Gastrointestinal Incretins—Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) beyond Pleiotropic Physiological Effects Are Involved in Pathophysiology of Atherosclerosis and Coronary Artery Disease—State of the Art
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Atherosclrosis and Coronary Artery Disease
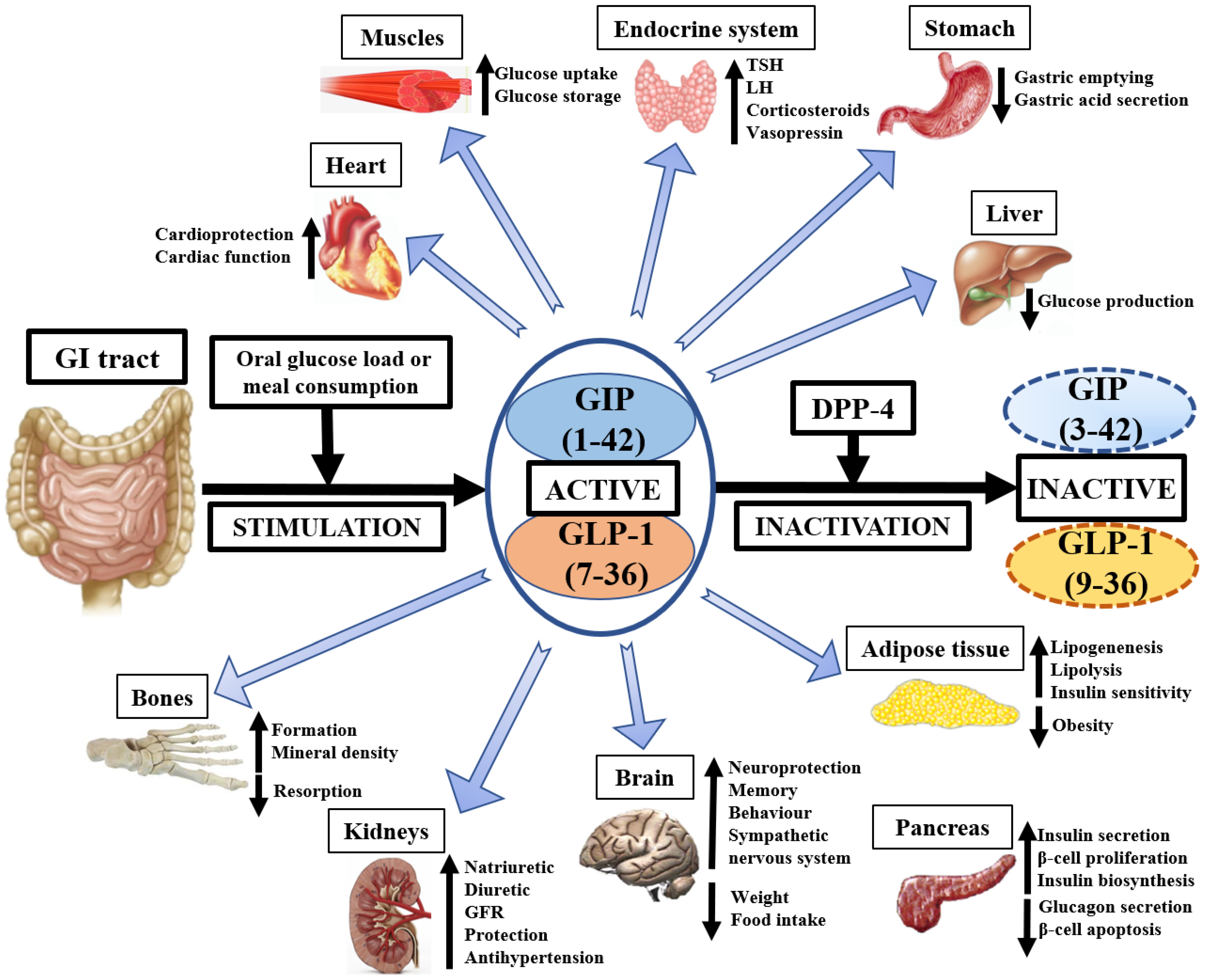
1.2. Physiology of GIP and GLP-1
1.3. GIP and GLP-1—Importance in Medicine
1.3.1. Liver
1.3.2. Kidneys
1.3.3. Nervous System
1.3.4. Adipose Tissue
1.3.5. Bones and Muscles
1.3.6. Endocrine System
1.3.7. Inflammation
1.4. Dipeptidyl Peptidase-4 (DPP-4) Role
2. Current Knowledge in Atherosclerosis and Coronary Artery Disease Insights from Animal Studies
2.1. Atherosclerosis
2.2. Myocardial Ischemia
3. Current Knowledge in Atherosclerosis and Coronary Artery Disease—Insights from Human Studies
3.1. Atherosclerosis
3.2. Coronary Artery Disease
3.3. Some Studies Have Additionally Focused on the Association between GLP-1 and Prognosis of Non-Diabetic Patients with ACS
4. The Dual GIP/GLP-1 Agonism
5. Practical Implications and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuster, V.; Stein, B.; Ambrose, J.A.; Badimon, L.; Badimon, J.J.; Chesebro, J.H. Atherosclerotic plaque rupture and thrombosis. Evolving concepts. Circulation 1990, 82 (Suppl. 3), II47–II59. [Google Scholar]
- Manduteanu, I.; Simionescu, M. Inflammation in atherosclerosis: A cause or a result of vascular disorders? J. Cell. Mol. Med. 2012, 16, 1978–1990. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2019, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Canto, J.G.; Shlipak, M.G.; Rogers, W.J.; Malmgren, J.A.; Frederick, P.; Lambrew, C.T.; Ornato, J.P.; Barron, H.V.; Kiefe, C.I. Prevalence, Clinical Characteristics, and Mortality Among Patients with Acute Myocardial Infarction Presenting without Chest Pain. J. Am. Med. Assoc. 2000, 283, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014, 35, 2950–2959. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Reimann, F.; Gribble, F.M. Glucose-Sensing in Glucagon-Like Peptide-1-Secreting Cells. Diabetes 2002, 51, 2757–2763. [Google Scholar] [CrossRef]
- Gribble, F.M.; Williams, L.; Simpson, A.K.; Reimann, F. A Novel Glucose-Sensing Mechanism Contributing to Glucagon-Like Peptide-1 Secretion From the GLUTag Cell Line. Diabetes 2003, 52, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Dhanvantari, S.; Seidah, N.G.; Brubaker, P.L. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol. Endocrinol. 1996, 10, 342–355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ørskov, C.; Wettergren, A.; Holst, J.J. Biological Effects and Metabolic Rates of Glucagonlike Peptide-1 7-36 Amide and Glucagonlike Peptide-1 7-37 in Healthy Subjects Are Indistinguishable. Diabetes 1993, 42, 658–661. [Google Scholar] [CrossRef]
- Ugleholdt, R.; Poulsen, M.L.; Holst, P.J.; Irminger, J.C.; Orskov, C.; Pedersen, J.; Rosenkilde, M.M.; Zhu, X.; Steiner, D.F.; Holst, J.J. Prohormone Convertase 1/3 Is Essential for Processing of the Glucose-dependent Insulinotropic Polypeptide Precursor. J. Biol. Chem. 2006, 281, 11050–11057. [Google Scholar] [CrossRef]
- Usdin, T.B.; Mezey, E.; Button, D.C.; Brownstein, M.J.; Bonner, T.I. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993, 133, 2861–2870. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Krarup, T.; Deacon, C.F.; Madsbad, S.; Holst, J.J. Reduced Postprandial Concentrations of Intact Biologically Active Glucagon-Like Peptide 1 in Type 2 Diabetic Patients. Diabetes 2001, 50, 609–613. [Google Scholar] [CrossRef]
- Brown, J.C.; Mutt, V.; Pederson, R.A. Further purification of a polypeptide demonstrating enterogastrone activity. J. Physiol. 1970, 209, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, G.; Gros, P.; Habener, J.F. Glucagon gene sequence. Four of six exons encode separate functional domains of rat pre-proglucagon. J. Biol. Chem. 1984, 259, 14082–14087. [Google Scholar] [CrossRef]
