Lipid-Derived Aldehydes: New Key Mediators of Plant Growth and Stress Responses
Abstract
Simple Summary
Abstract
1. Introduction
2. Lipid Hydroperoxide Biosynthesis and Aldehydes Formation
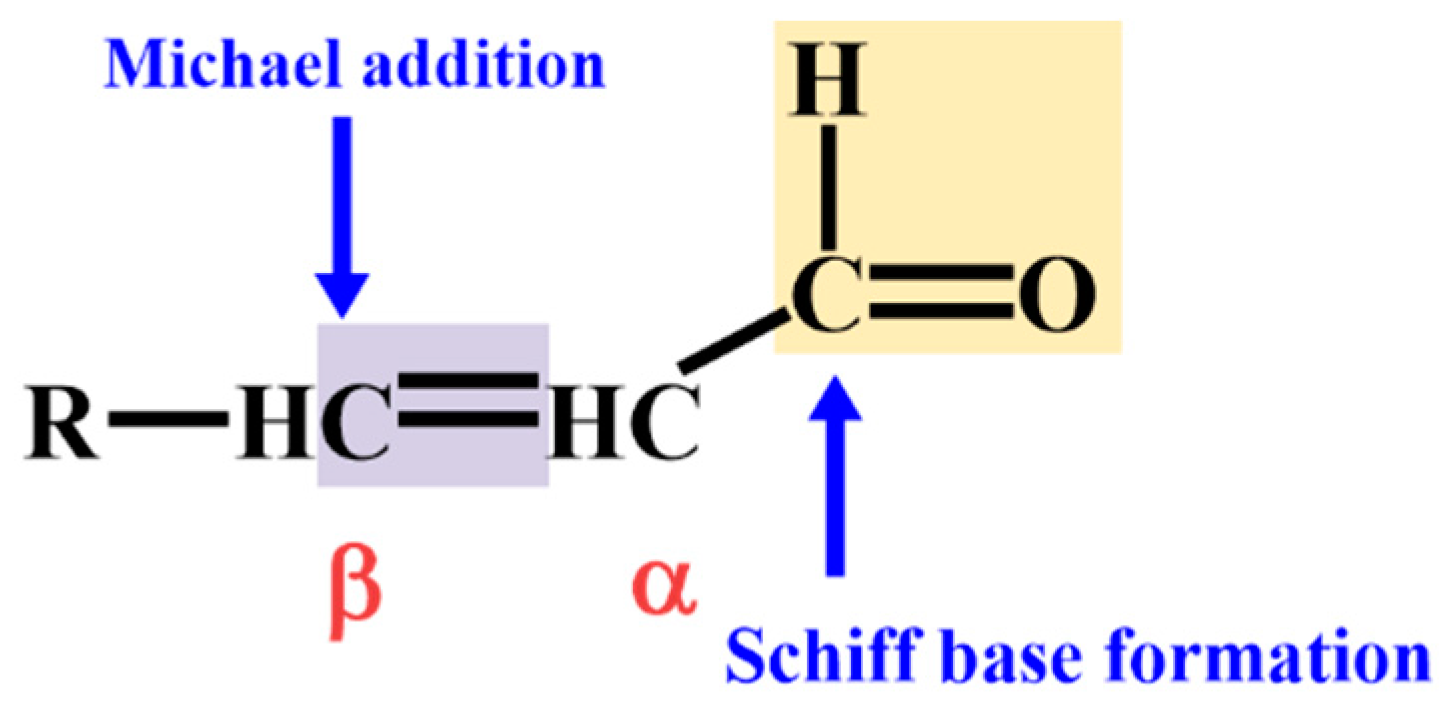
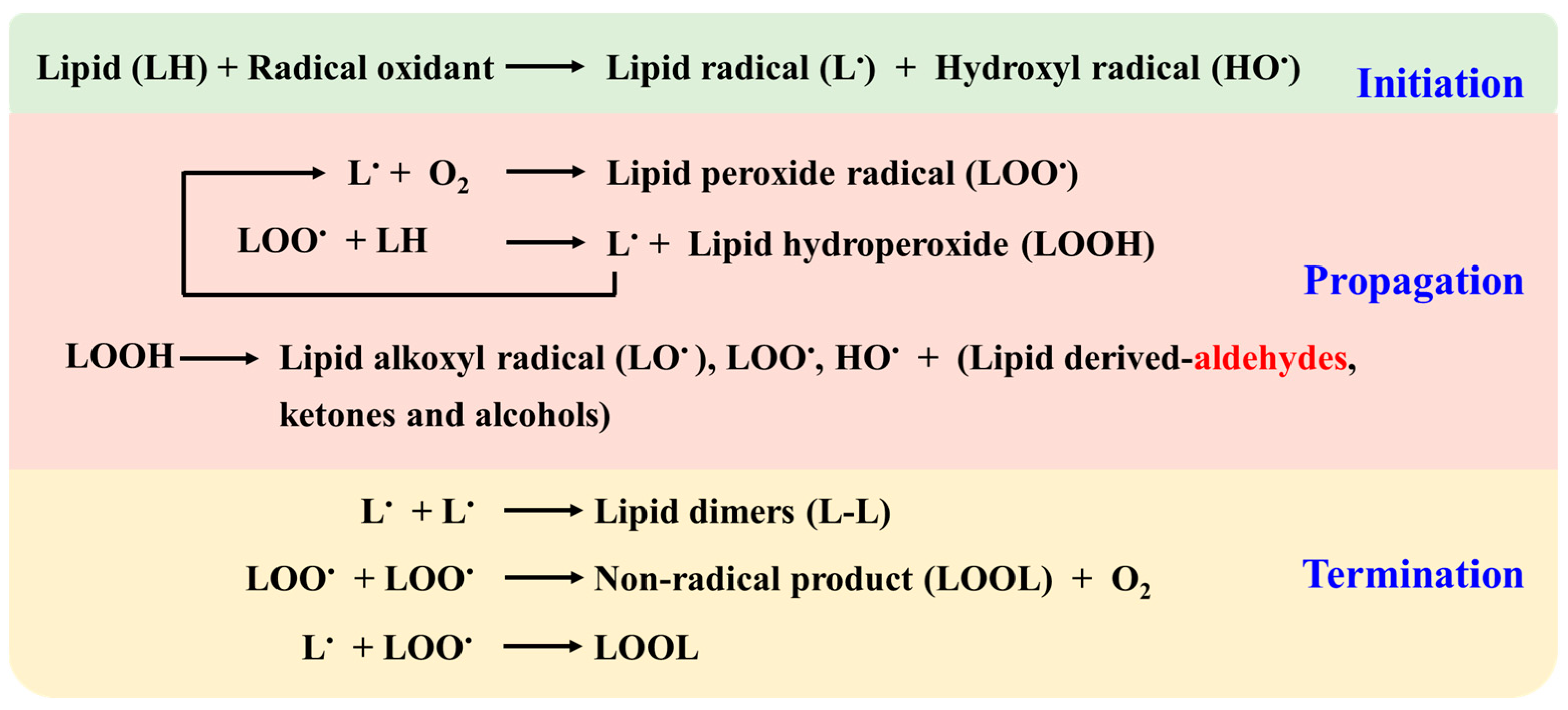
2.1. Non-Enzymatic Lipid Peroxidation Pathways and Aldehyde Generation
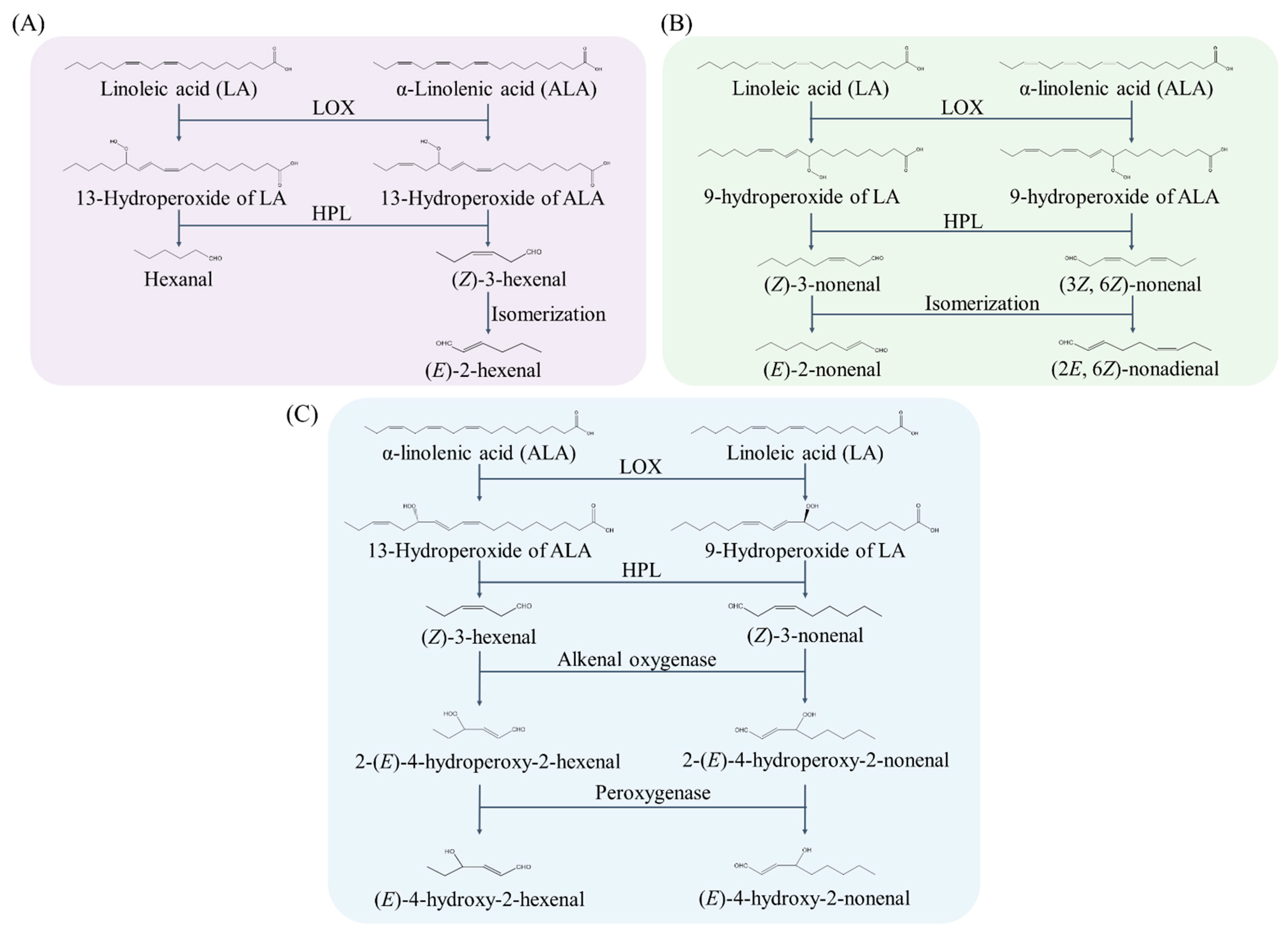
2.2. Enzymatic Generation Pathways of Lipid-Derived Aldehyde
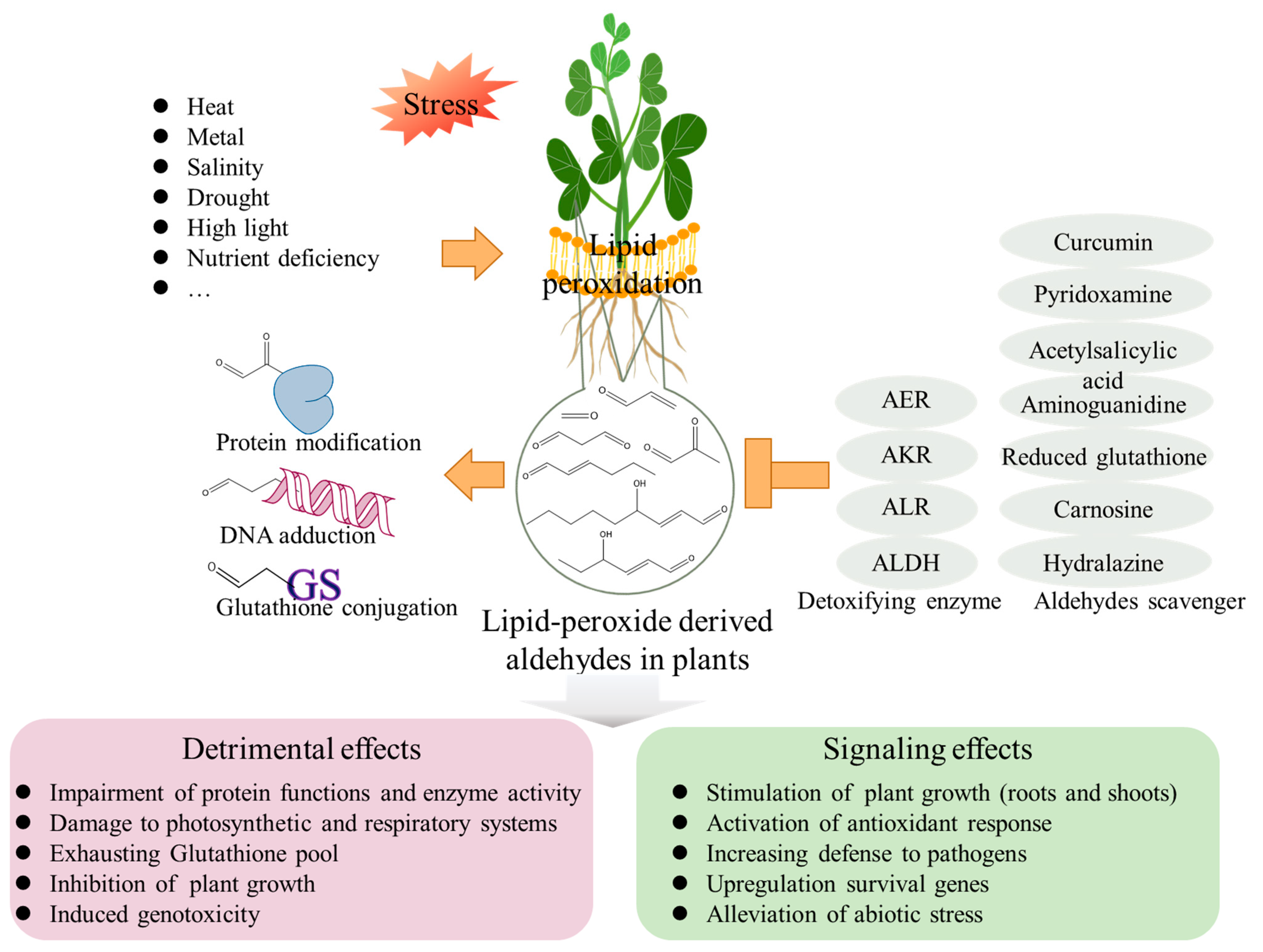
3. Aldehydes Induce Cell Injury in Plants
4. Endogenous Aldehyde Levels in Plants under Abiotic Stress Conditions
5. Signaling Effects of Aldehydes on Plants
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LoPachin, R.M.; Gavin, T. Molecular mechanisms of aldehyde toxicity: A chemical perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Lin, J.L.; Guo, X.X.; Tian, X.; Tian, Y.; Shangguan, X.X.; Wang, L.J.; Fang, X.; Chen, X.Y. RES transformation for biosynthesis and detoxification. Sci. China Life Sci. 2020, 63, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Fritz, K.S.; Petersen, D.R. An overview of the chemistry and biology of reactive aldehydes. Free Radic. Biol. Med. 2013, 59, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, S.; Mariani, M.; Alberti, J.-C.; Jacopini, S.; Brunini-Bronzini de Caraffa, V.; Berti, L.; Maury, J. Biocatalytic synthesis of natural green leaf volatiles using the lipoxygenase metabolic pathway. Catalysts 2019, 9, 873. [Google Scholar] [CrossRef]
- Mano, J.; Biswas, M.S.; Sugimoto, K. Reactive carbonyl species: A missing link in ROS signaling. Plants 2019, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Altomare, A.; Baron, G.; Gianazza, E.; Banfi, C.; Carini, M.; Aldini, G. Lipid peroxidation derived reactive carbonyl species in free and conjugated forms as an index of lipid peroxidation: Limits and perspectives. Redox Biol. 2021, 42, 101899. [Google Scholar] [CrossRef]
- Mano, J. Reactive carbonyl species: Their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol. Biochem. 2012, 59, 90–97. [Google Scholar] [CrossRef]
- Mano, J.i.; Khorobrykh, S.; Matsui, K.; Iijima, Y.; Sakurai, N.; Suzuki, H.; Shibata, D. Acrolein is formed from trienoic fatty acids in chloroplast: A targeted metabolomics approach. Plant Biotechnol. 2014, 31, 535–543. [Google Scholar] [CrossRef]
- Saxena, A.; Sonowal, H.; Ramana, K.V. Transcriptional factor modulation by lipid peroxidation-derived aldehydes. In The Molecular Nutrition of Fats; Academic Press: Cambridge, MA, USA, 2019; pp. 419–431. [Google Scholar] [CrossRef]
- Zarkovic, N. 4-Hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Asp. Med. 2003, 24, 281–291. [Google Scholar] [CrossRef]
- Brocker, C.; Cantore, M.; Failli, P.; Vasiliou, V. Aldehyde dehydrogenase 7A1 (ALDH7A1) attenuates reactive aldehyde and oxidative stress induced cytotoxicity. Chem. Biol. Interact. 2011, 191, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Wang, S.; Liu, J.; Wang, X.; Han, Y.; Liu, F. Up-regulated 2-alkenal reductase expression improves low-nitrogen tolerance in maize by alleviating oxidative stress. Plant Cell Environ. 2021, 44, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 2000, 28, 1685–1696. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef]
- Di Domenico, F.; Tramutola, A.; Butterfield, D.A. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med. 2017, 111, 253–261. [Google Scholar] [CrossRef]
- Zhang, S.; Eitan, E.; Wu, T.Y.; Mattson, M.P. Intercellular transfer of pathogenic alpha-synuclein by extracellular vesicles is induced by the lipid peroxidation product 4-hydroxynonenal. Neurobiol. Aging 2018, 61, 52–65. [Google Scholar] [CrossRef]
- Chung, F.L.; Tanaka, T.; Hecht, S.S. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986, 46, 1285–1289. [Google Scholar]
- Cohen, S.M.; Garland, E.M.; St John, M.; Okamura, T.; Smith, R.A. Acrolein initiates rat urinary bladder carcinogenesis. Cancer Res. 1992, 52, 3577–3581. [Google Scholar]
