Potential Effect of Enzymatic Porcine Placental Hydrolysate (EPPH) to Improve Alcoholic Liver Disease (ALD) by Promoting Lipolysis in the Liver
Abstract
Simple Summary
Abstract
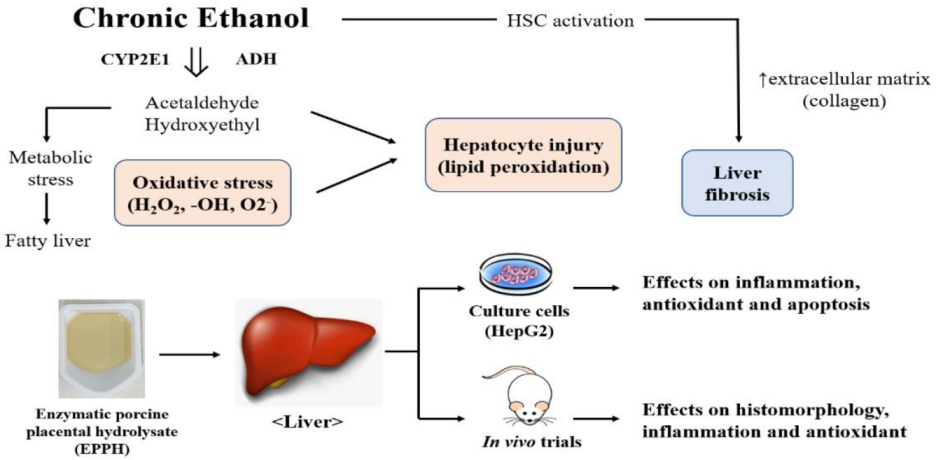
1. Introduction
2. Materials and Methods
2.1. Placenta Banding and Basic Composition Analysis
2.2. Preparation of Enzymatic Porcine Placenta Hydrolysate (EPPH)
2.3. Cell Culture and Viability Assay
2.4. Effect of EPPH on Antioxidant Activity in HepG2 Cell
2.5. Animals and Experimental Design
2.6. Serum Biochemical Parameters
2.7. Western Blot Analysis
2.8. Histological Evaluation
2.9. Statistical Analysis
3. Results
3.1. Effect of EPPH on Antioxidant Activity in HepG2 Cell
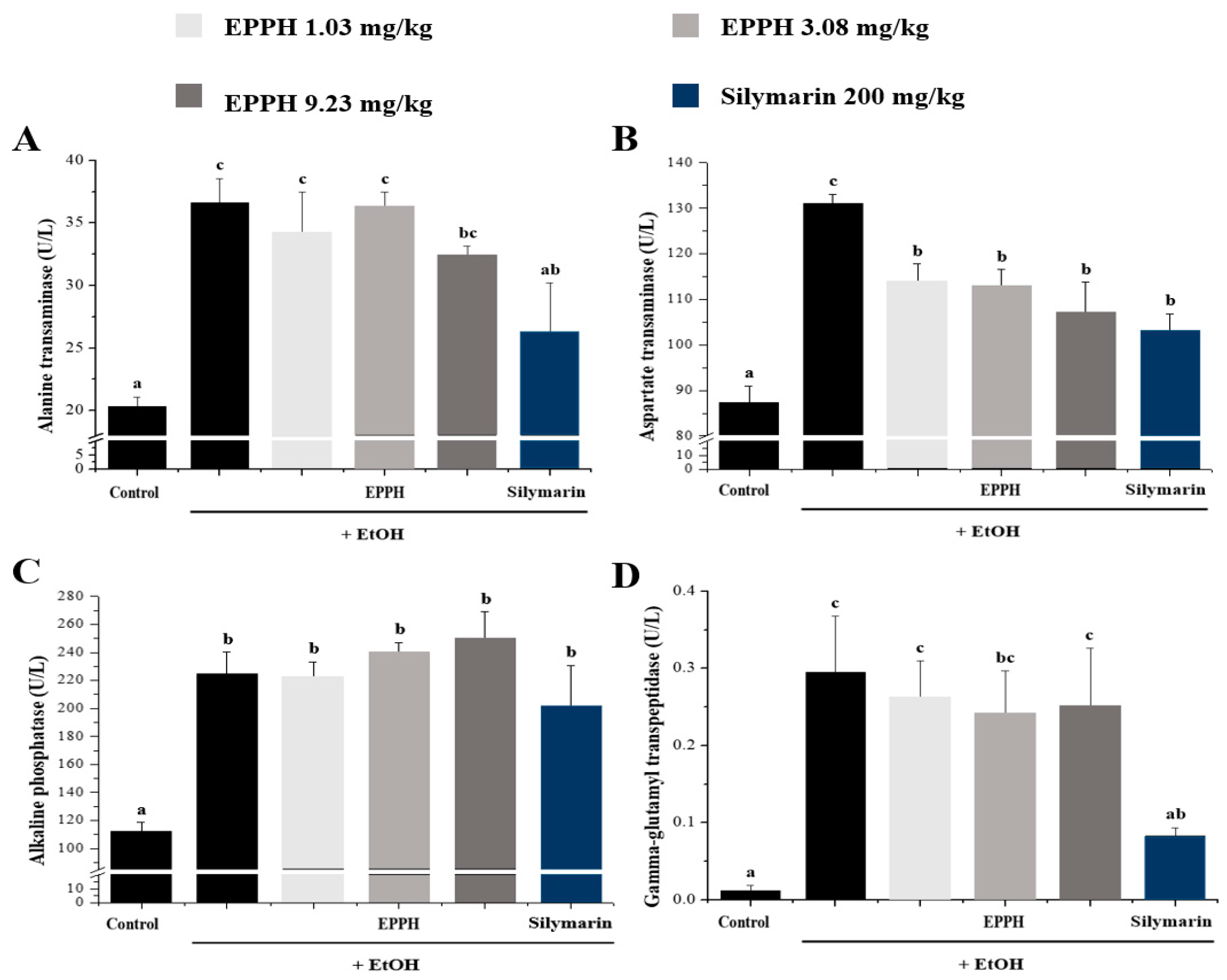
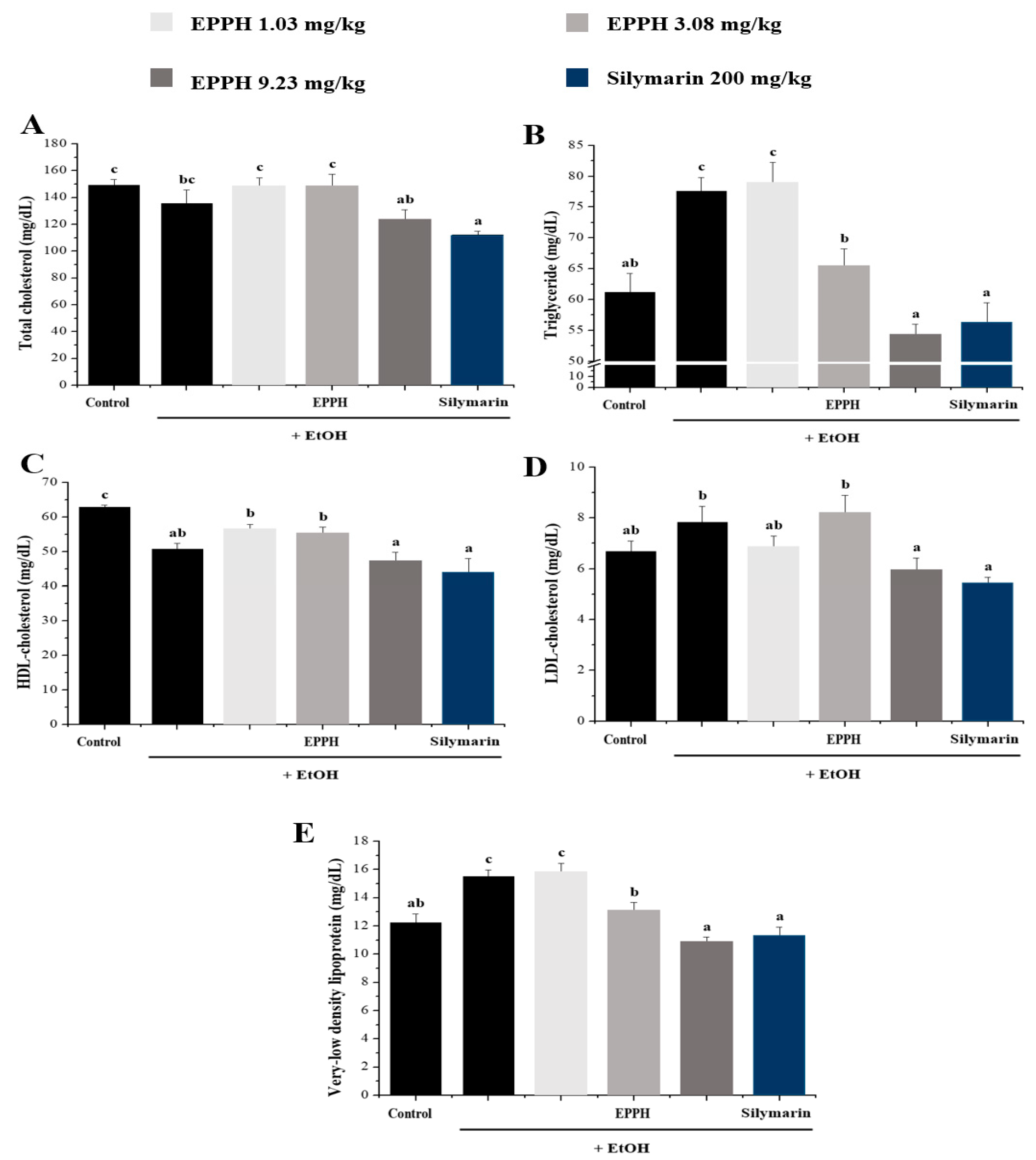
3.2. Serum Biochemical Parameters
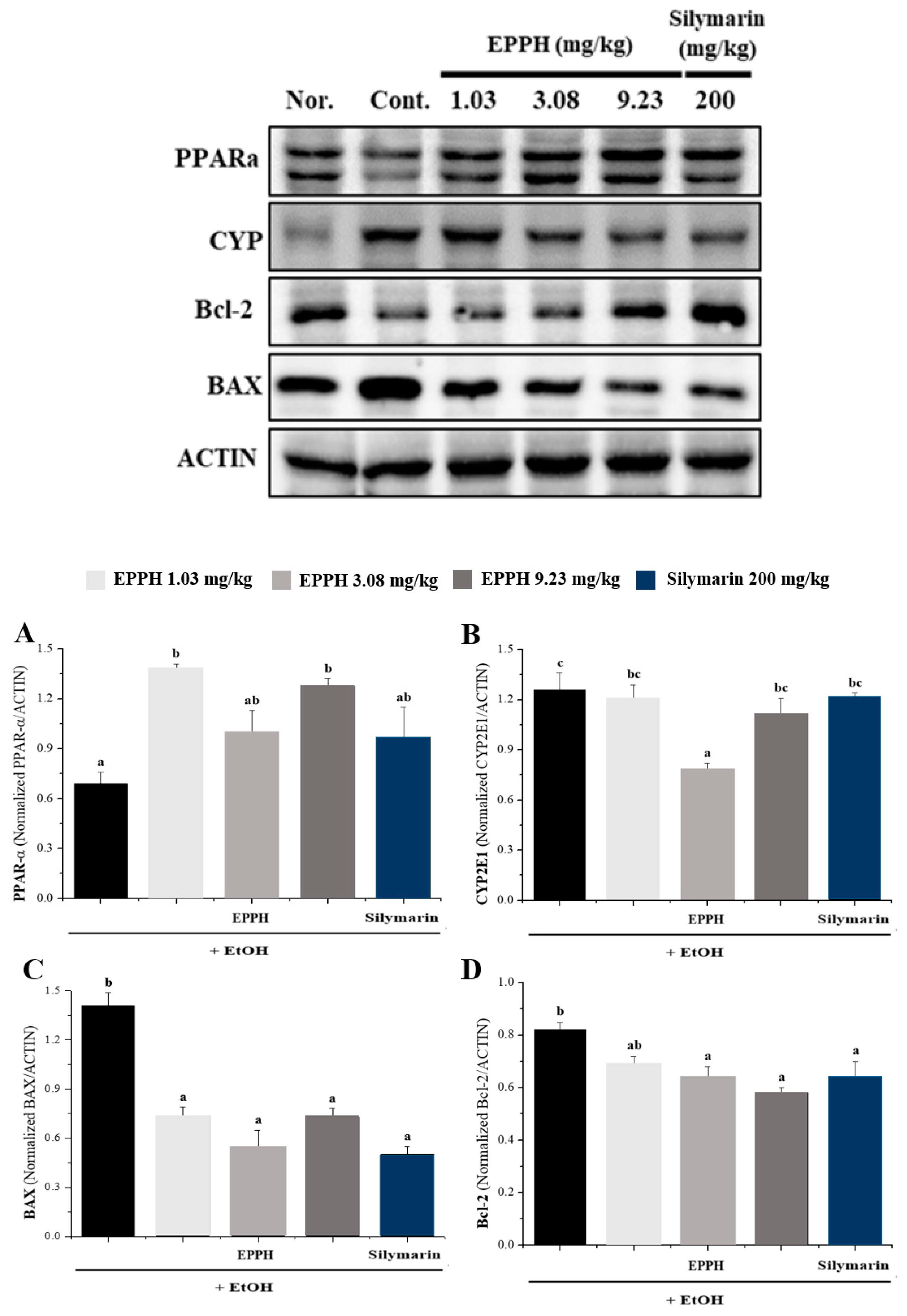
3.3. Western Blot Analysis
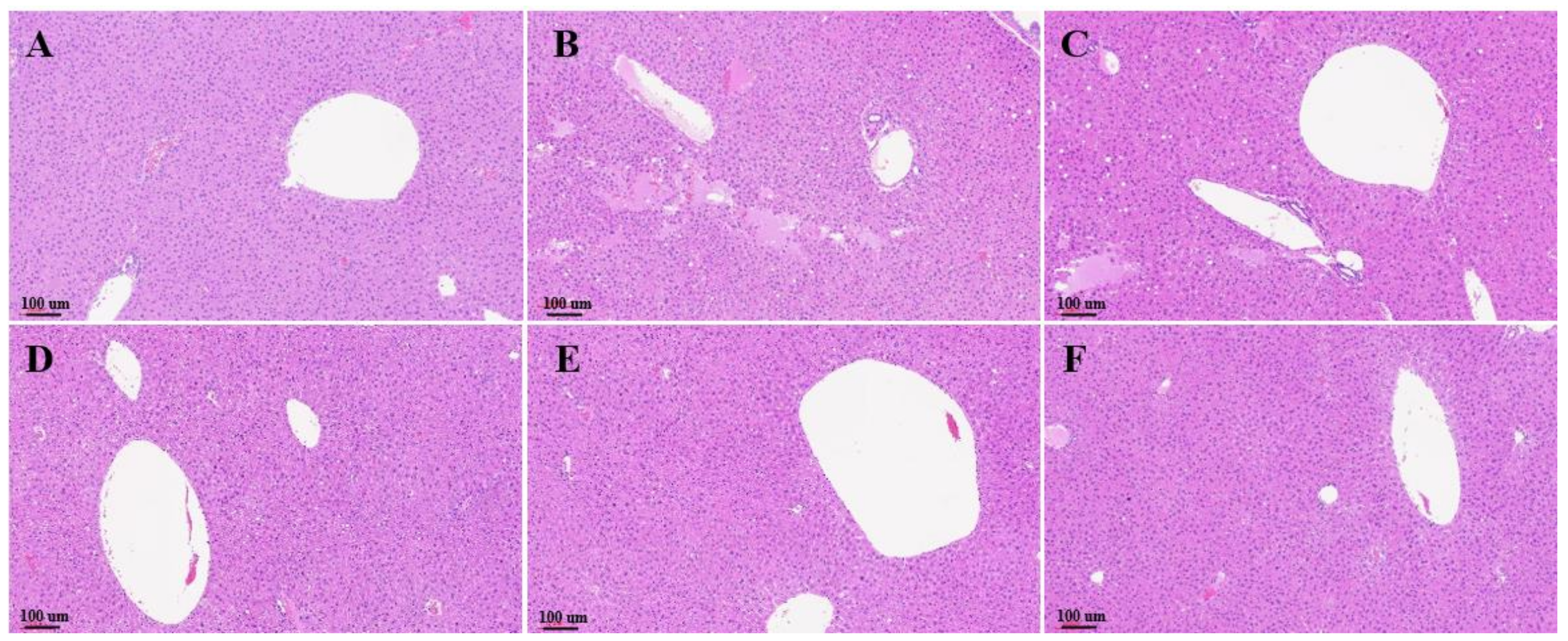
3.4. Histological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, C.; Fernandez-Checa, J.C. Mitochondrial glutathione: Hepatocellular survival–death switch. J. Gastroenterol. Hepatol. 2006, 21, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef]
- Pérez, M.J.; I Cederbaum, A. Metallothionein 2A induction by zinc protects HEPG2 cells against CYP2E1-dependent toxicity. Free Radic. Biol. Med. 2003, 34, 443–455. [Google Scholar] [CrossRef]
