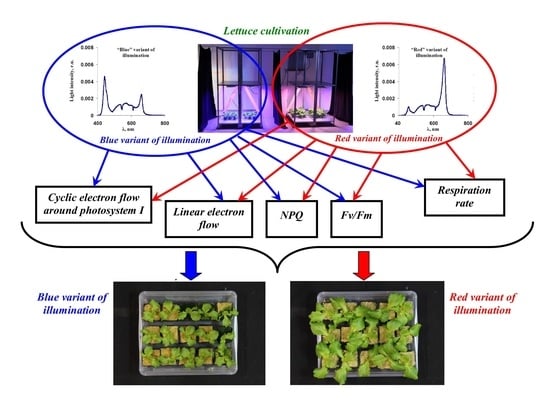
Ratio of Intensities of Blue and Red Light at Cultivation Influences Photosynthetic Light Reactions, Respiration, Growth, and Reflectance Indices in Lettuce
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
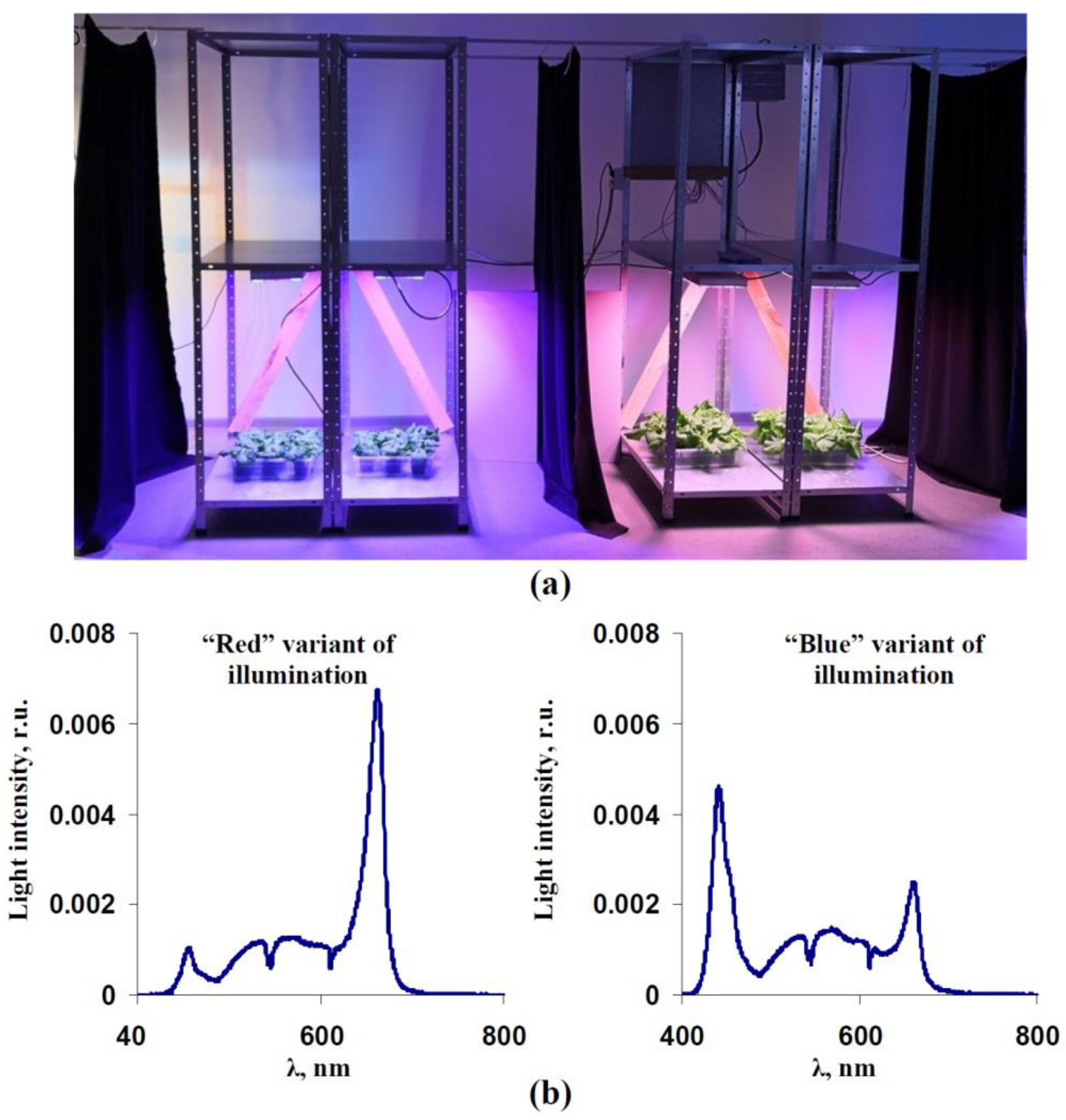
2.1. Plant Materials and Light Treatment
