Antimicrobial and Methicillin Resistance Pattern of Potential Mastitis-Inducing Staphylococcus aureus and Coagulase-Negative Staphylococci Isolates from the Mammary Secretion of Dairy Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Experimental Design
2.3. Bacterial Isolation
2.4. Identification of Bacterial Isolates
2.5. Phenotypic Methods for Antimicrobial Resistance Detection in Staphylococcus species
2.5.1. Disk Diffusion Method (Kirby–Bauer)
2.5.2. Automated Commercial Phenotypic Method—VITEK 2 COMPACT System
2.6. Detection of mecA and mecC genes in Presumptive Methicillin-Resistant Strains
2.6.1. DNA Extraction
2.6.2. Real-Time PCR Reactions
3. Results
3.1. Staphylococcus spp. Isolation and Species Identification by MALDI-TOF MS
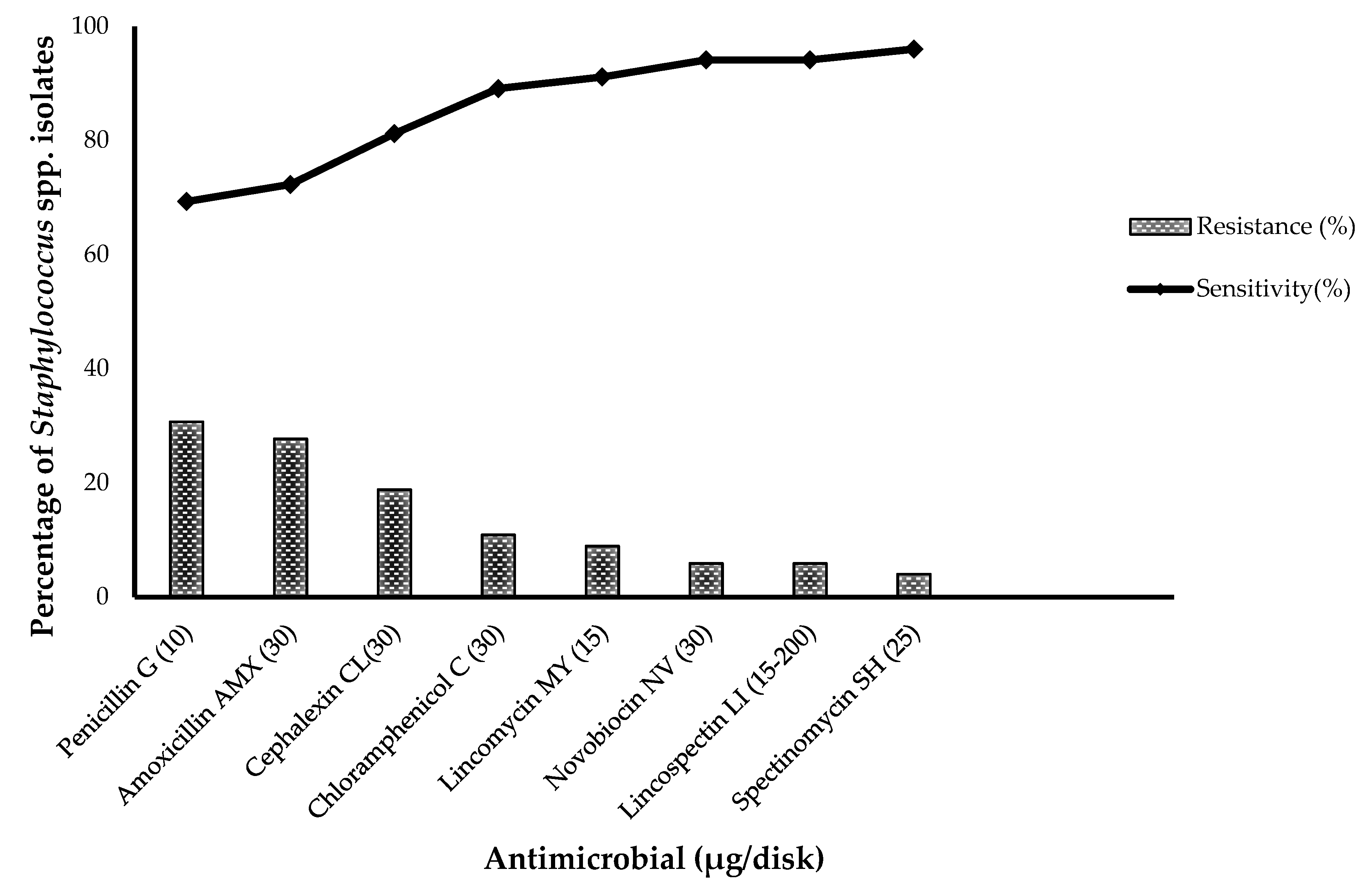
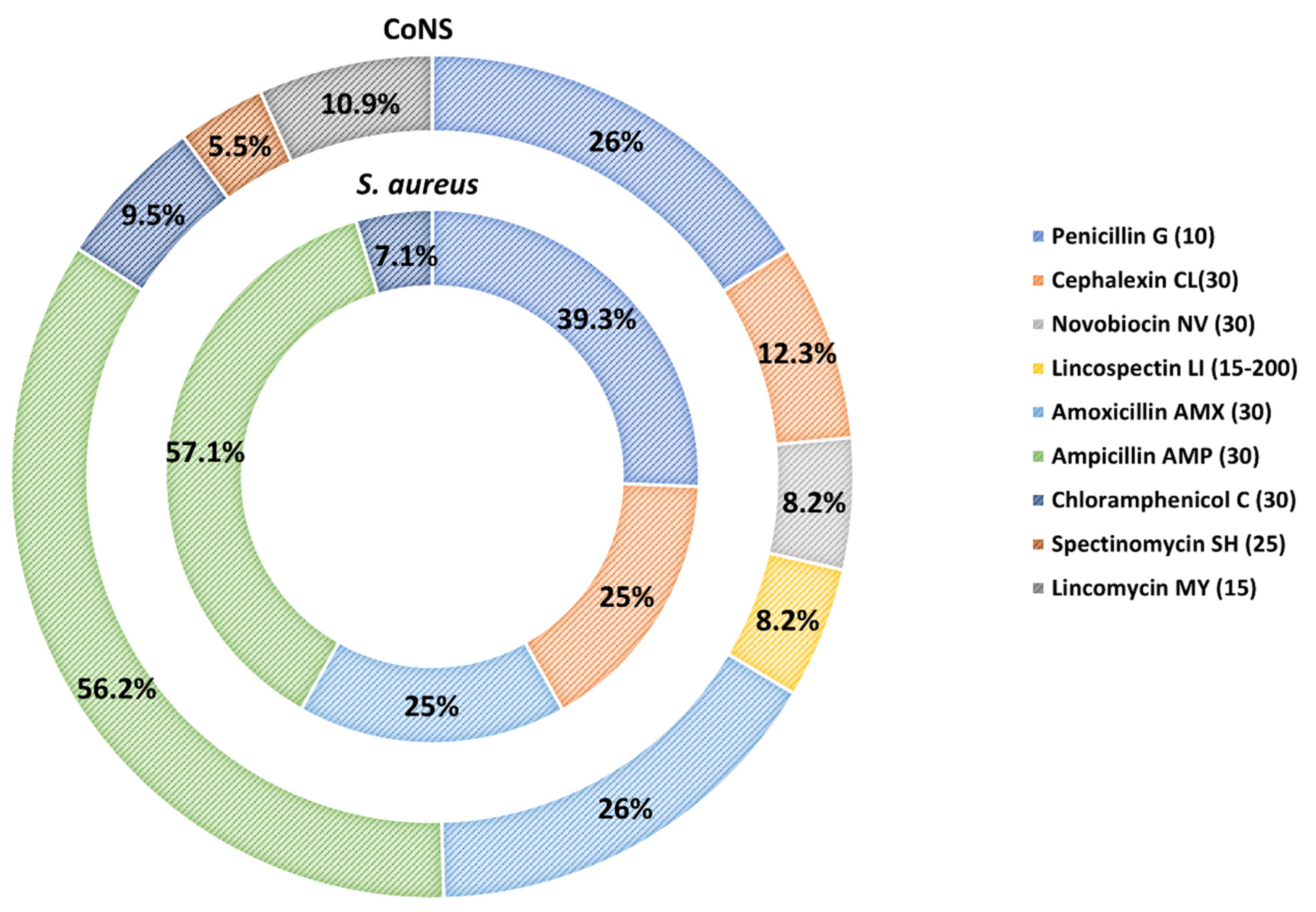
3.2. Antimicrobial Susceptibility of S. aureus and CoNS Strains
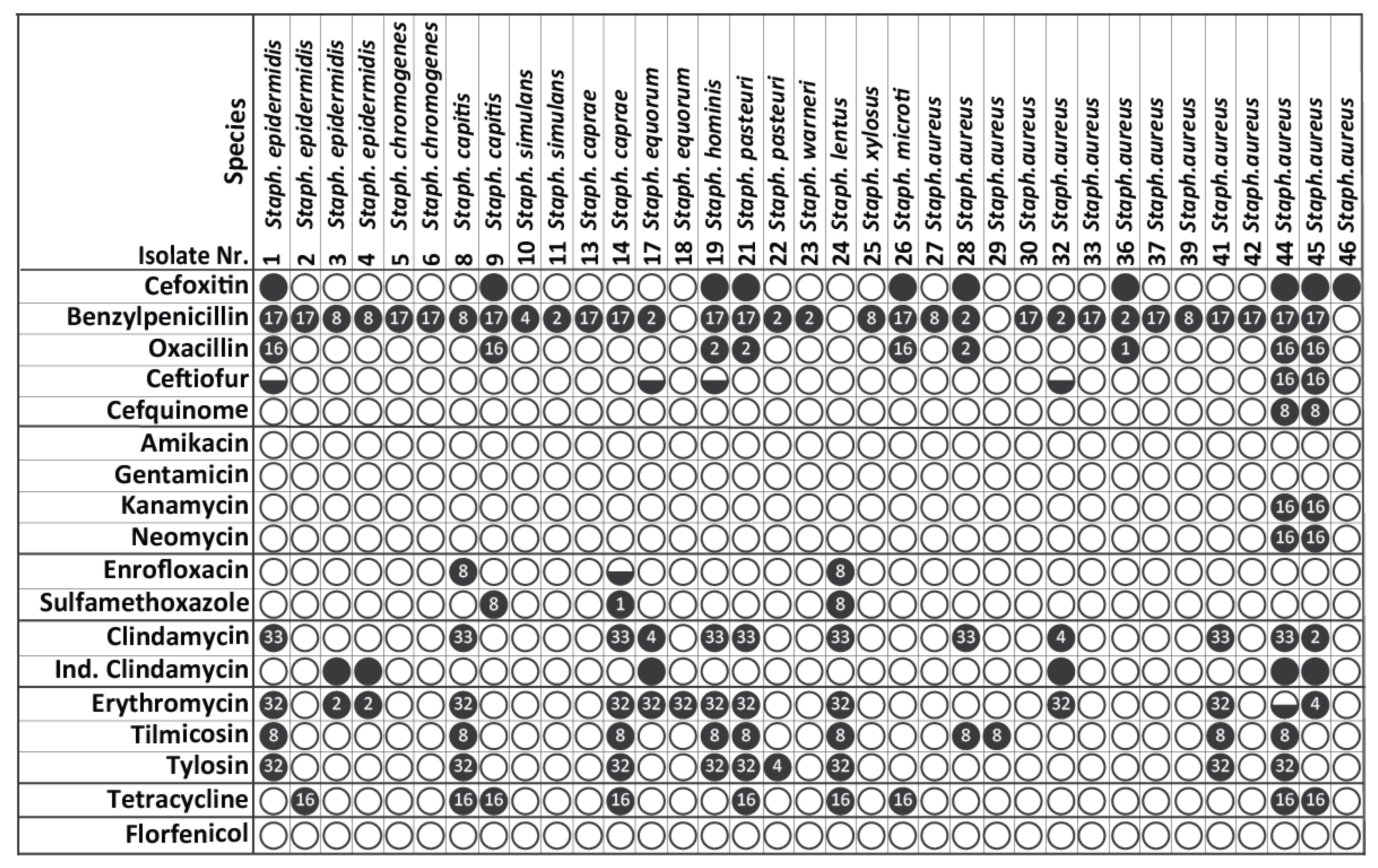
3.3. MICs Determination of Resistant S. aureus and CoNS Strains
3.4. Identification of MRSA and MR-CoNS via Detection of Methicillin Resistance-Associated Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Contreras, A.; Sierra, D.; Sánchez, A.; Corrales, J.C.; Marco, J.C.; Paape, M.J.; Gonzalo, C. Mastitis in small ruminants. Small Rumin. Res. 2007, 68, 145–153. [Google Scholar] [CrossRef]
- Le Maréchal, C.; Thiéry, R.; Vautor, E.; Le Loir, Y. Mastitis impact on technological properties of milk and quality of milk products—A review. Dairy Sci. Technol. 2011, 91, 247–282. [Google Scholar] [CrossRef] [Green Version]
- Silanikove, N.; Merin, U.; Leitner, G. On effects of subclinical mastitis and stage of lactation on milk quality in goats. Small Rumin. Res. 2014, 122, 76–82. [Google Scholar] [CrossRef]
- Gonzalo, C. Milk hygiene in small ruminants: A review. Span. J. Agric. Res. 2018, 15, e05R02. [Google Scholar] [CrossRef] [Green Version]
- Fragkou, I.A.; Skoufos, J.; Cripps, P.J.; Kyriazakis, I.; Papaioannou, N.; Boscos, C.M.; Tzora, A.; Fthenakis, G.C. Differences in susceptibility to Mannheimia haemolytica-associated mastitis between two breeds of dairy sheep. J. Dairy Res. 2007, 74, 349–355. [Google Scholar] [CrossRef]
