Sexual Dimorphism in Extracellular Matrix Composition and Viscoelasticity of the Healthy and Inflamed Mouse Brain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and EAE Model
2.2. MRE Acquisition
2.3. Data Reconstruction
2.4. Tissue Processing
2.5. Gene Expression Analysis
2.6. Histology
2.7. Statistical Analysis
3. Results
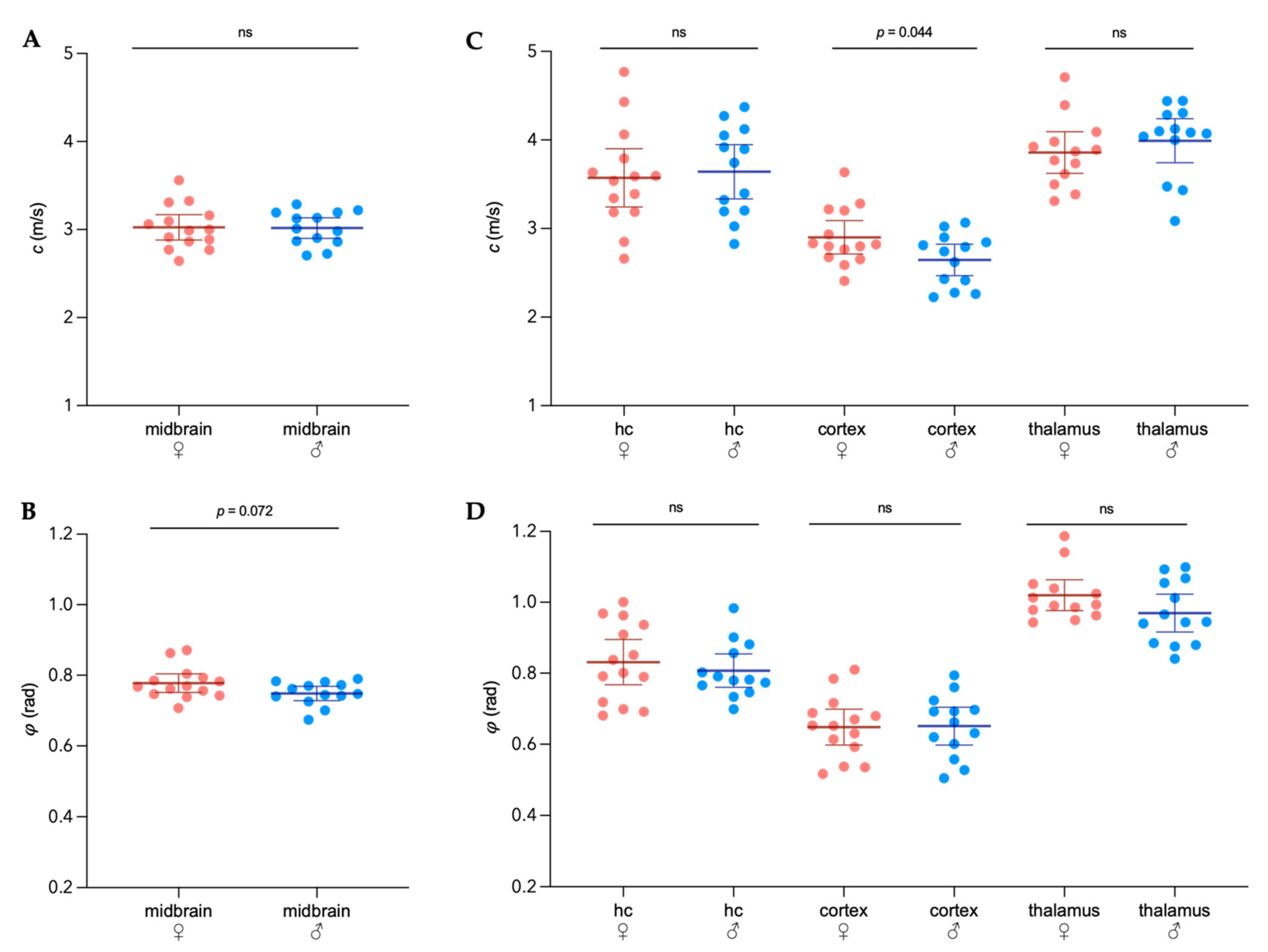
3.1. Sex-Specific Viscoelastic Properties of Healthy Mouse Brain
 ) and male (
) and male (  ) midbrains were compared. The predefined regions of interest were averaged for mean values of shear wave speed c (in m/s) and fluidity φ (in rad). Representative MRE parameter maps for the whole midbrain slice covering the cortex, hippocampus, and thalamus are depicted in Figure 1.
