The Role of Mesothelin in Activation of Portal Fibroblasts in Cholestatic Liver Injury
Abstract
:Simple Summary
Abstract
1. Introduction
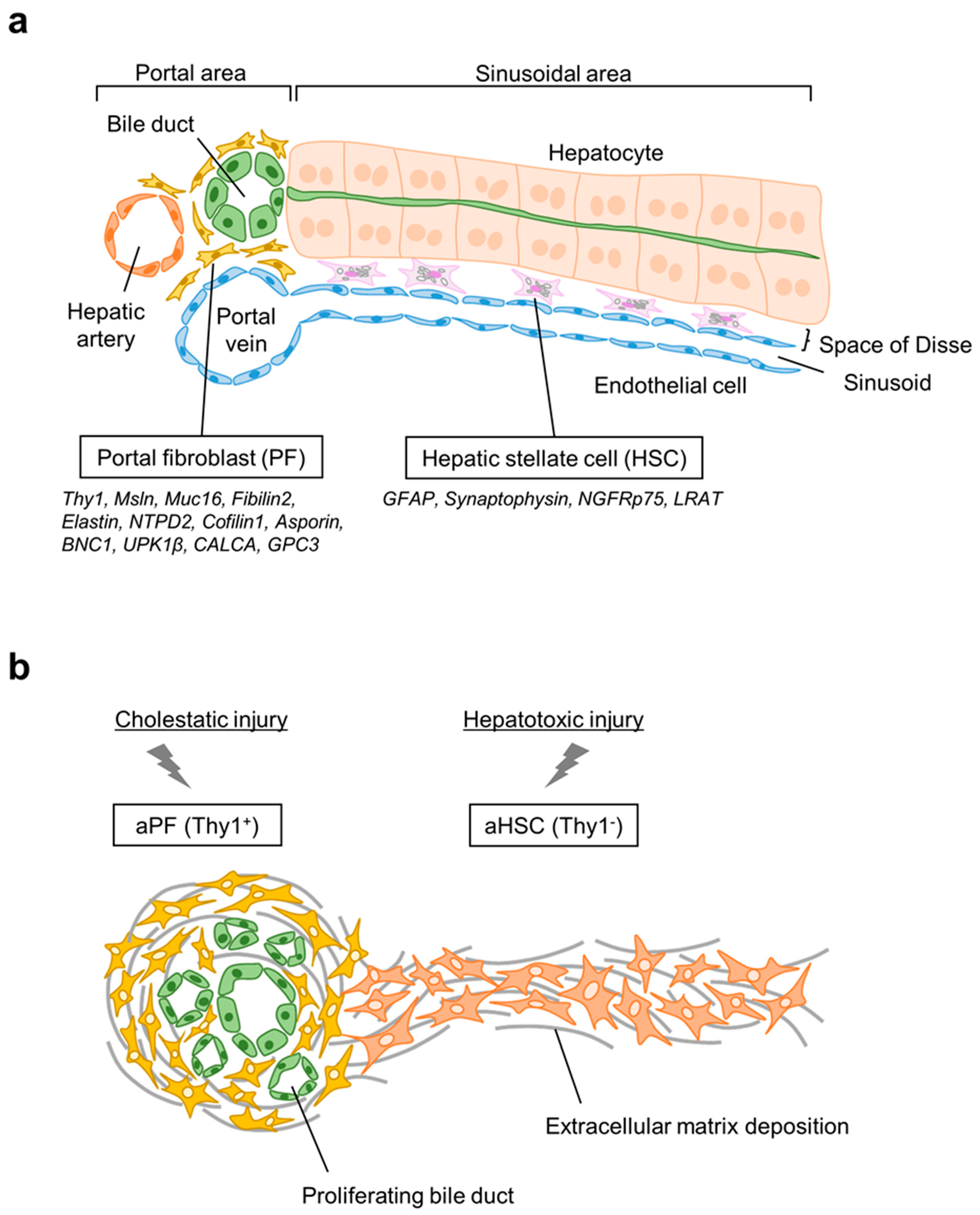
2. Origin of Hepatic Myofibroblasts
2.1. Hepatic Stellate Cells
2.2. Portal Fibroblasts
3. Activation of HSCs in Response to Cholestatic Injury
4. Activated Portal Fibroblasts in Cholestatic Fibrosis
5. Biological Function of Mesothelin, Muc16, and Thy1
5.1. Mesothelin
5.2. Mucin 16 (CA125)
5.3. Thy1 (CD90)
6. Msln, Thy1, and Muc16 Signaling in Activation of Portal Fibroblasts
6.1. Msln and Muc16 Regulate TGFβ1-Inducible Activation of aPFs
6.2. Ablation of Thy1 Exacerbates Cholestatic Fibrosis
6.3. Msln-Muc16-Thy1 Complex Regulates TGFβ/TGFβRI-Mediated Signaling in aPFs
6.4. Msln-Deficiency Suppresses TGFβ1-TGFβRI-Induced Activation of PFs
6.5. Ablation of Thy1 Accelerates TGFβ1-TGFβRI-Induced Activation of PFs
7. Common Fibrogenic Function of Msln in Tissue Fibroblasts across Organs
8. Anti-Fibrotic Therapy Targeting Msln
Anti-MSLN Ab-Immunotoxin Targeting MSLN+ aPFs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kisseleva, T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology 2017, 65, 1039–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, J.I.; Lau, L.F. Resolution of organ fibrosis. J. Clin. Investig. 2018, 128, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G.; Schwabe, R.F.; Schwabe, R. Origin and function of myofibroblasts in the liver. Semin. Liver Dis. 2015, 35, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, J.A.; Wells, R.G. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology 2010, 51, 1438–1444. [Google Scholar] [CrossRef] [Green Version]
- Kisseleva, T.; Uchinami, H.; Feirt, N.; Quintana-Bustamante, O.; Segovia, J.C.; Schwabe, R.F.; Brenner, D.A. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 2006, 45, 429–438. [Google Scholar] [CrossRef]
- Iwaisako, K.; Jiang, C.; Zhang, M.; Cong, M.; Moore-Morris, T.J.; Park, T.J.; Liu, X.; Xu, J.; Wang, P.; Paik, Y.H.; et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E3297–E3305. [Google Scholar] [CrossRef] [Green Version]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef] [Green Version]
- Wells, R.G. The portal fibroblast: Not just a poor man’s stellate cell. Gastroenterology 2014, 147, 41–47. [Google Scholar] [CrossRef]
- Wen, J.W.; Olsen, A.L.; Perepelyuk, M.; Wells, R.G. Isolation of rat portal fibroblasts by in situ liver perfusion. J. Vis. Exp. 2012, 64, e3669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchio, K.; Tuchweber, B.; Manabe, N.; Gabbiani, G.; Rosenbaum, J.; Desmouliere, A. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab. Investig. 2002, 82, 619–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruglov, E.A.; Jain, D.; Dranoff, J.A. Isolation of primary rat liver fibroblasts. J. Investig. Med. 2002, 50, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Wang, P.; Liang, S.; Iwaisako, K.; Liu, X.; Xu, J.; Zhang, M.; Sun, M.; Cong, M.; Karin, D.; et al. Mesothelin/mucin 16 signaling in activated portal fibroblasts regulates cholestatic liver fibrosis. J. Clin. Investig. 2017, 127, 1254–1270. [Google Scholar] [CrossRef] [Green Version]
- Fuji, H.; Miller, G.; Nishio, T.; Koyama, Y.; Lam, K.; Zhang, V.; Loomba, R.; Brenner, D.; Kisseleva, T. The role of Mesothelin signaling in Portal Fibroblasts in the pathogenesis of cholestatic liver fibrosis. Front. Mol. Biosci. 2021, 8, 790032. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Mori, T.; Sahoo, D.; Xu, P.X.; Bermingham, J.R.; Weissman, I.L. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat. Cell Biol. 2012, 14, 1251–1260. [Google Scholar] [CrossRef] [Green Version]