- Weltman, M.D.; Farrell, G.C.; Hall, P.; Ingelman-Sundberg, M.; Liddle, C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology 1998, 27, 128–133. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Lu, S.C. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001, 15, 1335–1349. [Google Scholar] [CrossRef]
- Lieber, C.S. Cytochrome P-4502E1: Its physiological and pathological role. Physiol. Rev. 1997, 77, 517–544. [Google Scholar] [CrossRef]
- Lieber, C.S.; Rubin, E.; DeCarli, L.M. Hepatic microsomal ethanol oxidizing system (MEOS): Differentiation from alcohol dehydrogenase and NADPH oxidase. Biochem. Biophys. Res. Commun. 1970, 40, 858–865. [Google Scholar] [CrossRef]
- Kessova, I.; Cederbaum, A.I. CYP2E1: Biochemistry, Toxicology, Regulation and Function in Ethanol-Induced Liver Injury. Curr. Mol. Med. 2003, 3, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Adas, F.; Salaün, J.; Berthou, F.; Picart, D.; Simon, B.; Amet, Y. Requirement for ω and (ω–1)-hydroxylations of fatty acids by human cytochromes P450 2E1 and 4A11. J. Lipid Res. 1999, 40, 1990–1997. [Google Scholar] [CrossRef]
- Fernandez-Checa, J.C.; Kaplowitz, N. Hepatic mitochondrial glutathione: Transport and role in disease and toxicity. Toxicol. Appl. Pharmacol. 2005, 204, 263–273. [Google Scholar] [CrossRef]
- Hirano, A.; Kaplowitz, N.; Tsukamoto, H.; Kamimura, S.; Fernández-Checa, J.C. Hepatic mitochondrial glutathione depletion and progression of experimental alcoholic liver disease in rats. Hepatology 1992, 16, 1423–1427. [Google Scholar] [CrossRef]
