The Effects of EMMPRIN/CD147 on Late Function and Histopathological Lesions of the Renal Graft
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
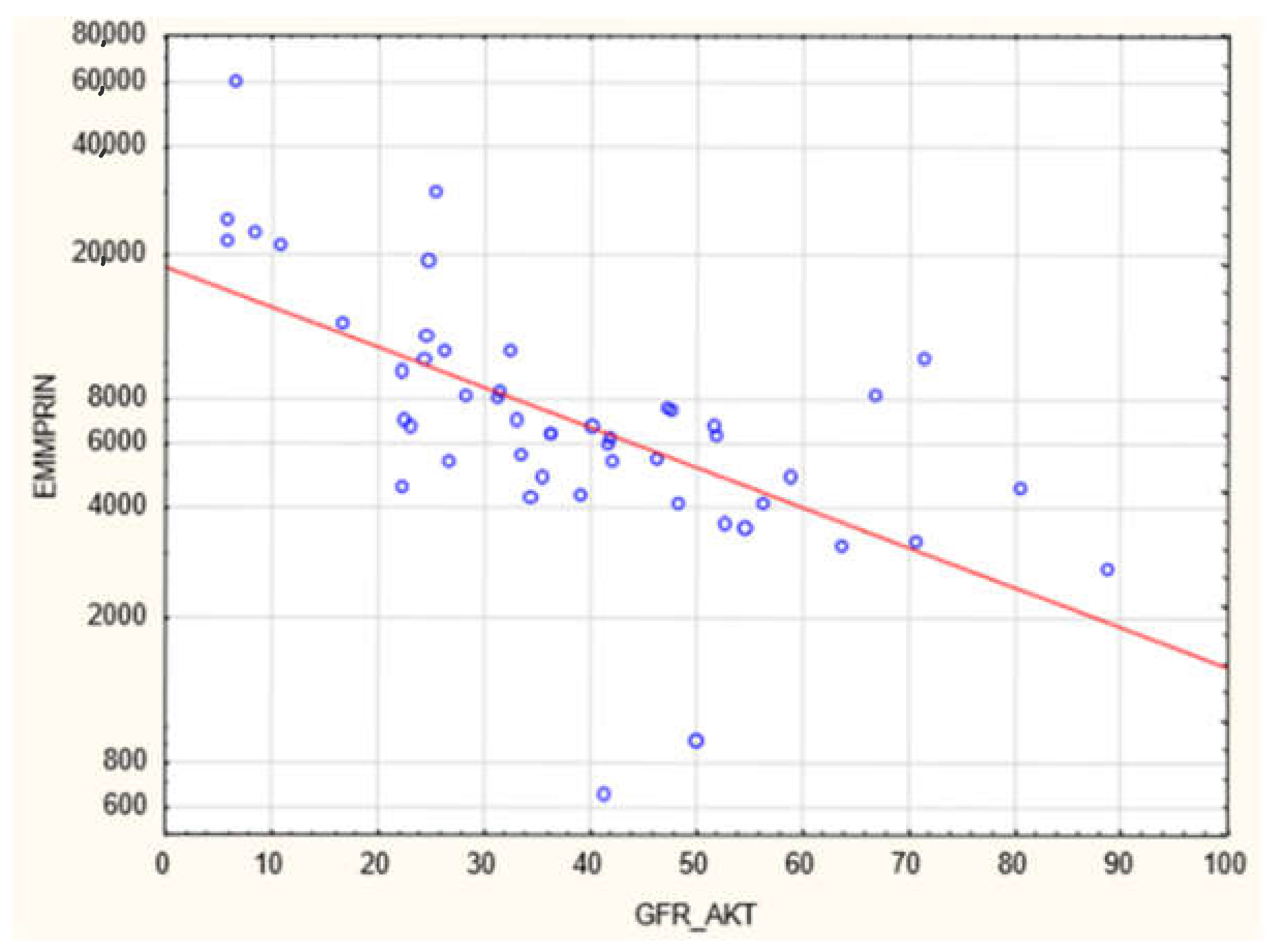
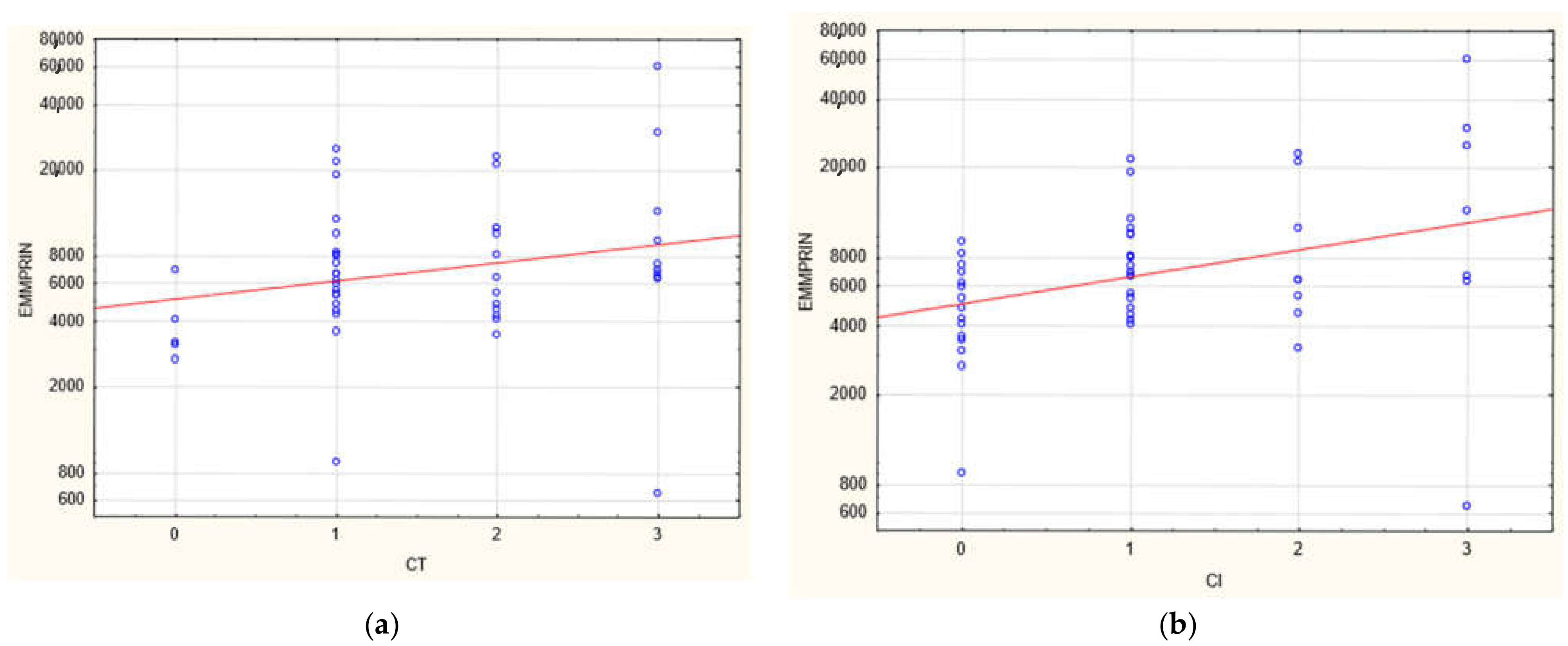
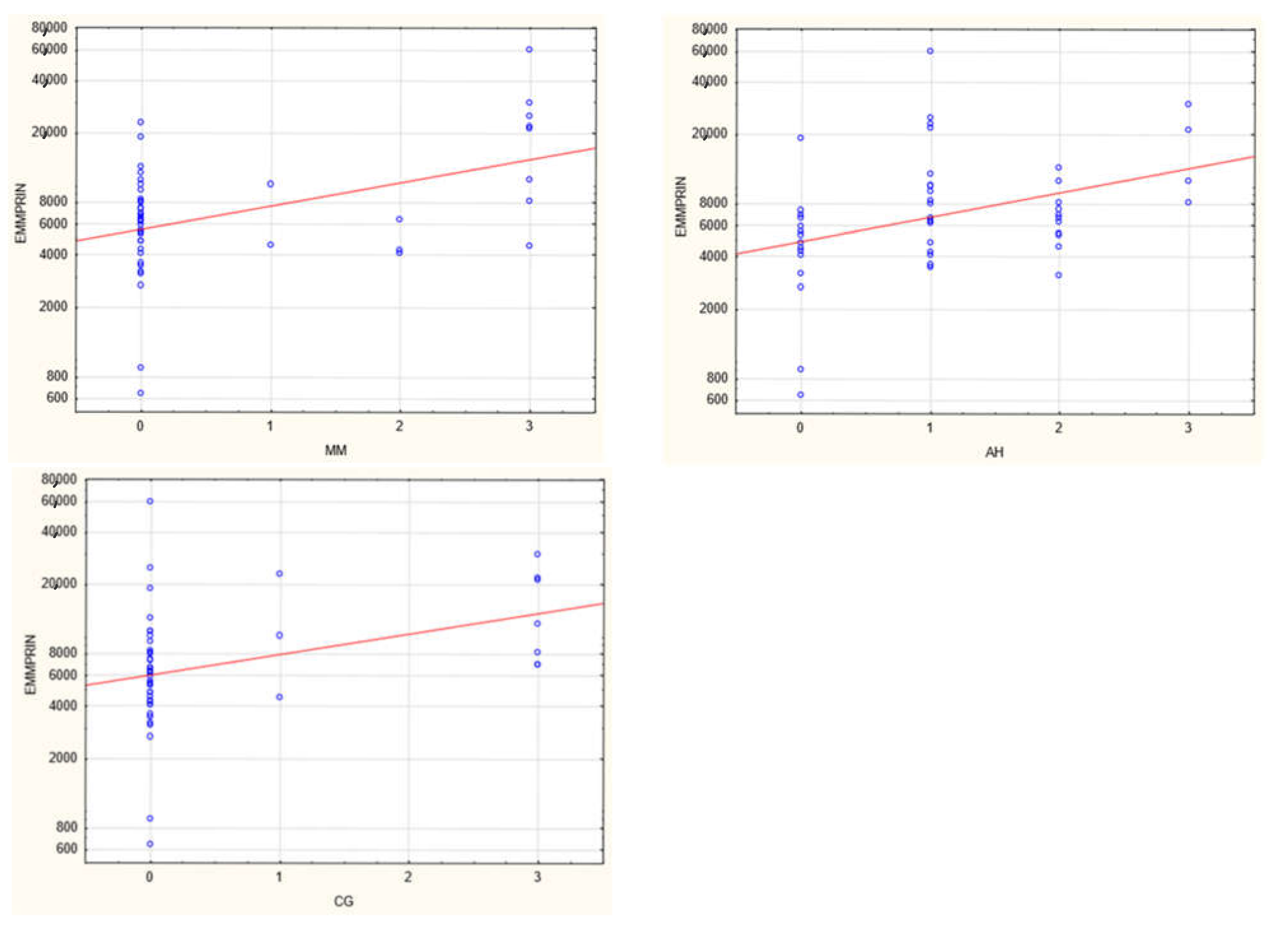
3. Results
4. Discussion
4.1. EMMPRIN/CD147
4.2. CD147/AKI
4.3. CKD/EMT
4.4. EMT/CD147/EMMPRIN
4.5. Future Perspectives
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, D.; Liu, Y. Renal Fibrosis in 2015: Understanding the Mechanisms of Kidney Fibrosis. Nat. Rev. Nephrol. 2016, 12, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.B.Y.; Qu, X.; Caruana, G.; Li, J. The Origin of Renal Fibroblasts/Myofibroblasts and the Signals That Trigger Fibrosis. Differentiation 2016, 92, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.D.; Toole, B.P. How, with Whom and When: An Overview of CD147-Mediated Regulatory Networks Influencing Matrix Metalloproteinase Activity. Biosci. Rep. 2016, 36, e00283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. New Insights into Epithelial-Mesenchymal Transition in Kidney Fibrosis. J. Am. Soc. Nephrol. 2010, 21, 212–222. [Google Scholar] [CrossRef]
- Sheng, L.; Zhuang, S. New Insights Into the Role and Mechanism of Partial Epithelial-Mesenchymal Transition in Kidney Fibrosis. Front. Physiol. 2020, 11, 569322. [Google Scholar] [CrossRef]
- Kemmner, S.; Schulte, C.; Hann von Weyhern, C.; Schmidt, R.; Baumann, M.; Heemann, U.; Renders, L.; Schmaderer, C. EMMPRIN Expression Is Involved in the Development of Interstitial Fibrosis and Tubular Atrophy in Human Kidney Allografts. Clin. Transplant. 2016, 30, 218–225. [Google Scholar] [CrossRef]
- Earley, A.; Miskulin, D.; Lamb, E.J.; Levey, A.S.; Uhlig, K. Estimating Equations for Glomerular Filtration Rate in the Era of Creatinine Standardization: A Systematic Review. Ann. Intern. Med. 2012, 156, 785–795. [Google Scholar] [CrossRef]