2.2. Measurements of Reflectance Indices in Lettuce Leaves
2.3. Measurements of Parameters of Photosynthesis, Respiration, and Transpiration in Lettuce Leaves
2.4. Measurements of Growth Parameters
2.5. Statistics
3. Results
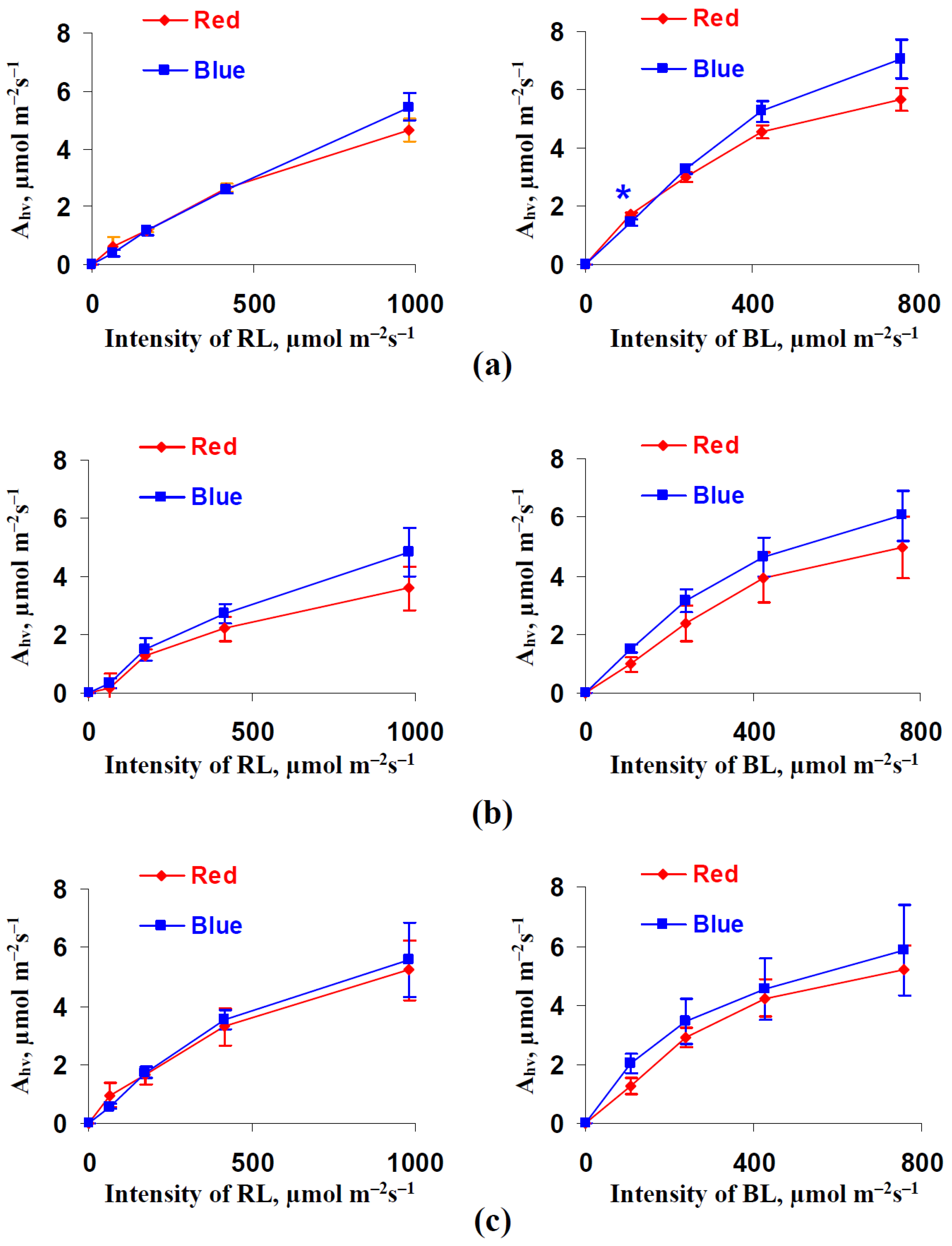
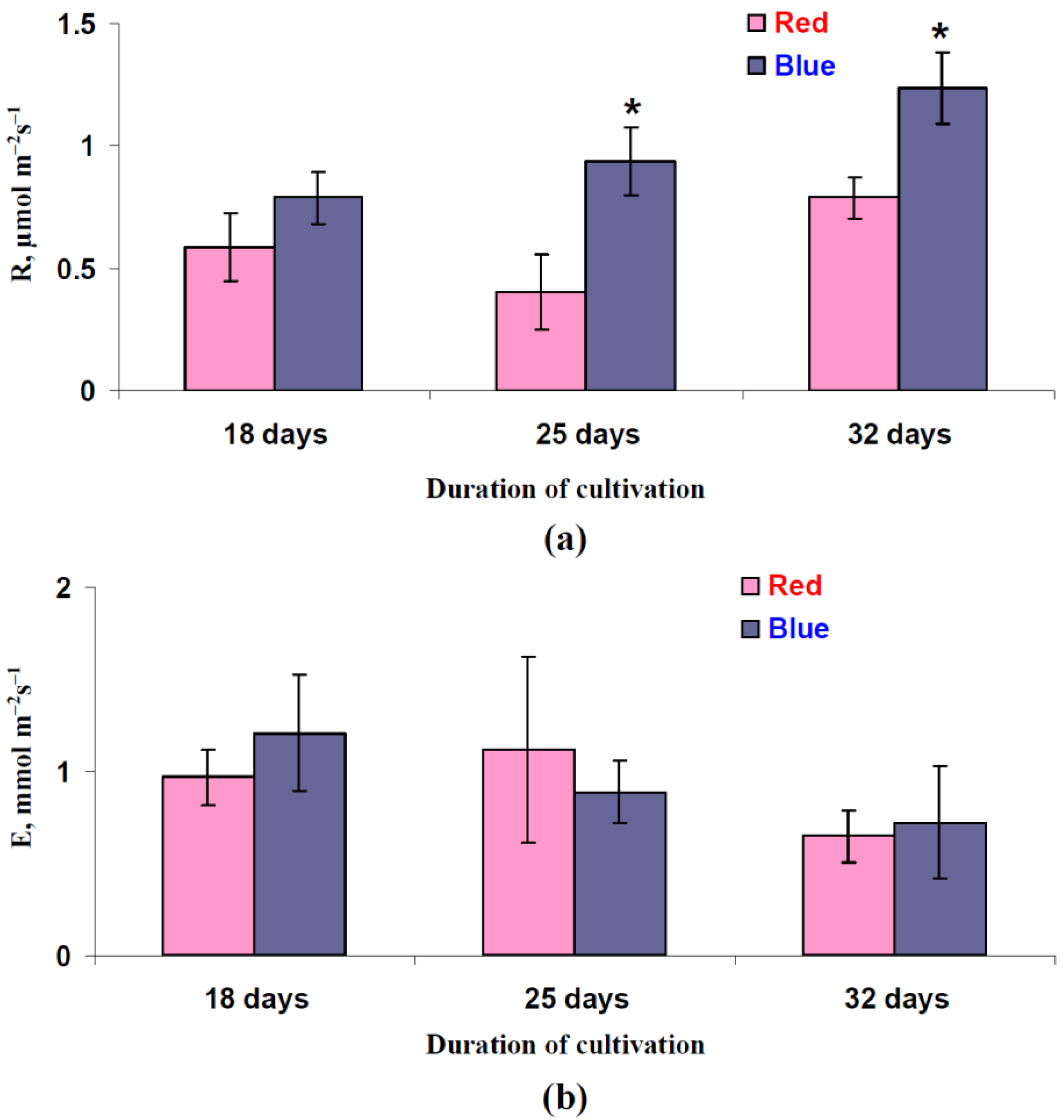
3.1. Influence of Red and Blue Variants of Illumination on