Simple Summary
Periodontal disease and atherosclerotic cardiovascular disease are very common around the world. Coronary artery disease is the leading cause of death. The main factor involved in the pathogenesis of atherosclerosis is inflammation. Therefore, a number of studies have indicated that periodontal disease (causes chronic inflammation) is a risk factor for the progression of atherosclerosis. The presence of periodontal pathogens has been found in human atherosclerotic plaques. A number of pathomechanisms have been demonstrated, thanks to which periodontal pathogens, especially Porphyromonas gingivalis, can directly increase the progression of atherosclerosis and the risk of cardiovascular disease. Observational studies found that patients with periodontal disease were at higher risk of developing atherosclerotic cardiovascular disease. Moreover, periodontal treatment leads to a reduction in cardiovascular risk therefore taking care of oral hygiene should be an important cardiovascular disease preventive measure.
Abstract
Atherosclerotic cardiovascular disease (ASCVD) and periodontal disease (PD) are global health problems. High frequency of ASCVD is associated with the spread of many risk factors, including poor diet, sedentary lifestyle, diabetes, hyperlipidemia, obesity, smoking, hypertension, chronic kidney disease, hypertension, hyperhomocysteinemia, hyperuricemia, excessive stress, virus infection, genetic predisposition, etc. The pathogenesis of ASCVD is complex, while inflammation plays an important role. PD is a chronic, multifactorial inflammatory disease caused by dysbiosis of the oral microbiota, causing the progressive destruction of the bone and periodontal tissues surrounding the teeth. The main etiological factor of PD is the bacteria, which are capable of activating the immune response of the host inducing an inflammatory response. PD is associated with a mixed microbiota, with the evident predominance of anaerobic bacteria and microaerophilic. The “red complex” is an aggregate of three oral bacteria: Tannerella forsythia Treponema denticola and Porphyromonas gingivalis responsible for severe clinical manifestation of PD. ASCVD and PD share a number of risk factors, and it is difficult to establish a causal relationship between these diseases. The influence of PD on ASCVD should be treated as a factor increasing the risk of atherosclerotic plaque destabilization and cardiovascular events. The results of observational studies indicate that PD significantly increases the risk of ASCVD. In interventional studies, PD treatment was found to have a beneficial effect in the prevention and control of ASCVD. This comprehensive review summarizes the current knowledge of the relationship between PD and ASCVD.
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) and periodontal disease (PD) are global health problems. ASCVD is defined as coronary artery disease (CAD), cerebrovascular disease (stroke), or peripheral arterial disease (PAD) of atherosclerotic origin. ASCVD represents the number one cause of morbidity and mortality worldwide [1,2]. The incidence of CAD in 2017 worldwide was 126 million (1.655 per 100,000), which is 1.72% of the human population. It is estimated that by 2030 the incidence of CAD will increase to 1.845 per 100,000. Moreover, CAD is the number one cause of death, disability, and human suffering globally [3]. The incidence of PAD and stroke in 2019 was 113 million and 101 million, respectively [4,5]. Such a high frequency of ASCVD is associated with the spread of many risk factors for these diseases. ASCVD risk factors include poor diet, sedentary lifestyle, diabetes, hyperlipidemia, obesity, smoking, hypertension, non-alcoholic fatty liver disease, chronic kidney disease, arterial hypertension, hyperhomocysteinemia, hyperuricemia, excessive stress, rheumatological diseases (systemic lupus erythematosus and rheumatoid arthritis), inflammatory bowel disease, human immunodeficiency virus infection, thyroid disease, menopause, testosterone and genetic predisposition [6,7,8,9]. The pathogenesis of atherosclerosis and thus ASCVD is complex, while inflammation plays an important role [10]. It is worth mentioning that almost 10–15% of myocardial infarction (MI) patients lack the presence of any classical ASCVD risk-factors, indicating the contribution of alternative mechanisms [11,12]. It points to an important role of chronic inflammation, and immune activation play a central role in atherosclerotic plaque instability, triggering a thromboembolic episode [13]. Hence, PD (leads to inflammation) has been assigned an important role in the pathogenesis of ASCVD for some time [14,15,16,17].
Assessment of the global prevalence of PD was the subject of a study by Nazir et al. using data from 27 countries. It was shown that only 9.3% of adults, 9.7% of older persons, and 21.2% of adolescents had no PD (p = 0.005). It was found that the incidence of PD increased with age [18]. Overall, PD occurs in 20–50% of people worldwide (severe forms of PD affect approximately 11% of the world’s population), and is more common among men [19,20,21]. Moreover, PD poses a significant economic problem. As indicated by Botelho et al. PD caused a €149.52 B loss in Europe and €122.65 B in the USA, in 2018. Moreover, the economic burden of PD is increasing [22].
PD is a chronic, multifactorial inflammatory disease caused by dysbiosis of the oral microbiota, causing the progressive destruction of the bone and periodontal tissues surrounding the teeth. In the early stages of PD, gums can become swollen and red and they may bleed. In advanced stages, PD can lead to sore, bleeding gums; painful chewing problems; and even tooth loss [23]. The main etiological factor of PD are the bacteria, caused of capable of activating the innate immune response of the host inducing an inflammatory response. The progression of this inflammatory response culminates in the destruction of periodontal tissue [24]. More than 700 species of microorganisms have been found in the oral cavity, including bacteria, viruses, fungi and protozoa. Not all oral bacteria are pathogenic, and most of them are saprophytes under health conditions. PD are associated with a mixed microbiota, with the evident predominance of anaerobic bacteria and microaerophilic. Bacteria causing PD include, among others, Porphyromonas gingivalis, Tannerella forsythia (Bacteroides forsythus), Treponema denticola, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Streptococcus sanguis, etc. [19]. It is worth mentioning that the “red complex” is an aggregate of three oral bacteria (T. forsythia, P. gingivalis and T. denticola) responsible for severe clinical manifestation of PD [25]. In the oral cavity, when hygiene is neglected, bacteria colonize the cervical areas of the tooth crowns, creating a bacterial biofilm together with dental plaque, from which, after mineralization, tartar is formed, often noticeable from the lingual side of the front teeth of the mandible. This biofilm is a specific ecological niche for bacteria, protecting them against the action of antiseptics and antibiotics [19]. The risk factors for PD include poor oral hygiene, male gender, older age, excessive stress, obesity, type 2 diabetes, smoking, excessive alcohol consumption, poor diet (vitamin C, D and calcium deficiencies), socioeconomic status, family history of PD, the use of certain medications (eg immunosuppression) and genetic predisposition [26,27,28]. Thus, a number of risk factors are common to PD and ASCVD [28]. Cumulative evidence from literature over the last decades have supported the role of PD as a risk factor for ASCVD [29,30].
The main stages in the etiopathogenesis of PD are shown in Figure 1.
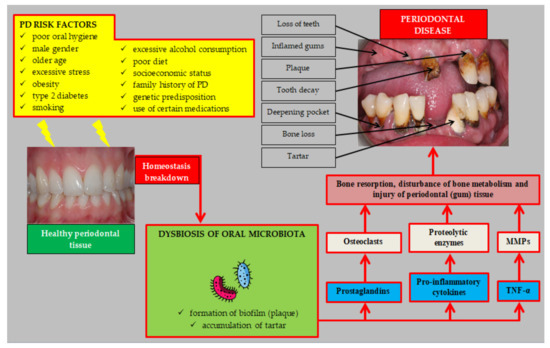
Figure 1.
Etiopathogenesis of periodontal disease. Abbreviations: PD (periodontal disease); TNF-α (tumor necrosis factor α); MMPs (matrix metalloproteinases).
Thus, ASCVD and PD are widespread diseases throughout the world. The important role of PD in the pathogenesis of atherosclerosis and ASCVD is indicated.
2. Periodontal Disease and Atherosclerosis: Pathogenesis
Atherosclerotic changes appear from childhood [31]. Therefore, the influence of PD on ASCVD should be treated as a factor accelerating the progression of atherosclerosis and increasing the risk of atherosclerotic plaque destabilization and the occurrence of a cardiovascular event. As mentioned earlier, ASCVD and PD share many risk factors. Moreover, inflammatory pathways, including increased levels of: C-reactive protein (CRP), white blood cells, fibrinogen, intercellular adhesion molecules, and proinflammatory cytokines, play a pivotal role in both ASCVD and PD pathogenesis [32]. It is worth pointing out here that the estimated periodontium area is equal to the area of the hand. The influence of local inflammation of such a large extent occurring during generalized PD may significantly contribute to systemic inflammation [33]. The effect of PD on the progression of atherosclerosis is complex and unclear. There are includes direct and indirect pathogenetic mechanisms [34]. A number of review articles discuss in detail the pathogenesis of ASCVD in patients with PD [28,32,35,36,37,38,39,40,41,42]. The most important pathogenetic mechanisms linking PD to ASCVD are summarized in Figure 2.
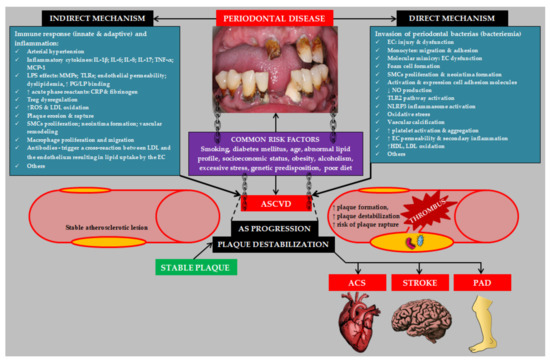
Figure 2.
Etiopathogenesis of atherosclerotic cardiovascular disease induced by periodontal disease. Abbreviations: IL-1β (interleukin 1β); IL-6 (interleukin 6); IL-8 (interleukin 8); IL-17 (interleukin 17); TNF-α (tumor necrosis factor α); MCP-1 (monocyte chemoattractant protein-1); MMPs (matrix metalloproteinases); TLRs (toll-like receptors); PG (proteoglycan); LP (lipoprotein); SMCs (smooth muscle cells); LDL (low density lipoprotein); EC (endothelial cell); ASCVD (atherosclerotic cardiovascular disease); AS (atherosclerosis); NO (nitric oxide); TLR2 (toll-like receptor 2); NLRP3 (NLR family pyrin domain containing 3); HDL (high density lipoprotein); ACS (acute coronary syndrome); PAD (peripheral arterial disease).