- Rognant, N.; Lemoine, S.; Laville, M.; Hadj-Aïssa, A.; Dubourg, L. Performance of the Chronic Kidney Disease Epidemiology Collaboration Equation to Estimate Glomerular Filtration Rate in Diabetic Patients. Diabetes Care 2011, 34, 1320–1322. [Google Scholar] [CrossRef]
- Schwandt, A.; Denkinger, M.; Fasching, P.; Pfeifer, M.; Wagner, C.; Weiland, J.; Zeyfang, A.; Holl, R.W. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault Equation in Relation to Measured Glomerular Filtration Rate among a Large Cohort with Diabetes. J. Diabetes Complicat. 2017, 31, 1376–1383. [Google Scholar] [CrossRef]
- Guindolet, D.; Gabison, E.E. Role of CD147 (EMMPRIN/Basigin) in Tissue Remodeling. Anat. Rec. 2020, 303, 1584–1589. [Google Scholar] [CrossRef]
- Ryzhakova, O.S.; Solov’eva, N.I. Matrix Metalloproteinases (MMP)–MMP-1,-2,-9 and Its Endogenous Activity Regulators in Transformed by E7 Oncogene HPV16 and HPV18 Cervical Carcinoma Cell Lines. Biomeditsinskaya Khimiya 2013, 59, 530–540. [Google Scholar] [CrossRef][Green Version]
- Kato, N.; Yuzawa, Y.; Kosugi, T.; Hobo, A.; Sato, W.; Miwa, Y.; Sakamoto, K.; Matsuo, S.; Kadomatsu, K. The E-Selectin Ligand Basigin/CD147 Is Responsible for Neutrophil Recruitment in Renal Ischemia/Reperfusion. J. Am. Soc. Nephrol. 2009, 20, 1565–1576. [Google Scholar] [CrossRef]
- Seizer, P.; Ochmann, C.; Schönberger, T.; Zach, S.; Rose, M.; Borst, O.; Klingel, K.; Kandolf, R.; MacDonald, H.R.; Nowak, R.A.; et al. Disrupting the EMMPRIN (CD147)-Cyclophilin a Interaction Reduces Infarct Size and Preserves Systolic Function after Myocardial Ischemia and Reperfusion. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1377–1386. [Google Scholar] [CrossRef]
- Kosugi, T.; Maeda, K.; Sato, W.; Maruyama, S.; Kadomatsu, K. CD147 (EMMPRIN/Basigin) in Kidney Diseases: From an Inflammation and Immune System Viewpoint. Nephrol. Dial. Transplant. 2015, 30, 1097–1103. [Google Scholar] [CrossRef]