- Arteche-Villasol, N.; Fernandez, M.; Gutierrez-Exposito, D.; Perez, V. Pathology of the Mammary Gland in Sheep and Goats. J. Comp. Pathol. 2022, 193, 37–49. [Google Scholar] [CrossRef]
- Mwenge Kahinda, C.T. Mastitis in Small Ruminants. In Mastitis in Dairy Cattle, Sheep and Goats; Dego, O.K., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Bergonier, D.; de Cremoux, R.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Vet. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef] [Green Version]
- Contreras, A.; Luengo, C.; Sánchez, A.; Corrales, J.C. The role of intramammary pathogens in dairy goats. Livest. Prod. Sci. 2003, 79, 273–283. [Google Scholar] [CrossRef]
- Kalogridou-Vassiliadou, D. Mastitis-related pathogens in goat milk. Small Rumin. Res. 1991, 4, 203–212. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Angelidis, A.S.; Giannakou, R.; Filioussis, G.; Kalamaki, M.S.; Arsenos, G. Bacterial subclinical mastitis and its effect on milk yield in low-input dairy goat herds. J. Dairy Sci. 2016, 99, 3698–3708. [Google Scholar] [CrossRef]
- Angelidis, A.S.; Komodromos, D.; Giannakou, R.; Arsenos, G.; Gelasakis, A.I.; Kyritsi, M.; Filioussis, G.; Hadjichristodoulou, C.; Torounidou, P.; Papa, A.; et al. Isolation and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) from milk of dairy goats under low-input farm management in Greece. Vet. Microbiol. 2020, 247, 108749. [Google Scholar] [CrossRef]
- Kotzamanidis, C.; Vafeas, G.; Giantzi, V.; Anastasiadou, S.; Mygdalias, S.; Malousi, A.; Loukia, E.; Daniel, S.; Zdragas, A. Staphylococcus aureus Isolated from Ruminants with Mastitis in Northern Greece Dairy Herds: Genetic Relatedness and Phenotypic and Genotypic Characterization. Toxins 2021, 13, 176. [Google Scholar] [CrossRef]
- Martins, A.; de Lourdes, R.S.; Cunha, M. Methicillin Resistance in Staphylococcus aureus and Coagulase-Negative Staphylococci: Epidemiological and Molecular Aspects. Microbiol. Immunol. 2007, 51, 787–795. [Google Scholar] [CrossRef]