2.1. Presence of Periodontal Pathogens in Atherosclerotic Plaques
Periodontal pathogens from subgingival microbiota can translocate within the bloodstream to atherosclerotic plaques located in various arteries [43]. This is confirmed by the results of clinical trials in which the presence of periodontal pathogens in atherosclerotic plaques was found. A very interesting study by Haraszthy et al. evaluated the composition of atherosclerotic plaques in the carotid arteries, using 50 samples of biological material collected from patients during the endarterectomy procedure. It was shown that 44% of the 50 atheromas were positive for at least one of the target periodontal pathogens. Thirty percent of the surgical specimens were positive for B. forsythus, 26% were positive for P. gingivalis, 18% were positive for A. actinomycetemcomitans, and 14% were positive for P. intermedia. It was found that periodontal pathogens present in the atherosclerotic plaque may be directly involved in the progression of atherosclerotic lesions [44]. These results were confirmed by Rath et al. demonstrating the presence of periodontal bacteria DNA in coronary atheromatous plaques samples of the patients who had undergone coronary endarterectomy for cardiovascular disease (CVD) [45]. Szulc et al. in their study involving 91 patients with CAD and PD found that P. gingivalis DNA was frequently found in carotid and coronary atheromatous plaques (1/5 patients) [46]. A case-control study by Mahendra et al. found that the number of periodontal bacteria (T. forsythia, C. rectus, P. gingivalis, P. intermedia and P. nigrescens) in subgingival plaque was significantly related to their number in atherosclerotic plaque in patients with CAD [47]. Mahendra et al. showed the occurrence of periodontal pathogens “red complex” in coronary plaque samples coming from 51 patients with chronic PD. The most frequently identified periodontal pathogens were T. denticola (51%) and P. gingivalis (45.1%) [48]. In a study by Rao et al., involving 81 patients scheduled for coronary artery bypass grafting or angioplasts, a significant relationship was demonstrated between the occurrence of P. gingivalis (p = 0.007) and T. forsythia (p = 0.001) in the subgingival and atherosclerotic plaques. Moreover, it was served that patients whose atherosclerotic plaques tested positive for one or more of the pathogens had chronic periodontitis [49]. A meta-analysis of 14 clinical trials by Joshi et al. summarized data on the occurrence of periodontal microorganisms in coronary atheromatous plaque specimens of myocardial infarction patients. The most common periodontal pathogens in coronary atheromatous plaque samples were: P. gingivalis (mean prevalence; MP = 0.4; 95% CI: 0.237–0.556, p = 0.00003) and A. actinomycetemcomitans (MP = 0.042; 95% CI: −0.398 to 0.282, p = 0.311) [50].
It should be mentioned that the periodontal pathogens in atherosclerotic plaques often have been detected using PCR technique, DNA hybridization, enzyme-linked immunosorbent assay, immunohistochemistry, and transmission or scanning electron microscopy. It is, however, only the fluorescence in situ hybridization technique and culture in media that permit detection of living microorganisms [51]. Therefore, in the study by Kędzia et al., which included 37 patients with carotid atherosclerosis, the presence of periodontal pathogens in atherosclerotic plaques was analyzed using the method of isolation and cultivation of anaerobic bacteria. It was shown that the most prevalent species were P. gingivalis and P. intermedia among Gram-negative rods, and P. acnes among Gram-positive rods [51].
From a clinical point of view, the results of the study by Kannosh et al., which found that the most colonized artery by periodontal pathogens was a. coronaria, followed by a. carotis, a. abdominalis, a. mammaria, and a. femoralis. Based on these results, it can be concluded that atherosclerotic plaques of the coronary arteries may be most prone to destabilization directly by periodontal pathogens [52].
Thus, numerous clinical studies have unequivocally demonstrated the presence of periodontal pathogens in atherosclerotic plaques of various arteries.
2.2. Pathogenetic Mechanisms—Brief Overview
The influence of inflammation caused by chronic PD (but also by other factors) on the progression of atherosclerosis has been well described [53].
2.2.1. Periodontal Pathogens and Lipids
The influence of P. gingivalis on the pathogenesis of atherosclerosis begins at the stage of promoting pathological changes in the lipid metabolism. In general, periodontal pathogens are characterized by the ability to oxidize lipoproteins [39]. Kim et al. found that P. gingivalis induced high density lipoprotein (HDL) oxidation, impairing the atheroprotective function of these lipoprotein [54]. Moreover, as indicated by Joo et al., P. gingivalis (more precisely peptide 19 [Pep19] of P. gingivalis heat shock protein 60 [HSP60]) was characterized by a greater ability to oxidize low density lipoprotein (LDL) than counterpart—C. pneumoniae and M. tuberculosis [55]. P. gingivalis and its different gingipain variants induced reactive oxygen species (ROS) and consumed antioxidants. Rgp and Kgp gingipains were involved in inducing lipid peroxidation [56]. Ljunggren et al. have shown that patients with PD have an altered plasma lipoprotein profile, defined by altered protein levels as well as post-translational and other structural modifications towards an atherogenic form [57].
2.2.2. Periodontal Pathogens and Vascular Endothelium
A study by Li et al. showed that lectin-type oxidized LDL receptor 1 (LOX-1) plays a key role in P. gingivalis-induced migration and adhesion of monocytes to human EC [58]. Moreover, in an in vivo and in vitro study by Reyes et al., it was found that P. gingivalis infection led to microvascular EC damage and that invasion of these cells via intercellular adhesion molecule 1 [ICAM-1] (anti-ICAM-1 antibodies reduced P. gingivalis invasion in EC) may be important for microbial persistence within tissues [59]. Periodontal pathogens are characterized by the ability to directly infect vascular endothelial cells (EC) [60]. The entry of P. gingivalis into EC is positively associated with bacterial load and some virulent proteins such as gingipains, fimbriae and haemagglutinin A [61]. Moreover, invasion of gingival epithelial and EC by P. gingivalis may be synergized by F. nucleatum and T. forsythia [62,63]. The EC infection by periodontal pathogens creates the possibility of direct damage to these cells. Recently, Xu et al.’s in vitro study showed that P. gingivalis infection induced mitochondrial fragmentation, increased the mitochondrial reactive oxygen species levels, and decreased the mitochondrial membrane potential and adenosine triphosphate concentration in vascular EC. Researchers indicate that mitochondrial dysfunction may represent the mechanism by which P. gingivalis accelerates the progression of atherosclerosis [64]. A very interesting mechanism connecting PD with endothelial dysfunction and progresion of atherosclerosis is molecular mimicry. The activation of the vascular endothelium leads to the expression of heat shock proteins (HSPs) on its surface. Owing to the homologous nature of HSPs among species, cross-reactivity of antibodies to bacterial HSP, termed GroEL (anti-P. gingivalis), with human HSP60 on EC may subsequently result in endothelial dysfunction and the development of atherosclerosis [65]. P. gingivalis infection also affects the permeability of the vascular endothelium. A study by Farrugia et al. assessed the effect of outer membrane vesicles (OMVs) produced by P. gingivalis on vascular endothelium. It was shown that human microvascular EC displayed an OMV-associated, gingipain-dependent decrease in cell surface levels of the intercellular adhesion molecule platelet endothelial cell adhesion molecule-1, leading to increased vascular endothelial permeability [66]. Probably gingipain led to the proteolytic cleavage of platelet endothelial cell adhesion molecule-1 and VE-cadherin [67]. Importantly, Song et al. showed that P. gingivalis significantly reduces plasminogen activator inhibitor-1 levels in human EC. They found that plasminogen activator inhibitor-1 was proteolyzed by lysine-specific gingipain-K, leading to deregulation of endothelial homeostasis, thus contributing to permeabilization and dysfunction of the vascular endothelial barrier [68]. The direct influence of P. gingivalis on EC was also demonstrated by Xie et al. These researchers found that P. gingivalis can impair endothelial integrity by inhibiting cell proliferation and inducing endothelial mesenchymal transformation and apoptosis of EC, which reduce the cell levels and cause the endothelium to lose its ability to repair itself. Moreover, the use of toll-like receptor (TLR) antagonist or nuclear factor-κB (NF-κB) signaling inhibitor significantly reduced the adverse effect of P. gingivalis on the EC, which indicates a significant role of TLR-NF-κB signaling pathway plays in the pathogenesis of EC damage by this periodontal pathogen [69]. P. gingivalis not only leads to EC damage but also reduces the regenerative capacity of the vascular endothelium. A study by Kebschull et al. found that P. gingivalis mediated TLR-2 to increase peripheral endothelial progenitor cells (EPCs) counts and decrease EPCs pools in the bone marrow, thereby possibly reducing overall endothelial regeneration capacity [70]. This was also confirmed in a clinical study by Isola et al. Patients with PD had significantly lower levels of EPCs (CD133+/KDR+) compared to healthy subjects (p < 0.001) [71]. Vascular endothelium dysfunction in PD was also demonstrated in a clinical study by Fujitani et al. [72]. It is worth noting that in the course of infection of the EC by P. gingivalis, the vicious circle effect develops. P. gingivalis stimulates the TLRs-NF-κB signaling pathway, which then leads, inter alia, to downregulation of brain and muscle Arnt-like 1 protein releases CLOCK, which phosphorylates p65 and further enhances NF-κB signaling, elevating oxidative stress and inflammatory response in human aortic EC [73]. In turn, a study by Charoensaensuk et al. was showed that P. gingivalis up-regulates IL-1β and tumor necrosis factor α expression, which consequently causes cell death of brain EC through the ROS/NF-κB pathway [74]. P. gingivalis is also characterized by the ability to directly increase the expression of adhesion molecules such as ICAM-1 in EC [75]. Bugueno et al. also found that P. gingivalis induced a pro-inflammatory and pro-oxidative EC response including up-regulation of tumor necrosis factor α, interleukin 6 and interleukin 8 as well as an altered expression of inducible and endothelial nitric oxide synthase at both mRNA and protein level. An increase of vascular cell adhesion protein 1 and ICAM-1 mRNA expression was also observed after P. gingivalis infection [76].
2.2.3. Periodontal Pathogens and Smooth Muscle Cells
Another stage of atherogenesis that may be potentiated by P. gingivalis is the proliferation and migration of smooth muscle cells (SMCs). The study by Cao et al. found that gingipains can promote phenotypic transformation and proliferation of rat SMCs [77]. Moreover, Zhang et al. found that activation of transforming growth factor β and Notch signaling pathways may be involved in the P. gingivalis-mediated proliferation of human aortic SMCs [78]. In turn, Kobayashi et al. found that P. gingivalis can promote neointimal formation in mice [79]. The results of experimental studies also indicate that P. gingivalis infection leads to vascular calcification [80,81].
