Nidus vespae Built by an Invasive Alien Hornet, Vespa velutina nigrithorax, Inhibits Adipose Tissue Expansion in High-Fat Diet-Induced Obese Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. NV Preparation
2.2. Reagents
2.3. Animals and NV Administration
2.4. Tissue Hematoxylin and Eosin (H&E) Staining
2.5. Blood Biochemical Analysis
2.6. Adipocyte Differentiation
2.7. ORO Staining and TG Assay
2.8. MTT Assay
2.9. Western Blot Analysis
2.10. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.11. Statistical Analysis
3. Results
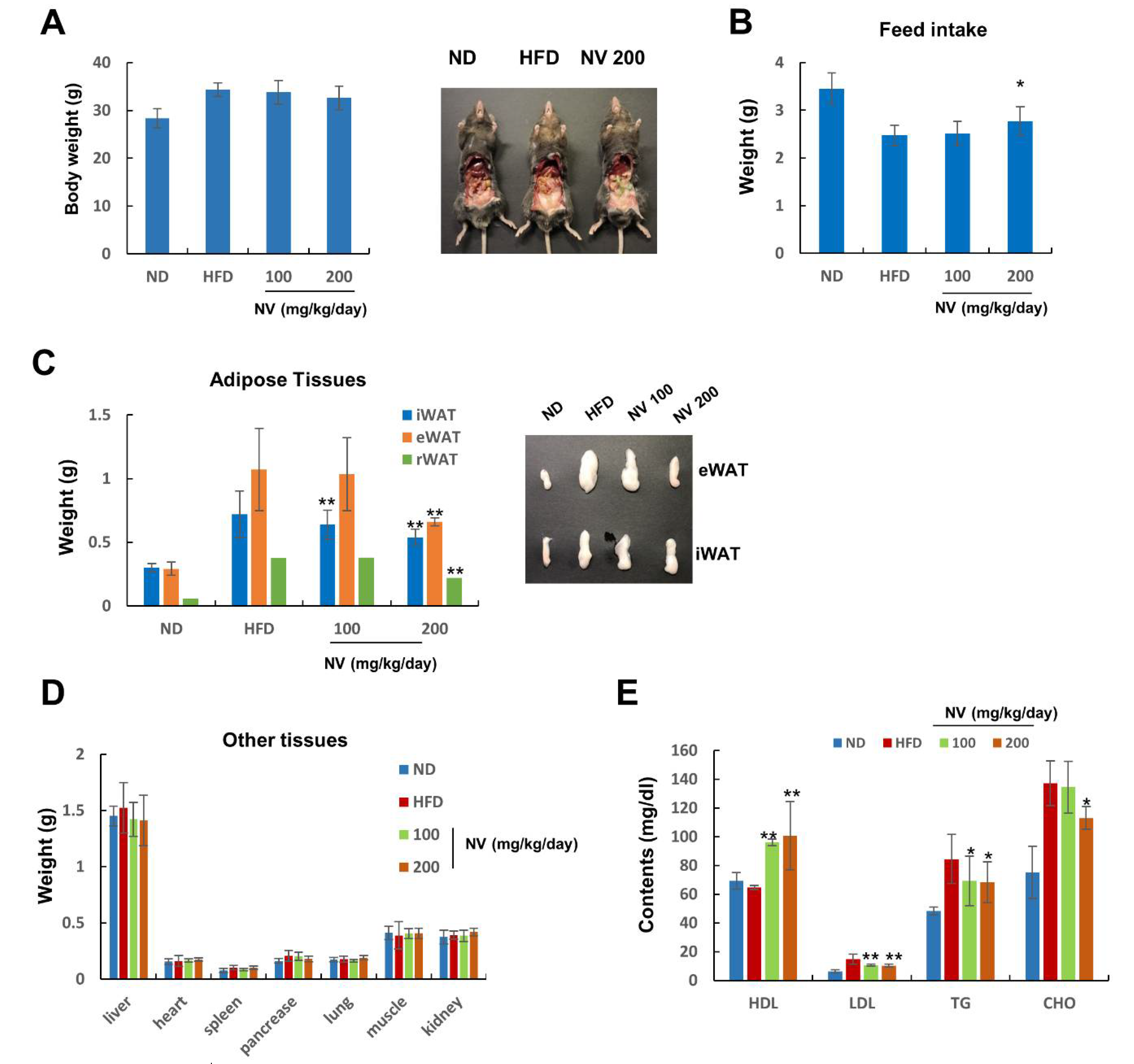
3.1. NV Suppressed Adipose Tissue Expansion and Reduced Plasma Lipid Levels in HFD-Induced Obese Mice
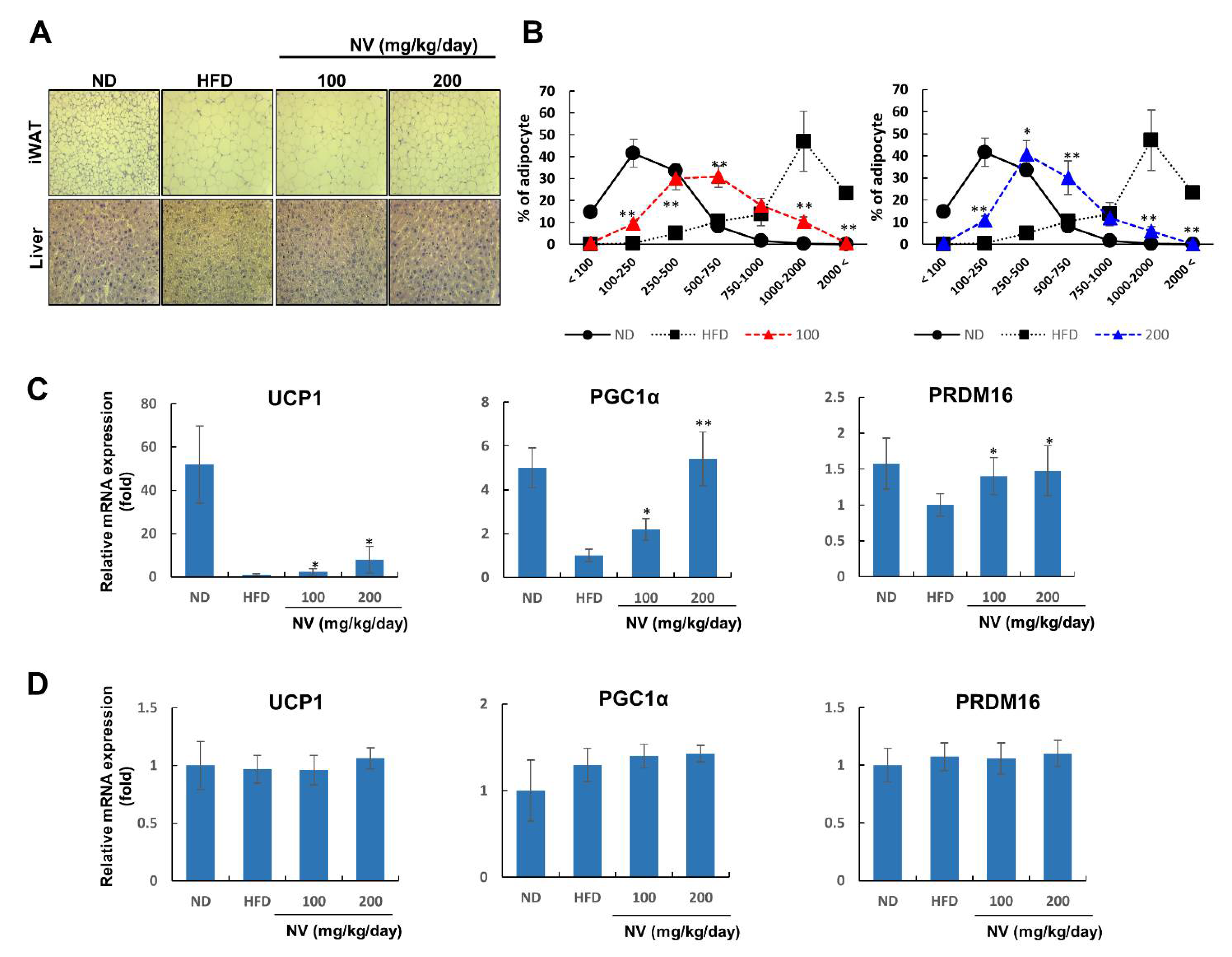
3.2. NV Induced Adipose Browning and Ameliorated Liver Steatosis
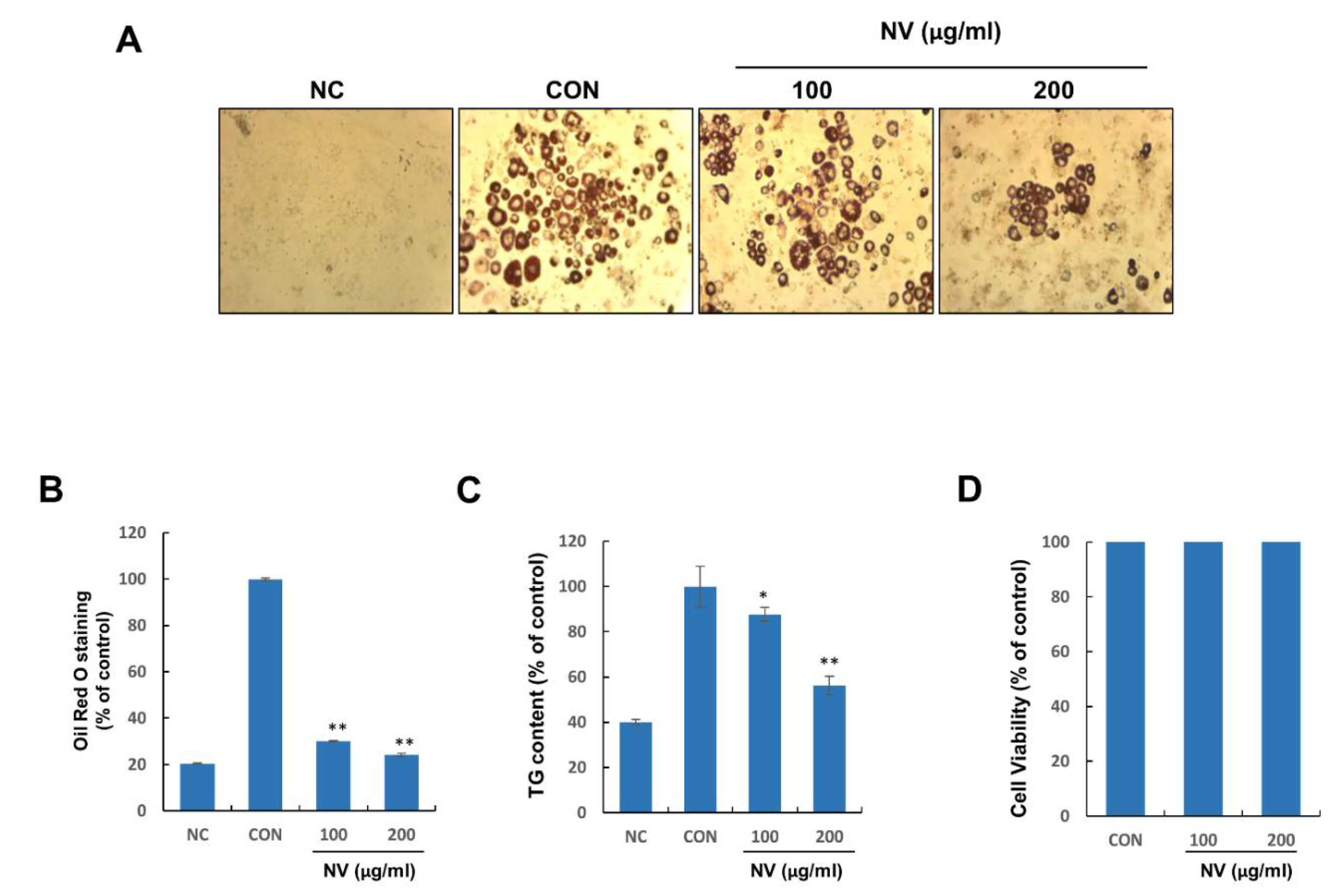
3.3. NV Inhibited Adipogenesis in 3T3-L1 Adipocytes without Inducing Cytotoxicity
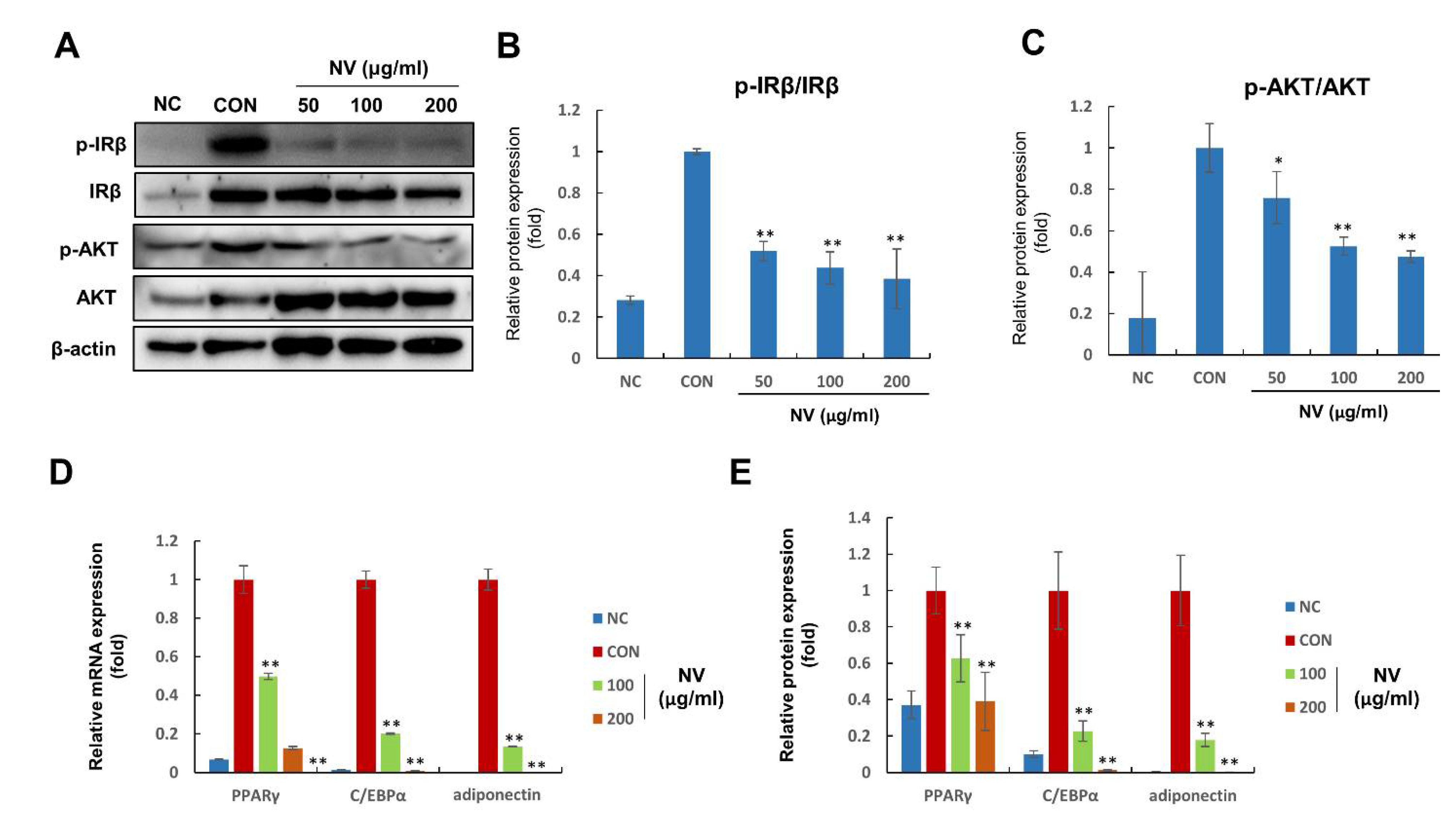
3.4. NV Suppressed the Activation of Insulin Signaling and the Expression of Adipogenesis-Related Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.G.; Kim, J.S.; Min, K.; Kwon, T.K.; Nam, J.-O. Hispidulin inhibits adipogenesis in 3T3-L1 adipocytes through PPARγ pathway. Chem. Biol. Interact. 2018, 293, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Kim, W.K.; Bae, K.-H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 2014, 6, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef]