- Lianou, D.T.; Fthenakis, G.C. Use of Antibiotics against Bacterial Infections on Dairy Sheep and Goat Farms: Patterns of Usage and Associations with Health Management and Human Resources. Antibiotics 2022, 11, 753. [Google Scholar] [CrossRef]
- Virdis, S.; Scarano, C.; Cossu, F.; Spanu, V.; Spanu, C.; De Santis, E.P. Antibiotic Resistance in Staphylococcus aureus and Coagulase Negative Staphylococci Isolated from Goats with Subclinical Mastitis. Vet. Med. Int. 2010, 2010, 517060. [Google Scholar] [CrossRef] [Green Version]
- Aragao, B.B.; Trajano, S.C.; de Oliveira, R.P.; Sobral da Silva, D.M.; de Carvalho, R.G.; Juliano, M.A.; Pinheiro Junior, J.W.; Mota, R.A. Multiresistant zoonotic pathogens isolated from goat milk in Northeastern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2021, 79, 101701. [Google Scholar] [CrossRef]
- Liu, H.; Dong, L.; Zhao, Y.; Meng, L.; Wang, J.; Wang, C.; Zheng, N. Antimicrobial Susceptibility, and Molecular Characterization of Staphylococcus aureus Isolated From Different Raw Milk Samples in China. Front. Microbiol. 2022, 13, 840670. [Google Scholar] [CrossRef]
- Coimbra, E.S.V.; Rossi, C.C.; Jesus-de Freitas, L.J.; Brito, M.; Laport, M.S.; Giambiagi-deMarval, M. Short communication: Diversity of species and transmission of antimicrobial resistance among Staphylococcus spp. isolated from goat milk. J. Dairy Sci. 2019, 102, 5518–5524. [Google Scholar] [CrossRef]
- Andrade, N.C.; Laranjo, M.; Costa, M.M.; Queiroga, M.C. Virulence Factors in Staphylococcus Associated with Small Ruminant Mastitis: Biofilm Production and Antimicrobial Resistance Genes. Antibiotics 2021, 10, 633. [Google Scholar] [CrossRef]
- Ma, F.; Xu, S.; Tang, Z.; Li, Z.; Zhang, L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf. Health 2021, 3, 32–38. [Google Scholar] [CrossRef]
- Lianou, D.T.; Petinaki, E.; Cripps, P.J.; Gougoulis, D.A.; Michael, C.K.; Tsilipounidaki, K.; Skoulakis, A.; Katsafadou, A.I.; Vasileiou, N.G.C.; Giannoulis, T.; et al. Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality and Risk Factors for Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Goat Herds. Antibiotics 2021, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Fotou, K.; Tzora, A.; Voidarou, C.; Alexopoulos, A.; Plessas, S.; Avgeris, I.; Bezirtzoglou, E.; Akrida-Demertzi, K.; Demertzis, P.G. Isolation of microbial pathogens of subclinical mastitis from raw sheep’s milk of Epirus (Greece) and their role in its hygiene. Anaerobe 2011, 17, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Fthenakis, G.C. Prevalence and aetiology of subclinical mastitis in ewes of Southern Greece. Small Rumin. Res. 1994, 13, 293–300. [Google Scholar] [CrossRef]
- Skoufos, I.; Tzora, A.; Karamoutsios, A.; Tsangaris, G.; Giannenas, I.; Fthenakis, G.C. Milk quality characteristics from Greek indigenous goats. J. Hell. Vet. Med. Soc. 2018, 67, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Vasileiou, N.G.C.; Cripps, P.J.; Ioannidi, K.S.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Orfanou, D.C.; Politis, A.P.; Gonzalez-Valerio, T.C.; Argyros, S.; et al. Extensive countrywide field investigation of subclinical mastitis in sheep in Greece. J. Dairy Sci. 2018, 101, 7297–7310. [Google Scholar] [CrossRef] [Green Version]
- Alatoom, A.A.; Cunningham, S.A.; Ihde, S.M.; Mandrekar, J.; Patel, R. Comparison of direct colony method versus extraction method for identification of gram-positive cocci by use of Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2011, 49, 2868–2873. [Google Scholar] [CrossRef] [Green Version]
- Barreiro, J.R.; Goncalves, J.L.; Grenfell, R.; Leite, R.F.; Juliano, L.; Santos, M.V. Direct identification of bovine mastitis pathogens by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry in pre-incubated milk. Braz. J. Microbiol. 2018, 49, 801–807. [Google Scholar] [CrossRef]
- Tzora, A.; Skoufos, S.; Bonos, E.; Fotou, K.; Karamoutsios, A.; Nelli, A.; Giannenas, I.; Tsinas, A.; Skoufos, I. Identification by MALDI-TOF MS and Antibiotic Resistance of Riemerella anatipestifer, Isolated from a Clinical Case in Commercial Broiler Chickens. Vet. Sci. 2021, 8, 29. [Google Scholar] [CrossRef]
