Stenling, R.; Öberg, Å.; Palmqvist, R. High intratumoural expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumour Biol. 2013, 34, 1013–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandberg, T.P.; Stuart, M.P.; Oosting, J.; Tollenaar, R.A.; Sier, C.F.; Mesker, W.E. Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumour-stroma ratio in colorectal cancer. BMC Cancer 2019, 19, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-Y.; Sung, R.; Lee, S.-J.; Lee, T.-G.; Kim, N.; Yoon, S.M.; Lee, E.J.; Chae, H.B.; Youn, S.J.; Park, S.M. Podoplanin, α-smooth muscle actin or S100A4 expressing cancer-associated fibroblasts are associated with different prognosis in colorectal cancers. J. Korean Med Sci. 2013, 28, 1293–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, S.; Garcia-Palmero, I.; Herrera, M.; Bartolomé, R.A.; Pena, C.; Fernandez-Aceñero, M.J.; Padilla, G.; Peláez-García, A.; Lopez-Lucendo, M.; Rodriguez-Merlo, R.; et al. LOXL2 is highly expressed in cancer-associated fibroblasts and associates to poor colon cancer survival. Clin. Cancer Res. 2015, 21, 4892–4902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsia, L.-T.; Ashley, N.; Ouaret, D.; Wang, L.M.; Wilding, J.; Bodmer, W.F. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc. Natl. Acad. Sci. USA 2016, 113, E2162–E2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, T.; Kikuchi, H.; Ishimatsu, H.; Iino, I.; Hirotsu, A.; Matsumoto, T.; Ozaki, Y.; Kawabata, T.; Hiramatsu, Y.; Ohta, M.; et al. Tenascin C in colorectal cancer stroma is a predictive marker for liver metastasis and is a potent target of miR-198 as identified by microRNA analysis. Br. J. Cancer 2017, 117, 1360–1370. [Google Scholar] [CrossRef] [Green Version]
- Kramer, N.; Schmöllerl, J.; Unger, C.; Nivarthi, H.; Rudisch, A.; Unterleuthner, D.; Scherzer, M.; Riedl, A.; Artaker, M.; Crncec, I.; et al. Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene 2017, 36, 5460–5472. [Google Scholar] [CrossRef]
- Mori, K.; Toiyama, Y.; Otake, K.; Fujikawa, H.; Saigusa, S.; Hiro, J.; Kobayashi, M.; Ohi, M.; Tanaka, K.; Inoue, Y.; et al. Proteomics analysis of differential protein expression identifies heat shock protein 47 as a predictive marker for lymph node metastasis in patients with colorectal cancer. Int. J. Cancer 2017, 140, 1425–1435. [Google Scholar] [CrossRef]
- Mohammadpour, S.; Esfahani, A.T.; Karimpour, R.; Bakhshian, F.; Tabatabaei, S.A.M.; Laleh, A.; Mojarad, E.N. High expression of Snail1 is associated with EMAST and poor prognosis in CRC patients. Gastroenterol. Hepatol. Bed Bench 2019, 12 (Suppl. S1), 30–36. [Google Scholar]
- Eckert, M.A.; Coscia, F.; Chryplewicz, A.; Chang, J.W.; Hernandez, K.M.; Pan, S.; Tienda, S.M.; Nahotko, D.A.; Li, G.; Blaženović, I.; et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 2019, 569, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Isella, C.; Terrasi, A.; Bellomo, S.E.; Petti, C.; Galatola, G.; Muratore, A.; Mellano, A.; Senetta, R.; Cassenti, A.; Sonetto, C.; et al. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 2015, 47, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, J.; Micke, P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin. Cancer Biol. 2014, 25, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Berral-González, A.; López-Cade, I.; Galindo-Pumariño, C.; Bueno-Fortes, S.; Martín-Merino, M.; Carrato, A.; Ocaña, A.; De La Pinta, C.; López-Alfonso, A.; et al. Cancer-associated fibroblast-derived gene signatures determine prognosis in colon cancer patients. Mol. Cancer 2021, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Bartolome, R.A.; Mendes, M.; Barderas, R.; Fernández-Aceñerp, M.J.; Peláez-García, A.; Peña, C.; Lopez-Lucendo, M.; Villar-Vázquez, R.; De Herreros, A.G.; et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin. Cancer Res. 2013, 19, 6006–6019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Yang, X.; Cheng, L.; Liu, R.; Lei, Y.; Dong, D.; Li, F.; Lau, Q.C.; Deng, L.; Nice, E.C.; et al. Chemokine CXCL14 is associated with prognosis in patients with colorectal carcinoma after curative resection. J. Transl. Med. 2013, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Karakasheva, T.A.; Lin, E.W.; Tang, Q.; Qiao, E.; Waldron, T.J.; Soni, M.; Klein-Szanto, A.J.; Sahu, V.; Basu, D.; Ohashi, S.; et al. IL-6 mediates cross-talk between tumour cells and activated fibroblasts in the tumour microenvironment. Cancer Res. 2018, 78, 4957–4970. [Google Scholar] [CrossRef] [Green Version]