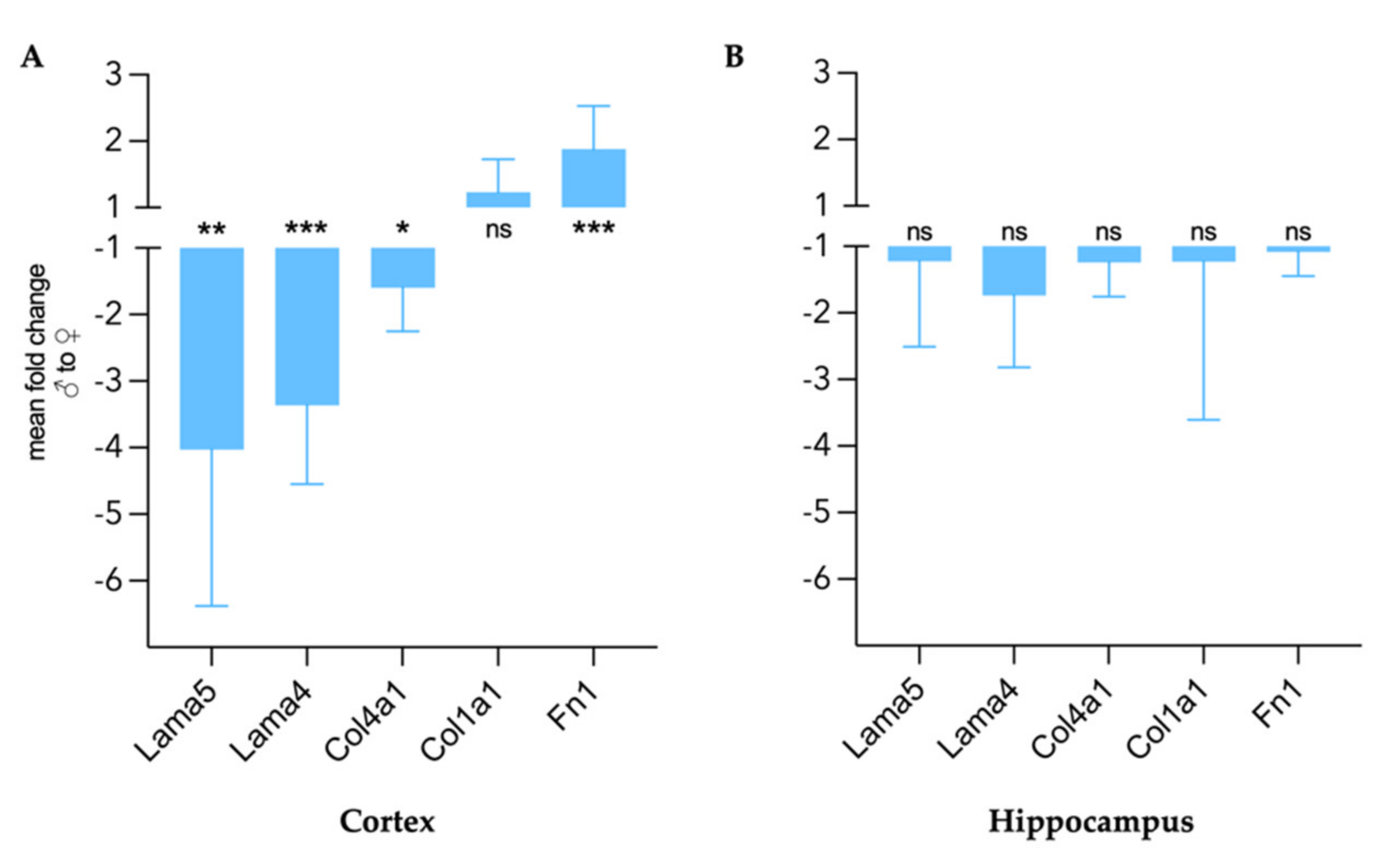
) midbrains were compared. The predefined regions of interest were averaged for mean values of shear wave speed c (in m/s) and fluidity φ (in rad). Representative MRE parameter maps for the whole midbrain slice covering the cortex, hippocampus, and thalamus are depicted in Figure 1.3.2. Differences in Extracellular Matrix Composition in the Healthy Brain of Males and Females
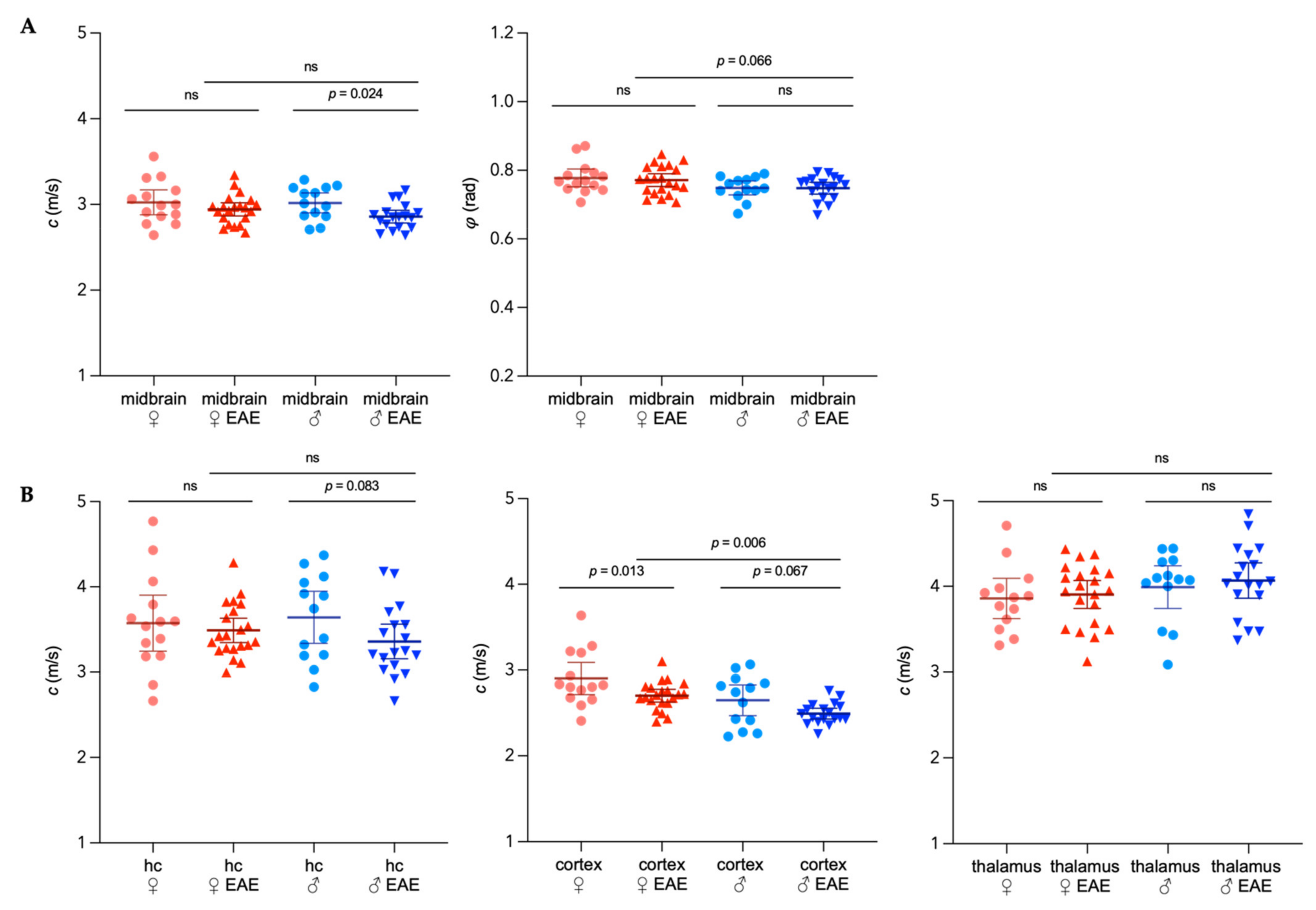
3.3. Sex-Specific Changes of Brain Viscoelastic Properties during EAE
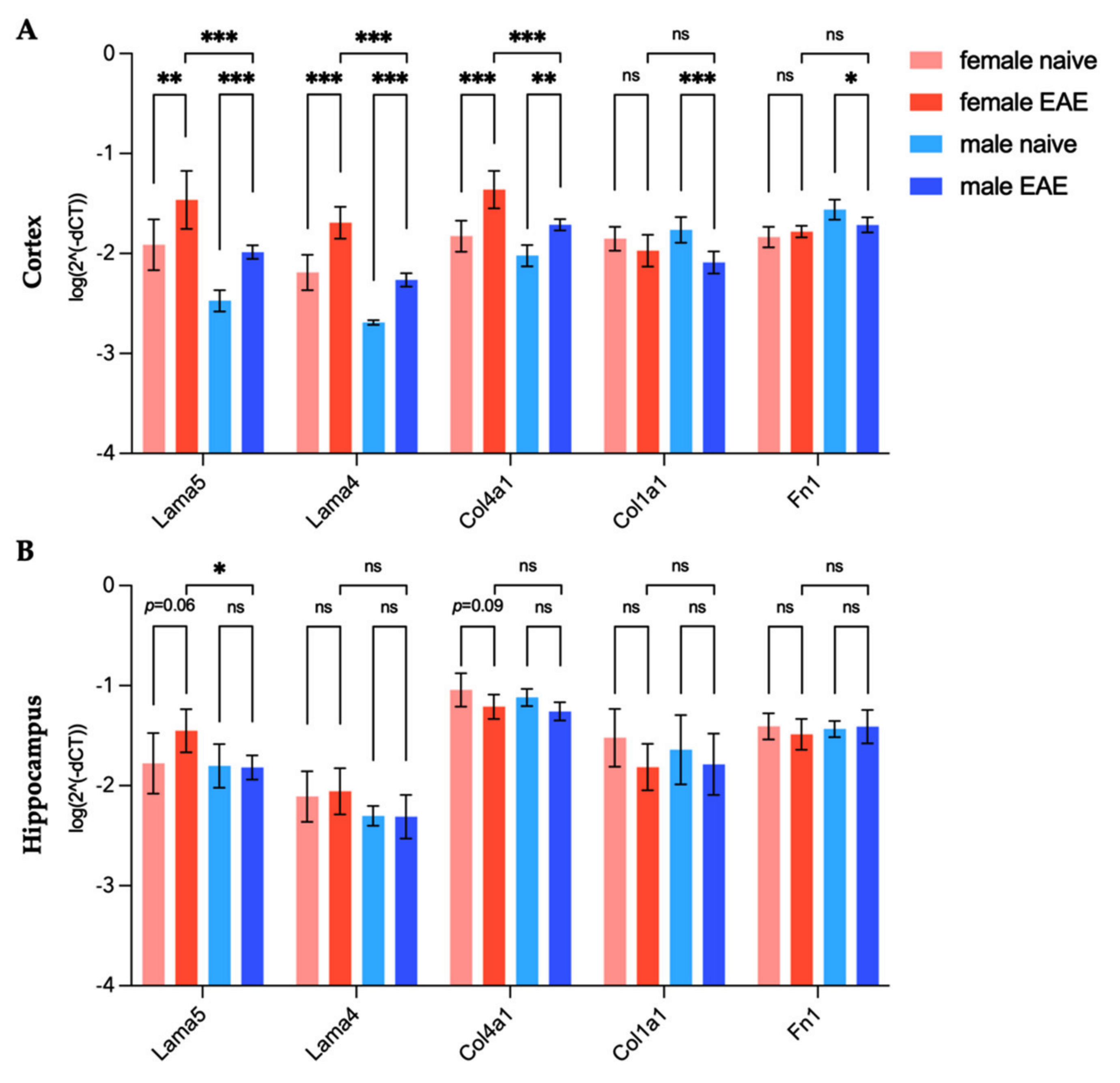
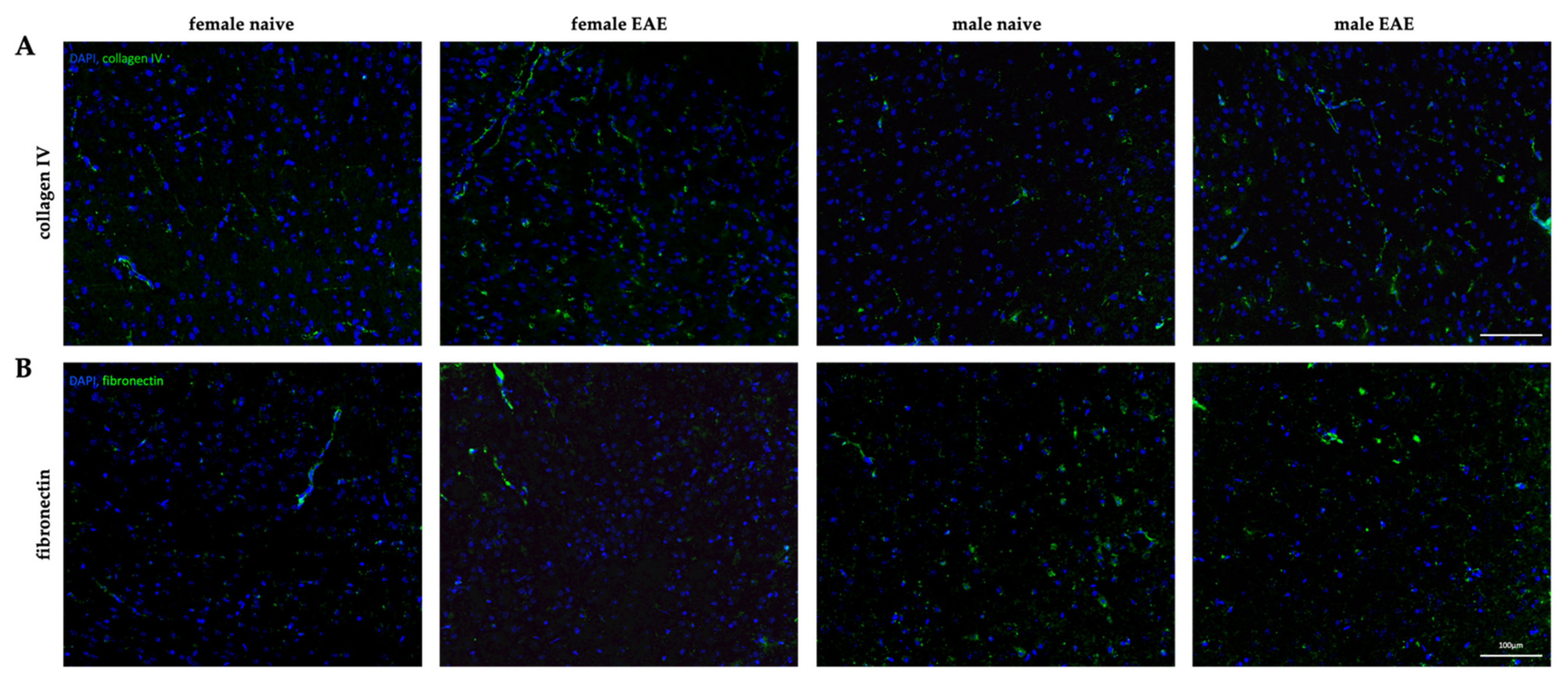
3.4. Extracellular Matrix Remodeling in Female and Male EAE Brains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Multiple Sclerosis International Federation, Atlas of MS, 3rd ed.; Multiple Sclerosis International Federation: London, UK, 2020.
- Gilli, F.; DiSano, K.D.; Pachner, A.R. SeXX Matters in Multiple Sclerosis. Front. Neurol. 2020, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, R. Prognostic Factors in Multiple Sclerosis. Int. Rev. Neurobiol. 2007, 79, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.R.; Patel, K.; Paul, F.; Gold, S.M.; Scheel, M.; Kuchling, J.; Cooper, G.; Asseyer, S.; Chien, C.; Brandt, A.U.; et al. Sex Differences in Brain Atrophy in Multiple Sclerosis. Biol. Sex Differ. 2020, 11, 49. [Google Scholar] [CrossRef]
- Bove, R.; Chitnis, T. The Role of Gender and Sex Hormones in Determining the Onset and Outcome of Multiple Sclerosis. Mult. Scler. J. 2014, 20, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; Gunde, E.; Lee, H. Sex-Based Differences in Multiple Sclerosis (MS): Part II: Rising Incidence of Multiple Sclerosis in Women and the Vulnerability of Men to Progression of This Disease. Curr. Top. Behav. Neurosci. 2015, 26, 57–86. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; Lee, H.; Pavri, F.R.; Zhang, M.A. Sex-Based Differences in Multiple Sclerosis (Part I): Biology of Disease Incidence. Curr. Top. Behav. Neurosci. 2015, 26, 29–56. [Google Scholar] [CrossRef]
- Magyari, M. Gender Differences in Multiple Sclerosis Epidemiology and Treatment Response. Dan. Med. J. 2016, 63, B5212. [Google Scholar]
- Golden, L.C.; Voskuhl, R. The Importance of Studying Sex Differences in Disease: The Example of Multiple Sclerosis. J. Neurosci. Res. 2017, 95, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Koch-Henriksen, N.; Sørensen, P.S. The Changing Demographic Pattern of Multiple Sclerosis Epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef]
- Hiscox, L.V.; Johnson, C.L.; Barnhill, E.; McGarry, M.D.J.; Huston, J.; van Beek, E.J.R.; Starr, J.M.; Roberts, N. Magnetic Resonance Elastography (MRE) of the Human Brain: Technique, Findings and Clinical Applications. Phys. Med. Biol. 2016, 61, R401–R437. [Google Scholar] [CrossRef]