- Tsuchida, S.; Nakayama, T. MALDI-Based Mass Spectrometry in Clinical Testing: Focus on Bacterial Identification. Appl. Sci. 2022, 12, 2814. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Guideline M100-S28; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI Document VET01S; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Morar, A.; Ban-Cucerzan, A.; Herman, V.; Tirziu, E.; Sallam, K.I.; Abd-Elghany, S.M.; Imre, K. Multidrug Resistant Coagulase-Positive Staphylococcus aureus and Their Enterotoxins Detection in Traditional Cheeses Marketed in Banat Region, Romania. Antibiotics 2021, 10, 1458. [Google Scholar] [CrossRef]
- Sanchini, A. Recent Developments in Phenotypic and Molecular Diagnostic Methods for Antimicrobial Resistance Detection in Staphylococcus aureus: A Narrative Review. Diagnostics 2022, 12, 208. [Google Scholar] [CrossRef]
- MacLowry, J.D.; Marsh, H.H. Semiautomatic microtechnique for serial dilution-antibiotic sensitivity testing in the clinical laboratory. J. Lab. Clin. Med. 1968, 72, 685–687. [Google Scholar]
- Gerlach, E.H. Microdilution 1: A Comparative Study. In Current Techniques for Antibiotic Susceptibility Testing; Balows, A., Ed.; Charles C. Thomas: Springfield, IL, USA, 1974; pp. 63–76. [Google Scholar]
- Stegger, M.; Andersen, P.S.; Kearns, A.; Pichon, B.; Holmes, M.A.; Edwards, G.; Laurent, F.; Teale, C.; Skov, R.; Larsen, A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin. Microbiol. Infect. 2012, 18, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Tzora, A.; Skoufos, J.; Tsinas, A.; Fotou, K.; Karamoutsios, A.; Kalyva, Z.; Nikolaou, K.; Fthenakis, G.C. The bacterial flora of the udder of goats. J. Hell. Vet. Med. Soc. 2018, 67, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Bernier Gosselin, V.; Lovstad, J.; Dufour, S.; Adkins, P.R.F.; Middleton, J.R. Use of MALDI-TOF to characterize staphylococcal intramammary infections in dairy goats. J. Dairy Sci. 2018, 101, 6262–6270. [Google Scholar] [CrossRef] [Green Version]
- Croxatto, A.; Prod’hom, G.; Greub, G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol. Rev. 2012, 36, 380–407. [Google Scholar] [CrossRef]