- Nishio, T.; Koyama, Y.; Liu, X.; Rosenthal, S.B.; Yamamoto, G.; Fuji, H.; Baglieri, J.; Li, N.; Brenner, L.N.; Iwaisako, K.; et al. Immunotherapy-based targeting of MSLN+ activated portal fibroblasts is a strategy for treatment of cholestatic liver fibrosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2101270118. [Google Scholar] [CrossRef]
- Duffield, J.S. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Investig. 2014, 124, 2299–2306. [Google Scholar] [CrossRef] [Green Version]
- Mack, M.; Yanagita, M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015, 87, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Yata, Y.; Scanga, A.; Gillan, A.; Yang, L.; Reif, S.; Breindl, M.; Brenner, D.A.; Rippe, R.A. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology 2003, 37, 267–276. [Google Scholar] [CrossRef]
- Nishio, T.; Hu, R.; Koyama, Y.; Liang, S.; Rosenthal, S.B.; Yamamoto, G.; Karin, D.; Baglieri, J.; Ma, H.Y.; Xu, J.; et al. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J. Hepatol. 2019, 71, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Graff, A.; Kruger, S.; Bochard, F.; Gabbiani, G.; Denk, H. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am. J. Pathol. 1991, 138, 1233–1242. [Google Scholar] [PubMed]
- Ueno, T.; Sata, M.; Sakata, R.; Torimura, T.; Sakamoto, M.; Sugawara, H.; Tanikawa, K. Hepatic stellate cells and intralobular innervation in human liver cirrhosis. Hum. Pathol. 1997, 28, 953–959. [Google Scholar] [CrossRef]
- Goddard, C.J.; Smith, A.; Hoyland, J.A.; Baird, P.; McMahon, R.F.; Freemont, A.J.; Shomaf, M.; Haboubi, N.Y.; Warnes, T.W. Localisation and semiquantitative assessment of hepatic procollagen mRNA in primary biliary cirrhosis. Gut 1998, 43, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisseleva, T.; Cong, M.; Paik, Y.; Scholten, D.; Jiang, C.; Benner, C.; Iwaisako, K.; Moore-Morris, T.; Scott, B.; Tsukamoto, H.; et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9448–9453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troeger, J.S.; Mederacke, I.; Gwak, G.Y.; Dapito, D.H.; Mu, X.; Hsu, C.C.; Pradere, J.P.; Friedman, R.A.; Schwabe, R.F. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 2012, 143, 1073–1083.e22. [Google Scholar] [CrossRef] [Green Version]
- Forbes, S.J.; Parola, M. Liver fibrogenic cells. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 207–217. [Google Scholar] [CrossRef]
- Geerts, A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 2001, 21, 311–336. [Google Scholar] [CrossRef]
- Senoo, H.; Kojima, N.; Sato, M. Vitamin a-storing cells (stellate cells). Vitam. Horm. 2007, 75, 131–159. [Google Scholar]
- Sachs, B.D.; Baillie, G.S.; McCall, J.R.; Passino, M.A.; Schachtrup, C.; Wallace, D.A.; Dunlop, A.J.; Mackenzie, K.F.; Klussmann, E.; Lynch, M.J.; et al. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J. Cell Biol. 2007, 177, 1119–1132. [Google Scholar] [CrossRef]
- Kendall, T.J.; Hennedige, S.; Aucott, R.L.; Hartland, S.N.; Vernon, M.A.; Benyon, R.C.; Iredale, J.P. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology 2009, 49, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Karin, D.; Koyama, Y.; Brenner, D.; Kisseleva, T. The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis. Differentiation 2016, 92, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, S.B.; Liu, X.; Ganguly, S.; Dhar, D.; Pasillas, M.P.; Ricciardelli, E.; Li, R.Z.; Troutman, T.D.; Kisseleva, T.; Glass, C.K.; et al. Heterogeneity of HSC s in a Mouse Model of NASH. Hepatology 2021, 74, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Rosenthal, S.; Zhang, L.J.; McCubbin, R.; Meshgin, N.; Shang, L.; Koyama, Y.; Ma, H.Y.; Sharma, S.; et al. Identification of Lineage-specific Transcription Factors That Prevent Activation of Hepatic Stellate Cells and Promote Fibrosis Resolution. Gastroenterology 2020, 158, 1728–1744.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisseleva, T.; Brenner, D.A. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. S1), S73–S78. [Google Scholar] [CrossRef]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Meyer, C.; Muller, A.; Herweck, F.; Li, Q.; Mullenbach, R.; Mertens, P.R.; Dooley, S.; Weng, H.L. IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-beta-independent Smad signaling. J. Immunol. 2011, 187, 2814–2823. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Koyama, Y.; Taura, K.; Kudoh, A.; Echizen, K.; Nakamura, D.; Li, X.; Nam, N.H.; Uemoto, Y.; Nishio, T.; et al. Characterization and role of collagen gene expressing hepatic cells following partial hepatectomy in mice. Hepatology 2022. [Google Scholar] [CrossRef]
- Beaussier, M.; Wendum, D.; Schiffer, E.; Dumont, S.; Rey, C.; Lienhart, A.; Housset, C. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab. Investig. 2007, 87, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Fickert, P.; Fuchsbichler, A.; Wagner, M.; Zollner, G.; Kaser, A.; Tilg, H.; Krause, R.; Lammert, F.; Langner, C.; Zatloukal, K.; et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 2004, 127, 261–274. [Google Scholar] [CrossRef]
- Knittel, T.; Kobold, D.; Saile, B.; Grundmann, A.; Neubauer, K.; Piscaglia, F.; Ramadori, G. Rat liver myofibroblasts and hepatic stellate cells: Different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology 1999, 117, 1205–1221. [Google Scholar] [CrossRef]
- Dudas, J.; Mansuroglu, T.; Batusic, D.; Saile, B.; Ramadori, G. Thy-1 is an in vivo and in vitro marker of liver myofibroblasts. Cell Tissue Res. 2007, 329, 503–514. [Google Scholar] [CrossRef]
- Yovchev, M.I.; Zhang, J.; Neufeld, D.S.; Grozdanov, P.N.; Dabeva, M.D. Thymus cell antigen-1-expressing cells in the oval cell compartment. Hepatology 2009, 50, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, T.; Legesse-Miller, A.; Hameed, M.R.; Aisner, S.C.; Randolph-Habecker, J.; Coller, H.A. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J. Histochem. Cytochem. 2008, 56, 347–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dranoff, J.A.; Kruglov, E.A.; Robson, S.C.; Braun, N.; Zimmermann, H.; Sevigny, J. The ecto-nucleoside triphosphate diphosphohydrolase NTPDase2/CD39L1 is expressed in a novel functional compartment within the liver. Hepatology 2002, 36, 1135–1144. [Google Scholar] [CrossRef]
- Bosselut, N.; Housset, C.; Marcelo, P.; Rey, C.; Burmester, T.; Vinh, J.; Vaubourdolle, M.; Cadoret, A.; Baudin, B. Distinct proteomic features of two fibrogenic liver cell populations: Hepatic stellate cells and portal myofibroblasts. Proteomics 2010, 10, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Koyama, Y.; Wang, P.; Lan, T.; Kim, I.G.; Kim, I.H.; Ma, H.Y.; Kisseleva, T. The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies. Front. Pharmacol. 2014, 5, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwaisako, K.; Brenner, D.A.; Kisseleva, T. What’s new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J. Gastroenterol. Hepatol. 2012, 27 (Suppl. S2), 65–68. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Munker, S.; Mullenbach, R.; Weng, H.L. IL-13 Signaling in Liver Fibrogenesis. Front. Immunol. 2012, 3, 116. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Koyama, Y.; Liu, X.; Xu, J.; Ma, H.Y.; Liang, S.; Kim, I.H.; Brenner, D.A.; Kisseleva, T. Promising Therapy Candidates for Liver Fibrosis. Front. Physiol. 2016, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Lazaridis, K.N.; LaRusso, N.F. Primary Sclerosing Cholangitis. N. Engl. J. Med. 2016, 375, 1161–1170. [Google Scholar] [CrossRef]
- Wells, R.G. Portal Fibroblasts in Biliary Fibrosis. Curr. Pathobiol. Rep. 2014, 2, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Desmouliere, A.; Darby, I.; Costa, A.M.; Raccurt, M.; Tuchweber, B.; Sommer, P.; Gabbiani, G. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab. Investig. 1997, 76, 765–778. [Google Scholar] [PubMed]