- Vella, A. Effect of glucagon-like peptide1 (7-36) amide on glucose effectiveness and insulin action in people with type 2 diabetes. Diabetes 2000, 49, 611–617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Ward, W.K.; Bolgiano, D.C.; McKnight, B.; Halter, J.B.; Porte, D. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J. Clin. Investig. 1984, 74, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Nauck, M.A. Is the Diminished Incretin Effect in Type 2 Diabetes Just an Epi-Phenomenon of Impaired β-Cell Function? Diabetes 2010, 59, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, A.H.; Holcombe, J.H.; Kendall, D.M. Management of Type 2 diabetes: The role of incretin mimetics. Expert Opin. Pharmacother. 2006, 7, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Heimesaat, M.M.; Orskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kjems, L.L.; Holst, J.J.; Vølund, A.; Madsbad, S. The Influence of GLP-1 on Glucose-Stimulated Insulin Secretion: Effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003, 52, 380–386. [Google Scholar] [CrossRef]
- Drucker, D.J.; Sherman, S.I.; Gorelick, F.S.; Bergenstal, R.M.; Sherwin, R.S.; Buse, J.B. Incretin-Based Therapies for the Treatment of Type 2 Diabetes: Evaluation of the Risks and Benefits. Diabetes Care 2010, 33, 428–433. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Hansen, K.B.; Knop, F.K.; Holst, J.J.; Vilsbøll, T. Treatment of type 2 diabetes with glucagon-like peptide-1 receptor agonists. Int. J. Clin. Pract. 2009, 63, 1154–1160. [Google Scholar] [CrossRef]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Standards of Medical Care in Diabetes—2012. Diabetes Care 2012, 35 (Suppl. 1), S11–S63. [CrossRef]
- Holst, J.J. From the Incretin Concept and the Discovery of GLP-1 to Today’s Diabetes Therapy. Front. Endocrinol. 2019, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Prigeon, R.L.; Quddusi, S.; Paty, B.; D’Alessio, D.A. Suppression of endogenous glucose production by glucagon-like peptide1 independent of islet hormones: An extrapancreatic effect of an incretin hormone. Am. J. Physiol. 2003, 285, 701–707. [Google Scholar] [CrossRef]
- Bernsmeier, C.; Meyer-Gerspach, A.C.; Blaser, L.S.; Jeker, L.; Steinert, R.E.; Heim, M.; Beglinger, C. Glucose-Induced Glucagon-Like Peptide 1 Secretion Is Deficient in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2014, 9, e87488. [Google Scholar] [CrossRef]
- Yu, M.; Moreno, C.; Hoagland, K.M.; Dahly, A.; Ditter, K.; Mistry, M.; Roman, R.J. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J. Hypertens. 2003, 21, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Gutzwiller, J.P.; Tschopp, S.; Bock, A.; Zehnder, C.E.; Huber, A.R.; Kreyenbuehl, M.; Gutmann, H.; Drewe, J.; Henzen, C.; Goeke, B.; et al. Glucagon-Like Peptide 1 Induces Natriuresis in Healthy Subjects and in Insulin-Resistant Obese Men. J. Clin. Endocrinol. Metab. 2004, 89, 3055–3061. [Google Scholar] [CrossRef]
- Turton, M.D.; O’Shea, D.; Gunn, I.; Beak, S.A.; Edwards, C.M.B.; Meeran, K.; Choi, S.J.; Taylor, G.M.; Heath, M.M.; Lambert, P.D.; et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Meeran, K.; O’Shea, D.; Edwards, C.M.B.; Turton, M.D.; Heath, M.M.; Gunn, I.; Abusnana, S.; Rossi, M.; Small, C.J.; Goldstone, A.P.; et al. Repeated Intracerebroventricular Administration of Glucagon-Like Peptide-1-(7-36) Amide or Exendin-(9-39) Alters Body Weight in the Rat. Endocrinology 1999, 140, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.R.; Monteiro, M.; Small, C.J.; Sajedi, A.; Smith, K.L.; Parkinson, J.R.; Ghatei, M.A.; Bloom, S.R. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005, 1044, 127–131. [Google Scholar] [CrossRef]
- During, M.J.; Cao, L.; Zuzga, D.S.; Francis, J.S.; Fitzsimons, H.L.; Jiao, X.; Bland, R.J.; Klugmann, M.; Banks, W.A.; Drucker, D.J.; et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 2003, 9, 1173–1179. [Google Scholar] [CrossRef]