- Latvala, J.; Hietala, J.; Koivisto, H.; Jarvi, K.; Anttila, P.; Niemela, O. Immune Responses to Ethanol Metabolites and Cytokine Profiles Differentiate Alcoholics with or without Liver Disease. Am. J. Gastroenterol. 2005, 100, 1303–1310. [Google Scholar] [CrossRef]
- Bird, G.L.; Sheron, N.; Goka, A.J.; Alexander, G.J.; Williams, R.S. Increased Plasma Tumor Necrosis Factor in Severe Alcoholic Hepatitis. ACP J. 1990, 112, 917–920. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 2006, 387, 349–360. [Google Scholar] [CrossRef]
- Setshedi, M.; Wands, J.R.; De La Monte, S.M. Acetaldehyde Adducts in Alcoholic Liver Disease. Oxidative Med. Cell. Longev. 2010, 3. [Google Scholar] [CrossRef]
- Nair, J.; Srivatanakul, P.; Haas, C.; Jedpiyawongse, A.; Khuhaprema, T.; Seitz, H.K.; Bartsch, H. High urinary excretion of lipid peroxidation-derived DNA damage in patients with cancer-prone liver diseases. Mutat. Res. Mol. Mech. Mutagen. 2010, 683, 23–28. [Google Scholar] [CrossRef]
- Chen, C.; Aplin, J. Placental Extracellular Matrix: Gene Expression, Deposition by Placental Fibroblasts and the Effect of Oxygen. Placenta 2003, 24, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-L.; Zhang, M.; Adhikari, B.; Mujumdar, A.S. Effects of Preparation and Drying Methods on the Antioxidant Activity of Enzymatically Hydrolyzed Porcine Placenta Hydrolysates. Dry. Technol. 2013, 31, 1600–1610. [Google Scholar] [CrossRef]