- Smit, J.J.; Schinkel, A.H.; Oude Elferink, R.P.; Groen, A.K.; Wagenaar, E.; van Deemter, L.; Mol, C.A.; Ottenhoff, R.; van der Lugt, N.M.; van Roon, M.A.; et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993, 75, 451–462. [Google Scholar] [CrossRef]
- Fickert, P.; Zollner, G.; Fuchsbichler, A.; Stumptner, C.; Weiglein, A.H.; Lammert, F.; Marschall, H.U.; Tsybrovskyy, O.; Zatloukal, K.; Denk, H.; et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology 2002, 123, 1238–1251. [Google Scholar] [CrossRef]
- Baghdasaryan, A.; Claudel, T.; Kosters, A.; Gumhold, J.; Silbert, D.; Thuringer, A.; Leski, K.; Fickert, P.; Karpen, S.J.; Trauner, M. Curcumin improves sclerosing cholangitis in Mdr2-/- mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut 2010, 59, 521–530. [Google Scholar] [CrossRef]
- Fickert, P.; Fuchsbichler, A.; Moustafa, T.; Wagner, M.; Zollner, G.; Halilbasic, E.; Stoger, U.; Arrese, M.; Pizarro, M.; Solis, N.; et al. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am. J. Pathol. 2009, 175, 2392–2405. [Google Scholar] [CrossRef] [Green Version]
- Mair, M.; Zollner, G.; Schneller, D.; Musteanu, M.; Fickert, P.; Gumhold, J.; Schuster, C.; Fuchsbichler, A.; Bilban, M.; Tauber, S.; et al. Signal transducer and activator of transcription 3 protects from liver injury and fibrosis in a mouse model of sclerosing cholangitis. Gastroenterology 2010, 138, 2499–2508. [Google Scholar] [CrossRef]
- Popov, Y.; Patsenker, E.; Fickert, P.; Trauner, M.; Schuppan, D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J. Hepatol. 2005, 43, 1045–1054. [Google Scholar] [CrossRef]
- Chang, K.; Pastan, I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA 1996, 93, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Qian, M.; Ho, M. The role of mesothelin in tumor progression and targeted therapy. Anticancer Agents Med. Chem. 2013, 13, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K.; Pastan, I. Mesothelin is not required for normal mouse development or reproduction. Mol. Cell Biol. 2000, 20, 2902–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, M.; Bera, T.K.; Willingham, M.C.; Onda, M.; Hassan, R.; FitzGerald, D.; Pastan, I. Mesothelin expression in human lung cancer. Clin. Cancer Res. 2007, 13, 1571–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordóñez, N.G. Application of mesothelin immunostaining in tumor diagnosis. Am. J. Surg. Pathol. 2003, 27, 1418–1428. [Google Scholar] [CrossRef]
- Yu, L.; Feng, M.; Kim, H.; Phung, Y.; Kleiner, D.E.; Gores, G.J.; Qian, M.; Wang, X.W.; Ho, M. Mesothelin as a potential therapeutic target in human cholangiocarcinoma. J. Cancer 2010, 1, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Pastan, I.; Hassan, R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014, 74, 2907–2912. [Google Scholar] [CrossRef] [Green Version]
- Alewine, C.; Ahmad, M.; Peer, C.J.; Hu, Z.I.; Lee, M.-J.; Yuno, A.; Kindrick, J.D.; Thomas, A.; Steinberg, S.M.; Trepel, J.B.; et al. Phase I/II Study of the Mesothelin-targeted Immunotoxin LMB-100 with Nab-Paclitaxel for Patients with Advanced Pancreatic Adenocarcinoma. Clin. Cancer Res. 2020, 26, 828–836. [Google Scholar] [CrossRef]
- Anwar, M.Y.; Williams, G.R.; Paluri, R.K. CAR T Cell Therapy in Pancreaticobiliary Cancers: A Focused Review of Clinical Data. J. Gastrointest. Cancer 2021, 52, 1–10. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Johnson, M.L.; Cobo, M.; Viteri, S.; Sarto, J.C.; Sukari, A.; Awad, M.M.; Salgia, R.; Papadimitrakopoulou, V.A.; Rajan, A.; et al. JNJ-64041757 (JNJ-757), a Live, Attenuated, Double-Deleted Listeria monocytogenes–Based Immunotherapy in Patients With NSCLC: Results from Two Phase 1 Studies. JTO Clin. Res. Rep. 2021, 2, 100103. [Google Scholar] [CrossRef]