- Perry, T.; Haughey, N.J.; Mattson, M.P.; Egan, J.M.; Greig, N.H. Protection and Reversal of Excitotoxic Neuronal Damage by Glucagon-Like Peptide-1 and Exendin-4. J. Pharmacol. Exp. Ther. 2002, 302, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, J.; Anderson, M.F.; Meister, B.; Alborn, A.M.; Ström, A.K.; Brederlau, A.; Illerskog, A.C.; Nilsson, O.; Kieffer, T.J.; Hietala, M.A.; et al. Glucose-Dependent Insulinotropic Polypeptide Is Expressed in Adult Hippocampus and Induces Progenitor Cell Proliferation. J. Neurosci. 2005, 25, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Salera, M.; Giacomoni, P.; Pironi, L.; Cornia, G.; Capelli, M.; Marini, A.; Benfenati, F.; Miglioli, M.; Barbara, L. Gastric Inhibitory Polypeptide Release after Oral Glucose: Relationship to Glucose Intolerance, Diabetes Mellitus, and Obesity. J. Clin. Endocrinol. Metab. 1982, 55, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Creutzfeldt, W.; Ebert, R.; Willms, B.; Frerichs, H.; Brown, J.C. Gastric inhibitory polypeptide (GIP) and insulin in obesity: Increased response to stimulation and defective feedback control of serum levels. Diabetologia 1978, 14, 15–24. [Google Scholar] [CrossRef]
- Gault, V.A.; Irwin, N.; Green, B.D.; McCluskey, J.T.; Greer, B.; Bailey, C.J.; Harriott, P.; O’Harte, F.P.; Flatt, P.R. Chemical Ablation of Gastric Inhibitory Polypeptide Receptor Action by Daily (Pro3)GIP Administration Improves Glucose Tolerance and Ameliorates Insulin Resistance and Abnormalities of Islet Structure in Obesity-Related Diabetes. Diabetes 2005, 54, 2436–2446. [Google Scholar] [CrossRef]
- Bollag, R.J.; Zhong, Q.; Phillips, P.; Min, L.; Zhong, L.; Cameron, R.; Mulloy, A.L.; Rasmussen, H.; Qin, F.; Ding, K.H.; et al. Osteoblast-Derived Cells Express Functional Glucose-Dependent Insulinotropic Peptide Receptors1. Endocrinology 2000, 141, 1228–1235. [Google Scholar] [CrossRef]
- Zhong, Q.; Itokawa, T.; Sridhar, S.; Ding, K.H.; Xie, D.; Kang, B.; Bollag, W.B.; Bollag, R.J.; Hamrick, M.; Insogna, K.; et al. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am. J. Physiol. 2007, 292, 543–548. [Google Scholar] [CrossRef]
- Fürnsinn, C.; Ebner, K.; Waldhäusl, W. Failure of GLP-1(7-36)amide to affect glycogenesis in rat skeletal muscle. Diabetologia 1995, 38, 864–867. [Google Scholar] [CrossRef][Green Version]
- Larsen, P.J.; Tang-Christensen, M.; Jessop, D.S. Central Administration of Glucagon-Like Peptide-1 Activates Hypothalamic Neuroendocrine Neurons in the Rat. Endocrinology 1997, 138, 4445–4455. [Google Scholar] [CrossRef]
- Lacroix, A.; Bolté, E.; Tremblay, J.; Dupré, J.; Poitras, P.; Fournier, H.; Garon, J.; Garrel, D.; Bayard, F.; Taillefer, R.; et al. Gastric Inhibitory Polypeptide-Dependent Cortisol Hypersecretion—A New Cause of Cushing’s Syndrome. N. Engl. J. Med. 1992, 327, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Ghanim, H.; Vora, M.; Sia, C.L.; Korzeniewski, K.; Dhindsa, S.; Makdissi, A.; Dandona, P. Exenatide Exerts a Potent Antiinflammatory Effect. J. Clin. Endocrinol. Metab. 2012, 97, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Yabe, D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J. Diabetes Investig. 2013, 4, 108–130. [Google Scholar] [CrossRef] [PubMed]
- Kahles, F.; Meyer, C.; Möllmann, J.; Diebold, S.; Findeisen, H.M.; Lebherz, C.; Trautwein, C.; Koch, A.; Tacke, F.; Marx, N.; et al. GLP-1 Secretion Is Increased by Inflammatory Stimuli in an IL-6-Dependent Manner, Leading to Hyperinsulinemia and Blood Glucose Lowering. Diabetes 2014, 63, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Hopsu-Havu, V.K.; Glenner, G.G. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-beta-naphthylamide. Histochemie 1966, 7, 197–201. [Google Scholar] [CrossRef]
- Sell, H.; Blüher, M.; Klöting, N.; Schlich, R.; Willems, M.; Ruppe, F.; Knoefel, W.T.; Dietrich, A.; Fielding, B.A.; Arner, P.; et al. Adipose Dipeptidyl Peptidase-4 and Obesity: Correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care 2013, 36, 4083–4090. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Biochemical Properties of Recombinant Prolyl Dipeptidases DPP-IV and DPP8. Dipeptidyl Aminopeptidases 2006, 575, 27–32. [Google Scholar] [CrossRef]