- Togashi, S.-I.; Takahashi, N.; Iwama, M.; Watanabe, S.; Tamagawa, K.; Fukui, T. Antioxidative Collagen-Derived Peptides In Human-Placenta Extract. Placenta 2002, 23, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.K.; Auddy, B.; Bhattacharya, N.P.; Bhattacharya, S.; Mukherjee, B. Wound healing activity of human placental extracts in rats. Acta Pharmacol. Sin. 2001, 22, 1113–1136. [Google Scholar]
- Park, N.J. Safety, Efficacy and Limitations of Medical Use of Placental Extract. J. Korean Med Assoc. 2005, 48, 1013–1021. [Google Scholar] [CrossRef][Green Version]
- Kanuri, G.; Weber, S.; Volynets, V.; Spruss, A.; Bischoff, S.C.; Bergheim, I. Cinnamon Extract Protects against Acute Alcohol-Induced Liver Steatosis in Mice. J. Nutr. 2009, 139, 482–487. [Google Scholar] [CrossRef]
- Laosam, P.; Panpipat, W.; Yusakul, G.; Cheong, L.-Z.; Chaijan, M. Porcine placenta hydrolysate as an alternate functional food ingredient: In vitro antioxidant and antibacterial assessments. PLoS ONE 2021, 16, e0258445. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Arida, A.U.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef]
- Nanji, A.A.; Zhao, S.; Sadrzadeh, S.M.H.; Dannenberg, A.J.; Tahan, S.R.; Waxman, D.J. Markedly Enhanced Cytochrome P450 2E1 Induction and Lipid Peroxidation Is Associated with Severe Liver Injury in Fish Oil-Ethanol-Fed Rats. Alcohol. Clin. Exp. Res. 1994, 18, 1280–1285. [Google Scholar] [CrossRef]