2.2.4. Periodontal Pathogens and Foam Cells
It should also be mentioned that P. gingivalis directly promotes the formation of foam cells. It has been suggested that lipopolysaccharides (LPS) P. gingivalis leads to acetyl-coenzyme A acetyltransferase 1 up-regulation [82]. Moreover, it was shown that LPS P. gingivalis led, via heme oxygenase-1, to upregulation of scavenger receptor class B member 3 and down-regulation of ATP binding cassette subfamily A member 1 protein, which also favored the accumulation of cholesterol in macrophages [83]. Moreover, Xu et al. found that P. gingivalis enhanced expression of macrophage migration inhibitory factors in the EC. Increasing the expression of macrophage migration inhibitory factor stimulates macrophages to secrete pro-inflammatory cytokines, prolongs the survival time of macrophages and thus maintains inflammation [84]. macrophage migration inhibitory factor enhances the formation of foam cells [85].
2.2.5. Periodontal Pathogens and Atherosclerotic Plaque Destabilization
P. gingivalis can also destabilize atherosclerotic plaque. In the study by Mubarokah et al., it was found that P. gingivalis induced macrophage to secrete matrix metallopeptidase 9 that led to fragmentation of vascular type IV collagen. This mechanism plays an important role in pathogenesis of atherosclerotic plaque rupture [86].
2.2.6. Periodontal Pathogens and Platelets
An important factor involved in atherothrombosis is the activation and aggregation of platelets. Experimental and clinical studies showed that P. gingivalis infection led to the activation of platelets and their aggregation [87,88,89]. P. gingivalis infection increased the expression of P-selectin and increased the binding of fibrinogen to platelets [87].
2.2.7. Other
Other periodontal pathogens are also characterized by properties favoring the progression of atherosclerosis [90,91,92].
In addition, there are common genetic factors leading to PD and ASCVD. A significant role is indicated as genetic variant at vesicle-associated membrane protein gene (VAMP3 and VAMP8) [93,94].
Thus, there is evidence that periodontal pathogens, mainly P. gingivalis, are directly involved in all major stages of atherosclerotic lesion formation, progression and instability [95].
3. Periodontal Disease and Risk of Atherosclerosis Cardiovascular Diseases
The influence of the presence of PD on the risk of ASCVD has been assessed in a number of studies. A meta-analysis of 32 studies by Larvin et al. summarized the effect of PD on the risk of various ASCVDs. It was shown that the occurrence of PD was associated with a 23% increase in the risk of developing ASCVD (RR = 1.23; 95% CI: 1.13–1.35). Subgroup analysis showed that PD increased the risk of ASCVD more in men than in women (RR = 1.16; 95% CI: 1.08–1.25 vs. RR = 1.11; 95% CI: 1.02–1.22, respectively). The risk of ASCVD was found to be related to the severity of PD (mild: RR = 1.09; 95% CI: 1.05–1.14 vs. moderate: RR = 1.23; 95%: 1.14–1.32 vs. severe: RR = 1.25; 95% CI: 1.15−1.35). Among all types of ASCVD, the risk of stroke was highest (RR = 1.24; 95% CI: 1.12–1.38), the risk of CAD was also increased (RR = 1.14; 95% CI: 1.08–1.21) [96]. In turn, a meta-analysis of 11 prospective studies conducted by Gao et al. showed that the presence of PD increased the risk of CAD by 18% (RR = 1.18; 95% CI: 1.10–1.26). Moreover, it was found that the risk of CAD was higher the smaller the number of teeth was (17–24 teeth: CAD risk ↑ by 12%; 11–16 teeth: CAD risk ↑ by 28%, and <10 teeth: CAD risk ↑ by 55%) [97]. Qin et al. in a meta-analysis of 10 cohort studies, showed that patients with PD were characterized by a 13% higher risk of myocardial infarction (MI) (RR = 1.13; 95% CI: 1.04–1.21, p = 0.004) [98].
In meta-analysis by Joshi et al. was assessed the relationship between the occurrence of serum antibody response against periodontal bacteria and risk of CAD. It showed a significant association between elevated serum IgG antibody responses (anti-p. gingivalis and anti-A. actinomycetemcomitans) and CAD, with pooled OR of 1.23 (95% CI: 1.09–1.38, p = 0.001) and 1.25 (95% CI: 1.04–1.47, p = 0.0004), respectively [99]. Moreover, in the Atherosclerosis Risk in Communities (ARIC study) by Arsiwala et al., which included 9793 subjects with an average follow-up of 20.1 years, the impact of PD on the risk of PAD was assessed. It was shown that PD increased the risk of PAD by 38% (HR = 1.38; 95% CI: 1.09–1.74) [100]. In a meta-analysis of 25 studies, including 22,090 participants, by Wang et al. showed that PD increased the risk of PAD by 60% (OR = 1.60; 95% CI: 1.41–1.82, p < 0.001). Subgroup analysis showed that PD significantly increased the occurrence of lower extremity arterial disease and carotid artery disease (OR = 3.00; 95% CI: 2.23–4.04, p < 0.001 and OR = 1.39, 95% CI: 1.24–1.56, respectively, p < 0.001) [101]. Zeng et al., in a meta-analysis of 17,330 participants, showed that PD increased the risk of carotid atherosclerosis by 27% (OR: 1.27; 95% CI: 1.14–1.41, p < 0.001). This risk depended on the severity of PD [102]. In the study by González-Navarro et al. including 83 patients suffering a cardiovascular event and 48 patients without cardiovascular events, it was shown that number of teeth with apical periodontitis and total dental index scores was significantly correlated with risk of cardiovascular events (OR = 2.3; 95% CI: 1.3–4.3, p = 0.006 and OR = 1.5; 95% CI: 1.2–1.9, p = 0.001, respectively) [103]. An interesting meta-analysis of 20 studies by Wang et al. summarized the relationship between PD and the risk of carotid artery calcification. The risk of carotid artery calcification was significantly higher by 102% in patients with PD (OR = 2.02; 95% CI: 1.18–3.45) [104]. It is also worth mentioning that the study by Rodean et al. showed that the occurrence of PD was associated with a higher risk of the presence of high-risk atherosclerotic plaques in the coronary arteries [105]. In a study by Cowan et al. including 8092 participants who were followed for an average of 12.9 years, PD was shown to independently increase the risk of venous thromboembolism by 29% (HR = 1.65; 95% CI: 1.25–2.18), with this association it weakened because confounding factors were taken into account [106]. From a clinical point of view, the results of a prospective study by Sánchez-Siles et al., showed that patients with the venous thromboembolic disease and PD could have more risk of a new thromboembolism episode are also important [107].
In a study by Kotronia et al. involving 5222 elderly subjects who were followed for an average of 9–15 years, it was shown that the presence of PD increased the risk of cardiovascular mortality by 49% (HR = 1.49; 95% CI: 1.01–2.20) [108]. Similar results were obtained by Bengtsson et al. in a study involving 858 subjects over 60 years of age who were followed for an average of 17 years, it was shown that PD increased the risk of CAD (HR = 1.5; CI: 1.1–2.1) and cardiovascular death (HR = 1.4, 95% CI: 1.2–1.8) [109]. It is worth mentioning that Qi et al. found a significant relationship between the occurrence of periodontal antibodies (P. melaninogenica, P. intermedia, P. nigrescens, and P. gingivalis) and the risk of cardiovascular disease mortality [110]. The impact of PD on the risk of cardiovascular death was summarized in a meta-analysis of 57 studies by Romandini et al. involving 5.71 million participants. It was shown that PD increases the risk of mortality due to cardiovascular diseases by 47% (RR = 1.47; 95% CI: 1.14–1.90) [111]. Moreover, the number of remaining natural teeth also affects the risk of disease-specific mortality. Only having 0–9 natural teeth was shown to have the highest risk of mortality from heart diseases [HR = 1.92; 95% CI: 1.33–2.77) and from diabetes [HR = 1.67; 95% CI: 1.05–2.66) [112]. The relationship between tooth loss and cardiovascular risk was also demonstrated in a meta-analysis of 17 studies by Cheng et al. These researchers found that compared with the lowest tooth loss, tooth loss was significantly associated with a higher risk of cardiovascular disease (RR = 1.52; 95% CI: 1.37–1.69; p < 0.001) [113].
Thus, observational studies and their meta-analyzes have shown that PD increases the risk of ASCVD. Figure 3 summarizes the effect of PD on the risk of the most important ASCVDs.
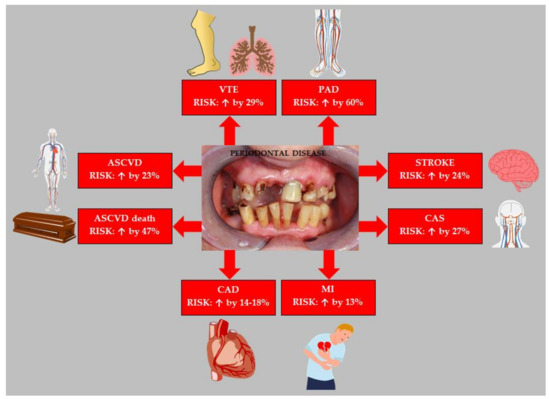
Figure 3.
Effect of periodontal disease on the risk of atherosclerotic cardiovascular disease. Based on data from [96,97,98,101,102,106,111]. Abbreviations: ASCVD (atherosclerotic cardiovascular disease); VTE (venous thromboembolism); PAD (peripheral arterial disease); CAS (carotid artery stenosis); MI (myocardial infarction); CAD (coronary artery disease).
At this point, however, it is worth noting that the results of observational studies only allow for the formulation of a research hypothesis, but cannot prove a cause-and-effect relationship. The cause and effect relationship between PD and ASCVD is currently under discussion. In Mendelian randomization studies by Zhou et al. did not provide evidence for dental caries and PD as the causes of ASCVD [114]. Moreover, the position paper from the Canadian Dental Hygienists Association indicates that the Bradford Hill criteria analysis failed to support a causal relationship between PD and ASCVD [115]. However, this issue remains controversial and debatable because, as presented later in this article, there is no doubt that periodontal treatment significantly improves cardiovascular parameters and the control of ASCVD risk factors. Therefore, more research is needed to assess the causal relationship between PD and ASCVD.