- Havre, P.A.; Abe, M.; Urasaki, Y.; Ohnuma, K.; Morimoto, C.; Dang, N.H. The role of CD26/dipeptidyl peptidase IV in cancer. Front. Biosci. 2008, 13, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.; Kampfrath, T.; Deiuliis, J.; Zhong, J.; Pineda, C.; Ying, Z.; Xu, X.; Lu, B.; Moffatt-Bruce, S.; Durairaj, R.; et al. Long-Term Dipeptidyl-Peptidase 4 Inhibition Reduces Atherosclerosis and Inflammation via Effects on Monocyte Recruitment and Chemotaxis. Circulation 2011, 124, 2338–2349. [Google Scholar] [CrossRef]
- Aini, K.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Sata, M. Vildagliptin, a DPP-4 Inhibitor, Attenuates Endothelial Dysfunction and Atherogenesis in Nondiabetic Apolipoprotein E-Deficient Mice. Int. Heart J. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Barnett, A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int. J. Clin. Pract. 2006, 60, 1454–1470. [Google Scholar] [CrossRef]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Boniol, M.; Franchi, M.; Bota, M.; Leclercq, A.; Guillaume, J.; van Damme, N.; Corrao, G.; Autier, P.; Boyle, P. Incretin-Based Therapies and the Short-term Risk of Pancreatic Cancer: Results from Two Retrospective Cohort Studies. Diabetes Care 2017, 41, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Mosenzon, O.; Alguwaihes, A.; Leon, J.L.A.; Bayram, F.; Darmon, P.; Davis, T.M.E.; Dieuzeide, G.; Eriksen, K.T.; Hong, T.; Kaltoft, M.S.; et al. CAPTURE: A multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc. Diabetol. 2021, 20, 154. [Google Scholar] [CrossRef]
- Arakawa, M.; Mita, T.; Azuma, K.; Ebato, C.; Goto, H.; Nomiyama, T.; Fujitani, Y.; Hirose, T.; Kawamori, R.; Watada, H. Inhibition of Monocyte Adhesion to Endothelial Cells and Attenuation of Atherosclerotic Lesion by a Glucagon-like Peptide-1 Receptor Agonist, Exendin-4. Diabetes 2010, 59, 1030–1037. [Google Scholar] [CrossRef]
- Nagashima, M.; Watanabe, T.; Terasaki, M.; Tomoyasu, M.; Nohtomi, K.; Kim-Kaneyama, J.; Miyazaki, A.; Hirano, T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 2011, 54, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Nogi, Y.; Nagashima, M.; Terasaki, M.; Nohtomi, K.; Watanabe, T.; Hirano, T. Glucose-Dependent Insulinotropic Polypeptide Prevents the Progression of Macrophage-Driven Atherosclerosis in Diabetic Apolipoprotein E-Null Mice. PLoS ONE 2012, 7, e35683. [Google Scholar] [CrossRef]
- Terasaki, M.; Nagashima, M.; Watanabe, T.; Nohtomi, K.; Mori, Y.; Miyazaki, A.; Hirano, T. Effects of PKF275-055, a dipeptidyl peptidase–4 inhibitor, on the development of atherosclerotic lesions in apolipoprotein E–null mice. Metabolism 2012, 61, 974–977. [Google Scholar] [CrossRef]
- Burgmaier, M.; Liberman, A.; Möllmann, J.; Kahles, F.; Reith, S.; Lebherz, C.; Marx, N.; Lehrke, M. Glucagon-like peptide-1 (GLP-1) and its split products GLP-1(9-37) and GLP-1(28-37) stabilize atherosclerotic lesions in ApoE−/− mice. Atherosclerosis 2013, 231, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Kahles, F.; Liberman, A.; Halim, C.; Rau, M.; Möllmann, J.; Mertens, R.W.; Rückbeil, M.; Diepolder, I.; Walla, B.; Diebold, S.; et al. The incretin hormone GIP is upregulated in patients with atherosclerosis and stabilizes plaques in ApoE−/− mice by blocking monocyte/macrophage activation. Mol. Metab. 2018, 14, 150–157. [Google Scholar] [CrossRef]
- Mori, Y.; Kushima, H.; Koshibu, M.; Saito, T.; Hiromura, M.; Kohashi, K.; Terasaki, M.; Seino, Y.; Yamada, Y.; Hirano, T. Glucose-Dependent Insulinotropic Polypeptide Suppresses Peripheral Arterial Remodeling in Male Mice. Endocrinology 2018, 159, 2717–2732. [Google Scholar] [CrossRef]
- Bose, A.K.; Mocanu, M.M.; Carr, R.D.; Brand, C.L.; Yellon, D.M. Glucagon-like Peptide 1 Can Directly Protect the Heart against Ischemia/Reperfusion Injury. Diabetes 2005, 54, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Parikh, P.; Bhashyam, S.; Bolukoglu, H.; Poornima, I.; Shen, Y.-T.; Shannon, R.P. Direct Effects of Glucagon-Like Peptide-1 on Myocardial Contractility and Glucose Uptake in Normal and Postischemic Isolated Rat Hearts. J. Pharmacol. Exp. Ther. 2006, 317, 1106–1113. [Google Scholar] [CrossRef]