- Hagerty, B.L.; Pegna, G.J.; Xu, J.; Tai, C.-H.; Alewine, C. Mesothelin-Targeted Recombinant Immunotoxins for Solid Tumors. Biomolecules 2020, 10, 973. [Google Scholar] [CrossRef]
- Hassan, R.; Alewine, C.; Mian, I.; Spreafico, A.; Siu, L.L.; Gomez-Roca, C.; Delord, J.P.; Italiano, A.; Lassen, U.; Soria, J.C.; et al. Phase 1 study of the immunotoxin LMB-100 in patients with mesothelioma and other solid tumors expressing mesothelin. Cancer 2020, 126, 4936–4947. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Bullock, S.; Premkumar, A.; Kreitman, R.J.; Kindler, H.; Willingham, M.C.; Pastan, I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin. Cancer Res. 2007, 13, 5144–5149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Q.; Ghafoor, A.; Mian, I.; Rathkey, D.; Thomas, A.; Alewine, C.; Sengupta, M.; Ahlman, M.A.; Zhang, J.; Morrow, B.; et al. Enhanced efficacy of mesothelin-targeted immunotoxin LMB-100 and anti-PD-1 antibody in patients with mesothelioma and mouse tumor models. Sci. Transl. Med. 2020, 12, eaaz7252. [Google Scholar] [CrossRef]
- Hassan, R.; Thomas, A.; Alewine, C.; Le, D.T.; Jaffee, E.M.; Pastan, I. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J. Clin. Oncol. 2016, 34, 4171–4179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMullen, M.R.; Pritchard, M.T.; Wang, Q.; Millward, C.A.; Croniger, C.M.; Nagy, L.E. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology 2005, 128, 2066–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubbels, J.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 2006, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, O.; Gong, L.; Zhang, J.; Hansen, J.K.; Hassan, R.; Lee, B.; Ho, M. A binding domain on mesothelin for CA125/MUC16. J. Biol. Chem. 2009, 284, 3739–3749. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, A.; Hirono, S.; Tani, M.; Kawai, M.; Okada, K.; Miyazawa, M.; Kitahata, Y.; Nakamura, Y.; Noda, T.; Yokoyama, S.; et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci. 2012, 103, 739–746. [Google Scholar] [CrossRef]
- Hassan, R.; Sharon, E.; Thomas, A.; Zhang, J.; Ling, A.; Miettinen, M.; Kreitman, R.J.; Steinberg, S.M.; Hollevoet, K.; Pastan, I. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014, 120, 3311–3319. [Google Scholar] [CrossRef]
- Einama, T.; Yamagishi, Y.; Takihata, Y.; Suzuki, T.; Yamasaki, T.; Hirose, Y.; Kobayashi, K.; Yonamine, N.; Fujinuma, I.; Tsunenari, T.; et al. Co-expression of mesothelin and CA125/MUC16 is a prognostic factor for breast cancer, especially in luminal-type breast cancer patients. Biomark. Res. 2021, 9, 78. [Google Scholar] [CrossRef]
- Chen, S.-H.; Hung, W.-C.; Wang, P.; Paul, C.; Konstantopoulos, K. Mesothelin Binding to CA125/MUC16 Promotes Pancreatic Cancer Cell Motility and Invasion via MMP-7 Activation. Sci. Rep. 2013, 3, 1870. [Google Scholar] [CrossRef] [PubMed]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholler, N.; Urban, N. CA125 in ovarian cancer. Biomark. Med. 2007, 1, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Schöniger-Hekele, M.; Müller, C. The Combined Elevation of Tumor Markers CA 19-9 and CA 125 in Liver Disease Patients Is Highly Specific for Severe Liver Fibrosis. Dig. Dis. Sci. 2006, 51, 338–345. [Google Scholar] [CrossRef]
- Maher, T.M.; Oballa, E.; Simpson, J.K.; Porte, J.; Habgood, A.; Fahy, W.A.; Flynn, A.; Molyneaux, P.L.; Braybrooke, R.; Divyateja, H.; et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: An analysis from the multicentre PROFILE cohort study. Lancet Respir. Med. 2017, 5, 946–955. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhang, Y.; Fu, J.; Zhang, L. Serum CA125 levels are decreased in rectal cancer but increased in fibrosis-associated diseases and in most types of cancers. Prog. Mol. Biol. Transl. Sci. 2019, 162, 241–252. [Google Scholar] [CrossRef]