- Heichler, C.; Scheibe, K.; Schmied, A.; Geppert, C.I.; Schmid, B.; Wirtz, S.; Thoma, O.M.; Kramer, V.; Waldner, M.J.; Büttner, C.; et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut 2020, 69, 1269–1282. [Google Scholar] [CrossRef]
- Allam, A.; Yakou, M.; Pang, L.; Ernst, M.; Huynh, J. Exploiting the STAT3 nexus in cancer-associated fibroblasts to improve cancer therapy. Front. Immunol. 2021, 12, 767939. [Google Scholar] [CrossRef]
- Tan, H.X.; Gong, W.Z.; Zhou, K.; Xiao, Z.G.; Hou, F.T.; Huang, T.; Zhang, L.; Dong, H.Y.; Zhang, W.L.; Liu, Y.; et al. CXCR4/TGF-β1 mediated hepatic stellate cells differentiation into carcinoma-associated fibroblasts and promoted liver metastasis of colon cancer. Cancer Biol. Ther. 2020, 21, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.F.; Iglesias, M.; Céspedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.-F.; et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthy, A.; Khan, L.; Bensler, N.P.; Bose, P.; De Carvalho, D.D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 2018, 9, 4692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Lai, Q.; Fang, Y.; Wu, C.; Liu, Y.; Li, Q.; Wang, X.; Gu, C.; Chen, J.; et al. Cancer-associated fibroblasts-derived exosomal miR-17-5p promotes colorectal cancer aggressive phenotype by initiating a RUNX3/MYC/TGF-β1 positive feedback loop. Cancer Lett. 2020, 491, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Rupp, C.; Scherzer, M.; Rudisch, A.; Unger, C.; Haslinger, C.; Schweifer, N.; Artaker, M.; Nivarthi, H.; Moriggl, R.; Hengstschläger, M.; et al. IGFBP7, a novel tumour stroma marker, with growth-promoting effects in colon cancer through a paracrine tumour-stroma interaction. Oncogene 2015, 34, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Flashner-Abramson, E.; Shalapour, S.; Zhong, Z.; Taniguchi, K.; Levitzki, A.; Karin, M. Targeting colorectal cancer via its microenvironment by inhibiting IGF-1 receptor-insulin receptor substrate and STAT3 signaling. Oncogene 2015, 35, 2634–2644. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Chen, J.; Zhou, B.; Xu, K.; Wang, K.; Fang, Z.; Shao, Y.; Yuan, Y.; Zheng, S.; Hu, W. HomeoboxC6 promotes metastasis by orchestrating the DKK1/Wnt/β-catenin axis in right-sided colon cancer. Cell Death Dis. 2021, 12, 337. [Google Scholar] [CrossRef]
- Unterleuthner, D.; Neuhold, P.; Schwarz, K.; Janker, L.; Neuditschko, B.; Nivarthi, H.; Crncec, I.; Kramer, N.; Unger, C.; Hengstschläger, M.; et al. Cancer-associated fibroblast-derived WNT2 increases tumour angiogenesis in colon cancer. Angiogenesis 2020, 23, 159–177. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Yu, S.; Xie, Y.; Dai, X.; Dong, M.; Sheng, C.; Hu, J. Cancer-associated fibroblasts-derived exosomes upregulate microRNA-135b-5p to promote colorectal cancer cell growth and angiogenesis by inhibiting thioredoxin-interacting protein. Cell. Signal. 2021, 84, 110029. [Google Scholar] [CrossRef]
- Mochizuki, S.; Ao, T.; Sugiura, T.; Yonemura, K.; Shiraishi, T.; Kajiwara, Y.; Okamoto, K.; Shinto, E.; Okada, Y.; Ueno, H. Expression and function of a disintegrin and metalloproteinases in cancer-associated fibroblasts of colorectal cancer. Digestion 2019, 101, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, H.; Ge, Y.; Wang, X. Integrated single-cell and bulk RNA sequencing analysis identifies a cancer associated fibroblast-related signature for predicting prognosis and therapeutic responses in colorectal cancer. Cancer Cell Int. 2021, 21, 552. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Ali, A. Cancer-associated fibroblasts of colorectal cancer and their markers: Updates, challenges and translational outlook. Future Oncol. 2020, 16, 2329–2344. [Google Scholar] [CrossRef] [PubMed]