- Timmers, L.; Henriques, J.P.; de Kleijn, D.P.; DeVries, J.H.; Kemperman, H.; Steendijk, P.; Verlaan, C.W.; Kerver, M.; Piek, J.J.; Doevendans, P.A.; et al. Exenatide Reduces Infarct Size and Improves Cardiac Function in a Porcine Model of Ischemia and Reperfusion Injury. J. Am. Coll. Cardiol. 2009, 53, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-M.; Chen, W.-T.; Chang, N.-C. Sitagliptin decreases ventricular arrhythmias by attenuated glucose-dependent insulinotropic polypeptide (GIP)-dependent resistin signalling in infarcted rats. Biosci. Rep. 2016, 36, e00307. [Google Scholar] [CrossRef]
- Ussher, J.R.; Campbell, J.E.; Mulvihill, E.E.; Baggio, L.L.; Bates, H.E.; McLean, B.A.; Gopal, K.; Capozzi, M.; Yusta, B.; Cao, X.; et al. Inactivation of the Glucose-Dependent Insulinotropic Polypeptide Receptor Improves Outcomes following Experimental Myocardial Infarction. Cell Metab. 2018, 27, 450–460.e6. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.; Moellmann, J.; Kahles, F.; Haj-Yehia, E.; Liehn, E.A.; Nickel, A.; Lebherz, C.; Maack, C.; Marx, N.; Lehrke, M.; et al. Myocardial infarction is sufficient to increase GLP-1 secretion, leading to improved left ventricular contractility and mitochondrial respiratory capacity. Diabetes Obes. Metab. 2018, 20, 2911–2918. [Google Scholar] [CrossRef]
- Bunck, M.C.; Cornér, A.; Eliasson, B.; Heine, R.J.; Shaginian, R.M.; Wu, Y.; Yan, P.; Smith, U.; Yki-Järvinen, H.; Diamant, M.; et al. One-year treatment with exenatide vs. Insulin Glargine: Effects on postprandial glycemia, lipid profiles, and oxidative stress. Atherosclerosis 2010, 212, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Chandalia, M.; Patti, A.M.; Di Bartolo, V.; Rizvi, A.A.; Montalto, G.; Abate, N. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovasc. Diabetol. 2014, 13, 49. [Google Scholar] [CrossRef]
- Zhang, J.; Xian, T.-Z.; Wu, M.-X.; Li, C.; Pan, Q.; Guo, L.-X. Comparison of the effects of twice-daily exenatide and insulin on carotid intima-media thickness in type 2 diabetes mellitus patients: A 52-week randomized, open-label, controlled trial. Cardiovasc. Diabetol. 2020, 19, 48. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Testa, R.; Bonfigli, A.R.; Marra, M.; Giugliano, D. The Possible Protective Role of Glucagon-Like Peptide 1 on Endothelium During the Meal and Evidence for an “Endothelial Resistance” to Glucagon-Like Peptide 1 in Diabetes. Diabetes Care 2011, 34, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.M.; Lyssenko, V.; Ladenvall, C.; Kotova, O.; Edsfeldt, A.; Pilgaard, K.; Alkayyali, S.; Brøns, C.; Forsblom, C.; Jonsson, A.; et al. Glucose-Dependent Insulinotropic Polypeptide (GIP) Stimulates Osteopontin Expression in the Vasculature via Endothelin-1 and CREB. Diabetes 2016, 65, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, L.A.; Mankad, S.; Sokos, G.G.; Miske, G.; Shah, A.; Elahi, D.; Shannon, R.P. Effects of Glucagon-Like Peptide-1 in Patients With Acute Myocardial Infarction and Left Ventricular Dysfunction After Successful Reperfusion. Circulation 2004, 109, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Lønborg, J.; Vejlstrup, N.; Kelbæk, H.; Bøtker, H.E.; Kim, W.Y.; Mathiasen, A.B.; Jørgensen, E.; Helqvist, S.; Saunamaki, K.; Clemmensen, P.; et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur. Heart J. 2012, 33, 1491–1499. [Google Scholar] [CrossRef]
- Woo, J.S.; Kim, W.; Ha, S.J.; Kim, J.B.; Kim, S.-J.; Kim, W.-S.; Seon, H.J.; Kim, K.S. Cardioprotective Effects of Exenatide in Patients With ST-Segment–Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: Results of exenatide myocardial protection in revascularization study. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2252–2260. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.; Chan, J.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef]
- Trevisan, M.; Fu, E.; Szummer, K.; Norhammar, A.; Lundman, P.; Wanner, C.; Sjölander, A.; Jernberg, T.; Carrero, J.J. Glucagon-like peptide-1 receptor agonists and the risk of cardiovascular events in diabetes patients surviving an acute myocardial infarction. Eur. Heart J.-Cardiovasc. Pharmacother. 2021, 7, 104–111. [Google Scholar] [CrossRef]