- Liebler, D.C. Protein damage by reactive electrophiles: Targets and consequences. Chem. Res. Toxicol. 2008, 21, 117–128. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Furutera, A.; Seki, K.; Toyoda, Y.; Tanaka, K.; Sugimoto, Y. Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol. Biochem. 2008, 46, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Mano, J.; Wang, S.; Tsuji, W.; Tanaka, K. The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol. 2010, 152, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ. Exp. Bot. 2015, 112, 44–54. [Google Scholar] [CrossRef]
- Majlath, I.; Eva, C.; Tajti, J.; Khalil, R.; Elsayed, N.; Darko, E.; Szalai, G.; Janda, T. Exogenous methylglyoxal enhances the reactive aldehyde detoxification capability and frost-hardiness of wheat. Plant Physiol. Biochem. 2020, 149, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Mano, J.; Tanaka, K.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S. High level of reduced glutathione contributes to detoxification of lipid peroxide-derived reactive carbonyl species in transgenic Arabidopsis overexpressing glutathione reductase under aluminum stress. Physiol. Plant 2017, 161, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Chetelat, A.; Reymond, P.; Farmer, E.E. Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J. 2004, 37, 877–888. [Google Scholar] [CrossRef]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I.; Mano, J.I. Lipid peroxidation-derived reactive carbonyl species (RCS): Their interaction with ROS and cellular redox during environmental stresses. Environ. Exp. Bot. 2019, 165, 139–149. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef]
- Ramu, V.S.; Preethi, V.; Nisarga, K.N.; Srivastava, K.R.; Sheshshayee, M.S.; Mysore, K.S.; Udayakumar, M. Carbonyl cytotoxicity affects plant cellular processes and detoxifying enzymes scavenge these compounds to improve stress tolerance. J. Agric. Food Chem. 2020, 68, 6237–6247. [Google Scholar] [CrossRef]
- Biswas, M.S.; Mano, J. Lipid peroxide-derived reactive carbonyl species as mediators of oxidative stress and signaling. Front. Plant Sci. 2021, 12, 720867. [Google Scholar] [CrossRef]
- Alche, J.D. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kapoor, A.; Bhatnagar, A. Oxidative and reductive metabolism of lipid-peroxidation derived carbonyls. Chem. Biol. Interact. 2015, 234, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic. Biol. Med. 2013, 56, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ou, Y.; Zhao, H.; Zhou, W.; Sun, C.; Lin, X. Lipid peroxide-derived short-chain aldehydes are involved in aluminum toxicity of wheat (Triticum aestivum) roots. J. Agric. Food Chem. 2021, 69, 10496–10505. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Takamura, H.; Gardner, H.W. Oxygenation of (3Z)-alkenal to (2E)-4-hydroxy-2-alkenal in soybean seed (Glycine max L). Biochim. Biophys. Acta-Lipids Lipid Metab. 1996, 1303, 83–91. [Google Scholar] [CrossRef]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 2012, 7, e36433. [Google Scholar] [CrossRef]
- Gardner, H.W. Formation of (2E)-4-hydroxy-2-nonenal and (2E)-4-hydroxy-2-hexenal by plant enzymes: A review suggests a role in the physiology of plants. Adv. Enzym Res. 2016, 4, 56–61. [Google Scholar] [CrossRef][Green Version]
- Liao, H.; Zhu, M.; Chen, Y. 4-Hydroxy-2-nonenal in food products: A review of the toxicity, occurrence, mitigation strategies and analysis methods. Trends Food Sci. Technol. 2020, 96, 188–198. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Veldink, G.A.; Vliegenthart, J.F.G. Fatty acid hydroperoxide lyase: A plant cytochrome P450 enzyme involved in wound healing and pest resistance. Chembiochem 2001, 2, 494–504. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; Alkhoori, M.A.; Hag-Ali, M.; Cheng, W.H.; Lim, S.H.; Loh, J.Y.; Lai, K.S. Contribution of aldehydes and their derivatives to antimicrobial and immunomodulatory activities. Molecules 2022, 27, 3589. [Google Scholar] [CrossRef] [PubMed]