- Wuerfel, J.; Paul, F.; Beierbach, B.; Hamhaber, U.; Klatt, D.; Papazoglou, S.; Zipp, F.; Martus, P.; Braun, J.; Sack, I. MR-Elastography Reveals Degradation of Tissue Integrity in Multiple Sclerosis. NeuroImage 2010, 49, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Sack, I.; Beierbach, B.; Wuerfel, J.; Klatt, D.; Hamhaber, U.; Papazoglou, S.; Martus, P.; Braun, J. The Impact of Aging and Gender on Brain Viscoelasticity. NeuroImage 2009, 46, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Arani, A.; Murphy, M.C.; Glaser, K.J.; Manduca, A.; Lake, D.S.; Kruse, S.A.; Jack, C.R.; Ehman, R.L.; Huston, J. Measuring the Effects of Aging and Sex on Regional Brain Stiffness with MR Elastography in Healthy Older Adults. NeuroImage 2015, 111, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streitberger, K.-J.; Sack, I.; Krefting, D.; Pfüller, C.; Braun, J.; Paul, F.; Wuerfel, J. Brain Viscoelasticity Alteration in Chronic-Progressive Multiple Sclerosis. PLoS ONE 2012, 7, e29888. [Google Scholar] [CrossRef] [Green Version]
- Fehlner, A.; Behrens, J.R.; Streitberger, K.-J.; Papazoglou, S.; Braun, J.; Bellmann-Strobl, J.; Ruprecht, K.; Paul, F.; Würfel, J.; Sack, I. Higher-Resolution MR Elastography Reveals Early Mechanical Signatures of Neuroinflammation in Patients with Clinically Isolated Syndrome. J. Magn. Reson. Imaging JMRI 2016, 44, 51–58. [Google Scholar] [CrossRef]
- Riek, K.; Millward, J.M.; Hamann, I.; Mueller, S.; Pfueller, C.F.; Paul, F.; Braun, J.; Infante-Duarte, C.; Sack, I. Magnetic Resonance Elastography Reveals Altered Brain Viscoelasticity in Experimental Autoimmune Encephalomyelitis. NeuroImage Clin. 2012, 1, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Millward, J.M.; Hanke-Vela, L.; Malla, B.; Pilch, K.; Gil-Infante, A.; Waiczies, S.; Mueller, S.; Boehm-Sturm, P.; Guo, J.; et al. MR Elastography-Based Assessment of Matrix Remodeling at Lesion Sites Associated With Clinical Severity in a Model of Multiple Sclerosis. Front. Neurol. 2019, 10, 1382. [Google Scholar] [CrossRef]
- Silva, R.V.; Morr, A.S.; Mueller, S.; Koch, S.P.; Boehm-Sturm, P.; Rodriguez-Sillke, Y.; Kunkel, D.; Tzschätzsch, H.; Kühl, A.A.; Schnorr, J.; et al. Contribution of Tissue Inflammation and Blood-Brain Barrier Disruption to Brain Softening in a Mouse Model of Multiple Sclerosis. Front. Neurosci. 2021, 15, 701308. [Google Scholar] [CrossRef]
- Schregel, K.; Wuerfel nee Tysiak, E.; Garteiser, P.; Gemeinhardt, I.; Prozorovski, T.; Aktas, O.; Merz, H.; Petersen, D.; Wuerfel, J.; Sinkus, R. Demyelination Reduces Brain Parenchymal Stiffness Quantified in Vivo by Magnetic Resonance Elastography. Proc. Natl. Acad. Sci. USA 2012, 109, 6650–6655. [Google Scholar] [CrossRef] [Green Version]
- Weickenmeier, J.; de Rooij, R.; Budday, S.; Steinmann, P.; Ovaert, T.C.; Kuhl, E. Brain Stiffness Increases with Myelin Content. Acta Biomater. 2016, 42, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Bertalan, G.; Meierhofer, D.; Klein, C.; Schreyer, S.; Steiner, B.; Wang, S.; Vieira da Silva, R.; Infante-Duarte, C.; Koch, S.; et al. Brain Maturation Is Associated with Increasing Tissue Stiffness and Decreasing Tissue Fluidity. Acta Biomater. 2019, 99, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Yong, V.W. The Extracellular Matrix as Modifier of Neuroinflammation and Remyelination in Multiple Sclerosis. Brain 2021, 144, 1958–1973. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Dijkstra, C.D.; De Vries, H.E. The Extracellular Matrix in Multiple Sclerosis Pathology. J. Neurochem. 