- Assiri, M.A.; Roy, S.R.; Harris, P.S.; Ali, H.; Liang, Y.; Shearn, C.T.; Orlicky, D.J.; Roede, J.R.; Hirschey, M.D.; Backos, D.S.; et al. Chronic Ethanol Metabolism Inhibits Hepatic Mitochondrial Superoxide Dismutase via Lysine Acetylation. Alcohol. Clin. Exp. Res. 2017, 41, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; DeCarli, L.M. Ethanol Oxidation by Hepatic Microsomes: Adaptive Increase after Ethanol Feeding. Science 1968, 162, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, O.; Parkkila, S.; Juvonen, R.O.; Viitala, K.; Gelboin, H.V.; Pasanen, M. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J. Hepatol. 2000, 33, 893–901. [Google Scholar] [CrossRef]
- Liangpunsakul, S.; Kolwankar, D.; Pinto, A.; Gorski, J.C.; Hall, S.D.; Chalasani, N. Activity of CYP2E1 and CYP3A enzymes in adults with moderate alcohol consumption: A comparison with nonalcoholics. Hepatology 2005, 41, 1144–1150. [Google Scholar] [CrossRef]
- Lieber, C.S. The Discovery of the Microsomal Ethanol Oxidizing System and Its Physiologic and Pathologic Role. Drug Metab. Rev. 2004, 36, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hall, S.D.; Maya, J.F.; Li, L.; Asghar, A.; Gorski, J.C. Diabetes mellitus increases thein vivoactivity of cytochrome P450 2E1 in humans. Br. J. Clin. Pharmacol. 2003, 55, 77–85. [Google Scholar] [CrossRef]
- Hong, J.; Pan, J.; Gonzalez, F.J.; Gelboin, H.V.; Yang, C.S. The induction of a specific form of cytochrome P-450 (P-450j) by fasting. Biochem. Biophys. Res. Commun. 1987, 142, 1077–1083. [Google Scholar] [CrossRef]