- Bethel, M.A.; Patel, R.A.; Merrill, P.; Lokhnygina, Y.; Buse, J.B.; Mentz, R.J.; Pagidipati, N.J.; Chan, J.C.; Gustavson, S.M.; Iqbal, N.; et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: A meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 105–113. [Google Scholar] [CrossRef]
- Marsico, F.; Paolillo, S.; Gargiulo, P.; Bruzzese, D.; Dell’Aversana, S.; Esposito, I.; Renga, F.; Esposito, L.; Marciano, C.; Dellegrottaglie, S.; et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: A meta-analysis of randomized controlled trials. Eur. Heart J. 2020, 41, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- Anholm, C.; Kumarathurai, P.; Pedersen, L.R.; Samkani, A.; Walzem, R.L.; Nielsen, O.W.; Kristiansen, O.P.; Fenger, M.; Madsbad, S.; Sajadieh, A.; et al. Liraglutide in combination with metformin may improve the atherogenic lipid profile and decrease C-reactive protein level in statin treated obese patients with coronary artery disease and newly diagnosed type 2 diabetes: A randomized trial. Atherosclerosis 2019, 288, 60–66. [Google Scholar] [CrossRef]
- Blatt, A.; Shiloah, E.; Mincha, S.; Bloch, O.; Rapoport, M.J. Incretin hormone glucagon-like peptide-1 is increased in patients with acute-phase ST-elevation myocardial infarction treated with a primary percutaneous coronary intervention: A pilot study. Cardiovasc. Endocrinol. 2013, 2, 98–102. [Google Scholar] [CrossRef]
- Kahles, F.; Rückbeil, M.V.; Mertens, R.W.; Foldenauer, A.C.; Arrivas, M.C.; Moellmann, J.; Lebherz, C.; Biener, M.; Giannitsis, E.; Katus, H.A.; et al. Glucagon-like peptide 1 levels predict cardiovascular risk in patients with acute myocardial infarction. Eur. Heart J. 2020, 41, 882–889. [Google Scholar] [CrossRef]
- Elbaz-Greener, G.; Bloch, O.; Kumets, I.; Blatt, A.; Rapoport, M.J. Endogenous glucagon-like peptide-1 system response is impaired during ST-elevation myocardial infarction in type 2 diabetes patients. Diabetes, Obes. Metab. 2019, 21, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Gault, V.A.; Kerr, B.D.; Harriott, P.; Flatt, P.R. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with Type 2 diabetes and obesity. Clin. Sci. 2011, 121, 107–117. [Google Scholar] [CrossRef]
- Bergmann, N.C.; Lund, A.; Gasbjerg, L.S.; Meessen, E.C.E.; Andersen, M.M.; Bergmann, S.; Hartmann, B.; Holst, J.J.; Jessen, L.; Christensen, M.; et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: A randomised, crossover study. Diabetologia 2019, 62, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Irwin, N.; McClean, P.L.; Cassidy, R.S.; O’Harte, F.P.M.; Green, B.D.; Gault, V.A.; Harriott, P.; Flatt, P.R. Comparison of the anti-diabetic effects of GIP- and GLP-1-receptor activation in obese diabetic (ob/ob) mice: Studies with DPP IV resistantN-AcGIP and exendin(1-39)amide. Diabetes/Metab. Res. Rev. 2007, 23, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Irwin, N.; Hunter, K.; Frizzell, N.; Flatt, P.R. Antidiabetic effects of sub-chronic activation of the GIP receptor alone and in combination with background exendin-4 therapy in high fat fed mice. Regul. Pept. 2009, 153, 70–76. [Google Scholar] [CrossRef]
- Irwin, N.; McClean, P.L.; Flatt, P.R. Comparison of the subchronic antidiabetic effects of DPP IV–resistant GIP and GLP-1 analogues in obese diabetic (ob/ob) mice. J. Pept. Sci. 2007, 13, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Bartels, E.; Orskov, C.; Ebert, R.; Creutzfeldt, W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J. Clin. Endocrinol. Metab. 1993, 76, 912–917. [Google Scholar] [CrossRef]
- Elahi, D.; McAloon-Dyke, M.; Fukagawa, N.K.; Meneilly, G.S.; Sclater, A.L.; Minaker, K.L.; Habener, J.F.; Andersen, D.K. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul. Pept. 1994, 51, 63–74. [Google Scholar] [CrossRef]
- Daousi, C.; Wilding, J.P.H.; Aditya, S.; Durham, B.H.; Cleator, J.; Pinkney, J.H.; Ranganath, L.R. Effects of peripheral administration of synthetic human glucose-dependent insulinotropic peptide (GIP) on energy expenditure and subjective appetite sensations in healthy normal weight subjects and obese patients with type 2 diabetes. Clin. Endocrinol. 2009, 71, 195–201. [Google Scholar] [CrossRef]