- Moroni, P.; Pisoni, G.; Antonini, M.; Ruffo, G.; Carli, S.; Varisco, G.; Boettcher, P. Subclinical Mastitis and Antimicrobial Susceptibility of Staphylococcus caprae and Staphylococcus epidermidis Isolated from Two Italian Goat Herds. J. Dairy Sci. 2005, 88, 1694–1704. [Google Scholar] [CrossRef] [Green Version]
- Koop, G.; De Vliegher, S.; De Visscher, A.; Supre, K.; Haesebrouck, F.; Nielen, M.; van Werven, T. Differences between coagulase-negative Staphylococcus species in persistence and in effect on somatic cell count and milk yield in dairy goats. J. Dairy Sci. 2012, 95, 5075–5084. [Google Scholar] [CrossRef] [Green Version]
- Bernier Gosselin, V.; Dufour, S.; Adkins, P.R.F.; Middleton, J.R. Persistence of coagulase negative staphylococcal intramammary infections in dairy goats. J. Dairy Res. 2019, 86, 211–216. [Google Scholar] [CrossRef]
- Pantucek, R.; Svec, P.; Dajcs, J.J.; Machova, I.; Cernohlavkova, J.; Sedo, O.; Gelbicova, T.; Maslanova, I.; Doskar, J.; Zdrahal, Z.; et al. Staphylococcus petrasii sp. nov. including S. petrasii subsp. petrasii subsp. nov. and S. petrasii subsp. croceilyticus subsp. nov., isolated from human clinical specimens and human ear infections. Syst. Appl. Microbiol. 2013, 36, 90–95. [Google Scholar] [CrossRef] [PubMed]
- De Bel, A.; Svec, P.; Petras, P.; Sedlacek, I.; Pantucek, R.; Echahidi, F.; Pierard, D.; Vandamme, P. Reclassification of Staphylococcus jettensis De Bel et al. 2013 as Staphylococcus petrasii subsp. jettensis subsp. nov. and emended description of Staphylococcus petrasii Pantucek et al. 2013. Int. J. Syst. Evol. Microbiol. 2014, 64, 4198–4201. [Google Scholar] [CrossRef] [PubMed]
- Svec, P.; Bel, A.; Sedlacek, I.; Petras, P.; Gelbicova, T.; Cernohlavkova, J.; Maslan ova, I.; Cnockaert, M.; Varbanovova, I.; Echahidi, F.; et al. Staphylococcus petrasii subsp. pragensis subsp. nov., occurring in human clinical material. Int. J. Syst. Evol. Microbiol 2015, 65, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Wanecka, A.; Twardon, J.; Mrowiec, J.; Dropinska, A.; Bania, J.; Podkowik, M.; Korzeniowska-Kowal, A.; Pasciak, M. Isolation of Staphylococcus microti from milk of dairy cows with mastitis. Vet. Microbiol. 2016, 182, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Rana, E.A.; Das, T.; Dutta, A.; Rahman, M.; Bostami, M.B.; Akter, N.; Barua, H. Coagulase-positive methicillin-resistant Staphylococcus aureus circulating in clinical mastitic goats in Bangladesh. Vet. World 2020, 13, 1303–1310. [Google Scholar] [CrossRef]