- Derbassi, N.B.; Pedrosa, M.C.; Heleno, S.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Plant volatiles: Using Scented molecules as food additives. Trends Food Sci. Technol. 2022, 122, 97–103. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, Z.; Liu, W.; Liu, B.; Zhang, R.; Wang, W.; Zheng, W.; Xu, F.; Wang, J.; Chen, Y. Protective effect of ALDH2 against cyclophosphamide-induced acute hepatotoxicity via attenuating oxidative stress and reactive aldehydes. Biochem. Biophys. Res. Commun. 2018, 499, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Leonarduzzi, G.; Testa, G.; Gargiulo, S.; Poli, G.; Biasi, F. Lipid oxidation derived aldehydes and oxysterols between health and disease. Eur. J. Lipid Sci. Technol. 2019, 121, 1700047. [Google Scholar] [CrossRef]
- Mano, J.; Nagata, M.; Okamura, S.; Shiraya, T.; Mitsui, T. Identification of oxidatively modified proteins in salt-stressed Arabidopsis: A carbonyl-targeted proteomics approach. Plant Cell Physiol. 2014, 55, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Nareshkumar, A.; Subbarao, S.; Vennapusa, A.R.; Ashwin, V.; Banarjee, R.; Kulkarni, M.J.; Ramu, V.S.; Udayakumar, M. Enzymatic and Non-enzymatic Detoxification of reactive carbonyl compounds improves the oxidative stress tolerance in cucumber, tobacco and rice seedlings. J. Plant Growth Regul. 2020, 39, 1359–1372. [Google Scholar] [CrossRef]
- Winger, A.M.; Taylor, N.L.; Heazlewood, J.L.; Day, D.A.; Millar, A.H. The cytotoxic lipid peroxidation product 4-hydroxy-2-nonenal covalently modifies a selective range of proteins linked to respiratory function in plant mitochondria. J. Biol. Chem. 2007, 282, 37436–37447. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Sugimoto, Y. Effect of protein modification by malondialdehyde on the interaction between the oxygen-evolving complex 33 kDa protein and photosystem II core proteins. Planta 2010, 231, 1077–1088. [Google Scholar] [CrossRef]
- Biswas, M.S.; Mano, J.i. Lipid peroxide-derived short-chain carbonyls mediate hydrogen peroxide-induced and salt-induced programmed cell death in plants. Plant Physiol. 2015, 168, 885–898. [Google Scholar] [CrossRef]
- Reynolds, T. Comparative effects of aliphatic compounds on inhibition of lettuce fruit germination. Ann. Bot. 1977, 41, 637–648. [Google Scholar] [CrossRef]
- Mano, J.I.; Miyatake, F.; Hiraoka, E.; Tamoi, M. Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. Planta 2009, 230, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.H.; Leaver, C.J. The cytotoxic lipid peroxidation product, 4-hydroxy-2-nonenal specifically inhibits decarboxylating dehydrogenases in the matrix of plant mitochondria. FEBS Lett. 2000, 481, 117–121. [Google Scholar] [CrossRef]
- Almeras, E.; Stolz, S.; Vollenweider, S.; Reymond, P.; Mene-Saffrane, L.; Farmer, E.E. Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 2003, 34, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, R.; Rauwerda, H.; Struys, E.A.; Jakobs, C.; Triantaphylides, C.; Haring, M.A.; Schuurink, R.C. The Arabidopsis her1 mutant implicates GABA in E-2-hexenal responsiveness. Plant J. 2008, 53, 197–213. [Google Scholar] [CrossRef]
- Srivastava, S.; Brychkova, G.; Yarmolinsky, D.; Soltabayeva, A.; Samani, T.; Sagi, M. Aldehyde oxidase 4 plays a critical role in delaying silique senescence by catalyzing aldehyde detoxification. Plant Physiol. 2017, 173, 1977–1997. [Google Scholar] [CrossRef]
- Biswas, M.S.; Fukaki, H.; Mori, I.C.; Nakahara, K.; Mano, J. Reactive oxygen species and reactive carbonyl species constitute a feed-forward loop in auxin signaling for lateral root formation. Plant J. 2019, 100, 536–548. [Google Scholar] [CrossRef]
- Mano, J.I.; Tokushige, K.; Mizoguchi, H.; Fujii, H.; Khorobrykh, S. Accumulation of lipid peroxide-derived, toxic alpha,beta-unsaturated aldehydes (E)-2-pentenal, acrolein and (E)-2-hexenal in leaves under photoinhibitory illumination. Plant Biotechnol. 2010, 27, 193–197. [Google Scholar] [CrossRef]