- Almendro, V.; Marusyk, A.; Polyak, K. Cellular heterogeneity and molecular evolution in cancer. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 277–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGranahan, N.; Swanton, C. Clonal heterogeneity and tumour evolution: Past, present, and the future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [Green Version]
- Merlo, L.M.F.; Pepper, J.W.; Reid, B.J.; Maley, C.C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 2006, 6, 924–935. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Nik-Zainal, S.; Alexandrov, L.; Wedge, D.; Van Loo, P.; Greenman, C.D.; Raine, K.; Jones, D.; Hinton, J.; Marshall, J.; Stebbings, L.A.; et al. Mutational Processes Molding the Genomes of 21 Breast Cancers. Cell 2012, 149, 979–993. [Google Scholar] [CrossRef] [Green Version]
- Gatenby, R.A.; Smallbone, K.; Maini, P.K.; Rose, F.; Averill, J.; Nagle, R.B.; Worrall, L.; Gillies, R. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br. J. Cancer 2007, 97, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Beerenwinkel, N.; Schwarz, R.F.; Gerstung, M.; Markowetz, F. Cancer evolution: Mathematical models and computational inference. Syst. Biol. 2014, 64, e1–e25. [Google Scholar] [CrossRef]
- Martincorena, I.; Campbell, P.J. Somatic mutation in cancer and normal cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Greenman, C.; Stephens, P.; Smith, R.; Dalgliesh, G.L.; Hunter, C.; Bignell, G.; Davies, H.; Teague, J.; Butler, A.; Stevens, C.; et al. Patterns of somatic mutation in human cancer genomes. Nature 2007, 446, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlinger, M.; McGranahan, N.; Dewhurst, S.M.; Burrell, R.A.; Tomlinson, I.; Swanton, C. Cancer: Evolution within a lifetime. Annu. Rev. Genet. 2014, 48, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, W. The somatic evolution of cancer. The Harveian Oration of 1996. J. R. Coll. Physicians Lond. 1997, 31, 82–89. [Google Scholar] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Bodmer, W.; Bielas, J.H.; Beckman, R.A. Genetic instability is not a requirement for tumour development. Cancer Res. 2008, 68, 3558–3561. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-C.; Peterson, S.E.; Loring, J.F. Protein post-translational modifications and regulation of pluripotency in human stem cells. Cell Res. 2013, 24, 143–160. [Google Scholar] [CrossRef] [Green Version]
- Lis, H.; Sharon, N. Protein glycosylation. Structural and functional aspects. Eur. J. Biochem. 1993, 218, 1–27. [Google Scholar] [CrossRef]
- Lauc, G.; Krištić, J.; Zoldoš, V. Glycans—The third revolution in evolution. Front. Genet 2014, 5, 145. [Google Scholar] [CrossRef] [Green Version]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Reymond, N.; D’Agua, B.B.; Ridley, A.J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 2013, 13, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Boccarelli, A.; Del Buono, N.; Esposito, F. Analysis of fibroblast genes selected by NMF to reveal the potential crosstalk between ulcerative colitis and colorectal cancer. Exp. Mol. Pathol. 2021, 123, 104713. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Balliet, R.M.; Rivadeneira, D.; Chiavarina, B.; Pavlides, S.; Wang, C.; Whitaker-Menezes, D.; Daumer, K.; Lin, Z.; Witkiewicz, A.; et al. Oxidative stress in cancer associated fibroblasts drives tumour-stroma co-evolution: A new paradigm for understanding tumour metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 2010, 9, 3256–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcucci, A.; Ruocco, M.R.; Granato, G.; Sacco, A.M.; Montagnani, S. Cancer: An oxidative crosstalk between solid tumour cells and cancer associated fibroblasts. BioMed. Res. Int. 2016, 2016, 4502846. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Cancer Facts & Figures 2012. Available online: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf (accessed on 4 March 2022).