- Gizaw, F.; Kekeba, T.; Teshome, F.; Kebede, M.; Abreham, T.; Hayishe, H.; Waktole, H.; Tufa, T.B.; Edao, B.M.; Ayana, D.; et al. Distribution and antimicrobial resistance profile of coagulase-negative staphylococci from cattle, equipment, and personnel on dairy farm and abattoir settings. Heliyon 2020, 6, e03606. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, A.J.; Loeffler, A.; Witney, A.A.; Gould, K.A.; Lloyd, D.H.; Lindsay, J.A. Extensive horizontal gene transfer during Staphylococcus aureus co-colonization in vivo. Genome Biol. Evol. 2014, 6, 2697–2708. [Google Scholar] [CrossRef]
- Fisarova, L.; Pantucek, R.; Botka, T.; Doskar, J. Variability of resistance plasmids in coagulase-negative staphylococci and their importance as a reservoir of antimicrobial resistance. Res. Microbiol. 2019, 170, 105–111. [Google Scholar] [CrossRef]
- Smith, J.T.; Andam, C.P. Extensive Horizontal Gene Transfer within and between Species of Coagulase-Negative Staphylococcus. Genome Biol. Evol. 2021, 13, evab206. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020–18. [Google Scholar] [CrossRef] [Green Version]
- Fessler, A.T.; Billerbeck, C.; Kadlec, K.; Schwarz, S. Identification and characterization of methicillin-resistant coagulase-negative staphylococci from bovine mastitis. J. Antimicrob. Chemother. 2010, 65, 1576–1582. [Google Scholar] [CrossRef] [Green Version]
- Klibi, A.; Maaroufi, A.; Torres, C.; Jouini, A. Detection and characterization of methicillin-resistant and susceptible coagulase-negative staphylococci in milk from cows with clinical mastitis in Tunisia. Int. J. Antimicrob. Agents 2018, 52, 930–935. [Google Scholar] [CrossRef]
| Target Gene | Nucleotide Sequence (5′-3′) | PCR Product Size (bp) |
|---|---|---|
| mecA | F: TCCAGATTACAACTTCACCAGG | 162 |
| R: CCACTTCATATCTTGTAACG | ||