- Vemanna, R.S.; Babitha, K.C.; Solanki, J.K.; Amarnatha Reddy, V.; Sarangi, S.K.; Udayakumar, M. Aldo-keto reductase-1 (AKR1) protect cellular enzymes from salt stress by detoxifying reactive cytotoxic compounds. Plant Physiol. Biochem. 2017, 113, 177–186. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Hasegawa, A.; Mizutani, M.; Sugimoto, Y. Chloroplastic NADPH-dependent alkenal/one oxidoreductase contributes to the detoxification of reactive carbonyls produced under oxidative stress. FEBS Lett. 2012, 586, 1208–1213. [Google Scholar] [CrossRef]
- Yang, G.-H.; Yang, L.-T.; Jiang, H.-X.; Li, Y.; Wang, P.; Chen, L.-S. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees-Struct. Funct. 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Jaafar, H.Z.; Ibrahim, M.H.; Mohamad Fakri, N.F. Impact of soil field water capacity on secondary metabolites, phenylalanine ammonia-lyase (PAL), maliondialdehyde (MDA) and photosynthetic responses of Malaysian kacip fatimah (Labisia pumila Benth). Molecules 2012, 17, 7305–7322. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ou, Y.; Zhao, H.; Qian, R.; Sun, C.; Lin, X. Short-chain aldehydes increase aluminum retention and sensitivity by enhancing cell wall polysaccharide contents and pectin demethylation in wheat seedlings. J. Hazard. Mater. 2022, 433, 128743. [Google Scholar] [CrossRef]
- Eva, C.; Solti, A.; Oszvald, M.; Toemoeskoezi-Farkas, R.; Nagy, B.; Horvath, G.V.; Tamas, L. Improved reactive aldehyde, salt and cadmium tolerance of transgenic barley due to the expression of aldo-keto reductase genes. Acta Physiol. Plant. 2016, 38, 99. [Google Scholar] [CrossRef]
- Shimakawa, G.; Iwamoto, T.; Mabuchi, T.; Saito, R.; Yamamoto, H.; Amako, K.; Sugimoto, T.; Makino, A.; Miyake, C. Acrolein, an alpha,beta-unsaturated carbonyl, inhibits both growth and PSII activity in the cyanobacterium Synechocystis sp. PCC 6803. Biosci. Biotechnol. Biochem. 2013, 77, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Nagy, T.; Oberschall, A.; Dudits, D.; Vass, I. Detoxification function of aldose/aldehyde reductase during drought and ultraviolet-B (280–320 nm) stresses. Plant Cell Environ. 2003, 26, 513–522. [Google Scholar] [CrossRef]
- Mano, J.I.; Torii, Y.; Hayashi, S.-I.; Takimoto, K.; Matsui, K.; Nakamura, K.; Inzé, D.; Babiychuk, E.; Kushnir, S.; Asada, K. The NADPH:quinone oxidoreductase P1-ζ-crystallin in Arabidopsis catalyzes the α,β-hydrogenation of 2-alkenals: Detoxication of the lipid peroxide-derived reactive aldehydes. Plant Cell Physiol. 2002, 43, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Oberschall, A.; Deak, M.; Torok, K.; Sass, L.; Vass, I.; Kovacs, I.; Feher, A.; Dudits, D.; Horvath, G.V. A novel aldose/aldehyde reductase protects transgenic plants against lipid peroxidation under chemical and drought stresses. Plant J. 2000, 24, 437–446. [Google Scholar] [CrossRef]
- Basu, U.; Good, A.G.; Taylor, G.J. Transgenic Brassica napus plants overexpressing aluminium-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminium. Plant Cell Environ. 2001, 24, 1269–1278. [Google Scholar] [CrossRef]
- Singla-Pareek, S.L.; Yadav, S.K.; Pareek, A.; Reddy, M.K.; Sopory, S.K. Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol. 2006, 140, 613–623. [Google Scholar] [CrossRef]
- Sunkar, R.; Bartels, D.; Kirch, H.H. Overexpression of a stress-inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J. 2003, 35, 452–464. [Google Scholar] [CrossRef]
- Xu, X.; Guo, R.; Cheng, C.; Zhang, H.; Zhang, Y.; Wang, X. Overexpression of ALDH2B8, an aldehyde dehydrogenase gene from grapevine, sustains Arabidopsis growth upon salt stress and protects plants against oxidative stress. Plant Cell Tissue Organ Cult. 2013, 114, 187–196. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, D.; Li, H.; Dong, L.; Lan, H. Ectopic overexpression of the aldehyde dehydrogenase ALDH21 from Syntrichia caninervis in tobacco confers salt and drought stress tolerance. Plant Physiol. Biochem. 2015, 95, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.F.; Kucukgergin, C.; Ozdemirler-Erata, G.; Kocak-Toker, N.; Uysal, M. The effect of carnosine treatment on prooxidant-antioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology 2010, 11, 103–109. [Google Scholar] [CrossRef]