- van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Èntomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Elferink, E.; Nonhebel, S.; Moll, H. Feeding livestock food residue and the consequences for the environmental impact of meat. J. Clean. Prod. 2008, 16, 1227–1233. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Nisbett, N.; Pretty, J.; Robinson, S.; Toulmin, C.; Whiteley, R. The future of the global food system. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2769–2777. [Google Scholar] [CrossRef] [Green Version]
- Luan, Y.; Cui, X.; Ferrat, M. Historical trends of food self-sufficiency in Africa. Food Secur. 2013, 5, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Rutaro, K.; Malinga, G.M.; Lehtovaara, V.J.; Opoke, R.; Valtonen, A.; Kwetegyeka, J.; Nyeko, P.; Roininen, H. The fatty acid composition of edible grasshopper Ruspolia differens (Serville) (Orthoptera: Tettigoniidae) feeding on diversifying diets of host plants. Èntomol. Res. 2018, 48, 490–498. [Google Scholar] [CrossRef]
- Jeong, D.; Min, N.; Kim, Y.; Kim, S.R.; Kwon, O. The effects of feed materials on the nutrient composition of Protaetia brevitarsis larvae. Èntomol. Res. 2020, 50, 23–27. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jo, M.J.; Nam, S.Y.; Kim, K.M.; Choi, M.B.; Lee, Y.H. Evaluating the effects of honey bee (Apis mellifera L.) venom on the expression of insulin sensitivity and inflammation-related genes in co-culture of adipocytes and macrophages. Entomol. Res. 2020, 50, 236–244. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, J.M.; Kim, B.; Nam, J.-O.; Hahn, D.; Choi, M.B. Nutritional Value of the Larvae of the Alien Invasive Wasp Vespa velutina nigrithorax and Amino Acid Composition of the Larval Saliva. Foods 2020, 9, 885. [Google Scholar] [CrossRef]