- Ballester, B.; Milara, J.; Montero, P.; Cortijo, J. MUC16 Is Overexpressed in Idiopathic Pulmonary Fibrosis and Induces Fibrotic Responses Mediated by Transforming Growth Factor-β1 Canonical Pathway. Int. J. Mol. Sci. 2021, 22, 6502. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Kaese, D.; Williams, A.W.; Rakela, J.; Klee, G.G.; Kamath, P.S. Cancer antigen 125 in patients with chronic liver disease. Mayo Clin. Proc. 2002, 77, 538–541. [Google Scholar] [CrossRef]
- Nosten-Bertrand, M.; Errington, M.L.; Murphy, K.P.; Tokugawa, Y.; Barboni, E.; Kozlova, E.; Michalovich, D.; Morris, R.G.; Silver, J.; Stewart, C.L.; et al. Normal spatial learning despite regional inhibition of LTP in mice lacking Thy-1. Nature 1996, 379, 826–829. [Google Scholar] [CrossRef]
- Rege, T.A.; Hagood, J.S. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006, 20, 1045–1054. [Google Scholar] [CrossRef] [Green Version]
- Hagood, J.S. Thy-1 as an Integrator of Diverse Extracellular Signals. Front. Cell Dev. Biol. 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Haeryfar, S.M.; Hoskin, D.W. Thy-1: More than a mouse pan-T cell marker. J. Immunol. 2004, 173, 3581–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagood, J.S.; Prabhakaran, P.; Kumbla, P.; Salazar, L.; MacEwen, M.W.; Barker, T.H.; Ortiz, L.A.; Schoeb, T.; Siegal, G.P.; Alexander, C.B.; et al. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am. J. Pathol. 2005, 167, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Hagood, J.S.; Murphy-Ullrich, J.E. Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic stimuli. Am. J. Pathol. 2004, 165, 659–669. [Google Scholar] [CrossRef]
- Fiore, V.F.; Wong, S.S.; Tran, C.; Tan, C.; Xu, W.; Sulchek, T.; White, E.S.; Hagood, J.S.; Barker, T.H. αvβ3 Integrin drives fibroblast contraction and strain stiffening of soft provisional matrix during progressive fibrosis. JCI Insight 2018, 3, e97597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wong, S.S.; Taype, C.A.; Kim, J.; Shentu, T.P.; Espinoza, C.R.; Finley, J.C.; Bradley, J.E.; Head, B.P.; Patel, H.H.; et al. Thy-1 interaction with Fas in lipid rafts regulates fibroblast apoptosis and lung injury resolution. Lab. Investig. 2017, 97, 256–267. [Google Scholar] [CrossRef] [Green Version]
- Bradley, J.E.; Ramirez, G.; Hagood, J.S. Roles and regulation of Thy-1, a context-dependent modulator of cell phenotype. Biofactors 2009, 35, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Hagood, J.S.; Lu, B.; Merryman, W.D.; Murphy-Ullrich, J.E. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J. Biol. Chem. 2010, 285, 22382–22393. [Google Scholar] [CrossRef] [Green Version]
- Fiore, V.F.; Strane, P.W.; Bryksin, A.V.; White, E.S.; Hagood, J.S.; Barker, T.H. Conformational coupling of integrin and Thy-1 regulates Fyn priming and fibroblast mechanotransduction. J. Cell Biol. 2015, 211, 173–190. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Jiang, M.; Wong, S.S.; Espinoza, C.R.; Kim, C.; Li, X.; Connors, E.; Hagood, J.S. Soluble Thy-1 reverses lung fibrosis via its integrin-binding motif. JCI Insight 2019, 4, e131152. [Google Scholar] [CrossRef] [Green Version]