2007, 103, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.R.; Gold, S.M. Sex-Related Factors in Multiple Sclerosis Susceptibility and Progression. Nat. Rev. Neurol. 2012, 8, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Sasidhar, M.V.; Morales, L.B.; Du, S.; Sicotte, N.L.; Tiwari-Woodruff, S.K.; Voskuhl, R.R. Estrogen Treatment Decreases Matrix Metalloproteinase (MMP)-9 in Autoimmune Demyelinating Disease through Estrogen Receptor Alpha (ERalpha). Lab. Investig. J. Tech. Methods Pathol. 2009, 89, 1076–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, L.W.; Cua, R.; Keough, M.B.; Haylock-Jacobs, S.; Yong, V.W. Pathophysiology of the Brain Extracellular Matrix: A New Target for Remyelination. Nat. Rev. Neurosci. 2013, 14, 722–729. [Google Scholar] [CrossRef]
- Miller, R.T. Mechanical Properties of Basement Membrane in Health and Disease. Matrix Biol. J. Int. Soc. Matrix Biol. 2017, 57–58, 366–373. [Google Scholar] [CrossRef]
- Bertalan, G.; Guo, J.; Tzschätzsch, H.; Klein, C.; Barnhill, E.; Sack, I.; Braun, J. Fast Tomoelastography of the Mouse Brain by Multifrequency Single-Shot MR Elastography. Magn. Reson. Med. 2019, 81, 2676–2687. [Google Scholar] [CrossRef]
- Teuscher, C.; Bunn, J.Y.; Fillmore, P.D.; Butterfield, R.J.; Zachary, J.F.; Blankenhorn, E.P. Gender, Age, and Season at Immunization Uniquely Influence the Genetic Control of Susceptibility to Histopathological Lesions and Clinical Signs of Experimental Allergic Encephalomyelitis. Am. J. Pathol. 2004, 165, 1593–1602. [Google Scholar] [CrossRef]
- Papenfuss, T.L.; Rogers, C.J.; Gienapp, I.; Yurrita, M.; McClain, M.; Damico, N.; Valo, J.; Song, F.; Whitacre, C.C. Sex Differences in Experimental Autoimmune Encephalomyelitis in Multiple Murine Strains. J. Neuroimmunol. 2004, 150, 59–69. [Google Scholar] [CrossRef]
- Millward, J.M.; Guo, J.; Berndt, D.; Braun, J.; Sack, I.; Infante-Duarte, C. Tissue Structure and Inflammatory Processes Shape Viscoelastic Properties of the Mouse Brain. NMR Biomed. 2015, 28, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Millward, J.M.; Ariza de Schellenberger, A.; Berndt, D.; Hanke-Vela, L.; Schellenberger, E.; Waiczies, S.; Taupitz, M.; Kobayashi, Y.; Wagner, S.; Infante-Duarte, C. Application of Europium-Doped Very Small Iron Oxide Nanoparticles to Visualize Neuroinflammation with MRI and Fluorescence Microscopy. Neuroscience 2019, 403, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Powell, K.L.; May, A.; Semple, B.D. Validation of Reference Genes for Gene Expression Analysis Following Experimental Traumatic Brain Injury in a Pediatric Mouse Model. Brain Res. Bull. 2020, 156, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wu, Z.; Cai, D.; Lu, B. Evaluation of Reference Genes for Gene Expression Studies in Mouse and N2a Cell Ischemic Stroke Models Using Quantitative Real-Time PCR. BMC Neurosci. 