- Baek, M.; Kim, M.; Kwon, Y.; Hwang, J.; Goo, T.; Jun, M.; Yun, E. Effects of processing methods on nutritional composition and antioxidant activity of mealworm (Tenebrio molitor) larvae. Èntomol. Res. 2019, 49, 284–293. [Google Scholar] [CrossRef]
- Hwang, D.; Lee, W.S.; Goo, T.; Park, S.; Yun, E. Effect of pretreatment with paste and sauce extract made using Tenebrio molitor larvae on ethanol-damaged HepG2 cells. Èntomol. Res. 2019, 49, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, M.; Lee, M.; Lee, Y.; Kim, H.; Nam, J.; Choi, M.B.; Hahn, D. Antibacterial potential of Nidus vespae built by invasive alien hornet, Vespa velutina nigrithorax, against food-borne pathogenic bacteria. Èntomol. Res. 2020, 50, 28–33. [Google Scholar] [CrossRef]
- SM, H.; HY, K.; SO, W.; SG, K.; HM, C.; HJ, M. Inhibitory effect of purified bee venom (Apis mellifera L.) on adipogenesis in Korea. J. Apic. 2020, 35, 49–54. [Google Scholar]
- Choi, M.B.; Martin, S.J.; Lee, J.W. Distribution, spread, and impact of the invasive hornet Vespa velutina in South Korea. J. Asia-Pac. Èntomol. 2012, 15, 473–477. [Google Scholar] [CrossRef]
- Choi, M.B.; Kim, T.G.; Kwon, O. Recent Trends in Wasp Nest Removal and Hymenoptera Stings in South Korea. J. Med. Entomol. 2019, 56, 254–260. [Google Scholar] [CrossRef]
- Cini, A.; Cappa, F.; Petrocelli, I.; Pepiciello, I.; Bortolotti, L.; Cervo, R. Competition between the native and the introduced hornets Vespa crabro and Vespa velutina: A comparison of potentially relevant life-history traits. Ecol. Entomol. 2018, 43, 351–362. [Google Scholar]
- Monceau, K.; Maher, N.; Bonnard, O.; Thiéry, D. Evaluation of competition between a native and an invasive hornet species: Do seasonal phenologies overlap? Bull. Entomol. Res. 2015, 105, 462. [Google Scholar] [PubMed] [Green Version]