- Liu, Y.H.; Offler, C.E.; Ruan, Y.L. Cell wall invertase promotes fruit set under heat stress by suppressing ROS-independent cell death. Plant Physiol. 2016, 172, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.X.; Zang, J.L.; Wang, T.; Xie, Y.L.; Zhang, J.; Hu, J.J. Aldehyde dehydrogenase gene superfamily in populus: Organization and expression divergence between paralogous gene pairs. PLoS ONE 2015, 10, e0124669. [Google Scholar] [CrossRef] [PubMed]
- Turoczy, Z.; Kis, P.; Torok, K.; Cserhati, M.; Lendvai, A.; Dudits, D.; Horvath, G.V. Overproduction of a rice aldo-keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol. Biol. 2011, 75, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Missihoun, T.D.; Bartels, D. The role of Arabidopsis aldehyde dehydrogenase genes in response to high temperature and stress combinations. J. Exp. Bot. 2017, 68, 4295–4308. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Andrade, M.O.; Gomes, A.P.; Damatta, F.M.; Baracat-Pereira, M.C.; Fontes, E.P. Arabidopsis and tobacco plants ectopically expressing the soybean antiquitin-like ALDH7 gene display enhanced tolerance to drought, salinity, and oxidative stress. J. Exp. Bot. 2006, 57, 1909–1918. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Wei, L.; Wang, L.; Yang, Y.; Wang, P.; Guo, T.; Kang, G. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front. Plant Sci. 2015, 6, 458. [Google Scholar] [CrossRef] [PubMed]
- Bowes, G.; Ogren, W.L.; Hageman, R.H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem. Biophys. Res. Commun. 1971, 45, 716–722. [Google Scholar] [CrossRef]
- Fischer, B.B.; Hideg, E.; Krieger-Liszkay, A. Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid. Redox Signal. 2013, 18, 2145–2162. [Google Scholar] [CrossRef] [PubMed]
- Roach, T.; Baur, T.; Stoggl, W.; Krieger-Liszkay, A. Chlamydomonas reinhardtii responding to high light: A role for 2-propenal (acrolein). Physiol. Plant 2017, 161, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.; Belles-Boix, E.; Babiychuk, E.; Inze, D.; Torii, Y.; Hiraoka, E.; Takimoto, K.; Slooten, L.; Asada, K.; Kushnir, S. Protection against photooxidative injury of tobacco leaves by 2-alkenal reductase. Detoxication of lipid peroxide-derived reactive carbonyls. Plant Physiol. 2005, 139, 1773–1783. [Google Scholar] [CrossRef]
- Machado, R.; Serralheiro, R. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Tola, A.J.; Jaballi, A.; Germain, H.; Missihoun, T.D. Recent development on plant aldehyde dehydrogenase enzymes and their functions in plant development and stress signaling. Genes 2020, 12, 51. [Google Scholar] [CrossRef]
- Vasiliou, V.; Pappa, A.; Petersen, D.R. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem. Biol. Interact. 2000, 129, 1–19. [Google Scholar] [CrossRef]
- Kotchoni, S.O.; Kuhns, C.; Ditzer, A.; Kirch, H.H.; Bartels, D. Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant Cell Environ. 2006, 29, 1033–1048. [Google Scholar] [CrossRef]
- Tagnon, M.D.; Simeon, K.O. Aldehyde dehydrogenases may modulate signaling by lipid peroxidation-derived bioactive aldehydes. Plant Signal. Behav. 2017, 12, e1387707. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, W.; Li, X.; Li, M.; Zhang, D.; Hao, Z.; Weng, J.; Xu, Y.; Bai, L.; Zhang, S.; et al. Low-nitrogen stress tolerance and nitrogen agronomic efficiency among maize inbreds: Comparison of multiple indices and evaluation of genetic variation. Euphytica 2011, 180, 281–290. [Google Scholar] [CrossRef]