- Johansson, I.; Lindros, K.O.; Eriksson, H.; Ingelman-Sundberg, M. Transcriptional control of CYP2E1 in the perivenous liver region and during starvation. Biochem. Biophys. Res. Commun. 1990, 173, 331–338. [Google Scholar] [CrossRef]
- Orellana, M.; Rodrigo, R.; Valdés, E. Peroxisomal and Microsomal Fatty Acid Oxidation in Liver of Rats after Chronic Ethanol Consumption. Gen. Pharmacol. 1998, 31, 817–820. [Google Scholar] [CrossRef]
- Thomasson, H.R.; Crabb, D.W.; Edenberg, H.J.; Li, T.-K. Alcohol and aldehyde dehydrogenase polymorphisms and alcoholism. Behav. Genet. 1993, 23, 131–136. [Google Scholar] [CrossRef]



| Histological Criteria | Severity | Description | Score |
|---|---|---|---|
| Steatosis | Absent | <10% | 0 |
| Mild | 10~30% | 1 | |
| Marked | 31~60% | 2 | |
| Servere | >60% | 3 | |
| Inflammation | None | 0 | |
| Moderate | Scattered | 1 | |
| Marked | Foci | 2 | |
| Severe | Diffuse | 3 | |
| Hepatocyte Ballooning | None | 0 | |
| Few balloon cells | 1 | ||
| Many cells/prominent ballooning | 2 |
| (n = 10) | NAS Score | Oil-Red-Positive Area (%) |
|---|---|---|
| Normal | 0.2 ± 0.26 * | 5.13 ± 2.53 * |
| Control | 1.8 ± 0.64 | 11.51 ± 4.35 |
| EPPH (1.03) | 1 ± 0.49 | 13.09 ± 4.22 |
| EPPH (3.08) | 0.8 ± 0.46 | 4.56 ± 2.32 * |
| EPPH (9.23) | 0.2 ± 0.26 * | 4.31 ±1.75 * |