- Yamasaki, K.; Takahashi, R.; Harada, R.; Matsuo, Y.; Nakamura, M.; Takahashi, J.-I. Reproductive interference by alien hornet Vespa velutina threatens the native populations of Vespa simillima in Japan. Sci. Nat. 2019, 106, 15. [Google Scholar] [CrossRef]
- Monceau, K.; Bonnard, O.; Thiéry, D. Vespa velutina: A new invasive predator of honeybees in Europe. J. Pest Sci. 2014, 87, 1–16. [Google Scholar] [CrossRef]
- Turchi, L.; Derijard, B. Options for the biological and physical control of Vespa velutina nigrithorax (Hym.: Vespidae) in Europe: A review. J. Appl. Entomol. 2018, 142, 553–562. [Google Scholar] [CrossRef]
- Zhu, M.; Han, W.; Ling, Y.; Qi, Q.; Zhang, Y.; Peng, Y.; Chen, L.; Bao, Y.; Liu, Y. Preliminary Study on the In Vitro Antitumor Effects of Nidus Vespae on Gastric Cancer. Evid.-Based Complement. Altern. Med. 2021, 2021, 1549359. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, J.S.; Lee, H.-S.; Lim, Y.-M.; So, J.-H.; Hahn, D.; Ha, Y.S.; Nam, J.-O. Bioconverted Orostachys japonicas Extracts Suppress Angiogenic Activity of Ms-1 Endothelial Cells. Int. J. Mol. Sci. 2017, 18, 2615. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.G.; Lee, Y.J.; Jang, M.-H.; Kwon, T.R.; Nam, J.-O. Panax ginseng Leaf Extracts Exert Anti-Obesity Effects in High-Fat Diet-Induced Obese Rats. Nutrients 2017, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Mosqueda-Solís, A.; Sánchez, J.; Portillo, M.P.; Palou, A.; Picó, C. Combination of Capsaicin and Hesperidin Reduces the Effectiveness of Each Compound to Decrease the Adipocyte Size and to Induce Browning Features in Adipose Tissue of Western Diet Fed Rats. J. Agric. Food Chem. 2018, 66, 9679–9689. [Google Scholar] [CrossRef]