- Nagaya, H.; Kosugi, T.; Maeda-Hori, M.; Maeda, K.; Sato, Y.; Kojima, H.; Hayashi, H.; Kato, N.; Ishimoto, T.; Sato, W.; et al. CD147/Basigin Reflects Renal Dysfunction in Patients with Acute Kidney Injury. Clin. Exp. Nephrol. 2014, 18, 746–754. [Google Scholar] [CrossRef]
- Mannon, R.B. Delayed Graft Function: The AKI of Kidney Transplantation. Nephron 2018, 140, 94–98. [Google Scholar] [CrossRef]
- Cruz-Solbes, A.S.; Youker, K. Epithelial to Mesenchymal Transition (EMT) and Endothelial to Mesenchymal Transition (EndMT): Role and Implications in Kidney Fibrosis. In Kidney Development and Disease; Results and Problems in Cell Differentiation book series 60; Springer: Cham, Switzerland, 2017; pp. 345–372. [Google Scholar] [CrossRef]
- Arora, K.; Gwinn, W.M.; Bower, M.A.; Watson, A.; Okwumabua, I.; MacDonald, H.R.; Bukrinsky, M.I.; Constant, S.L. Extracellular Cyclophilins Contribute to the Regulation of Inflammatory Responses. J. Immunol. 2005, 175, 517–522. [Google Scholar] [CrossRef]
- Lange-Sperandio, B.; Cachat, F.; Thornhill, B.A.; Chevalier, R.L. Selectins Mediate Macrophage Infiltration in Obstructive Nephropathy in Newborn Mice. Kidney Int. 2002, 61, 516–524. [Google Scholar] [CrossRef]
- Mori, Y.; Masuda, T.; Kosugi, T.; Yoshioka, T.; Hori, M.; Nagaya, H.; Maeda, K.; Sato, Y.; Kojima, H.; Kato, N.; et al. The Clinical Relevance of Plasma CD147/Basigin in Biopsy-Proven Kidney Diseases. Clin. Exp. Nephrol. 2018, 22, 815–824. [Google Scholar] [CrossRef]
- Wu, J.; Ru, N.Y.; Zhang, Y.; Li, Y.; Wei, D.; Ren, Z.; Huang, X.F.; Chen, Z.N.; Bian, H. HAb18G/CD147 Promotes Epithelial-Mesenchymal Transition through TGF-β Signaling and Is Transcriptionally Regulated by Slug. Oncogene 2011, 30, 4410–4427. [Google Scholar] [CrossRef]
- Guillot, S.; Delaval, P.; Brinchault, G.; Caulet-Maugendre, S.; Depince, A.; Lena, H.; Delatour, B.; Lagente, V.; Martin-Chouly, C. Increased Extracellular Matrix Metalloproteinase Inducer (EMMPRIN) Expression in Pulmonary Fibrosis. Exp. Lung Res. 2006, 32, 81–97. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, A.; Li, R.; Du, R.; He, L.; Sun, W.; Wang, H.; Huang, C. CD147 Renal Expression as a Biomarker for Progressive IgAN. J. Nephrol. 2015, 28, 307–314. [Google Scholar] [CrossRef]
