Increasing the Efficiency of Canola and Soybean GMO Detection and Quantification Using Multiplex Droplet Digital PCR
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Seeds and Reference Materials
2.2. DNA Extraction and Quantification
2.3. Droplet Digital PCR
3. Results and Discussion
3.1. Optimization of the Probe Concentrations
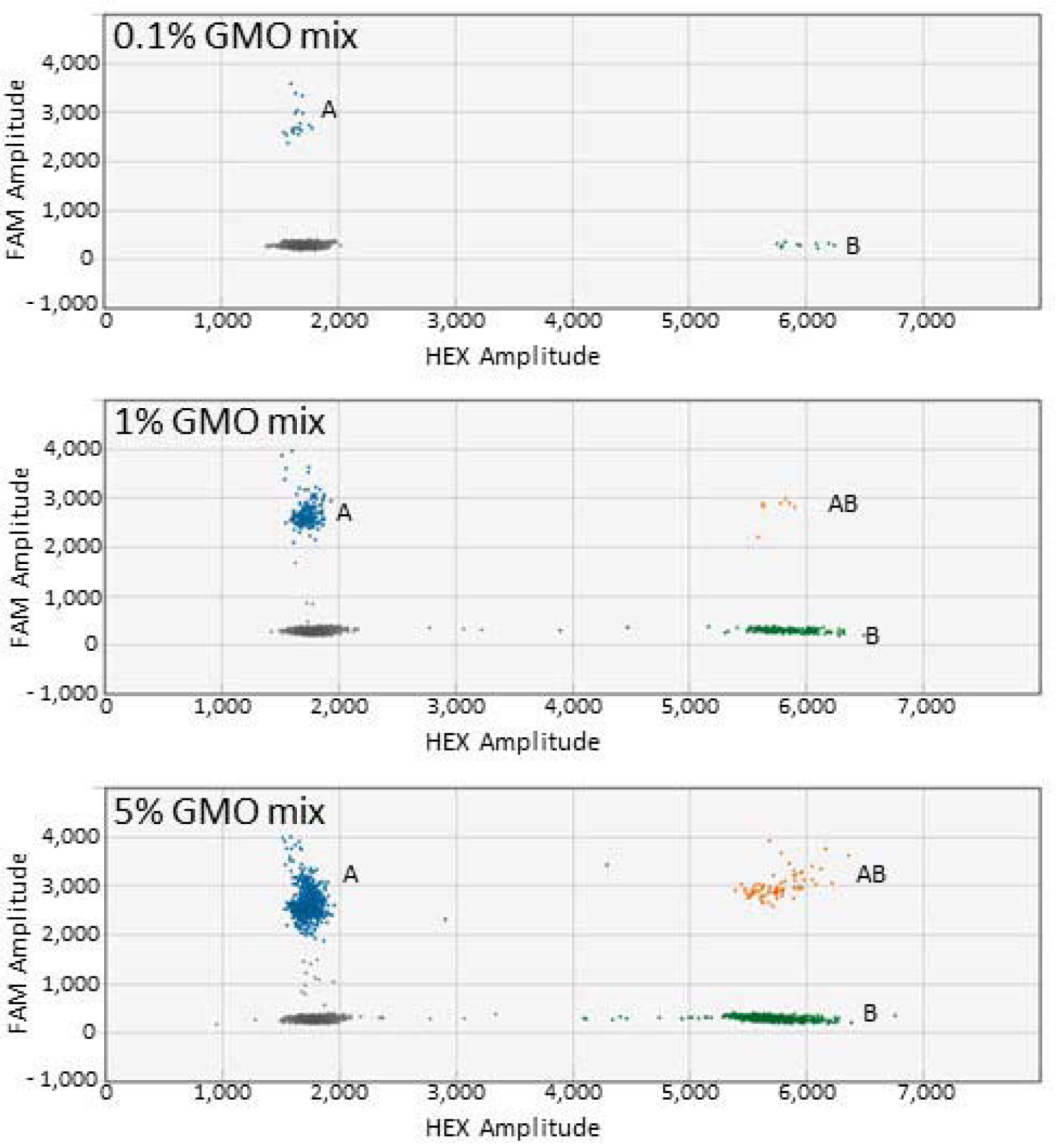
3.2. Detection of Two GM Events (Duplex ddPCR)
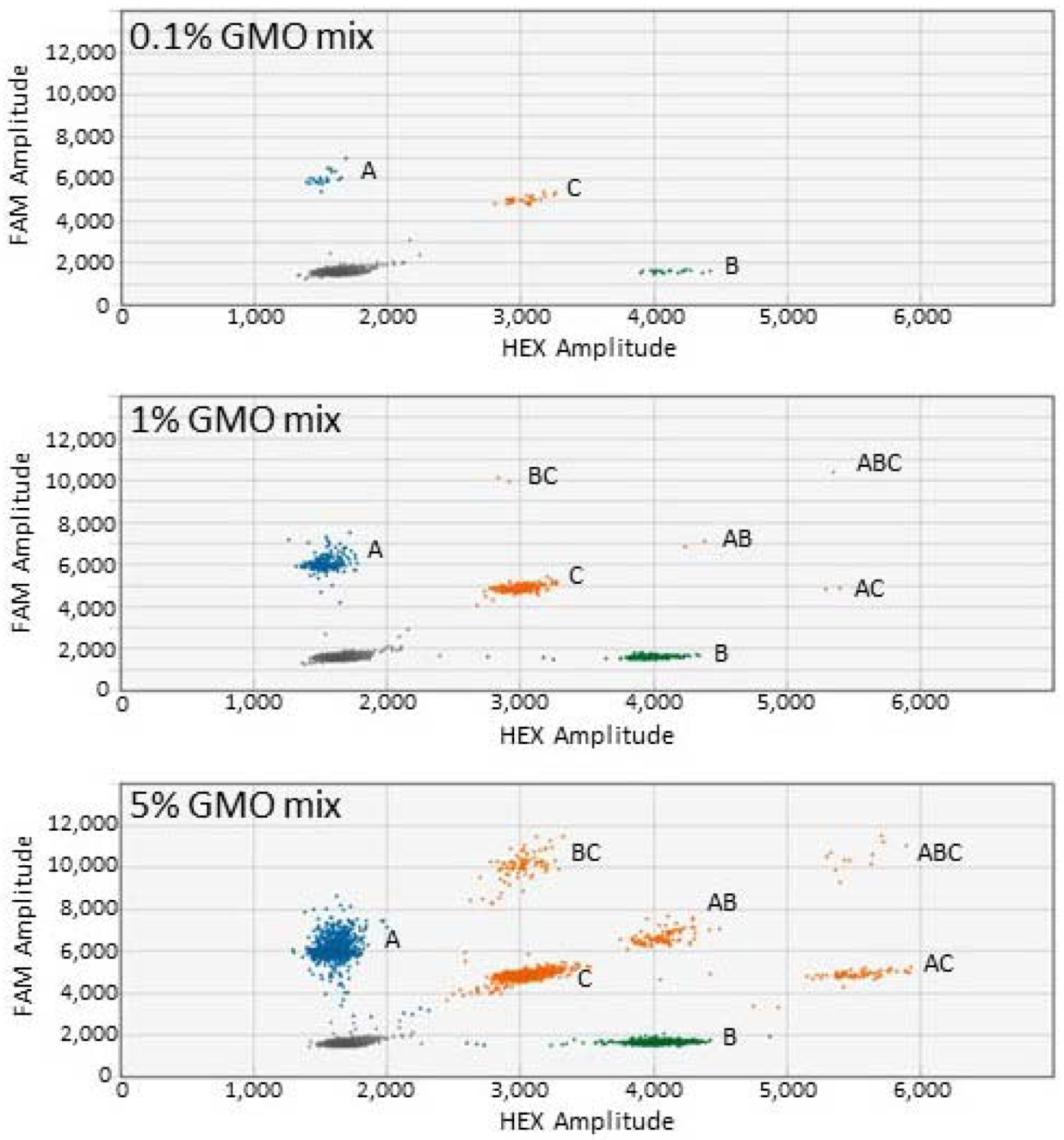
3.3. Detection of Three GM Events (Triplex ddPCR)
3.4. Detection of Four GM Events (Tetraplex ddPCR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, C. Global Status of Commercialized Biotech/GM Crops: International Service for Acquisition of Ari-Biotech Applications; ISAAA Brief 52; The International Service for the Acquisition of Agri-Biotech Applications (ISAAA): Manila, Philippines, 2016; Available online: https://www.isaaa.org/resources/publications/briefs/52/executivesummary/default.asp (accessed on 17 January 2022).