- Paulo, E.; Wang, B. Towards a Better Understanding of Beige Adipocyte Plasticity. Cells 2019, 8, 1552. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Park, J.; Lee, H.W.; Lee, Y.S.; Kim, J.B. Regulation of adipocyte differentiation and insulin action with rapamycin. Biochem. Biophys. Res. Commun. 2004, 321, 942–948. [Google Scholar] [CrossRef]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; García-Navarro, S.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Burrell, M.A.; et al. Insulin- and Leptin-Mediated Control of Aquaglyceroporins in Human Adipocytes and Hepatocytes Is Mediated via the PI3K/Akt/mTOR Signaling Cascade. J. Clin. Endocrinol. Metab. 2011, 96, E586–E597. [Google Scholar] [CrossRef] [Green Version]
- Kwak, D.H.; Lee, J.-H.; Song, K.H.; Ma, J.Y. Inhibitory effects of baicalin in the early stage of 3T3-L1 preadipocytes differentiation by down-regulation of PDK1/Akt phosphorylation. Mol. Cell. Biochem. 2014, 385, 257–264. [Google Scholar] [CrossRef]
- Mashmoul, M.; Azlan, A.; Khaza’Ai, H.; Yusof, B.N.M.; Noor, S.M. Saffron: A Natural Potent Antioxidant as a Promising Anti-Obesity Drug. Antioxidants 2013, 2, 293–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonk, M.; Vilcinskas, A.; Rahnamaeian, M. Insect antimicrobial peptides: Potential tools for the prevention of skin cancer. Appl. Microbiol. Biotechnol. 2016, 100, 7397–7405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Hwang, I.H.; Kim, J.H.; Kim, M.