- Qu, X.; Wang, C.; Zhang, J.; Qie, G.; Zhou, J. The Roles of CD147 and/or Cyclophilin a in Kidney Diseases. Mediat. Inflamm. 2014, 2014, 728673. [Google Scholar] [CrossRef]
- Zakiyanov, O.; Kalousová, M.; Zima, T.; Tesař, V. Matrix Metalloproteinases in Renal Diseases: A Critical Appraisal. Kidney Blood Press. Res. 2019, 44, 298–330. [Google Scholar] [CrossRef]
- Roufosse, C.; Simmonds, N.; Clahsen-Van Groningen, M.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, H.C.; Fogo, A.B. A Perspective on Chronic Kidney Disease Progression. Am. J. Physiol. Ren. Physiol. 2017, 312, F375–F384. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Lerut, E.; Emonds, M.P.; Herelixka, A.; Evenepoel, P.; Claes, K.; Bammens, B.; Sprangers, B.; Meijers, B.; Jochmans, I.; et al. Proteinuria as a Noninvasive Marker for Renal Allograft Histology and Failure: An Observational Cohort Study. J. Am. Soc. Nephrol. 2016, 27, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kosugi, T.; Masuda, T.; Watanabe, T.; Ryuge, A.; Nagaya, H.; Maeda, K.; Sato, Y.; Katsuno, T.; Kato, N.; et al. CD147/Basigin Deficiency Prevents the Development of Podocyte Injury through FAK Signaling. Am. J. Pathol. 2019, 189, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Ntrinias, T.; Papasotiriou, M.; Balta, L.; Kalavrizioti, D.; Vamvakas, S.; Papachristou, E.; Goumenos, D.S. Biomarkers in Progressive Chronic Kidney Disease. Still a Long Way to Go. Prilozi 2019, 40, 27–39. [Google Scholar] [CrossRef][Green Version]
- Kwiatkowska, E.; Domanski, L.; Bober, J.; Safranow, K.; Romanowski, M.; Pawlik, A.; Kwiatkowski, S.; Ciechanowski, K. Urinary Metalloproteinases-9 and -2 and Their Inhibitors TIMP-1 and TIMP-2 Are Markers of Early and Long-Term Graft Function after Renal Transplantation. Kidney Blood Press. Res. 2016, 41, 288–297. [Google Scholar] [CrossRef]