- European Commission. Regulation (EC) No. 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. Off. J. Eur. Union 2003, 268, 1–23. [Google Scholar]
- Vigani, M.; Raimondi, V.; Olper, A. International trade and endogenous standards: The case of GMO regulations. World Trade Rev. 2012, 11, 415–437. [Google Scholar] [CrossRef]
- Rosculete, E.; Bonciu, E.; Rosculete, C.A.; Teleanu, E. Detection and quantification of genetically modified soybean in some food and feed products. A case study on products available on Romanian market. Sustainability 2018, 10, 1325. [Google Scholar] [CrossRef]
- Peng, C.; Zheng, M.; Ding, L.; Chen, X.; Wang, X.; Feng, X.; Wang, J.; Xu, J. Accurate detection and evaluation of the gene-editing frequency in plants using droplet digital PCR. Front. Plant Sci. 2020, 11, 610790. [Google Scholar] [CrossRef]
- Cottenet, G.; Blancpain, C.; Sonnard, V.; Chuah, P.F. Development and validation of a multiplex real-time pCR method to simultaneously detect 47 targets for the identification of genetically modified organisms. Anal. Bioanal. Chem. 2013, 405, 6831–6844. [Google Scholar] [CrossRef]
- Mano, J.; Hatano, S.; Nagatomi, Y.; Futo, S.; Takabatake, R.; Kitta, K. Highly sensitive GMO detection using real-time PCR with a large amount of DNA template: Single-laboratory validation. J. AOAC Int. 2018, 101, 507–514. [Google Scholar] [CrossRef]
- Peng, C.; Wang, P.; Xu, X.; Wang, X.; Wei, W.; Chen, X.; Xu, J. Development of a qualitative real-time PCR method to detect 19 targets for identification of genetically modified organisms. SpringerPlus 2016, 5, 889. [Google Scholar] [CrossRef]
- Scholtens, I.M.J.; Kok, E.J.; Hougs, L.; Molenaar, B.; Thissen, J.T.N.M.; van der Voet, M. Increased efficacy for in-house validation of real-time PCR GMO detection methods. Anal. Bioanal. Chem. 2010, 396, 2213–2227. [Google Scholar] [CrossRef][Green Version]
- European Commission. EU Commission Regulation No. 619/2011. Laying down the methods of sampling and analysis for the official control of feed as regards presence of genetically modified material for which an authorisation procedure is pending or the authorisation of which has expired. Off. J. Eur. Union 2011, 166, 9–15. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0619&from=EN (accessed on 4 January 2022).
- Quan, P.L.; Sauzade, M.; Brouzes, E. dPCR: A technology review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef]
- The dMIQE Group; Huggett, J.F. The digital MIQE guidelines update: Minimum information for publication of quantitative digital PCR experiments for 2020. Clin. Chem. 2020, 66, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.B.; O’Brien, H.; Druce, J.; Do, H.; Kay, P.; Daniels, M.; You, J.; Burke, D.; Griffiths, K.; Emslie, R. Interlaboratory reproducibility of droplet digital polymerase chain reaction using a new DNA reference material format. Anal. Chem. 2017, 89, 11243–11251. [Google Scholar] [CrossRef] [PubMed]
- Whale, A.S.; Devonshire, A.S.; Karlin-Neumann, G.; Regan, J.; Javier, L.; Cowen, S.; Fernandez-Gonzalez, A.; Jones, J.M.; Redshaw, N.; Beck, J.; et al. International interlaboratory digital PCR study demonstrating high reproducibility for the measurement of a rare sequence variant. Anal. Chem. 2017, 89, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Iwobi, A.; Gerdes, L.; Busch, U.; Pecoraro, S. Droplet digital PCR for routine analysis of genetically modified foods (GMOs)—A comparison with real-time quantitative PCR. Food Control 2016, 69, 205–213. [Google Scholar] [CrossRef]
- Nixon, G.; Garson, J.A.; Grant, P.; Nastouli, E.; Foy, C.A.; Huggett, J.F. Comparative study of sensitivity, linearity, and resistance to inhibition of digital and nondigital polymerase chain reaction and loop mediated isothermal amplification assays for quantification of human cytomegalovirus. Anal Chem. 2014, 86, 4387–4394. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, Q.; Yin, Y.; Wang, Z. Comparison of droplet digital PCR and quantitative PCR assays for quantitative detection of Xanthomonas citri Subsp. citri. PLoS ONE 2016, 11, e0159004. [Google Scholar] [CrossRef]