- Mahiwal, S.; Pandey, G.K. Potassium: A vital nutrient mediating stress tolerance in plants. J. Plant Biochem. Biotechnol. 2022, 31, 705–719. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; López-Delacalle, M.; Ródenas, R.; Martínez, V.; Rubio, F.; Rivero, R.M. Critical responses to nutrient deprivation: A comprehensive review on the role of ROS and RNS. Environ. Exp. Bot. 2019, 161, 74–85. [Google Scholar] [CrossRef]
- Paradisone, V.; Navarro-Leon, E.; Albacete, A.; Ruiz, J.M.; Esposito, S.; Blasco, B. Improvement of the physiological response of barley plants to both Zinc deficiency and toxicity by the application of calcium silicate. Plant Sci. 2022, 319, 111259. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Wang, S.; Shi, L.; Ding, G.; Xu, F. Boron mediates nitrogen starvation-induced leaf senescence by regulating ROS production and C/N balance in Brassica napus. Environ. Exp. Bot. 2022, 200, 104905. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Tewari, N.; Srivastava, S.; Sharma, P.N. Macronutrient deficiencies and differential antioxidant responses—Influence on the activity and expression of superoxide dismutase in maize. Plant Sci. 2004, 166, 687–694. [Google Scholar] [CrossRef]
- Ding, Y.-C.; Chang, C.-R.; Luo, W.; Wu, Y.-S.; Ren, X.-L.; Wang, P.; Xu, G.-H. High potassium aggravates the oxidative stress inducedy by magnesium deficiency in rice leaves. Pedosphere 2008, 18, 316–327. [Google Scholar] [CrossRef]
- Garbisu, C.; Alkorta, I. Phytoextraction: A cost-effective plant-based technology for the removal of metals from the environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef]
- Shah, F.U.R.; Ahmad, N.; Masood, K.R.; Peralta-Videa, J.R.; Ahmad, F.U.D. Heavy metal toxicity in plants. In Plant Adaptation and Phytoremediation; Springer: Dordrecht, The Netherlands, 2010; pp. 71–97. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.; Prakash, N.B.; Lux, A.; Vaculik, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef]
- Islam, M.M.; Ye, W.; Akter, F.; Rhaman, M.S.; Matsushima, D.; Munemasa, S.; Okuma, E.; Nakamura, Y.; Biswas, M.S.; Mano, J.; et al. Reactive carbonyl species mediate methyl jasmonate-induced stomatal closure. Plant Cell Physiol. 2020, 61, 1788–1797. [Google Scholar] [CrossRef]
- Vollenweider, S.; Weber, H.; Stolz, S.; Chetelat, A.; Farmer, E.E. Fatty acid ketodienes and fatty acid ketotrienes: Michael addition acceptors that accumulate in wounded and diseased Arabidopsis leaves. Plant J. 2000, 24, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I. The roles of reactive carbonyl species in induction of antioxidant defence and ROS signalling in extreme halophytic model Eutrema parvulum and glycophytic model Arabidopsis thaliana. Environ. Exp. Bot. 2019, 160, 81–91. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Ye, W.; Matsushima, D.; Khokon, M.A.; Munemasa, S.; Nakamura, Y.; Murata, Y. Inhibition by acrolein of light-induced stomatal opening through inhibition of inward-rectifying potassium channels in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2015, 79, 59–62. [Google Scholar] [CrossRef][Green Version]
- Murakami, N.; Fuji, S.; Yamauchi, S.; Hosotani, S.; Mano, J.; Takemiya, A. Reactive carbonyl species inhibit blue-light-dependent activation of the plasma membrane H+-ATPase and stomatal opening. Plant Cell Physiol. 2022, 63, 1168–1176. [Google Scholar] [CrossRef]
- Liang, X.; Qian, R.; Ou, Y.; Wang, D.; Lin, X.; Sun, C. Lipid peroxide-derived short-chain aldehydes promote programmed cell death in wheat roots under aluminum stress. J. Hazard. Mater. 2022, 443, 130142. [Google Scholar] [CrossRef]
- Farmer, E.E.; Davoine, C. Reactive electrophile species. Curr. Opin. Plant Biol. 2007, 10, 380–386. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Woo, H.R.; Masclaux-Daubresse, C.; Lim, P.O. Plant senescence: How plants know when and how to die. J. Exp. Bot. 2018, 69, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indexes of lipid-peroxidation and peroxidative tissue-injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Deborah, S.D.; Palaniswami, A.; Velazhahan, R. The role of lipid peroxidation and lipoxygenase in the non-host disease resistance of rice. Cereal Res. Commun. 2002, 30, 149–155. [Google Scholar] [CrossRef]