- Kamb, A.; Wee, S.; Lengauer, C. Why is cancer drug discovery so difficult? Nat. Rev. Drug Discov. 2007, 6, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Pharoah, P.D.P.; Dunning, A.M.; Ponder, B.A.J.; Easton, D.F. Association studies for finding cancer-susceptibility genetic variants. Nat. Cancer 2004, 4, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.A. The Biology of Cancer, 2nd ed.; W.W. Norton & Company: New York, NY, USA, 2013. [Google Scholar]
- Hallatschek, O.; Nelson, D.R. Life at the front of an expanding population. Evolution 2010, 64, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Niida, A.; Mimori, K.; Shibata, T.; Miyano, S. Modeling colorectal cancer evolution. J. Hum. Genet. 2021, 66, 869–878. [Google Scholar] [CrossRef]
- Banerjee, S.; Zhang, X.; Kuang, S.; Wang, J.; Li, L.; Fan, G.; Luo, Y.; Sun, S.; Han, P.; Liu, X. Comparative analysis of clonal evolution among patients with right- and left-sided colon and rectal cancer. iScience 2021, 24, 102718. [Google Scholar] [CrossRef]
- Imperial, R.; Ahmed, Z.; Toor, O.M.; Erdoğan, C.; Khaliq, A.; Case, P.; Case, J.; Kennedy, K.; Cummings, L.S.; Melton, N.; et al. Comparative proteogenomic analysis of right-sided colon cancer, left-sided colon cancer and rectal cancer reveals distinct mutational profiles. Mol. Cancer 2018, 17, 177. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Zhang, Q.; Huang, R.; Gao, Z.; Yuan, Z.; Tang, Q.; Gao, F.; Wang, M.; Zhang, W.; Ma, T.; et al. Genomic analysis reveals heterogeneity between lesions in synchronous primary right-sided and left-sided colon cancer. Front. Mol. Biosci. 2021, 8, 689466. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Syulyukina, N.; Ramamoorthy, S.; Subramaniam, S. Right and left-sided colon cancers—Specificity of molecular mechanisms in tumourigenesis and progression. BMC Cancer 2020, 20, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwé, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype molding of stromal cells in the lung tumour microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, C.X.; Müller, S.; Keerthivasan, S.; Koeppen, H.; Hung, J.; Gierke, S.; Breart, B.; Foreman, O.; Bainbridge, T.W.; Castiglioni, A.; et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 2020, 10, 232–253. [Google Scholar] [CrossRef] [Green Version]
- Buechler, M.B.; Pradhan, R.N.; Krishnamurty, A.T.; Cox, C.; Calviello, A.K.; Wang, A.W.; Yang, Y.A.; Tam, L.; Caothien, R.; Roose-Girma, M.; et al. Cross-tissue organization of the fibroblast lineage. Nature 2021, 593, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Courtois, E.T.; Sengupta, D.; Tan, Y.; Chen, K.H.; Goh, J.J.; Kong, S.L.; Chua, C.; Hon, L.K.; Tan, W.S.; et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumours. Nat. Genet 2017, 49, 708–718. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, S.; Zhou, X.; Cui, Y.; Wang, W.; Wen, L.; Guo, L.; Fu, W.; Tang, F. Single-cell multiomics sequencing reveals prevalent genomic alterations in tumour stromal cells of human colorectal cancer. Cancer Cell 2020, 38, 818–828.e5. [Google Scholar] [CrossRef]
- Smillie, C.S.; Biton, M.; Ordovas-Montanes, J.; Sullivan, K.M.; Burgin, G.; Graham, D.B.; Herbst, R.H.; Rogel, N.; Slyper, M.; Waldman, J.; et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 2019, 178, 714–730.e22. [Google Scholar] [CrossRef]
- Muhl, L.; Genové, G.; Leptidis, S.; Liu, J.; He, L.; Mocci, G.; Sun, Y.; Gustafsson, S.; Buyandelger, B.; Chivukula, I.V.; et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat. Commun. 2020, 11, 3953. [Google Scholar] [CrossRef]