- Demeke, T.; Dobnik, D. Critical assessment of digital PCR for the detection and quantification of genetically modified organisms. Anal. Bioanal. Chem. 2018, 410, 4039–4050. [Google Scholar] [CrossRef]
- Whale, A.S.; Cowen, S.; Foy, C.A.; Huggett, J.F. Methods for applying accurate Digital PCR analysis on Low Copy DNA Samples. PLoS ONE 2013, 8, e58177. [Google Scholar] [CrossRef]
- Collier, R.; Dasgupta, K.; Xing, Y.P.; Hernandez, B.T.; Shao, M.; Rohozinski, D.; Kovak, E.; Lin, J.; de Oliveira, M.L.P.; Stover, E.; et al. Accurate measurement of transgene copy number in crop plants using droplet digital PCR. Plant J. 2017, 90, 1014–1025. [Google Scholar] [CrossRef]
- Long, L.; Yan, W.; He, Y.; Dong, L.; Xing, Z.; Li, C.; Xia, W.; Li, F. Development of a duplex digital PCR method to quantify five genetically modified soybean events. Food Anal. Methods 2021, 7, 8601. [Google Scholar] [CrossRef]
- Morisset, D.; Štebih, D.; Milavec, M.; Gruden, K.; Žel, J. Quantitative analysis of food and feed samples with droplet digital PCR. PLoS ONE 2013, 8, e62583. [Google Scholar] [CrossRef]
- Zhong, Q.; Bhattacharya, S.; Kotsopoulos, S.; Olson, J.; Taly, V.; Griffiths, A.D.; Link, D.R.; Larson, J.W. Multiplex digital PCR: Breaking the one target per color barrier of quantitative PCR. Lab Chip 2011, 11, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Dobnik, D.; Štebih, D.; Blejec, A.; Morisset, D.; Žel, J. Multiplex quantification of four DNA targets in one reaction with Bio-Rad droplet digital PCR system for GMO detection. Sci. Rep. 2016, 6, 35451. [Google Scholar] [CrossRef] [PubMed]
- Whale, A.S.; Huggett, J.F.; Tzonev, S. Fundamentals of multiplexing with digital PCR. Biomol. Detect. Quantif. 2016, 10, 15–23. [Google Scholar] [CrossRef]
- The International Service for the Acquisition of Agri-Biotech Applications (ISAAA). Global Status of Commercialized Biotech/GM Crops; ISAAA Brief 54; The International Service for the Acquisition of Agri-Biotech Applications: Manila, Philippines, 2018; Available online: https://www.isaaa.org/resources/publications/briefs/54/default.asp (accessed on 10 January 2020).
- Demeke, T.; Eng, M.; Holigroski, M.; Lee, S.-J. Effect of amount of DNA on Digital PCR assessment of genetically engineered canola and soybean events. Food Anal. Methods 2021, 14, 372–379. [Google Scholar] [CrossRef]
- Henderson, N.; Harmon, M.; Zhong, C.X. PCR-based detection and quantification of a transgenic glyphosate-tolerant canola using a novel reference gene system. Food Anal. Methods 2016, 9, 353–361. [Google Scholar] [CrossRef]
- European Union Reference Laboratory for Genetically Modified Food (EURL-GMFF). Event-Specific Method for Quantification of Soybean Line 40-3-2 Using Real-Time PCR; Joint Research Centre: Ispra, Italy, 2009; Available online: https://gmo-crl.jrc.ec.europa.eu/summaries/40-3-2_validated_Method.pdf (accessed on 10 January 2022).
- European Reference Laboratory for GM Food and Feed (EURL-GMFF). Method Validations. Available online: https://gmo-crl.jrc.ec.europa.eu/method-validations (accessed on 10 January 2022).
- Wu, G.; Wu, Y.; Xiao, L.; Lu, C. Event-specific qualitative and quantitative PCR methods for the detection of genetically modified rapeseed Oxy-235. Transgenic Res. 2008, 17, 851–862. [Google Scholar] [CrossRef]
- European Reference Laboratory for GM Food and Feed (EURL-GMFF). Event-Specific Method for the Quantification of Soybean Event DP305423-1 Using Real-Time PCR; Joint Research Centre: Ispra, Italy, 2009; Available online: https://gmo-crl.jrc.ec.europa.eu/summaries/CRLVL0707VP%20Corr2%20EURL%20web.pdf (accessed on 8 January 2022).