2018, 19, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennasroune, A.; Romier-Crouzet, B.; Blaise, S.; Laffargue, M.; Efremov, R.G.; Martiny, L.; Maurice, P.; Duca, L. Elastic Fibers and Elastin Receptor Complex: Neuraminidase-1 Takes the Center Stage. Matrix Biol. J. Int. Soc. Matrix Biol. 2019, 84, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Culav, E.M.; Clark, C.H.; Merrilees, M.J. Connective Tissues: Matrix Composition and Its Relevance to Physical Therapy. Phys. Ther. 1999, 79, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarnieri, D.; Battista, S.; Borzacchiello, A.; Mayol, L.; De Rosa, E.; Keene, D.R.; Muscariello, L.; Barbarisi, A.; Netti, P.A. Effects of Fibronectin and Laminin on Structural, Mechanical and Transport Properties of 3D Collageneous Network. J. Mater. Sci. Mater. Med. 2007, 18, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Storm, C.; Pastore, J.J.; MacKintosh, F.C.; Lubensky, T.C.; Janmey, P.A. Nonlinear Elasticity in Biological Gels. Nature 2005, 435, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zheng, Y.; Han, Y.L.; Cai, S.; Guo, M. Nonlinear Elasticity of Biological Basement Membrane Revealed by Rapid Inflation and Deflation. Proc. Natl. Acad. Sci. USA 2021, 118, e2022422118. [Google Scholar] [CrossRef]
- Bhave, G.; Colon, S.; Ferrell, N. The Sulfilimine Cross-Link of Collagen IV Contributes to Kidney Tubular Basement Membrane Stiffness. Am. J. Physiol. Ren. Physiol. 2017, 313, F596–F602. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Pareja, J.C.; Xu, T. Shaping Cells and Organs in Drosophila by Opposing Roles of Fat Body-Secreted Collagen IV and Perlecan. Dev. Cell 2011, 21, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candiello, J.; Balasubramani, M.; Schreiber, E.M.; Cole, G.J.; Mayer, U.; Halfter, W.; Lin, H. Biomechanical Properties of Native Basement Membranes. FEBS J. 2007, 274, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.B.; Phalan, F.C.; Breedveld, G.J.; van Mil, S.E.; Smith, R.S.; Schimenti, J.C.; Aguglia, U.; van der Knaap, M.S.; Heutink, P.; John, S.W.M. Mutations in Col4a1 Cause Perinatal Cerebral Hemorrhage and Porencephaly. Science 2005, 308, 1167–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, D.B.; Phalan, F.C.; van Mil, S.E.; Sundberg, J.P.; Vahedi, K.; Massin, P.; Bousser, M.G.; Heutink, P.; Miner, J.H.; Tournier-Lasserve, E.; et al. Role of COL4A1 in Small-Vessel Disease and Hemorrhagic Stroke. N. Engl. J. Med. 2006, 354, 1489–1496. [Google Scholar] [CrossRef] [Green Version]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV Is Essential for Basement Membrane Stability but Dispensable for Initiation of Its Assembly during Early Development. Dev. Camb. Engl. 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [Green Version]
- Leclech, C.; Natale, C.F.; Barakat, A.I. The Basement Membrane as a Structured Surface—Role in Vascular Health and Disease. J. Cell Sci. 2020, 133, jcs239889. [Google Scholar] [CrossRef]
- Yurchenco, P.D. Basement Membranes: Cell Scaffoldings and Signaling Platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, M.A.; Sherwood, D.R. An Active Role for Basement Membrane Assembly and Modification in Tissue Sculpting. J. Cell Sci. 2015, 128, 1661–1668. [Google Scholar] [CrossRef] [Green Version]