- Mentis, N.; Vardarli, I.; Köthe, L.D.; Holst, J.J.; Deacon, C.F.; Theodorakis, M.; Meier, J.J.; Nauck, M.A. GIP Does Not Potentiate the Antidiabetic Effects of GLP-1 in Hyperglycemic Patients With Type 2 Diabetes. Diabetes 2011, 60, 1270–1276. [Google Scholar] [CrossRef]
- Finan, B.; Ma, T.; Ottaway, N.; Müller, T.D.; Habegger, K.M.; Heppner, K.M.; Kirchner, H.; Holland, J.; Hembree, J.; Raver, C.; et al. Unimolecular Dual Incretins Maximize Metabolic Benefits in Rodents, Monkeys, and Humans. Sci. Transl. Med. 2013, 5, 209ra151. [Google Scholar] [CrossRef]
- Schmitt, C.; Portron, A.; Jadidi, S.; Sarkar, N.; DiMarchi, R. Pharmacodynamics, pharmacokinetics and safety of multiple ascending doses of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 in people with type 2 diabetes mellitus. Diabetes Obes. Metab. 2017, 19, 1436–1445. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Landó, L.F.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Landó, L.F.; Brown, K.; Bray, R.; Rodríguez, Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef]
- A Study of Tirzepatide (LY3298176) Versus Placebo in Participants with Type 2 Diabetes Inadequately Controlled on Insulin Glargine with or without Metformin. Available online: https://clinicaltrials.gov/ct2/show/NCT04039503 (accessed on 12 January 2022).
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.P.; Banerjee, Y.; Rizvi, A.A.; Rizzo, M. Diabetes and the COVID-19 Pandemic: How Insights from Recent Experience Might Guide Future Management. Metab. Syndr. Relat. Disord. 2020, 18, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Davies, M.J.; Kosiborod, M.N.; Nauck, M.A. Long COVID—Metabolic risk factors and novel therapeutic management. Nat. Rev. Endocrinol. 2021, 17, 379–380. [Google Scholar] [CrossRef]
| Study Type | Clinical Characteristics | Conclusions | Ref. No. |
|---|---|---|---|
| prospective | 6-week-old mice (n = 19) with apolipoprotein E knockout (ApoE−/−) |
| [65] |
| prospective | 17-week-old mice (n = 346) with apolipoprotein E knockout (ApoE−/−) |
| [66] |
| prospective | 21-week-old mice (n = 49) with apolipoprotein E knockout (ApoE−/−) |
| [67] |
| prospective | 17-week-old mice (n = 44) with apolipoprotein E knockout (ApoE−/−) |
| [68] |
| prospective | 6-week-old mice (n = 40) with apolipoprotein E knockout (ApoE−/−) |
| [69] |
| prospective | 6-week-old mice (n = 18–20) with apolipoprotein E knockout (ApoE−/−) |
| [70] |
| prospective | 9-week-old mice (n = 35) |
| [71] |
| prospective | 107 rats: 22—in vivo; 85—in vitro within 30 min of LM occlusion and 2 h of reperfusion |
| [72] |
| prospective | 75 rats within 30 min of low-flow ischemia and 30 min of reperfusion |
| [73] |
| prospective | 18 pigs after ischemia by LCx ligation and subsequent reperfusion |
| [74] |
| prospective | Rats (n = 22) with MI by LAD ligation |
| [75] |
| prospective | 10–12-week-old mice (n = 4–6) with MI by LAD ligation |
| [76] |
| prospective | 6-week-old mice (n = 12) with MI induced by LAD ligation |
| [77] |
| Study Type | Clinical Characteristics | Conclusions | Ref. No. |
|---|---|---|---|
| prospective, randomized | 69 DM 2 patients (60—completed) treated with metformin plus exenatide (30) or insulin glargine (30) |
| [78] |
| prospective | 64 DM 2 patients with no prior history of CAD |
| [79] |
| prospective, randomized | 66 DM 2 patients treated with exenatide or insulin aspartate |
| [80] |
| prospective, randomized | 28 patients (16 DM 2 and 12 healthy control) |
| [81] |
| retrospective | Patients with confirmed diagnosis of critical limb ischemia (n = 85) and healthy controls (n = 101). |
| [82] |
| prospective | 21 patients (42.9% diabetes) with MI and LVEF < 40% after successful PCI (GLP-1 = 10, controls = 11). |
| [83] |
| prospective, randomized | 172 patients (6.4% diabetes) undergoing PCI for STEMI (exenatide = 85, controls = 87). |
| [84] |
| prospective, randomized | 58 patients (25.9% diabetes) who underwent PCI for STEMI (exenatide = 18, controls = 40). |
| [85] |
| retrospective | 731 patients (32.7% diabetes) presented for elective coronary angiography. |