- Khaliq, A.M.; Kurt, Z.; Grunvald, M.W.; Erdogan, C.; Turgut, S.S.; Rand, T.; Khare, S.; Borgia, J.A.; Hayden, D.M.; Pappas, G. Redefining tumour classification and clinical stratification through a colorectal cancer single-cell atlas. bioRxiv 2021, 2021, 429256. [Google Scholar]
- Nishina, T.; Deguchi, Y.; Ohshima, D.; Takeda, W.; Ohtsuka, M.; Shichino, S.; Ueha, S.; Yamazaki, S.; Kawauchi, M.; Nakamura, E.; et al. Interleukin-11-expressing fibroblasts have a unique gene signature correlated with poor prognosis of colorectal cancer. Nat. Commun. 2021, 12, 2281. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Arai, Y.; Furukawa, E.; Narushima, D.; Matsuura, T.; Nakamura, H.; Shiokawa, D.; Nagai, M.; Imai, T.; Mimori, K.; et al. Single-cell DNA and RNA sequencing reveals the dynamics of intra-tumour heterogeneity in a colorectal cancer model. BMC Biol. 2021, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Amirouchene-Angelozzi, N.; Swanton, C.; Bardelli, A. Tumour evolution as a therapeutic target. Cancer Discov. 2017, 7, 805–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the antitumour immune response by cancer-associated fibroblasts. Front. Immunol. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2018, 18, 99–115. [Google Scholar] [CrossRef]
- Kobayashi, H.; Enomoto, A.; Woods, S.L.; Burt, A.D.; Takahashi, M.; Worthley, D.L. Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 282–295. [Google Scholar] [CrossRef]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.J.; Perera, R.M.; Wang, H.; Wu, D.-C.; Liu, X.S.; Han, S.; Fitamant, J.; Jones, P.D.; Ghanta, K.S.; Kawano, S.; et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc. Natl. Acad. Sci. USA 2014, 111, E3091–E3100. [Google Scholar] [CrossRef] [Green Version]
- Miyai, Y.; Esaki, N.; Takahashi, M.; Enomoto, A. Cancer-associated fibroblasts that restrain cancer progression: Hypotheses and perspectives. Cancer Sci. 2020, 111, 1047–1057. [Google Scholar] [CrossRef] [Green Version]
- Gerling, M.; Büller, N.V.J.A.; Kirn, L.M.; Joost, S.; Frings, O.; Englert, B.; Bergström, Å.; Kuiper, R.V.; Blaas, L.; Wielenga, M.C.B.; et al. Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nat. Commun. 2016, 7, 12321. [Google Scholar] [CrossRef] [PubMed]
- Pallangyo, C.K.; Ziegler, P.K.; Greten, F.R. IKKβ acts as a tumour suppressor in cancer-associated fibroblasts during intestinal tumourigenesis. J. Exp. Med. 2015, 212, 2253–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gieniec, K.A.; Butler, L.M.; Worthley, D.L.; Woods, S.L. Cancer-associated fibroblasts—Heroes or villains? Br. J. Cancer 2019, 121, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Hasbullah, H.H.; Musa, M. Gene therapy targeting p53 and KRAS for colorectal cancer treatment: A myth or the way forward? IJMS 2021, 22, 11941. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Xiong, Y.; Ye, Z.; Zhao, J.; Zhong, L.; Liu, Y.; Zhu, Y.; Tian, L.; Qiu, X.; Hong, X. Microbial community profiling distinguishes left-sided and right-sided colon cancer. Front. Cell. Infect. Microbiol. 2020, 10, 498502. [Google Scholar] [CrossRef]
- Phipps, O.; Quraishi, M.; Dickson, E.; Steed, H.; Kumar, A.; Acheson, A.; Beggs, A.; Brookes, M.; Al-Hassi, H. Differences in the on- and off-tumor microbiota between right- and left-Sided colorectal cancer. Microorganisms 2021, 9, 1108. [Google Scholar] [CrossRef]
- Royston, K.J.; Adedokun, B.; Olopade, O.I. Race, the microbiome and colorectal cancer. World J. Gastrointest Oncol. 2019, 11, 773–787. [Google Scholar] [CrossRef]