- Sarver, D.C.; Kharaz, Y.A.; Sugg, K.B.; Gumucio, J.P.; Comerford, E.; Mendias, C.L. Sex Differences in Tendon Structure and Function. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2017, 35, 2117–2126. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Shin, S.-C.; Park, G.-C.; Lee, J.-C.; Jeon, Y.K.; Ahn, S.J.; Thibeault, S.; Lee, B.-J. Effect of Sex Hormones on Extracellular Matrix of Lamina Propria in Rat Vocal Fold. Laryngoscope 2020, 130, 732–740. [Google Scholar] [CrossRef]
- Soldano, S.; Montagna, P.; Villaggio, B.; Parodi, A.; Gianotti, G.; Sulli, A.; Seriolo, B.; Secchi, M.E.; Cutolo, M. Endothelin and Sex Hormones Modulate the Fibronectin Synthesis by Cultured Human Skin Scleroderma Fibroblasts. Ann. Rheum. Dis. 2009, 68, 599–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehlet, S.N.; Willumsen, N.; Armbrecht, G.; Dietzel, R.; Brix, S.; Henriksen, K.; Karsdal, M.A. Age-Related Collagen Turnover of the Interstitial Matrix and Basement Membrane: Implications of Age- and Sex-Dependent Remodeling of the Extracellular Matrix. PLoS ONE 2018, 13, e0194458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, G.; Phillips, T.J. Estrogen and Skin: The Effects of Estrogen, Menopause, and Hormone Replacement Therapy on the Skin. J. Am. Acad. Dermatol. 2005, 53, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Freimann, F.B.; Müller, S.; Streitberger, K.-J.; Guo, J.; Rot, S.; Ghori, A.; Vajkoczy, P.; Reiter, R.; Sack, I.; Braun, J. MR Elastography in a Murine Stroke Model Reveals Correlation of Macroscopic Viscoelastic Properties of the Brain with Neuronal Density. NMR Biomed. 2013, 26, 1534–1539. [Google Scholar] [CrossRef]
- Pakkenberg, B.; Gundersen, H.J. Neocortical Neuron Number in Humans: Effect of Sex and Age. J. Comp. Neurol. 1997, 384, 312–320. [Google Scholar] [CrossRef]
- Rabinowicz, T.; Petetot, J.M.-C.; Gartside, P.S.; Sheyn, D.; Sheyn, T.; de Courten-Myers, G.M. Structure of the Cerebral Cortex in Men and Women. J. Neuropathol. Exp. Neurol. 2002, 61, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Mouton, P.R.; Long, J.M.; Lei, D.-L.; Howard, V.; Jucker, M.; Calhoun, M.E.; Ingram, D.K. Age and Gender Effects on Microglia and Astrocyte Numbers in Brains of Mice. Brain Res. 2002, 956, 30–35. [Google Scholar] [CrossRef]
- Cahill, L. Why Sex Matters for Neuroscience. Nat. Rev. Neurosci. 2006, 7, 477–484. [Google Scholar] [CrossRef]
- Chou, X.-L.; Fang, Q.; Yan, L.; Zhong, W.; Peng, B.; Li, H.; Wei, J.; Tao, H.W.; Zhang, L.I. Contextual and Cross-Modality Modulation of Auditory Cortical Processing through Pulvinar Mediated Suppression. eLife 2020, 9, e54157. [Google Scholar] [CrossRef]
- Fang, Q.; Chou, X.-L.; Peng, B.; Zhong, W.; Zhang, L.I.; Tao, H.W. A Differential Circuit via Retino-Colliculo-Pulvinar Pathway Enhances Feature Selectivity in Visual Cortex through Surround Suppression. Neuron 2020, 105, 355–369.e6. [Google Scholar] [CrossRef]
- Huang, A.S.; Rogers, B.P.; Sheffield, J.M.; Vandekar, S.; Anticevic, A.; Woodward, N.D. Characterizing Effects of Age, Sex and Psychosis Symptoms on Thalamocortical Functional Connectivity in Youth. NeuroImage 2021, 243, 118562. [Google Scholar] [CrossRef] [PubMed]
- Kipp, M.; Nyamoya, S.; Hochstrasser, T.; Amor, S. Multiple Sclerosis Animal Models: A Clinical and Histopathological Perspective. Brain Pathol. 2017, 27, 123–137. [Google Scholar] [CrossRef]