4. Periodontal Treatment and Atherosclerosis Cardiovascular Diseases
A primary goal of periodontal therapy (PT) is to reduce the burden of pathogenic bacteria and thereby reduce the potential for progressive inflammation and recurrence of PD [116]. A systematic review and meta-analysis of 10 clinical trials by Roca-Millan et al. summarized the effect of PT on cardiovascular risk. PT was shown to lead to: reduced CRP levels, tumor necrosis factor α, interleukin 6 and leukocytes. Fibrinogen levels after PT also improved considerably. Moreover, after PT there was a significant reduction in oxidized LDL (oxLDL) and oxidized HDL (oxHDL). A meta-analysis showed that non-surgical PT (NSPT) in contrast to receiving no treatment at all led to a significant reduction in CRP (p < 0.001) and leukocyte values (p < 0.001) [117]. An earlier meta-analysis of 25 clinical trials by Teeuw et al. indicated that PT improves the atherosclerotic profile: weighted mean difference (WMD) for hsCRP (−0.50 mg/L; 95% CI: −0.78 to −0.22), IL-6 (−0.48 ng/L; 95% CI: −0.90 to −0.06), tumor necrosis factor α (−0.75 pg/mL; 95% CI: −1.34 to −0.17), fibrinogen (−0.47 g/L; 95% CI: −0.76 to −0.17), total cholesterol (−0.11 mmol/L; 95% CI: −0.21 to −0.01) and HDL (0.04 mmol/L; 95% CI: 0.03–0.06). From a clinical point of view, it is important that the greatest benefits from PT were achieved by patients with concomitant cardiovascular and/or metabolic disease, which makes maintaining periodontal health very important and very beneficial in these patients [118]. A number of interventional studies (Table 1) have been conducted to assess the effect of periodontal therapy on ASCVD and the control of risk factors for these diseases.

Table 1.
Selected intervention studies assessing the effect of periodontal disease treatment on cardiovascular parameters. Abbreviations: RCT (randomized control trial); PD (periodontal disease); PT (periodontal therapy); CT (clinical trial); PAC-1 (first procaspase activating compound); CIMT (carotid intima-media thickness); BP (blood pressure); ABPM (ambulatory blood pressure monitoring); SBP (systolic blood pressure); DBP (diastolic blood pressure); EMP (endothelial microparticles); TG (trigliceride); HDL (hig density lipoprotein); TNF-α (tumor necrosis factor α); IL-1β (interleukine 1β); IL-6 (interleukine 6); T2DM (type 2 diabetes mellitus); HbA1C (glycated haemoglobin); FPG (fasting plasma glucose); MI (myocardial infarction); HF (heart failure); HR (hazard ratio); hsCRP (high sensitivity C-reactive protein); ESRD (end-stage renal disease); CVDs (cardiovascular diseases); PAD (peripheral arterial disease); OR (odds ratio); CAD (coronary artery disease); IL-8 (interleukin 8); sVCAM-1 (soluble vascular cell adhesion molecule-1); sICAM-1 (soluble intercellular cell adhesion molecule-1); MMP-9 (matrix metallopeptidase 9); FMD (flow-mediated dilation).
Thus, a number of clinical studies indicate that PT leads to a significant reduction ASCVD risk factors and biomarkers. It is worth citing the results of several more recent meta-analyzes that summarize the available data on the influence of PT on the control of ASCVD risk factors. A meta-analysis of nine randomized clinical trials (RCTs) by Baeza et al. showed that PT led to a significant reduction in HbA1C (p < 0.01) and CRP (p < 0.01) in patients with type 2 diabetes mellitus (T2DM) [141]. Similar results were obtained by Cao et al. in a meta-analysis of 14 RCTs, in which a significant effect of PT on the reduction of HbA1C was found [142]. Moreover, a meta-analysis of 18 clinical trials conducted by Lima et al. showed that PT led to a significant reduction in the level of TNF-α (p = 0.001) in patients with T2DM [143]. The beneficial effect of PT on the control of the lipid profile in T2DM patients was confirmed in a meta-analysis of seven clinical trials by Garde et al., showing a significant reduction in total cholesterol (p = 0.001) and triglycerides (p < 0.00001) [144]. In a very interesting meta-analysis of 8 RCTs Shrama et al. showed that intensive PT led to significant reduction in systolic blood pressure (WMD = −11.41 mmHg; 95% CI: 95% CI: −13.66 to −9.15, p < 0.00001) and diastolic blood pressure (WMD = −8.43 mmHg; 95% CI: −10.96 to −5.91, p < 0.00001) among prehypertensive/hypertensive patients. Moreover, significant reductions in CRP and improvement of endothelial function were found after PT in these patients [145].
It should also be mentioned that PT improves the control of other diseases that are a risk factor for ASCVD. For example, a meta-analysis by da Silva et al. showed that PD treatment, by increasing glomerular filtration rate, may improve renal function in patients with chronic kidney disease [146]. Moreover, as shown by Okada et al., PT in patients with rheumatoid arthritis led to a reduction in levels of antibodies to P. gingivalis and citrulline, which may contribute to better control of this disease [147]. In turn, Fabbri et al. found that PT improves systemic lupus erythematosus response to immunosupressive therapy, which may also contribute to better control of this disease [148].
It is important to control the effectiveness of PT because, as demonstrated by Holmlund et al. patients who did not respond well to PT had an increased risk for future ASCVD (incidence rate ratio [IRR] = 1.28; 95% CI: 1.07–1.53, p = 0.007 vs. to good responders) [149].
The most important drugs in the treatment of ASCVD are statins with many pleiotropic effects [150]. From the point of view of the effectiveness of ASCVD treatment with the use of drugs, it should be mentioned that in clinical trials these drugs significantly reduce periodontal inflammation, which is another anti-atherosclerotic pleiotropic effect [151]. In a comperhensive review by Tahamtan et al. was summarized the mechanisms of beneficial effects of statin use (local or systemic) on supporting PD treatment. These mechanisms include: anti-inflammatory and antioxidant activity, antibacterial activity, reduction of matrix metalloproteinases level, reduction of osteoclast activity and reduction of osteoclastogenesis while increasing osteoblast activity and increasing the expression of bone morphogenic protein-2 [152]. In a meta-analysis of the results of 10 clinical trials conducted by Muniz et al., it was found that statins significantly supported PD treatment (improved clinical attachment level, reduced probing pocket depth and improved intrabony defect) [153].
To sum up, PT in many clinical trials and their meta-analyzes had a very positive effect on the control of the most important risk factors of ASCVD. Moreover, PT may be beneficial in controlling other diseases that increase cardiovascular risk. From the clinical point of view, it is also important that statins reduce periodontal inflammation.
5. Conclusions
The authors of the paper, after analyzing the cited publications, came to the conclusion that at present it is not possible to unequivocally define PD as an independent factor generating ASCVD. This is due to the poor spectrum of biochemical inflammatory markers selectively referring to the oral cavity, and clinical periodontal examination remains the most reliable and unambiguous.
Experimental studies show that periodontal pathogens, especially P. gingivalis, can directly increase the progression of atherosclerosis at all its stages. The pathophysiological rationale is reflected in observational studies, which found that PD significantly increases the risk of various ASCVDs. Genetic analyzes do not confirm a cause-and-effect relationship between PD and ASCVD. The results of randomized clinical trials and their meta-analyzes stand in opposition to them, which clearly indicate that PD treatment significantly improves the control of the most important risk factors for ASCVD, contributing to the improvement of cardiovascular health. Taking all this into account, in the latest 2021 guidelines of the European Society of Cardiology (ESC) Guidelines on cardiovascular disease prevention in clinical practice, the inclusion of periodontal infection in the chapter “gap of evidence” indicates the need for further research [154].
It is worth noting that not only periodontal pathogens contribute to the development of atherosclerosis, but also infections with such pathogens as: H. pylori, pneumonia pathogens, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), cytomegalovirus (CMV), herpes simplex virus (HSV), hepatitis-C virus (HCV) and HIV [155].
There is no doubt that taking care of oral health should be the basis of ASCVD prevention. For this purpose, avoiding risk factors for PD should be recommended (Figure 1). An important recommendation combining the prevention of PD and ASCVD is to recommend a healthy diet. As indicated by Altun et al., the use of DASH diet/Mediterranean diet reduced the risk of PD (OR = 0.92; 95% CI: 0.87–0.97, p < 0.001 and OR = 0.93; 95% CI: 0.91–0.96, p < 0.001, respectively) [156]. These diets are also effective and recommended in the prevention of ASCVD [154]. Patients with PD can be encouraged to treatment by pointing to the results of a study by Vivek et al., which found that PT significantly increased the quality of life [157]. Moreover, it should be emphasized that the presence of PD is a significant risk factor for coronavirus disease 2019 (COVID-19) [158]. Therefore, in the current pandemic situation, the prevention and treatment of PD becomes even more important. It should be remembered that, without consulting a periodontist, not to recommend too frequent use of oral fluids containing chlorhexidine in order to prevent PD. Excessive use of oral hygiene fluids containing chlorhexidine may have the opposite effect and, by disrupting the oral microbiota, it may contribute to an increased risk of hypertension and T2DM [159,160]. A very important role in the prevention of ASCVD is educating the people about the adverse impact of PD on the risk of these diseases. The authors’ own research carried out in a group of medical students showed that their knowledge of the influence of PD on the risk of hypertension (an important risk factor for ASCVD) was insufficient. In a subgroup analysis, we found that 32.8% of final-years students said that PD was not a risk factor for hypertension, while 25.8% had no knowledge of it. Medical students of the last years of their studies should participate in the education of the society on the prevention of ASCVD, therefore more emphasis should be placed on their education in the field of non-classical risk factors for these diseases [161].
Taking into account that lifestyle modifications are the cornerstones in the prevention and treatment of ASCVD, in the assessment of cardiovascular risk, PD should be sought, considered and prevented, and, if necessary, periodontal treatment recommended.
Author Contributions
Conceptualization, A.W., K.J.F., M.R.C. and S.S.; methodology, M.R.C. and S.S.; formal analysis, M.R.C., S.S. and M.R.; investigation M.R.C., S.S. and M.R.; resources, M.R.C., S.S., M.R. and J.M.N.; data curation, M.R.C. and S.S.; writing—original draft preparation M.R.C., S.S., M.R. and J.M.N.; writing—review and editing M.R.C., S.S., M.R. and J.M.N.; visualization, M.R.C. and S.S.; supervision, A.W. and K.J.F.; project administration, M.R.C. and S.S.; funding acquisition, M.R.C. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sathiyakumar, V.; Kapoor, K.; Jones, S.R.; Banach, M.; Martin, S.S. Novel therapeutic targets for managing dyslipidemia. Trends Pharm. Sci. 2018, 39, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Banach, M. Fibrinogen and atherosclerotic cardiovascular diseases—review of literature and clinical studies. Int. J. Mol. Sci. 2022, 23, 193. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global epidemiology of ischemic heart disease: Results from the global burden of disease study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk factors for coronary artery disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/32119297/ (accessed on 15 January 2022).