| mecC | F: GAAAAAAAGGCTTAGAACGCCTC | 138 |
| R: GAAGATCTTTTCCGTTTTCAGC |
| Isolates | 1st Real Time PCR Assay | 2nd Real Time PCR Assay | |||
|---|---|---|---|---|---|
| nuc | SCCmec/orfX | mecA/mecC | mecA | mecC | |
| 1.CoNS * | + | + | + | + | − |
| 9.CoNS * | + | + | + | + | − |
| 19.CoNS * | + | + | + | + | − |
| 21.CoNS * | + | + | + | + | − |
| 26.CoNS * | + | + | + | + | − |
| 28.SA * | + | + | + | + | − |
| 36.SA * | + | − | − | − | − |
| 44.SA * | + | + | + | + | − |
| 45.SA * | + | + | + | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelli, A.; Voidarou, C.; Venardou, B.; Fotou, K.; Tsinas, A.; Bonos, E.; Fthenakis, G.C.; Skoufos, I.; Tzora, A. Antimicrobial and Methicillin Resistance Pattern of Potential Mastitis-Inducing Staphylococcus aureus and Coagulase-Negative Staphylococci Isolates from the Mammary Secretion of Dairy Goats. Biology 2022, 11, 1591. https://doi.org/10.3390/biology11111591
Nelli A, Voidarou C, Venardou B, Fotou K, Tsinas A, Bonos E, Fthenakis GC, Skoufos I, Tzora A. Antimicrobial and Methicillin Resistance Pattern of Potential Mastitis-Inducing Staphylococcus aureus and Coagulase-Negative Staphylococci Isolates from the Mammary Secretion of Dairy Goats. Biology. 2022; 11(11):1591. https://doi.org/10.3390/biology11111591
Chicago/Turabian StyleNelli, Aikaterini, Chrysoula (Chrysa) Voidarou, Brigkita Venardou, Konstantina Fotou, Anastasios Tsinas, Eleftherios Bonos, George C. Fthenakis, Ioannis Skoufos, and Athina Tzora. 2022. "Antimicrobial and Methicillin Resistance Pattern of Potential Mastitis-Inducing Staphylococcus aureus and Coagulase-Negative Staphylococci Isolates from the Mammary Secretion of Dairy Goats" Biology 11, no. 11: 1591. https://doi.org/10.3390/biology11111591
APA StyleNelli, A., Voidarou, C., Venardou, B., Fotou, K., Tsinas, A., Bonos, E., Fthenakis, G. C., Skoufos, I., & Tzora, A. (2022). Antimicrobial and Methicillin Resistance Pattern of Potential Mastitis-Inducing Staphylococcus aureus and Coagulase-Negative Staphylococci Isolates from the Mammary Secretion of Dairy Goats. Biology, 11(11), 1591. https://doi.org/10.3390/biology11111591