- Scheld, M.; Rüther, B.J.; Große-Veldmann, R.; Ohl, K.; Tenbrock, K.; Dreymüller, D.; Fallier-Becker, P.; Zendedel, A.; Beyer, C.; Clarner, T.; et al. Neurodegeneration Triggers Peripheral Immune Cell Recruitment into the Forebrain. J. Neurosci. 2016, 36, 1410. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hirsch, S.; Fehlner, A.; Papazoglou, S.; Scheel, M.; Braun, J.; Sack, I. Towards an Elastographic Atlas of Brain Anatomy. PLoS ONE 2013, 8, e71807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, J.; Guo, J.; Lützkendorf, R.; Stadler, J.; Papazoglou, S.; Hirsch, S.; Sack, I.; Bernarding, J. High-Resolution Mechanical Imaging of the Human Brain by Three-Dimensional Multifrequency Magnetic Resonance Elastography at 7T. NeuroImage 2014, 90, 308–314. [Google Scholar] [CrossRef]
- Streitberger, K.-J.; Fehlner, A.; Pache, F.; Lacheta, A.; Papazoglou, S.; Bellmann-Strobl, J.; Ruprecht, K.; Brandt, A.; Braun, J.; Sack, I.; et al. Multifrequency Magnetic Resonance Elastography of the Brain Reveals Tissue Degeneration in Neuromyelitis Optica Spectrum Disorder. Eur. Radiol. 2017, 27, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Moeendarbary, E.; Weber, I.P.; Sheridan, G.K.; Koser, D.E.; Soleman, S.; Haenzi, B.; Bradbury, E.J.; Fawcett, J.; Franze, K. The Soft Mechanical Signature of Glial Scars in the Central Nervous System. Nat. Commun. 2017, 8, 14787. [Google Scholar] [CrossRef]
- Embry, A.E.; Liu, Z.; Henderson, J.M.; Byfield, F.J.; Liu, L.; Yoon, J.; Wu, Z.; Cruz, K.; Moradi, S.; Gillombardo, C.B.; et al. Similar Biophysical Abnormalities in Glomeruli and Podocytes from Two Distinct Models. J. Am. Soc. Nephrol. JASN 2018, 29, 1501–1512. [Google Scholar] [CrossRef] [Green Version]
- Rempe, R.G.; Hartz, A.M.; Bauer, B. Matrix Metalloproteinases in the Brain and Blood-Brain Barrier: Versatile Breakers and Makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [Green Version]
- Sobel, R.A. The Extracellular Matrix in Multiple Sclerosis: An Update. Braz. J. Med. Biol. Res. 2001, 34, 603–609. [Google Scholar] [CrossRef]
- Zeis, T.; Kinter, J.; Herrero-Herranz, E.; Weissert, R.; Schaeren-Wiemers, N. Gene Expression Analysis of Normal Appearing Brain Tissue in an Animal Model for Multiple Sclerosis Revealed Grey Matter Alterations, but Only Minor White Matter Changes. J. Neuroimmunol. 2008, 205, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Pathogenic Mechanisms Associated With Different Clinical Courses of Multiple Sclerosis. Front. Immunol. 2019, 9, 3116. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, P.; Khoshneviszadeh, M.; Jandke, S.; Schreiber, S.; Dityatev, A. Interplay between Perivascular and Perineuronal Extracellular Matrix Remodelling in Neurological and Psychiatric Diseases. Eur. J. Neurosci. 2021, 53, 3811–3830. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.G.; Sim, H.J.; Song, E.K.; Kwon, T.; Park, T.J. Extracellular Matrixes and Neuroinflammation. BMB Rep. 2020, 53, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Streitberger, K.-J.; Lilaj, L.; Schrank, F.; Braun, J.; Hoffmann, K.