- Hörstrup, J.H.; Gehrmann, M.; Schneider, B.; Plöger, A.; Froese, P.; Schirop, T.; Kampf, D.; Frei, U.; Neumann, R.; Eckardt, K.U. Elevation of Serum and Urine Levels of TIMP-1 and Tenascin in Patients with Renal Disease. Nephrol. Dial. Transplant. 2002, 17, 1005–1013. [Google Scholar] [CrossRef]
- Musiał, K.; Zwolińska, D. Matrix Metalloproteinases (MMP-2,9) and Their Tissue Inhibitors (TIMP-1,2) as Novel Markers of Stress Response and Atherogenesis in Children with Chronic Kidney Disease (CKD) on Conservative Treatment. Cell Stress Chaperones 2011, 16, 97–103. [Google Scholar] [CrossRef]
- Mansour, S.G.; Puthumana, J.; Coca, S.G.; Gentry, M.; Parikh, C.R. Biomarkers for the Detection of Renal Fibrosis and Prediction of Renal Outcomes: A Systematic Review. BMC Nephrol. 2017, 18, 72. [Google Scholar] [CrossRef]





| Banff Lesion | Abbreviation | 0 | 1 | 2 | 3 |
|---|---|---|---|---|---|
| Interstitial inflammation | I | <10% | 10–25% | 26–50% | >50% |
| Tubulitis | T | none | 1–4/tubular cross section or 10 tubular epithelial cells | 5–10 | >10 or foci of tubular basement membrane destruction with i ≥ 2 and t2 elsewhere |
| Glomerulitis | G | none | <25% | 25–75% | >75% |
| Peritubular capillaritis | PTC | <3 leukocytes/PTC | ≥1 leukocyte in ≥10% of PTCs with max. of 3–4/PTCS | ≥1 leukocyte in ≥10% of PTCs with max. of 5–10/PTCS | ≥1 leukocyte in ≥10% of PTCs with max. of >10/PTCS |
| C4d | C4d | none | <10% | 10–50% | >50% |
| Interstitial fibrosis | CI | ≤5% | 6–25% | 26–50% | >50% |
| Tubular atrophy | CT | none | ≤25% | 26–50% | >50% |
| Vascular fibrous Intimal thickening | CV | none | ≤25% | 26–50% | >50% |
| double contours of GBM | CG | none | 1 a: only by EM 1 b: ≤25% by LM | 26–50% | >50% |
| Mesangial matrix expansion | MM | none | ≤25% | 26–50% | >50% |