- Borrello, K.; Lim, U.; Park, S.-Y.; Monroe, K.R.; Maskarinec, G.; Boushey, C.J.; Wilkens, L.R.; Randolph, T.W.; Le Marchand, L.; Hullar, M.A.; et al. Dietary intake mediates ethnic differences in gut microbial composition. Nutrients 2022, 14, 660. [Google Scholar] [CrossRef]
- Senghor, B.; Sokhna, C.; Ruimy, R.; Lagier, J.-C. Gut microbiota diversity according to dietary habits and geographical provenance. Hum. Microbiome J. 2018, 7–8, 1–9. [Google Scholar] [CrossRef]
- Aranda-Olmedo, I.; Rubio, L.A. Dietary legumes, intestinal microbiota, inflammation and colorectal cancer. J. Funct. Foods 2020, 64, 103707. [Google Scholar] [CrossRef]
| Secretome/Mediator | Expression | Influence on Carcinogenesis | Ref. | |
|---|---|---|---|---|
| Chemokine | CCL2; CCL8 | Up | Secreted CCL2 and CCL8 from CAFs induce proliferation and invasion of CRC cells | [67] |
| CXCL14 | Up | Stimulates CAF pro-tumourigenic activity via autocrine effects on CAFs and paracrine signalling on neoplastic cells, leading to higher cancer cell proliferation | [68] | |
| IL-6/IL-11 | Up | Induce tumour proliferation and CAF formation | [69] | |
| STAT3 activation facilitated by IL-6/IL-11 in CAFs drives CRC progression and is associated with poor prognosis | [70] | |||
| Intrinsic STAT3 activity in CAFs induces the release of IL-6, TGF-β and VEGF by CRC cells and promotes carcinogenesis, immune suppression and metastasis | [71] | |||
| CXCR4/CXCL12 | Up | CXCR4/TGF-β1 axis supports the differentiation from HSCs into CAFs and promotes metastasis | [72] | |
| Growth factor | TGF-β | Up | TGF-β activity on CAFs promotes colonisation of CRC cells. TGF-β-stimulated CAFs secrete IL-11, which induces STAT3 signalling that supports cancer metastasis | [73] |
| Decreases T-cell activity, leading to cancer immune evasion | [74] | |||
| Presence of upstream transcription factors, SMADs, which predict the failure of immune checkpoint (PD-1) blockade | [75] | |||
| Secreted by CRC cells, interacts with CAF-derived exosome miR-17-5p, resulting in tumour invasion and metastasis | [76] | |||
| IGF-1/IGF-1R | Up | IGFBP7 (TGF-β-target gene) promotes cancer cell proliferation through tumour-stroma paracrine signalling | [77] | |
| IGF-1 and STAT3 drive CRC progression through cell autonomous and pro-tumourigenic activity of CAFs | [78] | |||
| Wnt/β-catenin | Up | Induce tumour invasion and metastasis | [79] | |
| CAF-derived WNT2 induces angiogenesis and promotes carcinogenesis | [80] | |||
| MicroRNA | miR-135b-5p | Up | Upregulation of miR-135b-5p by CAF-derived exosomes to support CRC cell growth and angiogenesis via TXNIP inhibition | [81] |
| ECM components | ADAMs | Up | ADAMs expressed by CAFs drive tumour invasion and metastasis | [82] |
| TIMP-1 | High expression of TIMP-1 stimulates stromal cells growth and activation of ERK1/2 kinase | [83] | ||
| Purpose | Analysis | Model/Study Design | Finding | Ref. |
|---|---|---|---|---|
| Studying CRC cellular heterogeneity | scRNA-Seq | Human model | Two distinct subtypes of CAFs (CAF-A and CAF-B) were identified. CAF-B cells showed expression of cytoskeletal genes and other associated markers of activated myofibroblasts, whereas expression of ECM-related genes was found in CAF-A. | [120] |
| Studying genomic changes of CRC stromal cells | Single-cell multi-omics sequencing | Human model | Higher proportions of aneuploid fibroblasts in tumours compared to those in normal tissues, with significant clonal expansion of fibroblasts with an extra copy of chromosome 7. | [121] |