- Jhandier, M.N.; Kruglov, E.A.; Lavoie, E.G.; Sévigny, J.; Dranoff, J.A. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J. Biol. Chem. 2005, 280, 22986–22992. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Huang, X.R.; Chung, A.C.K.; Qin, W.; Shao, X.; Igarashi, P.; Ju, W.; Bottinger, E.P.; Lan, H.Y. Smad2 Protects against TGF-β/Smad3-Mediated Renal Fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1477–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.M.; Chung, A.C.; Lan, H.Y. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin. Sci. 2013, 124, 243–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Liu, C.; Meng, X.-M.; Huang, C.; Xu, F.; Li, J. Smad2 protects against TGF-β1/Smad3-mediated collagen synthesis in human hepatic stellate cells during hepatic fibrosis. Mol. Cell. Biochem. 2015, 400, 17–28. [Google Scholar] [CrossRef]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harb Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.L.; Kisseleva, T.; Brenner, D.A.; Duffield, J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008, 173, 1617–1627. [Google Scholar] [CrossRef] [Green Version]
- Boor, P.; Floege, J. The renal (myo-)fibroblast: A heterogeneous group of cells. Nephrol. Dial. Transplant. 2012, 27, 3027–3036. [Google Scholar] [CrossRef] [Green Version]
- Barron, L.; Gharib, S.A.; Duffield, J.S. Lung Pericytes and Resident Fibroblasts: Busy Multitaskers. Am. J. Pathol. 2016, 186, 2519–2531. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.; Linn, G.; Chow, Y.H.; Kobayashi, A.; Mittelsteadt, K.; Altemeier, W.A.; Gharib, S.A.; Schnapp, L.M.; Duffield, J.S. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2013, 188, 820–830. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Gupta, I.; Park, J.; Bram, Y.; Schwartz, R.E. Hedgehog Signaling Demarcates a Niche of Fibrogenic Peribiliary Mesenchymal Cells. Gastroenterology 2020, 159, 624–638.e9. [Google Scholar] [CrossRef]
- Kramann, R.; Schneider, R.K.; DiRocco, D.P.; Machado, F.; Fleig, S.; Bondzie, P.A.; Henderson, J.M.; Ebert, B.L.; Humphreys, B.D. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015, 16, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.A.; Spino, C.; Magee, J.C.; Shneider, B.L.; Rosenthal, P.; Wang, K.S.; Erlichman, J.; Haber, B.; Hertel, P.M.; Karpen, S.J.; et al. Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: The START randomized clinical trial. JAMA 2014, 311, 1750–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, J.P.; McKeever, T.M.; Fogarty, A.W.; Navaratnam, V.; Hubbard, R.B. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann. Am. Thorac. Soc. 2014, 11, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Watanabe, K.; Nabeshima, K.; Hamasaki, M.; Iwasaki, H. Prognostic significance of fibroblastic foci in usual interstitial pneumonia and non-specific interstitial pneumonia. Respirology 2013, 18, 278–283. [Google Scholar] [CrossRef]
- Li, C.; Li, M.; Li, S.; Xing, Y.; Yang, C.Y.; Li, A.; Borok, Z.; De Langhe, S.; Minoo, P. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. Stem Cells 2015, 33, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- Ntokou, A.; Klein, F.; Dontireddy, D.; Becker, S.; Bellusci, S.; Richardson, W.D.; Szibor, M.; Braun, T.; Morty, R.E.; Seeger, W.; et al. Characterization of the platelet-derived growth factor receptor-alpha-positive cell lineage during murine late lung development. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L942–L958. [Google Scholar] [CrossRef] [Green Version]
- Al Alam, D.; El Agha, E.; Sakurai, R.; Kheirollahi, V.; Moiseenko, A.; Danopoulos, S.; Shrestha, A.; Schmoldt, C.; Quantius, J.; Herold, S.; et al. Evidence for the involvement of fibroblast growth factor 10 in lipofibroblast formation during embryonic lung development. Development 2015, 142, 4139–4150. [Google Scholar] [CrossRef] [Green Version]
- Rinn, J.L.; Bondre, C.; Gladstone, H.B.; Brown, P.O.; Chang, H.Y. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006, 2, e119. [Google Scholar] [CrossRef]
- Sueblinvong, V.; Neujahr, D.C.; Mills, S.T.; Roser-Page, S.; Guidot, D.; Rojas, M.; Ritzenthaler, J.D.; Roman, J. Predisposition for disrepair in the aged lung. Am. J. Med. Sci. 2012, 344, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Hagood, J.S.; Guo, B.L.; Nesbitt, J.E.; Lasky, J.A. Differential expression and secretion of connective tissue growth factor by lung fibroblast subpopulations. Am. J. Respir. Crit. Care Med. 1999, 159, A195. [Google Scholar]