| Arteriolar hyalinosis | AA | none | Mild to moderate ≥1 | Moderate to severe in >1 | Severe in many |
| Hyaline arteriolar thickening | AAH | none | 1 without circumferential | ≥1 without circumferential | Severe in many circumferenial |
| N | ME | MIN | MAX | MEAN | SD | |
|---|---|---|---|---|---|---|
| Time from Tx (months) | 49 | 69 | 12 | 182 | 75.7 | 52.3 |
| Recipient age (years) | 49 | 42 | 24 | 71 | 45.7 | 13.6 |
| BMI at Tx | 49 | 23.8 | 16.1 | 34.8 | 24.4 | 4 |
| BMI at last appointment | 49 | 25.4 | 18.1 | 34 | 25.9 | 4.3 |
| eGFR at last appointment (mL/min/1.73 m2) | 49 | 36 | 15 | 89 | 38.6 | 19.1 |
| Urine protein (mg/dL) | 49 | 0 | 0 | 865.7 | 78.7 | 165 |
| Duration of dialysis prior to Tx (months) | 49 | 15 | 0 | 102 | 23.9 | 23.8 |
| CIT (min) | 49 | 1260 | 72 | 2100 | 1116.7 | 555 |
| PRA(%) | 49 | 3 | 0 | 56 | 8 | 15.9 |
| ZENITH eGFR (mL/min/1.73 m2) | 49 | 31.0 | 98.0 | 118.0 | 58 | 17 |
| eGFR at 1 year (mL/min/1.73 m2) | 49 | 45 | 9 | 96.0 | 47.7 | 17.9 |
| eGFR at 2 years (mL/min/1.73 m2) | 48 | 49 | 15 | 85 | 48 | 17.1 |
| eGFR at 3 years (mL/min/1.73 m2) | 43 | 47 | 17 | 87 | 47.3 | 15.4 |
| eGFR at 4 years (mL/min/1.73 m2) | 38 | 46 | 19 | 98 | 48 | 18.9 |
| eGFR at 5 years (mL/min/1.73 m2) | 35 | 41 | 17 | 109 | 45.5 | 20.1 |
| eGFR at 10 years (mL/min/1.73 m2) | 13 | 44 | 23 | 82 | 47.8 | 18.7 |
| EMMPRIN (pg/mL) | 49 | 6623.2 | 643.36 | 59,861.6 | 9419.67 | 9678.712 |
| sex | 49 (23 W,26 M) |
| Group without Chronic Changes N = 23 | Group with Chronic Changes N = 26 | ||||||
|---|---|---|---|---|---|---|---|
| ME | MIN | MAX | ME | MIN | MAX | p | |
| Time from Tx (months) | 44.9 | 3.16 | 162.1 | 82.7 | 9.53 | 181 | 0.004 |
| Recipient age (years) | 43 | 24 | 71 | 40,5 | 26 | 70 | NS |
| BMI at last appointment (kg/m2) | 26.2 | 18.7 | 33.7 | 24.4 | 18.1 | 34 | NS |
| eGFR at last appointment (mL/min/1.73 m2) | 41.9 | 6.1 | 88.9 | 33.7 | 6.02 | 71.62 | NS |