- Gohlke-Bärwolf, C. Coronary artery disease-is menopause a risk factor? Basic Res. Cardiol. 2000, 95, 177–183. [Google Scholar] [CrossRef]
- Nikpay, M.; Goel, A.; Won, H.H.; Hall, L.M.; Willenborg, C.; Kanoni, S.; Saleheen, D.; Kyriakou, T.; Nelson, C.P.; Hopewell, J.C.; et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015, 47, 1121–1130. [Google Scholar]
- Wolf, D.; Ley, K. Immunity and inflammation in atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014, 35, 2929–2933. [Google Scholar] [CrossRef]
- Khot, U.N.; Khot, M.B.; Bajzer, C.T.; Sapp, S.K.; Ohman, E.M.; Brener, S.J.; Ellis, S.G.; Lincoff, A.M.; Topol, E.J. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003, 290, 898–904. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Czerniuk, M.R.; Górska, R.; Filipiak, K.J.; Opolski, G. Inflammatory response to acute coronary syndrome in patients with coexistent periodontal disease. J. Periodontol. 2004, 75, 1020–1026. [Google Scholar] [CrossRef]
- Zaremba, M.; Górska, R.; Suwalski, P.; Czerniuk, M.R.; Kowalski, J. Periodontitis as a risk factor of coronary heart diseases? Adv. Med. Sci. 2006, 51, 34–39. [Google Scholar]
- Czerniuk, M.R.; Górska, R.; Filipiak, K.J.; Opolski, G. C-reactive protein in patients with coexistent periodontal disease and acute coronary syndromes. J. Clin. Periodontol. 2006, 33, 415–420. [Google Scholar] [CrossRef]
- Paul, O.; Arora, P.; Mayer, M.; Chatterjee, S. Inflammation in periodontal disease: Possible link to vascular disease. Front. Physiol. 2021, 11, 609614. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global prevalence of periodontal disease and lack of its surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Sanz, M.; D’Aiuto, F.; Deanfield, J.; Fernandez, A.F. European workshop in periodontal health and cardiovascular disease—scientific evidence on the association between periodontal and cardiovascular diseases: A review of the literature. Eur. Heart J. Suppl. 2010, 12, 3–12. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Ioannidou, E. The sex and gender intersection in chronic periodontitis. Front. Public Health 2017, 5, 189. [Google Scholar] [CrossRef]
- Botelho, J.; Proença, L.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. Economic burden of periodontal disease in Europe and the United States of America—An updated forecast. medRxiv 2021. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Lopez, M.; Villa-Islas, V.; Rocha Arriaga, C.; Villaseñor-Altamirano, A.B.; Guzmán-Solís, A.; Sandoval-Velasco, M.; Wesp, J.K.; Alcantara, K.; López-Corral, A.; Gómez-Valdés, J.; et al. Paleogenomic insights into the red complex bacteria Tannerella forsythia in Pre-Hispanic and Colonial individuals from Mexico. Philos. Trans. R Soc. Lond. B Biol. Sci. 2020, 375, 20190580. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, G.R.; Etchegaray, A.; Marcelino, L.; Sicard, A.; Villalpando, K.; Pinheiro, S.L. Periodontal disease: General aspects from biofilm to the immune response driven by periodontal pathogen. Adv. Microbiol. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Surma, S.; Romańczyk, M.; Witalińska-Łabuzek, J.; Czerniuk, M.R.; Łabuzek, K.; Filipiak, K.J. Periodontitis, blood pressure, and the risk and control of arterial hypertension: Epidemiological, clinical, and pathophysiological aspects-review of the literature and clinical trials. Curr. Hypertens. Rep. 2021, 23, 27. [Google Scholar] [CrossRef]
- Priyamvara, A.; Dey, A.K.; Bandyopadhyay, D.; Katikineni, V.; Zaghlol, R.; Basyal, B.; Barssoum, K.; Amarin, R.; Bhatt, D.L.; Lavie, C.J. Periodontal inflammation and the risk of cardiovascular disease. Curr. Atheroscler. Rep. 2020, 22, 28. [Google Scholar] [CrossRef]
- Gheorghita, D.; Eördegh, G.; Nagy, F.; Antal, M. Periodontal disease, a risk factor for atherosclerotic cardiovascular disease. Orv. Hetil. 2019, 160, 419–425. [Google Scholar] [CrossRef]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- McGill, H.C., Jr.; McMahan, C.A.; Herderick, E.E.; Malcom, G.T.; Tracy, R.E.; Strong, J.P. Origin of atherosclerosis in childhood and adolescence. Am. J. Clin. Nutr. 2000, 72, 1307–1315. [Google Scholar]
- Zardawi, F.; Gul, S.; Abdulkareem, A.; Sha, A.; Yates, J. Association between periodontal disease and atherosclerotic cardiovascular diseases: Revisited. Front. Cardiovasc. Med. 2021, 7, 625579. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Munoz-Aguilera, E.; D’Aiuto, F.; Czesnikiewicz-Guzik, M.; Monaco, A.; Guzik, T.J.; Ferri, C. Periodontitis and hypertension: Is the association causal? High Blood Press Cardiovasc. Prev. 2020, 27, 281–289. [Google Scholar] [CrossRef]
- Kholy, K.E.; Genco, R.J.; Van Dyke, T.E. Oral infections and cardiovascular disease. Trends Endocrinol. Metab. 2015, 26, 315–321. [Google Scholar] [CrossRef]
- Herrera, D.; Molina, A.; Buhlin, K.; Klinge, B. Periodontal diseases and association with atherosclerotic disease. Periodontology 2000 2020, 83, 66–89. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal disease: A risk factor for diabetes and cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Links between atherosclerotic and periodontal disease. Exp. Mol. Pathol. 2016, 100, 220–235. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; Falasca, F.; Turriziani, O.; Sessa, R. Infectious agents in atherosclerotic cardiovascular diseases through oxidative stress. Int. J. Mol. Sci. 2017, 18, 2459. [Google Scholar] [CrossRef]
- Gianos, E.; Jackson, E.A.; Tejpal, A.; Aspry, K.; O’Keefe, J.; Aggarwal, M.; Jain, A.; Itchhaporia, D.; Williams, K.; Batts, T.; et al. Oral health and atherosclerotic cardiovascular disease: A review. Am. J. Prev. Cardiol. 2021, 7, 100179. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontology 2000 2020, 83, 90–106. [Google Scholar] [CrossRef]
- Febbraio, M.; Roy, C.B.; Levin, L. Is there a causal link between periodontitis and cardiovascular disease? A concise review of recent findings. Int. Dent. J. 2022, 72, 37–51. [Google Scholar] [CrossRef]
- Figuero, E.; Lindahl, C.; Marín, M.J.; Renvert, S.; Herrera, D.; Ohlsson, O.; Wetterling, T.; Sanz, M. Quantification of periodontal pathogens in vascular, blood, and subgingival samples from patients with peripheral arterial disease or abdominal aortic aneurysms. J. Periodontol. 2014, 85, 1182–1193. [Google Scholar] [CrossRef]
- Haraszthy, V.I.; Zambon, J.J.; Trevisan, M.; Zeid, M.; Genco, R.J. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 2000, 71, 1554–1560. [Google Scholar] [CrossRef]
- Rath, S.K.; Mukherjee, M.; Kaushik, R.; Sen, S.; Kumar, M. Periodontal pathogens in atheromatous plaque. Indian J. Pathol. Microbiol. 2014, 57, 259–264. [Google Scholar] [CrossRef]
- Szulc, M.; Kustrzycki, W.; Janczak, D.; Michalowska, D.; Baczynska, D.; Radwan-Oczko, M. Presence of periodontopathic bacteria DNA in atheromatous plaques from coronary and carotid arteries. Biomed. Res. Int. 2015, 2015, 825397. [Google Scholar] [CrossRef]
- Mahendra, J.; Mahendra, L.; Nagarajan, A.; Mathew, K. Prevalence of eight putative periodontal pathogens in atherosclerotic plaque of coronary artery disease patients and comparing them with noncardiac subjects: A case-control study. Indian J. Dent. Res. 2015, 26, 189–195. [Google Scholar] [CrossRef]
- Mahendra, J.; Mahendra, L.; Felix, J.; Romanos, G.E. Genetic analysis of Porphyromonas gingivalis (fimA), Aggregatibacter actinomycetemcomitans, and red complex in coronary plaque. J. Investig. Clin. Dent. 2014, 5, 201–207. [Google Scholar] [CrossRef]
- Rao, A.; D’Souza, C.; Subramanyam, K.; Rai, P.; Thomas, B.; Gopalakrishnan, M.; Karunasagar, I.; Kumar, B.K. Molecular analysis shows the presence of periodontal bacterial DNA in atherosclerotic plaques from patients with coronary artery disease. Indian Heart J. 2021, 73, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.; Bapat, R.; Anderson, W.; Dawson, D.; Hijazi, K.; Cherukara, G. Detection of periodontal microorganisms in coronary atheromatous plaque specimens of myocardial infarction patients: A systematic review and meta-analysis. Trends Cardiovasc. Med. 2021, 31, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Kędzia, A.; Ciecierski, M.; Kufel, A.; Wierzbowska, M.; Kwapisz, E. Isolation of anaerobic bacteria from atherosclerotic plaques from carotid arteries. Acta Angiol. 2012, 18, 59–67. [Google Scholar]
- Kannosh, I.; Staletovic, D.; Toljic, B.; Radunovic, M.; Pucar, A.; Matic Petrovic, S.; Grubisa, I.; Lazarevic, M.; Brkic, Z.; Vukcevic, J.K.; et al. The presence of periopathogenic bacteria in subgingival and atherosclerotic plaques—An age related comparative analysis. J. Infect. Dev. Ctries. 2018, 12, 1088–1095. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Kim, H.J.; Cha, G.S.; Kim, H.J.; Kwon, E.Y.; Lee, J.Y.; Choi, J.; Joo, J.Y. Porphyromonas gingivalis accelerates atherosclerosis through oxidation of high-density lipoprotein. J. Periodontal. Implant. Sci. 2018, 48, 60–68. [Google Scholar] [CrossRef]
- Joo, J.Y.; Cha, G.S.; Chung, J.; Lee, J.Y.; Kim, S.J.; Choi, J. Peptide 19 of Porphyromonas gingivalis heat shock protein is a potent inducer of low-density lipoprotein oxidation. J. Periodontol. 2017, 88, 58–64. [Google Scholar] [CrossRef]