-T.; Reiss-Zimmermann, M.; Käs, J.A.; Sack, I. How Tissue Fluidity Influences Brain Tumor Progression. Proc. Natl. Acad. Sci. USA 2020, 117, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. The Impact of Extracellular Matrix Viscoelasticity on Cellular Behavior. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Elosegui-Artola, A. The Extracellular Matrix Viscoelasticity as a Regulator of Cell and Tissue Dynamics. Curr. Opin. Cell Biol. 2021, 72, 10–18. [Google Scholar] [CrossRef]
- Van Wageningen, T.A.; Antonovaite, N.; Paardekam, E.; Brevé, J.J.P.; Iannuzzi, D.; van Dam, A.-M. Viscoelastic Properties of White and Gray Matter-Derived Microglia Differentiate upon Treatment with Lipopolysaccharide but Not upon Treatment with Myelin. J. Neuroinflammation 2021, 18, 83. [Google Scholar] [CrossRef]
- Previtera, M.L.; Langhammer, C.G.; Firestein, B.L. Effects of Substrate Stiffness and Cell Density on Primary Hippocampal Cultures. J. Biosci. Bioeng. 2010, 110, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Georges, P.C.; Miller, W.J.; Meaney, D.F.; Sawyer, E.S.; Janmey, P.A. Matrices with Compliance Comparable to That of Brain Tissue Select Neuronal over Glial Growth in Mixed Cortical Cultures. Biophys. J. 2006, 90, 3012–3018. [Google Scholar] [CrossRef] [Green Version]
- Urbanski, M.M.; Kingsbury, L.; Moussouros, D.; Kassim, I.; Mehjabeen, S.; Paknejad, N.; Melendez-Vasquez, C.V. Myelinating Glia Differentiation Is Regulated by Extracellular Matrix Elasticity. Sci. Rep. 2016, 6, 33751. [Google Scholar] [CrossRef] [PubMed]

| Cortex | Hippocampus | |||||
|---|---|---|---|---|---|---|
| Gene |  EAE/Naive |  EAE/Naive | EAE  / /  |  EAE/Naive |  EAE/Naive EAE/Naive | EAE  / /  |
| Lama5 | 2.91 | 3.04 | −3.85 | 1.83 | −1.08 | −2.42 |
| Lama4 | 3.10 | 2.70 | −3.86 | 1.08 | 1.11 | −1.69 |
| Col4a1 | 2.99 | 2.01 | −2.38 | −1.51 | −1.37 | −1.12 |
| Col1a1 | −1.29 | −2.12 | −1.34 | −2.07 | −1.33 | 1.26 |
| Fn1 | 1.12 | −1.43 | 1.18 | −1.19 | 1.14 | 1.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batzdorf, C.S.; Morr, A.S.; Bertalan, G.; Sack, I.; Silva, R.V.; Infante-Duarte, C. Sexual Dimorphism in Extracellular Matrix Composition and Viscoelasticity of the Healthy and Inflamed Mouse Brain. Biology 2022, 11, 230. https://doi.org/10.3390/biology11020230
Batzdorf CS, Morr AS, Bertalan G, Sack I, Silva RV, Infante-Duarte C. Sexual Dimorphism in Extracellular Matrix Composition and Viscoelasticity of the Healthy and Inflamed Mouse Brain. Biology. 2022; 11(2):230. https://doi.org/10.3390/biology11020230
Chicago/Turabian StyleBatzdorf, Clara Sophie, Anna Sophie Morr, Gergely Bertalan, Ingolf Sack, Rafaela Vieira Silva, and Carmen Infante-Duarte. 2022. "Sexual Dimorphism in Extracellular Matrix Composition and Viscoelasticity of the Healthy and Inflamed Mouse Brain" Biology 11, no. 2: 230. https://doi.org/10.3390/biology11020230
APA StyleBatzdorf, C. S., Morr, A. S., Bertalan, G., Sack, I., Silva, R. V., & Infante-Duarte, C. (2022). Sexual Dimorphism in Extracellular Matrix Composition and Viscoelasticity of the Healthy and Inflamed Mouse Brain. Biology, 11(2), 230. https://doi.org/10.3390/biology11020230