| Silymarin | 0.4 ± 0.35 | 11.41 ± 3.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.Y.; Park, Y.M.; Shin, D.Y.; Park, K.H.; Kim, M.J.; Yoon, S.M.; Kim, K.N.; Yang, H.J.; Kim, M.J.; Choi, S.-C.; et al. Potential Effect of Enzymatic Porcine Placental Hydrolysate (EPPH) to Improve Alcoholic Liver Disease (ALD) by Promoting Lipolysis in the Liver. Biology 2022, 11, 1012. https://doi.org/10.3390/biology11071012
Lee HY, Park YM, Shin DY, Park KH, Kim MJ, Yoon SM, Kim KN, Yang HJ, Kim MJ, Choi S-C, et al. Potential Effect of Enzymatic Porcine Placental Hydrolysate (EPPH) to Improve Alcoholic Liver Disease (ALD) by Promoting Lipolysis in the Liver. Biology. 2022; 11(7):1012. https://doi.org/10.3390/biology11071012
Chicago/Turabian StyleLee, Hak Yong, Young Mi Park, Dong Yeop Shin, Kwang Hyun Park, Min Ju Kim, Sun Myung Yoon, Keun Nam Kim, Hye Jeong Yang, Min Jung Kim, Soo-Cheol Choi, and et al. 2022. "Potential Effect of Enzymatic Porcine Placental Hydrolysate (EPPH) to Improve Alcoholic Liver Disease (ALD) by Promoting Lipolysis in the Liver" Biology 11, no. 7: 1012. https://doi.org/10.3390/biology11071012
APA StyleLee, H. Y., Park, Y. M., Shin, D. Y., Park, K. H., Kim, M. J., Yoon, S. M., Kim, K. N., Yang, H. J., Kim, M. J., Choi, S.-C., & Lee, I.-A. (2022). Potential Effect of Enzymatic Porcine Placental Hydrolysate (EPPH) to Improve Alcoholic Liver Disease (ALD) by Promoting Lipolysis in the Liver. Biology, 11(7), 1012. https://doi.org/10.3390/biology11071012