- Lönn, J.; Ljunggren, S.; Klarström-Engström, K.; Demirel, I.; Bengtsson, T.; Karlsson, H. Lipoprotein modifications by gingipains of Porphyromonas gingivalis. J. Periodontal. Res. 2018, 53, 403–413. [Google Scholar] [CrossRef]
- Ljunggren, S.; Bengtsson, T.; Karlsson, H.; Starkhammar Johansson, C.; Palm, E.; Nayeri, F.; Ghafouri, B.; Davies, J.; Svensäter, G.; Lönn, J. Modified lipoproteins in periodontitis: A link to cardiovascular disease? Biosci. Rep. 2019, 39, BSR20181665. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Liu, W.; Chu, Y.; Zhong, J.; Xie, Y.; Lou, X.; Ouyang, X. LOX-1 regulates P. gingivalis-induced monocyte migration and adhesion to human umbilical vein endothelial cells. Front. Cell Dev. Biol. 2020, 8, 596. [Google Scholar] [CrossRef]
- Reyes, L.; Getachew, H.; Dunn, W.A.; Progulske-Fox, A. Porphyromonas gingivalis W83 traffics via ICAM1 in microvascular endothelial cells and alters capillary organization in vivo. J. Oral Microbiol. 2020, 12, 1742528. [Google Scholar] [CrossRef]
- Dorn, B.R.; Dunn, W.A., Jr.; Progulske-Fox, A. Invasion of human coronary artery cells by periodontal pathogens. Infect. Immun. 1999, 67, 5792–5798. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Wang, M.; Harokopakis, E.; Triantafilou, M.; Triantafilou, K. Porphyromonas gingivalis fimbriae proactively modulate beta2 integrin adhesive activity and promote binding to and internalization by macrophages. Infect. Immun. 2006, 74, 5658–5666. [Google Scholar] [CrossRef]
- Saito, A.; Inagaki, S.; Kimizuka, R.; Okuda, K.; Hosaka, Y.; Nakagawa, T.; Ishihara, K. Fusobacterium nucleatum enhances invasion of human gingival epithelial and aortic endothelial cells by Porphyromonas gingivalis. FEMS Immunol. Med. Microbiol. 2008, 54, 349–355. [Google Scholar] [CrossRef]
- Inagaki, S.; Onishi, S.; Kuramitsu, H.K.; Sharma, A. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by “Tannerella forsythia”. Infect. Immun. 2006, 74, 5023–5028. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Dong, Q.; Luo, Y.; Liu, Y.; Gao, L.; Pan, Y.; Zhang, D. Porphyromonas gingivalis infection promotes mitochondrial dysfunction through Drp1-dependent mitochondrial fission in endothelial cells. Int. J. Oral Sci. 2021, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.J.; Ford, P.J.; Cullinan, M.P.; Leishman, S.; West, M.J.; Yamazaki, K. Infection or inflammation: The link between periodontal and cardiovascular diseases. Future Cardiol. 2009, 5, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Stafford, G.P.; Murdoch, C. Porphyromonas gingivalis outer membrane vesicles increase vascular permeability. J. Dent. Res. 2020, 99, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Stafford, G.P.; Potempa, J.; Wilkinson, R.N.; Chen, Y.; Murdoch, C.; Widziolek, M. Mechanisms of vascular damage by systemic dissemination of the oral pathogen Porphyromonas gingivalis. FEBS J. 2021, 288, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Song, L.T.; Tada, H.; Nishioka, T.; Nemoto, E.; Imamura, T.; Potempa, J.; Li, C.Y.; Matsushita, K.; Sugawara, S. Porphyromonas gingivalis gingipains-mediated degradation of plasminogen activator inhibitor-1 leads to delayed wound healing responses in human endothelial cells. J. Innate Immun. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Tang, Q.; Yu, S.; Sun, J.; Mei, F.; Zhao, J.; Chen, L. Porphyromonas gingivalis disrupts vascular endothelial homeostasis in a TLR-NF-kappaB axis dependent manner. Int. J. Oral Sci. 2020, 12, 28. [Google Scholar] [CrossRef]
- Kebschull, M.; Haupt, M.; Jepsen, S.; Deschner, J.; Nickenig, G.; Werner, N. Mobilization of endothelial progenitors by recurrent bacteremias with a periodontal pathogen. PLoS ONE 2013, 8, e54860. [Google Scholar] [CrossRef]
- Isola, G.; Giudice, A.L.; Polizzi, A.; Alibrandi, A.; Patini, R.; Ferlito, S. Periodontitis and tooth loss have Negative systemic impact on circulating progenitor cell levels: A clinical study. Genes 2019, 10, 1022. [Google Scholar] [CrossRef]
- Fujitani, T.; Aoyama, N.; Hirata, F.; Minabe, M. Association between periodontitis and vascular endothelial function using noninvasive medical device-a pilot study. Clin. Exp. Dent. Res. 2020, 6, 576–582. [Google Scholar] [CrossRef]
- Xie, M.; Tang, Q.; Nie, J.; Zhang, C.; Zhou, X.; Yu, S.; Sun, J.; Cheng, X.; Dong, N.; Hu, Y.; et al. BMAL1-downregulation aggravates Porphyromonas gingivalis-induced atherosclerosis by encouraging oxidative stress. Circ. Res. 2020, 126, 15–29. [Google Scholar] [CrossRef]
- Charoensaensuk, V.; Chen, Y.C.; Lin, Y.H.; Ou, K.L.; Yang, L.Y.; Lu, D.Y. Porphyromonas gingivalis induces proinflammatory cytokine expression leading to apoptotic death through the oxidative stress/NF-κB pathway in brain endothelial cells. Cells 2021, 10, 3033. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, W.; Hou, J.; Liu, Y.; Li, R.; Liu, J.; Li, C.; Tang, X.; Lin, L.; Pan, Y.; et al. Porphyromonas gingivalis-induced MIF regulates intercellular adhesion molecule-1 expression in EA.hy926 cells and monocyte-endothelial cell adhesion through the receptors CD74 and CXCR4. Inflammation 2019, 42, 874–883. [Google Scholar] [CrossRef]
- Bugueno, I.M.; Zobairi El-Ghazouani, F.; Batool, F.; El Itawi, H.; Anglès-Cano, E.; Benkirane-Jessel, N.; Toti, F.; Huck, O. Porphyromonas gingivalis triggers the shedding of inflammatory endothelial microvesicles that act as autocrine effectors of endothelial dysfunction. Sci. Rep. 2020, 10, 1778. [Google Scholar] [CrossRef]
- Cao, C.; Ji, X.; Luo, X.; Zhong, L. Gingipains from Porphyromonas gingivalis promote the transformation and proliferation of vascular smooth muscle cell phenotypes. Int. J. Clin. Exp. Med. 2015, 8, 18327–18334. [Google Scholar]
- Zhang, B.; Elmabsout, A.A.; Khalaf, H.; Basic, V.T.; Jayaprakash, K.; Kruse, R.; Bengtsson, T.; Sirsjö, A. The periodontal pathogen Porphyromonas gingivalis changes the gene expression in vascular smooth muscle cells involving the TGFbeta/Notch signalling pathway and increased cell proliferation. BMC Genom. 2013, 14, 770. [Google Scholar] [CrossRef]
- Kobayashi, N.; Suzuki, J.; Ogawa, M.; Aoyama, N.; Hanatani, T.; Hirata, Y.; Nagai, R.; Izumi, Y.; Isobe, M. Porphyromonas gingivalis accelerates neointimal formation after arterial injury. J. Vasc. Res. 2012, 49, 417–424. [Google Scholar] [CrossRef]
- Li, J.; Deng, J.; Shang, S.; Liu, G.; Song, W.; Sun, P.; Jiang, W.; Pan, K. Effect of Porphyromonas gingivalis lipopolysaccharide on calcification of human umbilical artery smooth muscle cells co-cultured with human periodontal ligament cells. Exp. Med. 2021, 21, 655. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, Y.; Kim, M.K.; Park, H.R.; Kim, H.J.; Bae, S.K.; Bae, M.K. Infection of Porphyromonas gingivalis increases phosphate-induced calcification of vascular smooth muscle cells. Cells 2020, 9, 2694. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Xu, J.; Liu, F.; Hu, R.; Deng, H. Effects of Porphyromonas gingivalis lipopolysaccharide on the expression of key genes involved in cholesterol metabolism in macrophages. Arch. Med. Sci. 2016, 12, 959–967. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, C.; Xiang, X.R.; Chen, F.C.; Yang, C.M.; Wu, J. Porphyromonas gingivalis lipopolysaccharide increases lipid accumulation by affecting CD36 and ATP-binding cassette transporter A1 in macrophages. Oncol. Rep. 2013, 30, 1329–1336. [Google Scholar] [CrossRef]
- Xu, W.; Pan, Y.; Xu, Q.; Wu, Y.; Pan, J.; Hou, J.; Lin, L.; Tang, X.; Li, C.; Liu, J.; et al. Porphyromonas gingivalis ATCC 33277 promotes intercellular adhesion molecule-1 expression in endothelial cells and monocyte-endothelial cell adhesion through macrophage migration inhibitory factor. BMC Microbiol. 2018, 18, 16. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Liu, S.; Gu, L.; Zhou, X. MircroRNA-19a promotes vascular inflammation and foam cell formation by targeting HBP-1 in atherogenesis. Sci. Rep. 2017, 7, 12089. [Google Scholar] [CrossRef]
- Mubarokah, S.N.; Susilawati, I.D.A.; Sumarno, S.; Muliartha, I.K.G.; Sargowo, D. Porphyromonas gingivalis induced fragmentation of type IV collagen through macrophage-activated MMP-9: (in vitro study of collagenolytic mechanism in pathogenesis of atherosclerotic plaque rupture. Indones. Biomed. J. 2009, 1, 88–96. [Google Scholar] [CrossRef][Green Version]
- Yu, K.M.; Inoue, Y.; Umeda, M.; Terasaki, H.; Chen, Z.Y.; Iwai, T. The periodontal anaerobe Porphyromonas gingivalis induced platelet activation and increased aggregation in whole blood by rat model. Thromb. Res. 2011, 127, 418–425. [Google Scholar] [CrossRef]
- Zhan, Y.; Lu, R.; Meng, H.; Wang, X.; Hou, J. Platelet activation and platelet-leukocyte interaction in generalized aggressive periodontitis. J. Leukoc. Biol. 2016, 100, 1155–1166. [Google Scholar] [CrossRef]
- Klarström Engström, K.; Khalaf, H.; Kälvegren, H.; Bengtsson, T. The role of Porphyromonas gingivalis gingipains in platelet activation and innate immune modulation. Mol. Oral Microbiol. 2015, 30, 62–73. [Google Scholar] [CrossRef]
- Zhang, T.; Kurita-Ochiai, T.; Hashizume, T.; Du, Y.; Oguchi, S.; Yamamoto, M. Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol. Med. Microbiol. 2010, 59, 143–151. [Google Scholar] [CrossRef]