| [70] |
| prospective, randomized | 9340 DM 2 patients with high risk of CV events (liraglutide = 4668, placebo = 4672) from LEADER trial. Median follow-up: 3.8 years |
| [86] |
| prospective, randomized | 3297 DM 2 patients with high risk of CV events (semaglutide = 1648, placebo = 1649) from SUSTAIN-6 trial. Median follow-up: 2.1 years |
| [87] |
| prospective, randomized | 9901 DM 2 patients with either previous CVD or CV risk (dulaglutide = 4949, placebo = 4952) from REWIND trial. Median follow-up: 5.4 years |
| [88] |
| prospective, randomized | 9463 DM 2 patients with CVD (albiglutide = 4731, placebo = 4732) from HARMONY trial. Median follow-up: 1.6 years |
| [89] |
| prospective, randomized | 3183 DM 2 patients with high CV risk (semaglutide = 1591, placebo = 1592) from PIONEER-6 trial. Median follow-up: 15.9 months |
| [90] |
| prospective, randomized | 14,752 DM 2 patients and with or without CVD (exenatide = 7356, placebo = 7396) form EXSCEL trial. Median follow-up: 3.2 years |
| [91] |
| prospective, randomized | 6068 DM 2 patients with MI or hospitalized for UA within the previous 6 months (lixenatide = 3034, placebo = 3034) from ELIXA trial. Median follow-up: 25 months |
| [92] |
| prospective, registry | 17,868 patients with diabetes discharged alive after a first event of MI (365 (2%) using GLP-1 RAs) from nationwide SWEDEHEART registry. Median follow-up: 3.0 years |
| [93] |
| meta-analysis from randomized trials | 33,475 DM 2 patients with or without established CVD (but high/very high CV risk). Median follow-up: 2.1–3.8 years |
| [94] |
| meta-analysis from randomized trials | 56,004 DM 2 patients with or without established CVD (but high/very high CV risk). Median follow-up: 1.3–5.4 years |
| [95] |
| prospective, randomized | 41 patients (28 with complete data) with CAD and newly diagnosed DM 2 |
| [96] |
| prospective | 12 patients (10—nondiabetic) presenting with STEMI before and 24, 72 h, and 90 days after PCI. |
| [97] |
| retrospective | 918 patients (75.7%—nondiabetic) with MI (321 STEMI, 597 NSTEMI). Median follow-up: 310 days for primary endpoint and 311 days for all-cause mortality |
| [98] |
| retrospective | 41 patients presented with clinical indication for coronary angiography (26-STEMI; 15-controls (angiographic exclusion of CAD)). |
| [77] |
| retrospective | 103 patients (78 admitted for PCI) with STEMI (n = 33; 20—nondiabetic) and three control groups: NSTEMI (n = 27; 14—nondiabetic), stable angina pectoris (n = 18; 8—nondiabetic), and control-healthy subjects (n = 25). |
| [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonik, S.; Marchel, M.; Grabowski, M.; Opolski, G.; Mazurek, T. Gastrointestinal Incretins—Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) beyond Pleiotropic Physiological Effects Are Involved in Pathophysiology of Atherosclerosis and Coronary Artery Disease—State of the Art. Biology 2022, 11, 288. https://doi.org/10.3390/biology11020288
Jonik S, Marchel M, Grabowski M, Opolski G, Mazurek T. Gastrointestinal Incretins—Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) beyond Pleiotropic Physiological Effects Are Involved in Pathophysiology of Atherosclerosis and Coronary Artery Disease—State of the Art. Biology. 2022; 11(2):288. https://doi.org/10.3390/biology11020288
Chicago/Turabian StyleJonik, Szymon, Michał Marchel, Marcin Grabowski, Grzegorz Opolski, and Tomasz Mazurek. 2022. "Gastrointestinal Incretins—Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) beyond Pleiotropic Physiological Effects Are Involved in Pathophysiology of Atherosclerosis and Coronary Artery Disease—State of the Art" Biology 11, no. 2: 288. https://doi.org/10.3390/biology11020288
APA StyleJonik, S., Marchel, M., Grabowski, M., Opolski, G., & Mazurek, T. (2022). Gastrointestinal Incretins—Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) beyond Pleiotropic Physiological Effects Are Involved in Pathophysiology of Atherosclerosis and Coronary Artery Disease—State of the Art. Biology, 11(2), 288. https://doi.org/10.3390/biology11020288