- Sun, C.; Lu, L.; Liu, L.; Liu, W.; Yu, Y.; Liu, X.; Hu, Y.; Jin, C.; Lin, X. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol. 2014, 201, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munne-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, L.; Liang, X.; Dai, P.; Zhang, Y.; Li, B.; Lin, X.; Sun, C. Enhancement of polyphenolic metabolism as an adaptive response of lettuce (Lactuca sativa) roots to aluminum stress. Environ. Pollut. 2020, 261, 114230. [Google Scholar] [CrossRef]
- Farmer, E.E. Surface-to-air signals. Nature 2001, 411, 854–856. [Google Scholar] [CrossRef]
- Poltronieri, P.; De Domenico, S.; Bonsegna, S.; Santino, A. Oxylipins and green leaf volatiles: Application of enzymes from plant origin to produce flavors and antifungal aldehydes. In Enzymes in Food Biotechnology; Wiley-Blackwell: Singapore, 2019; pp. 551–567. [Google Scholar] [CrossRef]
| Growth Condition | Plant Species | Ways of Aldehydes Treatment | Detailed Information | References |
|---|---|---|---|---|
| Normal condition | Lettuce (Lactuca saliva L.) | Exogenous application | 14 kinds of aldehydes showed inhibition of germination of Lettuce | [51] |
| Potato tuber mitochondria | Exogenous application | HNE inhibited mitochondrial decarboxylating dehydrogenases and inhibited O2 consumption | [53] | |
| Arabidopsis cell | Exogenous application | Mitochondrial matrix proteins by HNE resulted in the reduction of oxygen consumption in mitochondria | [48] | |
| Arabidopsis thaliana | Exogenous application | Acrolein decreased the Fv/Fm ratio | [54] | |
| (E)-2-hexenal inhibited root elongation | [55] | |||
| (E)-2-hexenal and (Z)-3-hexenal decreased the Fv/Fm ratio | [38] | |||
| Benzaldehyde, citral, hexanal, naphthaldehyde, MDA, acrolein, or HNE caused significant tissue damage and enhanced MDA levels | [56] | |||
| High concentrations of acrolein and HNE caused leaf bleaching and high concentrations of (Z)-3-hexenal and n-hexanal caused anthocyanin accumulation | [57] | |||
| Tobacco (Nicotiana tabacum) | Exogenous application | HNE and (E)-2-hexenal inhibited root growth | [26] | |
| Wheat (Triticum aestivum L.) | Exogenous application | (E)-2-hexenal inhibited root growth | [35] | |
| Stress condition | Tobacco (Nicotiana tabacum) | Intracellular formation | 2-alkenals significantly increased after high-light illumination leading to inactivating CO2 photoreduction and GSH depletion | [52,58] |
| Roots accumulated higher levels of α,β-unsaturated aldehydes under Al stress | [26] | |||
| MDA significantly accumulated under salt stress | [59] | |||
| AKR1 overexpressing transgenics accumulated a lower level of MDA under glucose, NaCl and methyl viologen-induced oxidative stress, and showed higher seedling growth | [47] | |||
| Spinach thylakoid membrane and Arabidopsis thaliana | Exogenous application and intracellular formation | MDA modification proteins in heat-stressed plants leading to a loss of Rubisco activity | [22] | |
| Spinach (Spinacia oleracea) | Exogenous application and intracellular formation | MDA modification of PSII proteins caused the release of oxygen-evolving complex 33 kDa protein from PSII leading to inactivation of the oxygen-evolving complex, which is promoted in heat and oxidative conditions | [49] | |
| Arabidopsis thaliana | Intracellular formation | Methyl viologen treatment caused the inactivation of the photosystems due to enhanced acrolein and crotonaldehyde accumulation | [60] | |
| HNE, HHE, acrolein, crotonaldehyde and MDA-modified proteins accumulated in leaves under salt stress | [46] | |||
| Siliques of aldehyde oxidase 4-knockout lines accumulated elevated levels of MDA and acrolein, inducing a premature senescence phenotype under UV-C irradiation and dark stress | [56] | |||