- Lee, H.R.; Jun, H.K.; Choi, B.K. Tannerella forsythia BspA increases the risk factors for atherosclerosis in ApoE(−/−) mice. Oral Dis. 2014, 20, 803–808. [Google Scholar]
- Kesavalu, L.; Lucas, A.R.; Verma, R.K.; Liu, L.; Dai, E.; Sampson, E.; Progulske-Fox, A. Increased atherogenesis during Streptococcus mutans infection in ApoE-null mice. J. Dent. Res. 2012, 91, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, G.; Häsler, R.; El Mokhtari, N.E.; König, I.R.; Loos, B.G.; Jepsen, S.; Rosenstiel, P.; Schreiber, S.; Schaefer, A.S. The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum. Mol. Genet. 2013, 22, 4516–4527. [Google Scholar] [CrossRef] [PubMed]
- Munz, M.; Richter, G.M.; Loos, B.G.; Jepsen, S.; Divaris, K.; Offenbacher, S.; Teumer, A.; Holtfreter, B.; Kocher, T.; Bruckmann, C.; et al. Genome-wide association meta-analysis of coronary artery disease and periodontitis reveals a novel shared risk locus. Sci. Rep. 2018, 8, 13678. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kopra, E.; Lahdentausta, L.; Liljestrand, J.; Paju, S. Association between oral infections and cardiovascular diseases. Nor. Tannlegeforen Tid. 2020, 130, 122–127. [Google Scholar]
- Larvin, H.; Kang, J.; Aggarwal, V.R.; Pavitt, S.; Wu, J. Risk of incident cardiovascular disease in people with periodontal disease: A systematic review and meta-analysis. Clin. Exp. Dent. Res. 2021, 7, 109–122. [Google Scholar] [CrossRef]
- Gao, S.; Tian, J.; Li, Y.; Liu, T.; Li, R.; Yang, L.; Xing, Z. Periodontitis and number of teeth in the risk of coronary heart disease: An updated meta-analysis. Med. Sci. Monit. 2021, 27, e930112. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, Y.; Guo, Y. Periodontal disease and myocardial infarction risk: A meta-analysis of cohort studies. Am. J. Emerg. Med. 2021, 48, 103–109. [Google Scholar] [CrossRef]
- Joshi, C.; Bapat, R.; Anderson, W.; Dawson, D.; Cherukara, G.; Hijazi, K. Serum antibody response against periodontal bacteria and coronary heart disease: Systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 1570–1586. [Google Scholar] [CrossRef]
- Arsiwala, L.T.; Mok, Y.; Yang, C.; Ishigami, J.; Selvin, E.; Beck, J.D.; Allison, M.A.; Heiss, G.; Demmer, R.T.; Matsushita, K. Periodontal disease measures and risk of incident peripheral artery disease: The Atherosclerosis Risk in Communities (ARIC) study. J. Periodontol. 2021. [Google Scholar] [CrossRef]
- Wang, J.; Geng, X.; Sun, J.; Zhang, S.; Yu, W.; Zhang, X.; Liu, H. The risk of periodontitis for peripheral vascular disease: A systematic review. Rev. Cardiovasc. Med. 2019, 20, 81–89. [Google Scholar]
- Zeng, X.T.; Leng, W.D.; Lam, Y.Y.; Yan, B.P.; Wei, X.M.; Weng, H.; Kwong, J.S. Periodontal disease and carotid atherosclerosis: A meta-analysis of 17,330 participants. Int. J. Cardiol. 2016, 203, 1044–1051. [Google Scholar] [CrossRef]
- González-Navarro, B.; Segura-Egea, J.J.; Estrugo-Devesa, A.; Pintó-Sala, X.; Jane-Salas, E.; Jiménez-Sánchez, M.C.; Cabanillas-Balsera, D.; López-López, J. Relationship between apical periodontitis and metabolic syndrome and cardiovascular events: A cross-sectional study. J. Clin. Med. 2020, 9, 3205. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Z.; Wang, Y.; Gao, H.; Wang, Y.; Zhang, Q. Association between periodontitis and carotid artery calcification: A systematic review and meta-analysis. Biomed. Res. Int. 2021, 2021, 3278351. [Google Scholar] [CrossRef]
- Rodean, I.P.; Lazăr, L.; Halațiu, V.B.; Biriș, C.; Benedek, I.; Benedek, T. Periodontal disease is associated with increased vulnerability of coronary atheromatous plaques in patients undergoing coronary computed tomography angiography-results from the Atherodent Study. J. Clin. Med. 2021, 10, 1290. [Google Scholar] [CrossRef]
- Cowan, L.T.; Lakshminarayan, K.; Lutsey, P.L.; Folsom, A.R.; Beck, J.; Offenbacher, S.; Pankow, J.S. Periodontal disease and incident venous thromboembolism: The Atherosclerosis Risk in Communities study. J. Clin. Periodontol. 2019, 46, 12–19. [Google Scholar] [CrossRef]
- Sánchez-Siles, M.; Rosa-Salazar, V.; Salazar-Sánchez, N.; Camacho-Alonso, F. Periodontal disease as a risk factor of recurrence of venous thromboembolic disease: A prospective study. Acta Odontol. Scand. 2015, 73, 8–13. [Google Scholar] [CrossRef]
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Oral health and all-cause, cardiovascular disease, and respiratory mortality in older people in the UK and USA. Sci. Rep. 2021, 11, 16452. [Google Scholar] [CrossRef]
- Bengtsson, V.W.; Persson, G.R.; Berglund, J.S.; Renvert, S. Periodontitis related to cardiovascular events and mortality: A long-time longitudinal study. Clin. Oral Investig. 2021, 25, 4085–4095. [Google Scholar] [CrossRef]
- Qi, J.; Zihang, Z.; Zhang, J.; Park, Y.M.; Shrestha, D.; Jianling, B.; Merchant, A.T. Periodontal antibodies and all-cause and cardiovascular disease mortality. J. Dent. Res. 2020, 99, 51–59. [Google Scholar] [CrossRef]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, edentulism, and risk of mortality: A systematic review with meta-analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef]
- Yu, Y.H.; Cheung, W.S.; Steffensen, B.; Miller, D.R. Number of teeth is associated with all-cause and disease-specific mortality. BMC Oral Health 2021, 21, 568. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhang, M.; Wang, Q.; Xu, H.; Dong, X.; Gao, Z.; Chen, J.; Wei, Y.; Qin, F. Tooth loss and risk of cardiovascular disease and stroke: A dose-response meta-analysis of prospective cohort studies. PLoS ONE 2018, 13, e0194563. [Google Scholar]
- Zhou, M.; Dong, J.; Zha, L.; Liao, Y. Causal association between periodontal diseases and cardiovascular diseases. Genes 2022, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, S.E.; Forrest, J.L. An umbrella review of systematic reviews of the evidence of a causal relationship between periodontal disease and cardiovascular diseases: Position paper from the Canadian Dental Hygienists Association. Can. J. Dent. Hyg. 2020, 54, 32–41. [Google Scholar]
- Sweeting, L.A.; Davis, K.; Cobb, C.M. Periodontal Treatment Protocol (PTP) for the general dental practice. J. Dent. Hyg. 2008, 82, 16–26. [Google Scholar]
- Roca-Millan, E.; González-Navarro, B.; Sabater-Recolons, M.M.; Marí-Roig, A.; Jané-Salas, E.; López-López, J. Periodontal treatment on patients with cardiovascular disease: Systematic review and meta-analysis. Med. Oral. Patol. Oral Cir. Bucal. 2018, 23, 681–690. [Google Scholar] [CrossRef]
- Teeuw, W.J.; Slot, D.E.; Susanto, H.; Gerdes, V.E.; Abbas, F.; D’Aiuto, F.; Kastelein, J.J.; Loos, B.G. Treatment of periodontitis improves the atherosclerotic profile: A systematic review and meta-analysis. J. Clin. Periodontol. 2014, 41, 70–79. [Google Scholar] [CrossRef]
- Laky, M.; Anscheringer, I.; Wolschner, L.; Heber, S.; Haririan, H.; Schrottmaier, W.C.; Kral-Pointner, J.B.; Salzmann, M.; Volf, I.; Moritz, A.; et al. Periodontal treatment limits platelet activation in patients with periodontitis-a controlled-randomized intervention trial. J. Clin. Periodontol. 2018, 45, 1090–1097. [Google Scholar] [CrossRef]
- Arvanitidis, E.; Bizzarro, S.; Alvarez Rodriguez, E.; Loos, B.G.; Nicu, E.A. Reduced platelet hyper-reactivity and platelet-leukocyte aggregation after periodontal therapy. Thromb. J. 2017, 15, 5. [Google Scholar] [CrossRef]
- Toregeani, J.F.; Nassar, C.A.; Nassar, P.O.; Toregeani, K.M.; Gonzatto, G.K.; Vendrame, R.; Castilhos, J.S.; Rotta, L.S.; Reinheimer, A.C.; Longoni, A.; et al. Evaluation of periodontitis treatment effects on carotid intima-media thickness and expression of laboratory markers related to atherosclerosis. Gen. Dent. 2016, 64, 55–62. [Google Scholar]
- Czesnikiewicz-Guzik, M.; Osmenda, G.; Siedlinski, M.; Nosalski, R.; Pelka, P.; Nowakowski, D.; Wilk, G.; Mikolajczyk, T.P.; Schramm-Luc, A.; Furtak, A.; et al. Causal association between periodontitis and hypertension: Evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur. Heart J. 2019, 40, 3459–3470. [Google Scholar] [CrossRef]
- Zhou, Q.B.; Xia, W.H.; Ren, J.; Yu, B.B.; Tong, X.Z.; Chen, Y.B.; Chen, S.; Feng, L.; Dai, J.; Tao, J.; et al. Effect of intensive periodontal therapy on blood pressure and endothelial microparticles in patients with prehypertension and periodontitis: A randomized controlled trial. J. Periodontol. 2017, 88, 711–722. [Google Scholar] [CrossRef]
- Fu, Y.W.; Li, X.X.; Xu, H.Z.; Gong, Y.Q.; Yang, Y. Effects of periodontal therapy on serum lipid profile and proinflammatory cytokines in patients with hyperlipidemia: A randomized controlled trial. Clin. Oral Investig. 2016, 20, 1263–1269. [Google Scholar] [CrossRef]
- Mauri-Obradors, E.; Merlos, A.; Estrugo-Devesa, A.; Jané-Salas, E.; López-López, J.; Viñas, M. Benefits of non-surgical periodontal treatment in patients with type 2 diabetes mellitus and chronic periodontitis: A randomized controlled trial. J. Clin. Periodontol. 2018, 45, 345–353. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Gkranias, N.; Bhowruth, D.; Khan, T.; Orlandi, M.; Suvan, J.; Masi, S.; Tsakos, G.; Hurel, S.; Hingorani, A.D.; et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: A 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 954–965. [Google Scholar] [CrossRef]