| Single-cell analysis of colon biopsy | Droplet-based scRNA-Seq, SMART-Seq2 on colonic spheroids | Human model—normal and UC patients | Using clustering, 51 cell subsets were identified (epithelial: 15; fibroblast: 8; endothelial: 4; glial: 1; myeloid: 7; B: 4; T: 10 (CD4+ Tconv, Tregs, CD8+, and γδ); innate lymphoid cell (ILC): 1; NK cell: 1. The inflammatory fibroblast (IAF) subset expresses markers of CAFs unique to UC, suggesting an IAF expansion of CRC. IAFs are composed of WNT2B+ and WNT5B+ subsets. | [122] |
| Single-cell transcriptional profiles study | SmartSeq2 | Animal (murine) model—comparison between fibroblasts and vascular cells in muscular organs | Subpopulation of fibroblast cells (Tnc+ Cd34−) which are localised at the surface epithelium whereas Tnc− Cd34+ fibroblasts were found deeper down in the lamina propria and in the muscularis mucosa. Differential expression in BMP and WNT signalling pathways was also reported between the two populations. | [123] |
| Prediction of prognosis and therapeutic responses in CRC | GEO single-cell transcriptome, qPCR analyses | Bio-informatics analysis | Established the correlation between greater CAF risk scores with poor prognosis in CRC samples. Those with higher CAF risk scores indicated lower response to immunotherapy, but better sensitivity to conventional chemotherapeutics. | [83] |
| Classification of tumour cells and clinical stratification | Single-cell resolution transcriptomic analysis | Bio-informatics analysis | Identification of the transcriptional signature of specific subtypes of colorectal CAF (CAF-S1 and CAF-S4) that significantly indicate stratification of a patient’s survival. Two CAF-S1 subpopulations, ecm-myCAF and TGFß-myCAF, are linked to primary resistance to immunotherapies. | [124] |
| Association between presence of IL-11-expressing fibroblasts and CRC prognosis | Transcriptome analysis on human cancer database | Bio-informatics analysis | Expression of fibroblast markers and genes implicated in cell growth and repair in IL-11+ cells. Expression of genes enriched in IL-11+ fibroblasts is increased in colorectal tumours and associated with lower recurrence-free survival. | [125] |
| Dissecting ITH of CRC | Single-cell exome and transcriptome sequencing | Animal (mouse) model and metastatic human CRC model | Demonstrated the dynamics of ITH of CRC. The emergence of transcriptional subpopulations which lead to increased ITH may be vital for adaptation to drastic changes in the microenvironment when malignant cells have gained sufficient genetic alterations at the advanced stage of tumourigenesis. | [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad Zawawi, S.S.; Musa, M. Dynamic Co-Evolution of Cancer Cells and Cancer-Associated Fibroblasts: Role in Right- and Left-Sided Colon Cancer Progression and Its Clinical Relevance. Biology 2022, 11, 1014. https://doi.org/10.3390/biology11071014
Ahmad Zawawi SS, Musa M. Dynamic Co-Evolution of Cancer Cells and Cancer-Associated Fibroblasts: Role in Right- and Left-Sided Colon Cancer Progression and Its Clinical Relevance. Biology. 2022; 11(7):1014. https://doi.org/10.3390/biology11071014
Chicago/Turabian StyleAhmad Zawawi, Sahira Syamimi, and Marahaini Musa. 2022. "Dynamic Co-Evolution of Cancer Cells and Cancer-Associated Fibroblasts: Role in Right- and Left-Sided Colon Cancer Progression and Its Clinical Relevance" Biology 11, no. 7: 1014. https://doi.org/10.3390/biology11071014
APA StyleAhmad Zawawi, S. S., & Musa, M. (2022). Dynamic Co-Evolution of Cancer Cells and Cancer-Associated Fibroblasts: Role in Right- and Left-Sided Colon Cancer Progression and Its Clinical Relevance. Biology, 11(7), 1014. https://doi.org/10.3390/biology11071014