| Crop | Event Name | Primer/Probe Name | DNA Sequences (5′ to 3′) | Amplicon Length (bp) |
|---|---|---|---|---|
| CANOLA | GT73 | RT73-1 | CCA TAT TGA CCA TCA TAC TCA TTG CT | 108 |
| RT73-2 | GCT TAT ACG AAG GCA AGA AAA GGA | |||
| RT73 (P) | TTC CCG GAC ATG AAG ATC ATC CTC CTT-BHQ1 | |||
| OXY235 | Oxy RG | GAT AGA TGG TGG TGT GAG TCT TGT | 124 | |
| OXY RV | CCT AAC TTT TGG TGT GAT GAT GCT | |||
| Oxy RP | TGC CAT CAG CTG ACA CGC CGT GC-BHQ1 | |||
| HCN92 | MDB685 | GTT GCG GTT CTG TCA GTT CC | 95 | |
| KVM180 | CGA CCG GCG CTG ATA TAT GA | |||
| TM029 | TCC CGC GTC ATC GGC GG-BHQ1 | |||
| MON88302 | 88302QF | TCC TTG AAC CTT ATT TTA TAG TGC ACA | 101 | |
| 88302QR | TCA GAT TGT CGT TTC CCG CCT TCA | |||
| 88302QP | TAG TCA TCA TGT TGT ACC ACT TCA AAC ACT-BHQ1 | |||
| FatA(A) | 09-0-2824-F | ACA GAT GAA GTT CGG GAC GAG TAC | 84 | |
| 09-0-2825-R | CAG GTT GAG ATC CAC ATG CTT AAA TAT | |||
| 09-QP-87-P | AAG AAG AAT CAT CAT GCT TC-BHQ1 | |||
| SOYBEAN | DP305423 | DP305 F1 | CGT GTT CTC TTT TTG GCT AGC | 93 |
| DP305 R5 | GTG ACC AAT GAA TAC ATA ACA CAA ACT A | |||
| DP305423 P | TGA CAC AAA TGA TTT TCA TAC AAA AGT CGA GA-BHQ1 | |||
| A2704 | KVM175 | GCA AAA AAG CGG TTA GCT CCT | 74 | |
| SMO 001 | ATT CAG GCT GCG CAA CTG TT | |||
| TM021 | CGG TCC TCC GAT CGC CCT TCC-BHQ1 | |||
| MON89788 | MON89788 F | TCC CGC TCT AGC GCT TCA AT | 139 | |
| MON89788 R | TCG AGC AGG ACC TGC AGA A | |||
| MON89788 P | CTG AAG GCG GGA AAC GAC AAT CTG-BHQ1 | |||
| DAS81419 | DAS81419 F2 | TCT AGC TAT ATT TAG CAC TTG ATA TTC AT | 105 | |
| DAS81419 R1 | GCT TCA AGA TCC CAA CTT GCG | |||
| DAS81419 P3 | ATC AAC AGG CAC CGA TGC GCA CCG-BHQ1 | |||
| Lectin1 | lec-F | CCA GCT TCG CCG CTT CCT TC | 74 | |
| lec-R | GAA GGC AAG CCC ATC TGC AAG CC | |||
| lec-P | CTT CAC CTT CTA TGC CCC TGA CAC-BHQ1 |
| Crop | Duplex Events | Event | Experimental Results a | ||
|---|---|---|---|---|---|
| 0.1% | 1% | 5% | |||
| Canola | HCN92 and GT73 | HCN92 | 0.08 ± 0.01 | 1.12 ± 0.07 | 5.20 ± 0.48 |
| GT73 | 0.08 ± 0.03 | 1.00 ± 0.12 | 5.30 ± 0.40 | ||
| HCN92 and MON88302 | HCN92 | 0.07 ± 0.01 | 0.94 ± 0.08 | 4.53 ± 0.42 | |
| MON88302 | 0.10 ± 0.01 | 1.06 ± 0.06 | 5.10 ± 0.22 | ||
| GT73 and MON88302 | GT73 | 0.08 ± 0.03 | 0.94 ± 0.10 | 4.55 ± 0.25 | |
| MON88302 | 0.08 ± 0.02 | 0.93 ± 0.07 | 5.10 ± 0.25 | ||
| Soybean | A2704 and DP305423 | A2704 | 0.16 ± 0.02 | 1.26 ± 0.10 | 4.69 ± 0.68 |
| DP305423 | 0.18 ± 0.06 | 1.56 ± 0.07 | 5.57 ± 0.65 | ||
| A2704 and MON89788 | A2704 | 0.13 ± 0.03 | 1.16 ± 0.08 | 4.49 ± 0.65 | |
| MON89788 | 0.15 ± 0.02 | 1.35 ± 0.05 | 4.93 ± 0.69 | ||
| DP305423 and MON89788 | DP305423 | 0.15 ± 0.03 | 1.50 ± 0.02 | 5.65 ± 0.24 | |