- McIntosh, J.C.; Hagood, J.S.; Richardson, T.L.; Simecka, J.W. Thy1 (+) and (−) lung fibrosis subpopulations in LEW and F344 rats. Eur. Respir. J. 1994, 7, 2131–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, Y.Y.; Kumbla, P.; Hagood, J.S. Enhanced myofibroblastic differentiation and survival in Thy-1(-) lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2007, 36, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.; Hagood, J.S.; Sanders, Y.; Ramirez, R.; Becerril, C.; Segura, L.; Barrera, L.; Selman, M.; Pardo, A. Absence of Thy-1 results in TGF-beta induced MMP-9 expression and confers a profibrotic phenotype to human lung fibroblasts. Lab. Investig. 2011, 91, 1206–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onda, M.; Willingham, M.; Nagata, S.; Bera, T.K.; Beers, R.; Ho, M.; Hassan, R.; Kreitman, R.J.; Pastan, I. New monoclonal antibodies to mesothelin useful for immunohistochemistry, fluorescence-activated cell sorting, Western blotting, and ELISA. Clin. Cancer Res. 2005, 11, 5840–5846. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Onda, M.; Lee, B.; Kreitman, R.J.; Hassan, R.; Xiang, L.; Pastan, I. Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes. Proc. Natl. Acad. Sci. USA 2012, 109, 11782–11787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, R.; Bera, T.; Pastan, I. Mesothelin: A new target for immunotherapy. Clin. Cancer Res. 2004, 10, 3937–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, R.; Viner, J.L.; Wang, Q.C.; Margulies, I.; Kreitman, R.J.; Pastan, I. Anti-tumor activity of K1-LysPE38QQR, an immunotoxin targeting mesothelin, a cell-surface antigen overexpressed in ovarian cancer and malignant mesothelioma. J. Immunother. 2000, 23, 473–479. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Hassan, R.; Fitzgerald, D.J.; Pastan, I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin. Cancer Res. 2009, 15, 5274–5279. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, P.S.; Pastan, I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat. Biotechnol. 1999, 17, 568–572. [Google Scholar] [CrossRef]
- Alewine, C.; Xiang, L.; Yamori, T.; Niederfellner, G.; Bosslet, K.; Pastan, I. Efficacy of RG7787, a next-generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancers. Mol. Cancer Ther. 2014, 13, 2653–2661. [Google Scholar] [CrossRef] [Green Version]
- Pastan, I.; Hassan, R.; FitzGerald, D.J.; Kreitman, R.J. Immunotoxin treatment of cancer. Annu. Rev. Med. 2007, 58, 221–237. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishio, T.; Koyama, Y.; Fuji, H.; Ishizuka, K.; Iwaisako, K.; Taura, K.; Hatano, E.; Brenner, D.A.; Kisseleva, T. The Role of Mesothelin in Activation of Portal Fibroblasts in Cholestatic Liver Injury. Biology 2022, 11, 1589. https://doi.org/10.3390/biology11111589
Nishio T, Koyama Y, Fuji H, Ishizuka K, Iwaisako K, Taura K, Hatano E, Brenner DA, Kisseleva T. The Role of Mesothelin in Activation of Portal Fibroblasts in Cholestatic Liver Injury. Biology. 2022; 11(11):1589. https://doi.org/10.3390/biology11111589
Chicago/Turabian StyleNishio, Takahiro, Yukinori Koyama, Hiroaki Fuji, Kei Ishizuka, Keiko Iwaisako, Kojiro Taura, Etsuro Hatano, David A. Brenner, and Tatiana Kisseleva. 2022. "The Role of Mesothelin in Activation of Portal Fibroblasts in Cholestatic Liver Injury" Biology 11, no. 11: 1589. https://doi.org/10.3390/biology11111589
APA StyleNishio, T., Koyama, Y., Fuji, H., Ishizuka, K., Iwaisako, K., Taura, K., Hatano, E., Brenner, D. A., & Kisseleva, T. (2022). The Role of Mesothelin in Activation of Portal Fibroblasts in Cholestatic Liver Injury. Biology, 11(11), 1589. https://doi.org/10.3390/biology11111589