- Peng, C.H.; Yang, Y.S.; Chan, K.C.; Kornelius, E.; Chiou, J.Y.; Huang, C.N. Periodontal treatment and the risks of cardiovascular disease in patients with type 2 diabetes: A retrospective cohort study. Intern. Med. 2017, 56, 1015–1021. [Google Scholar] [CrossRef]
- Montero, E.; López, M.; Vidal, H.; Martínez, M.; Virto, L.; Marrero, J.; Herrera, D.; Zapatero, A.; Sanz, M. Impact of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: A randomized clinical trial. Diabetes Obes. Metab. 2020, 22, 2120–2132. [Google Scholar] [CrossRef]
- López, N.J.; Quintero, A.; Casanova, P.A.; Ibieta, C.I.; Baelum, V.; López, R. Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: A controlled clinical trial. J. Periodontol. 2012, 83, 267–278. [Google Scholar] [CrossRef]
- Santos-Paul, M.A.; Neves, R.S.; Gowdak, L.H.W.; de Paula, F.J.; David-Neto, E.; Bortolotto, L.A.; Ramires, J.A.F.; De Lima, J.J.G. Cardiovascular risk reduction with periodontal treatment in patients on the waiting list for renal transplantation. Clin. Transplant. 2019, 33, e13658. [Google Scholar] [CrossRef]
- Huang, S.T.; Yu, T.M.; Ke, T.Y.; Wu, M.J.; Chuang, Y.W.; Li, C.Y.; Chiu, C.W.; Lin, C.L.; Liang, W.M.; Chou, T.C.; et al. Intensive periodontal treatment reduces risks of hospitalization for cardiovascular disease and all-cause mortality in the hemodialysis population. J. Clin. Med. 2018, 7, 344. [Google Scholar] [CrossRef]
- Lin, H.W.; Chen, C.M.; Yeh, Y.C.; Chen, Y.Y.; Guo, R.Y.; Lin, Y.P.; Li, Y.C. Dental treatment procedures for periodontal disease and the subsequent risk of ischaemic stroke: A retrospective population-based cohort study. J. Clin. Periodontol. 2019, 46, 642–649. [Google Scholar] [CrossRef]
- Aarabi, G.; Raedel, M.; Kreutzburg, T.; Hischke, S.; Debus, E.S.; Marschall, U.; Seedorf, U.; Behrendt, C.A. Periodontal treatment and peripheral arterial disease severity—A retrospective analysis of health insurance claims data. Vasa 2020, 49, 128–132. [Google Scholar] [CrossRef]
- Montenegro, M.M.; Ribeiro, I.W.J.; Kampits, C.; Saffi, M.A.L.; Furtado, M.V.; Polanczyk, C.A.; Haas, A.N.; Rösing, C.K. Randomized controlled trial of the effect of periodontal treatment on cardiovascular risk biomarkers in patients with stable coronary artery disease: Preliminary findings of 3 months. J. Clin. Periodontol. 2019, 46, 321–331. [Google Scholar] [CrossRef]
- Saffi, M.A.L.; Rabelo-Silva, E.R.; Polanczyk, C.A.; Furtado, M.V.; Montenegro, M.M.; Ribeiro, I.W.J.; Kampits, C.; Rösing, C.K.; Haas, A.N. Periodontal therapy and endothelial function in coronary artery disease: A randomized controlled trial. Oral Dis. 2018, 24, 1349–1357. [Google Scholar] [CrossRef]
- Javed, F.; Kellesarian, S.V.; Al-Kheraif, A.A.; Ranna, V.; Qadri, T.; Yunker, M.; Malmstrom, H.; Romanos, G.E. Effect of Nd:YAG laser-assisted non-surgical periodontal therapy on clinical periodontal and serum biomarkers in patients with and without coronary artery disease: A short-term pilot study. Lasers Surg. Med. 2016, 48, 929–935. [Google Scholar] [CrossRef]
- Bokhari, S.A.; Khan, A.A.; Butt, A.K.; Azhar, M.; Hanif, M.; Izhar, M.; Tatakis, D.N. Non-surgical periodontal therapy reduces coronary heart disease risk markers: A randomized controlled trial. J. Clin. Periodontol. 2012, 39, 1065–1074. [Google Scholar] [CrossRef]
- Kao, Y.W.; Shia, B.C.; Chiang, H.C.; Chen, M.; Wu, S.Y. Association of tooth scaling with acute myocardial infarction and analysis of the corresponding medical expenditure: A nationwide population-based study. Int. J. Environ. Res. Public Health 2021, 18, 7613. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, N.; Gugnani, S. Can treatment of severe periodontitis in patients with ST-segment elevation myocardial infarction improve endothelial function? Evid. Based Dent. 2021, 22, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.G.; Schmidt, M.M.; Lopes, R.D.; Dipp, T.; Feijó, I.P.; Schmidt, K.E.S.; Gazeta, C.A.; Azeredo, M.L.; Markoski, M.; Pellanda, L.C.; et al. Treating periodontal disease in patients with myocardial infarction: A randomized clinical trial. Eur. J. Intern. Med. 2020, 71, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, Q.; Wu, Q.; Yao, M.; Chen, Y.; Zhou, H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: A systematic review and Bayesian network meta-analysis. BMC Oral Health 2019, 19, 176. [Google Scholar] [CrossRef]
- Esteves Lima, R.P.; Atanazio, A.R.S.; Costa, F.O.; Cunha, F.A.; Abreu, L.G. Impact of non-surgical periodontal treatment on serum TNF-alpha levels in individuals with type 2 diabetes: A systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2021, 21, 101546. [Google Scholar] [CrossRef]
- Garde, S.; Akhter, R.; Nguyen, M.A.; Chow, C.K.; Eberhard, J. Periodontal therapy for improving lipid profiles in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 3826. [Google Scholar] [CrossRef]
- Sharma, S.; Sridhar, S.; McIntosh, A.; Messow, C.M.; Aguilera, E.M.; Del Pinto, R.; Pietropaoli, D.; Gorska, R.; Siedlinski, M.; Maffia, P.; et al. Periodontal therapy and treatment of hypertension-alternative to the pharmacological approach. A systematic review and meta-analysis. Pharmacol. Res. 2021, 166, 105511. [Google Scholar] [CrossRef]
- Da Silva, T.A.; Abreu, L.G.; Esteves Lima, R.P. A meta-analysis on the effect of periodontal treatment on the glomerular filtration rate of chronic kidney disease individuals: A systematic review and meta-analysis was conducted to assess the impact of the periodontal treatment on the glomerular filtration rate of individuals with chronic kidney disease. Spec. Care Dent. 2021, 41, 670–678. [Google Scholar]
- Okada, M.; Kobayashi, T.; Ito, S.; Yokoyama, T.; Abe, A.; Murasawa, A.; Yoshie, H. Periodontal treatment decreases levels of antibodies to Porphyromonas gingivalis and citrulline in patients with rheumatoid arthritis and periodontitis. J. Periodontol. 2013, 84, 74–84. [Google Scholar] [CrossRef]
- Fabbri, C.; Fuller, R.; Bonfá, E.; Guedes, L.K.; D’Alleva, P.S.; Borba, E.F. Periodontitis treatment improves systemic lupus erythematosus response to immunosuppressive therapy. Clin. Rheumatol. 2014, 33, 505–509. [Google Scholar] [CrossRef]
- Holmlund, A.; Lampa, E.; Lind, L. Poor response to periodontal treatment may predict future cardiovascular disease. J. Dent. Res. 2017, 96, 768–773. [Google Scholar] [CrossRef]
- Surma, S.; Banach, M.; Lewek, J. COVID-19 and lipids. The role of lipid disorders and statin use in the prognosis of patients with SARS-CoV-2 infection. Lipids Health Dis. 2021, 20, 141. [Google Scholar] [CrossRef]
- Petit, C.; Batool, F.; Bugueno, I.M.; Schwinté, P.; Benkirane-Jessel, N.; Huck, O. Contribution of statins towards periodontal treatment: A review. Mediat. Inflamm. 2019, 2019, 6367402. [Google Scholar] [CrossRef]
- Tahamtan, S.; Shirban, F.; Bagherniya, M.; Johnston, T.P.; Sahebkar, A. The effects of statins on dental and oral health: A review of preclinical and clinical studies. J. Transl. Med. 2020, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Muniz, F.W.M.G.; Taminski, K.; Cavagni, J.; Celeste, R.K.; Weidlich, P.; Rösing, C.K. The effect of statins on periodontal treatment-a systematic review with meta-analyses and meta-regression. Clin. Oral Investig. 2018, 22, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Szwed, P.; Gąsecka, A.; Zawadka, M.; Eyileten, C.; Postuła, M.; Mazurek, T.; Szarpak, Ł.; Filipiak, K.J. Infections as novel risk factors of atherosclerotic cardiovascular diseases: Pathophysiological links and therapeutic implications. J. Clin. Med. 2021, 10, 2539. [Google Scholar] [CrossRef]
- Altun, E.; Walther, C.; Borof, K.; Petersen, E.; Lieske, B.; Kasapoudis, D.; Jalilvand, N.; Beikler, T.; Jagemann, B.; Zyriax, B.C.; et al. Association between dietary pattern and periodontitis-a cross-sectional study. Nutrients 2021, 13, 4167. [Google Scholar] [CrossRef]
- Vivek, B.; Ramesh, K.S.V.; Gautami, P.S.; Sruthima, G.N.V.S.; Dwarakanath, C.; Anudeep, M. Effect of periodontal treatment on oral health-related quality of life—a randomised controlled trial. J. Taibah Univ. Med. Sci. 2021, 16, 856–863. [Google Scholar] [CrossRef]
- Anand, P.S.; Jadhav, P.; Kamath, K.P.; Kumar, S.R.; Vijayalaxmi, S.; Anil, S. A case-control study on the association between periodontitis and coronavirus disease (COVID-19). J. Periodontol. 2021. [Google Scholar] [CrossRef]
- Joshipura, K.; Muñoz-Torres, F.; Fernández-Santiago, J.; Patel, R.; Lopez-Candales, A. Over-the-counter mouthwash use, nitric oxide and hypertension risk. Blood Press. 2020, 29, 103–112. [Google Scholar] [CrossRef]
- Joshipura, K.; Muñoz-Torres, F.; Morou-Bermudez, E.; Patel, R. Over-the-counter mouthwash use and risk of pre-diabetes/diabetes. Nitric. Oxide. 2017, 71, 14–20. [Google Scholar] [CrossRef]
- Sobierajski, T.; Surma, S.; Romańczyk, M.; Łabuzek, K.; Filipiak, K.J.; Oparil, S. What is or what is not a risk factor for arterial hypertension? Not Hamlet, but medical students answer that question. Curr. Hypertens. Rep. 2022, in press. Available online: https://www.editorialmanager.com/hypr/default1.aspx (accessed on 15 January 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).