-A.; Hwang, J.S.; Kim, Y.H.; Na, M. Quinoxaline-, dopamine-, and amino acid-derived metabolites from the edible insect Protaetia brevitarsis seulensis. Arch. Pharm. Res. 2017, 40, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, Y.; Zuo, Y.L.; Li, J.Y.; Ye, L.; Zhou, X.D. Effects of Nidus Vespae extract and chemical fractions on the growth and acidogenicity of oral microorganisms. Arch. Oral Biol. 2006, 51, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.; Andrews, Z.B.; Simonds, S.; Michael, N.; DeVeer, M.; Brüning, J.C.; Spanswick, D.; Cowley, M.; Tiganis, T. A Hypothalamic Phosphatase Switch Coordinates Energy Expenditure with Feeding. Cell Metab. 2017, 26, 375–393. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Baile, C.A.; Zhu, S.; Shoji, M. Bioactive flavonoid p-hydroxycinnamic acid stimulates osteoblastogenesis and suppresses adipogenesis in bone marrow culture. Cell Tissue Res. 2013, 354, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Chuang, C.-C.; Martinez, K.; Xie, G.; Kennedy, A.; Bumrungpert, A.; Overman, A.; Jia, W.; McIntosh, M.K. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-α-mediated inflammation and insulin resistance in primary human adipocytes. Am. J. Clin. Nutr. 2010, 92, 1511–1521. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| PPARγ | GTAATCAGCAACCATTGGGTCA | GAAAGGTTGGCTTGACCTGCT |
| C/EBPα | GGTCGTTTCTCCATTAAATTCTCAT | CTAGAAACTTTCCCAGAAATCTTCC |
| adiponectin | GATGGCACTCCTGGAGAGAA | GCGAAACTCGATGACTCCTCGG |
| β-actin | CGTGCGTGACATCAAAGAGAA | GCTCGTTGCCAATAGTGATGA. |
| ND | HFD | NV 100 | NV 200 | |
|---|---|---|---|---|
| GOT (U/L) | 62.4 ± 16.79 | 163.5 ± 9.27 | 119.4 ± 20.87 * | 86.9 ± 34.05 ** |