| MON89788 | 0.12 ± 0.00 | 1.33 ± 0.03 | 4.83 ± 0.14 | ||
| Crop | Triplex Events | Event | Experimental Results a | ||
|---|---|---|---|---|---|
| 0.1% | 1% | 5% | |||
| Canola | HCN92, GT73, | HCN92 | 0.10 ± 0.03 | 1.09 ± 0.08 | 5.15 ± 0.30 |
| and MON88302 | GT73 | 0.12 ± 0.01 | 1.07 ± 0.05 | 4.99 ± 0.12 | |
| MON88302 | 0.11 ± 0.01 | 1.05 ± 0.03 | 5.09 ± 0.32 | ||
| Canola | OXY235, GT73, and | OXY235 | 0.13 ± 0.01 | 1.38 ± 0.07 | 6.27 ± 0.19 |
| MON88302 | GT73 | 0.12 ± 0.01 | 1.17 ± 0.02 | 5.31 ± 0.06 | |
| MON88302 | 0.12 ± 0.01 | 1.12 ± 0.05 | 5.22 ± 0.18 | ||
| Soybean | A2704, DP305423, | A2704 | 0.11 ± 0.03 | 0.94 ± 0.09 | 3.91 ± 0.10 |
| and MON89788 | DP305423 | 0.17 ± 0.05 | 1.22 ± 0.04 | 5.21 ± 0.32 | |
| MON89788 | 0.12 ± 0.02 | 1.01 ± 0.14 | 4.51 ± 0.33 | ||
| Soybean | A2704, DAS81419, | A2704 | 0.10 ± 0.05 | 1.03 ± 0.04 | 4.90 ± 0.17 |
| and MON89788 | DAS81419 | 0.14 ± 0.03 | 1.35 ± 0.06 | 6.20 ± 0.17 | |
| MON89788 | 0.10 ± 0.01 | 1.15 ± 0.05 | 5.41 ± 0.22 | ||
| Crop | Tetraplex Events | Event | Experimental Results a | ||
|---|---|---|---|---|---|
| 0.1% | 1% | 5% | |||
| Canola | GT73, MON88302, | GT73 | 0.11 ± 0.02 | 1.07 ± 0.05 | 5.01 ± 0.19 |
| OXY235, and HCN92 | MON88302 | 0.12 ± 0.02 | 1.10 ± 0.04 | 5.07 ± 0.18 | |
| OXY235 | 0.12 ± 0.01 | 1.26 ± 0.10 | 5.75 ± 0.12 | ||
| HCN92 | 0.10 ± 0.03 | 1.13 ± 0.07 | 5.06 ± 0.05 | ||
| Soybean | A2704, DP305423, | A2704 | 0.14 ± 0.0 | 1.31 ± 0.13 | 6.03 ± 0.18 |
| DAS81419, MON89788 | DP305423 | 0.16 ± 0.02 | 1.50 ± 0.08 | 6.67 ± 0.20 | |
| DAS81419 | 0.11 ± 0.01 | 1.10 ± 0.08 | 4.74 ± 0.20 | ||
| MON89788 | 0.13 ± 0.03 | 1.22 ± 0.30 | 5.00 ± 0.17 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 Crown Copyright. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demeke, T.; Lee, S.-J.; Eng, M. Increasing the Efficiency of Canola and Soybean GMO Detection and Quantification Using Multiplex Droplet Digital PCR. Biology 2022, 11, 201. https://doi.org/10.3390/biology11020201
Demeke T, Lee S-J, Eng M. Increasing the Efficiency of Canola and Soybean GMO Detection and Quantification Using Multiplex Droplet Digital PCR. Biology. 2022; 11(2):201. https://doi.org/10.3390/biology11020201
Chicago/Turabian StyleDemeke, Tigst, Sung-Jong Lee, and Monika Eng. 2022. "Increasing the Efficiency of Canola and Soybean GMO Detection and Quantification Using Multiplex Droplet Digital PCR" Biology 11, no. 2: 201. https://doi.org/10.3390/biology11020201
APA StyleDemeke, T., Lee, S.-J., & Eng, M. (2022). Increasing the Efficiency of Canola and Soybean GMO Detection and Quantification Using Multiplex Droplet Digital PCR. Biology, 11(2), 201. https://doi.org/10.3390/biology11020201