| ZENITH eGFR (mL/min/1.73 m2) | 64 | 34.17 | 92.38 | 53.85 | 31.1 | 97.76 | 0.03 |
| G | 0 | 0 | 2 | 0 | 0 | 0 | NS |
| CG | 0 | 0 | 3 | 0 | 0 | 3 | NS |
| MM | 0 | 0 | 3 | 0 | 0 | 3 | 0.013 |
| I | 0 | 0 | 3 | 1 | 0 | 3 | 0.007 |
| T | 0 | 0 | 2 | 0 | 0 | 3 | NS |
| PTC | 0 | 0 | 10 | 0 | 0 | 3 | NS |
| CV | 0 | 0 | 3 | 0 | 0 | 2 | NS |
| AH | 1 | 0 | 3 | 1 | 0 | 3 | 0.027 |
| CI | 0 | 0 | 1 | 2 | 0 | 3 | 3.32 × 10−6 |
| CT | 1 | 0 | 1 | 2 | 0 | 3 | 4.06 × 10−8 |
| DGF Positive | DGF Negative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ME | MIN | MAX | MEAN | SD | ME | MIN | MAX | MEAN | SD | p | |
| EMMPRIN(pg/mL) | 7729.9 | 643.4 | 29,709.47 | 11,658.36 | 8370.301 | 5348.1 | 897.4 | 59,861.6 | 8047.789 | 10,064.92 | 0.00896 |
| N | 16 | 33 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalewajska, M.; Opara-Bajerowicz, M.; Safranow, K.; Pawlik, A.; Ciechanowski, K.; Kwiatkowski, S.; Kwiatkowska, E. The Effects of EMMPRIN/CD147 on Late Function and Histopathological Lesions of the Renal Graft. Biology 2022, 11, 232. https://doi.org/10.3390/biology11020232
Nalewajska M, Opara-Bajerowicz M, Safranow K, Pawlik A, Ciechanowski K, Kwiatkowski S, Kwiatkowska E. The Effects of EMMPRIN/CD147 on Late Function and Histopathological Lesions of the Renal Graft. Biology. 2022; 11(2):232. https://doi.org/10.3390/biology11020232
Chicago/Turabian StyleNalewajska, Magdalena, Martyna Opara-Bajerowicz, Krzysztof Safranow, Andrzej Pawlik, Kazimierz Ciechanowski, Sebastian Kwiatkowski, and Ewa Kwiatkowska. 2022. "The Effects of EMMPRIN/CD147 on Late Function and Histopathological Lesions of the Renal Graft" Biology 11, no. 2: 232. https://doi.org/10.3390/biology11020232
APA StyleNalewajska, M., Opara-Bajerowicz, M., Safranow, K., Pawlik, A., Ciechanowski, K., Kwiatkowski, S., & Kwiatkowska, E. (2022). The Effects of EMMPRIN/CD147 on Late Function and Histopathological Lesions of the Renal Graft. Biology, 11(2), 232. https://doi.org/10.3390/biology11020232