| GPT (U/L) | 21 ± 1.73 | 48 ± 8.14 | 32.7 ± 1.15 ** | 27.0 ± 1.0 ** |
| ALP (U/L) | 96 ± 1.15 | 73 ± 2.0 | 66.8 ± 2.30 | 66.7 ± 18.29 |
| T-BIL (mg/dL) | 0.1 ± 0.08 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.12 |
| BUN (mg/dL) | 21.8 ± 3.59 | 22.4 ± 0.4 | 23.8 ± 2.65 | 21.9 ± 2.38 |
| CREA (mg/dL) | 0.6 ± 0.08 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.G.; Kim, D.S.; Chae, J.; Lee, E.; Hahn, D.; Kim, I.-K.; Kim, C.-J.; Choi, M.B.; Nam, J.-O. Nidus vespae Built by an Invasive Alien Hornet, Vespa velutina nigrithorax, Inhibits Adipose Tissue Expansion in High-Fat Diet-Induced Obese Mice. Biology 2022, 11, 1013. https://doi.org/10.3390/biology11071013
Lee SG, Kim DS, Chae J, Lee E, Hahn D, Kim I-K, Kim C-J, Choi MB, Nam J-O. Nidus vespae Built by an Invasive Alien Hornet, Vespa velutina nigrithorax, Inhibits Adipose Tissue Expansion in High-Fat Diet-Induced Obese Mice. Biology. 2022; 11(7):1013. https://doi.org/10.3390/biology11071013
Chicago/Turabian StyleLee, Seul Gi, Dong Se Kim, Jongbeom Chae, Eunbi Lee, Dongyup Hahn, Il-Kwon Kim, Chang-Jun Kim, Moon Bo Choi, and Ju-Ock Nam. 2022. "Nidus vespae Built by an Invasive Alien Hornet, Vespa velutina nigrithorax, Inhibits Adipose Tissue Expansion in High-Fat Diet-Induced Obese Mice" Biology 11, no. 7: 1013. https://doi.org/10.3390/biology11071013
APA StyleLee, S. G., Kim, D. S., Chae, J., Lee, E., Hahn, D., Kim, I.-K., Kim, C.-J., Choi, M. B., & Nam, J.-O. (2022). Nidus vespae Built by an Invasive Alien Hornet, Vespa velutina nigrithorax, Inhibits Adipose Tissue Expansion in High-Fat Diet-Induced Obese Mice. Biology, 11(7), 1013. https://doi.org/10.3390/biology11071013