| Citrus | Intracellular formation | MDA significantly accumulated in leaves and root with a magnesium-deficiency condition | [61] | |
| Labisia pumila Benth | Intracellular formation | MDA content increased in drought-stressed plants | [62] | |
| Cucumber | Intracellular formation | MDA accumulation, protein carbonyls content increase under glucose, NaCl and methyl viologen-induced oxidative stress | [47] | |
| Wheat (Triticum aestivum L.) | Intracellular formation | Roots accumulated higher level of short-chain aldehydes under Al stress | [35,63] | |
| Exogenous application | (E)-2-hexenal exacerbated Al accumulation | [63] |
| Plant Species | Signaling Functions | Detailed Information | References |
|---|---|---|---|
| Tobacco BY-2 cell | Initiate programmed cell death (PCD) | Endogenous HNE and acrolein mediating hydrogen peroxide-induced and salt-induced PCD | [50] |
| Tobacco cells exposed to HNE and acrolein suffered PCD | [50] | ||
| Tobacco | Regulate stomatal movements | Acrolein and HNE mediated methyl jasmonate-induced stomatal closure | [103] |
| Arabidopsis thaliana | Activate antioxidant defense | Stomatal Closure | [104] |
| Exogenous MDA powerfully induced the expression of GST and APX genes | [27] | ||
| Exogenously applied HNE, HHE and acrolein elevated the activities of H2O2 scavenging enzymes and downregulated NADPH oxidase | [105] | ||
| Activate pathogen defense | Exogenous (E)-2-hexenal activated defense genes and induced resistance against a necrotrophic fungal pathogen | [106] | |
| Deter invaders | Endogenous C6-aldehydes accumulated to deter invaders in disrupted tissues | [38] | |
| Induce senescence | Siliques of aldehyde oxidase 4-knockout lines accumulated higher levels of MDA and acrolein, inducing a premature senescence phenotype under UV-C irradiation and dark stress | [56] | |
| Exogenous benzaldehyde, citral, hexanal, naphthaldehyde, MDA, acrolein, or HNE caused senescence symptoms | [56] | ||
| Promote lateral root formation | Reactive oxygen species and reactive aldehydesconstitute a feed-forward loop in auxin signaling for lateral root formation | [57] | |
| Regulate stomatal movements | Acrolein inhibited light-induced stomatal opening through inhibition of inward-rectifying potassium channels in guard cells | [107] | |
| Regulate stomatal movements | Acrolein and HNE mediated methyl jasmonate-induced stomatal closure | [103] | |
| Regulate stomatal movements | Acrolein and HNE inhibited blue-light-dependent activation of the plasma membrane H+-ATPase and stomatal opening | [108] | |
| Eutrema parvulum | Activate antioxidant defense | Exogenous HNE, HHE and acrolein increased root length and fresh weight under salt stress and might be acting as a downstream signal to activate H2O2 scavenging enzymes and regulate ion homeostasis | [105] |
| Wheat (Triticum aestivum L.) | Mediate PCD | Short-chain aldehydes (E)-2-hexenal promoted Al-triggered PCD probably through activating caspase-3-like protease in wheat roots | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Qian, R.; Wang, D.; Liu, L.; Sun, C.; Lin, X. Lipid-Derived Aldehydes: New Key Mediators of Plant Growth and Stress Responses. Biology 2022, 11, 1590. https://doi.org/10.3390/biology11111590
Liang X, Qian R, Wang D, Liu L, Sun C, Lin X. Lipid-Derived Aldehydes: New Key Mediators of Plant Growth and Stress Responses. Biology. 2022; 11(11):1590. https://doi.org/10.3390/biology11111590
Chicago/Turabian StyleLiang, Xin, Ruyi Qian, Dan Wang, Lijuan Liu, Chengliang Sun, and Xianyong Lin. 2022. "Lipid-Derived Aldehydes: New Key Mediators of Plant Growth and Stress Responses" Biology 11, no. 11: 1590. https://doi.org/10.3390/biology11111590
APA StyleLiang, X., Qian, R., Wang, D., Liu, L., Sun, C., & Lin, X. (2022). Lipid-Derived Aldehydes: New Key Mediators of Plant Growth and Stress Responses. Biology, 11(11), 1590. https://doi.org/10.3390/biology11111590