Photosynthetic Assimilation of CO2, Respiration, and Transpiration in Leaves of Lettuce Plants
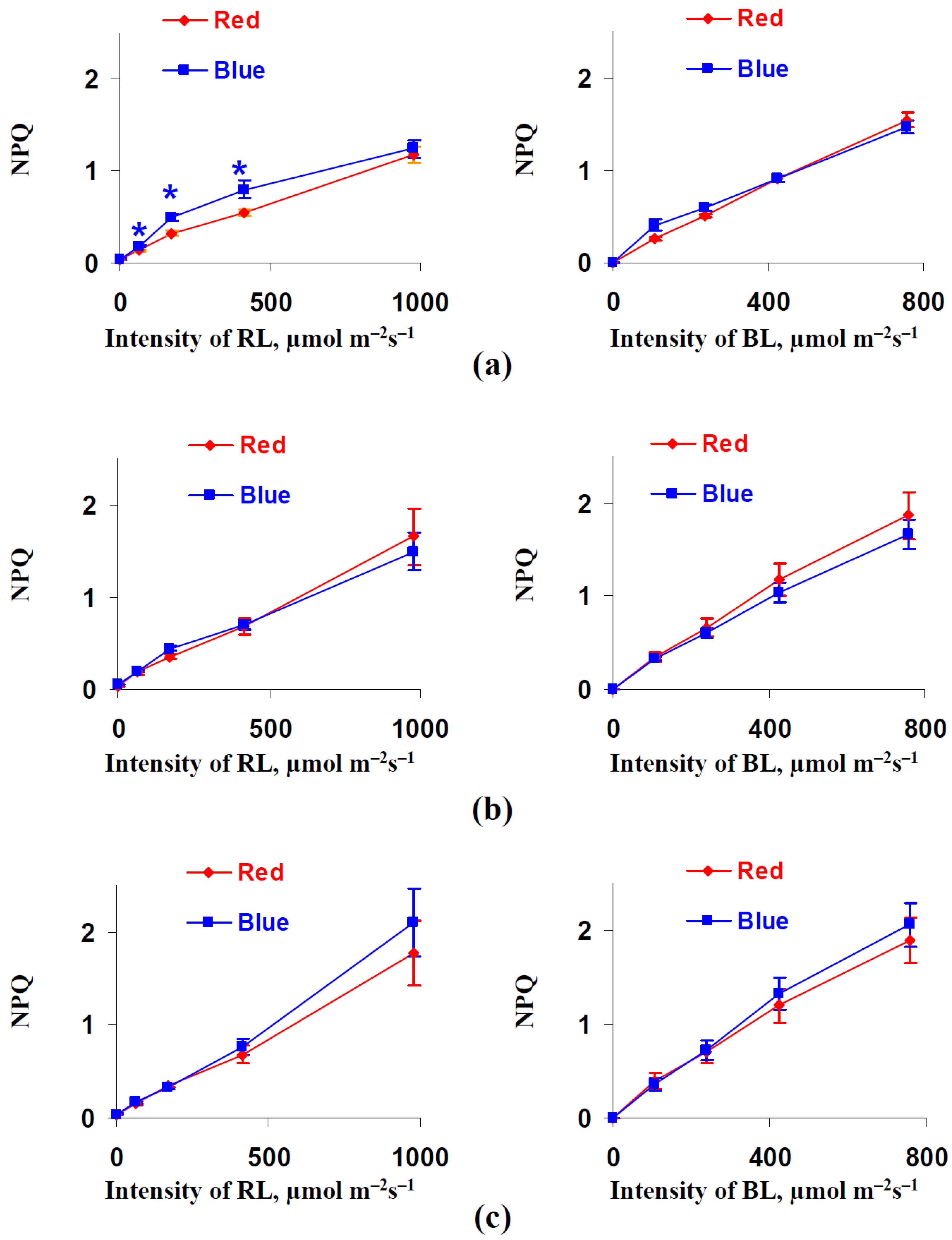
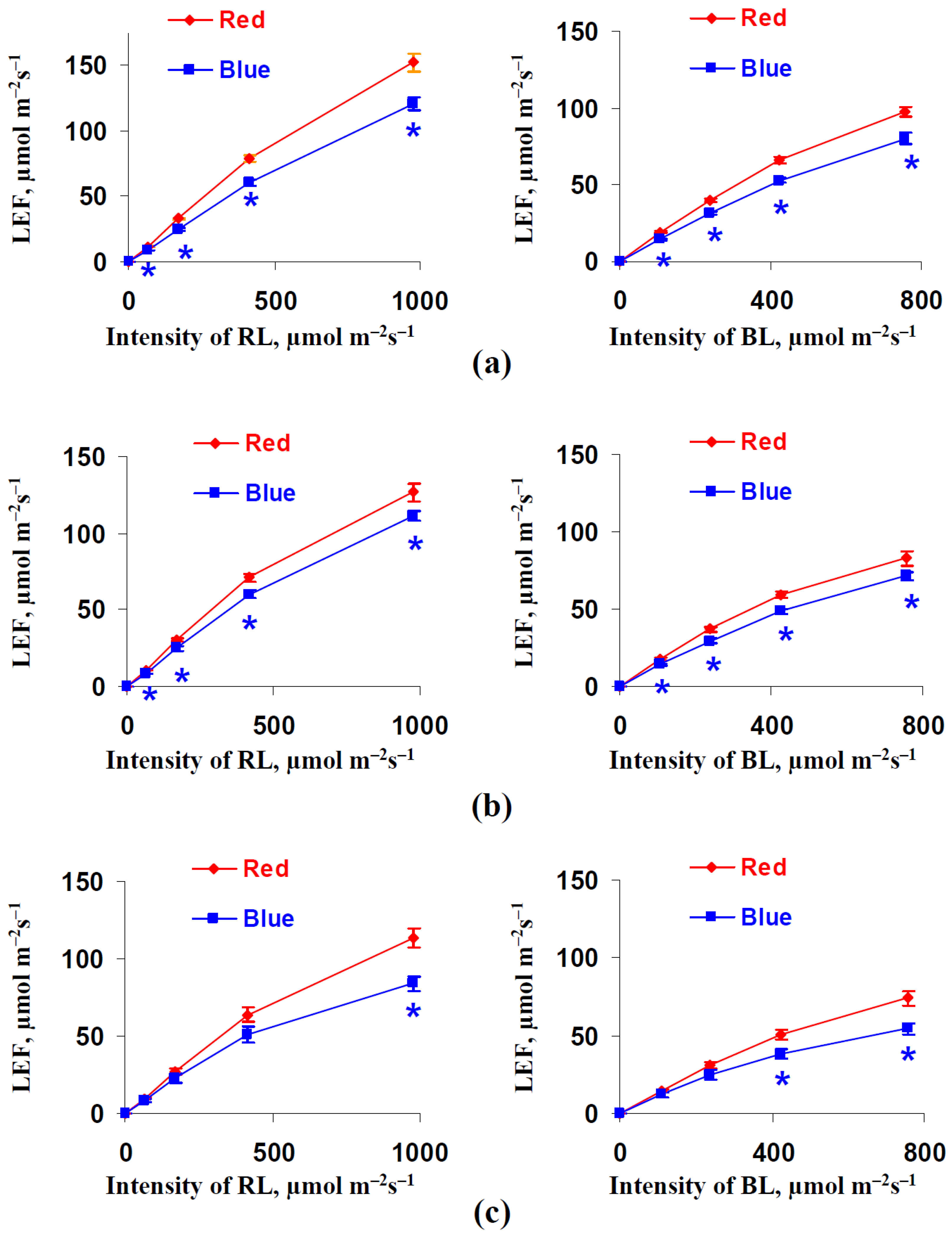
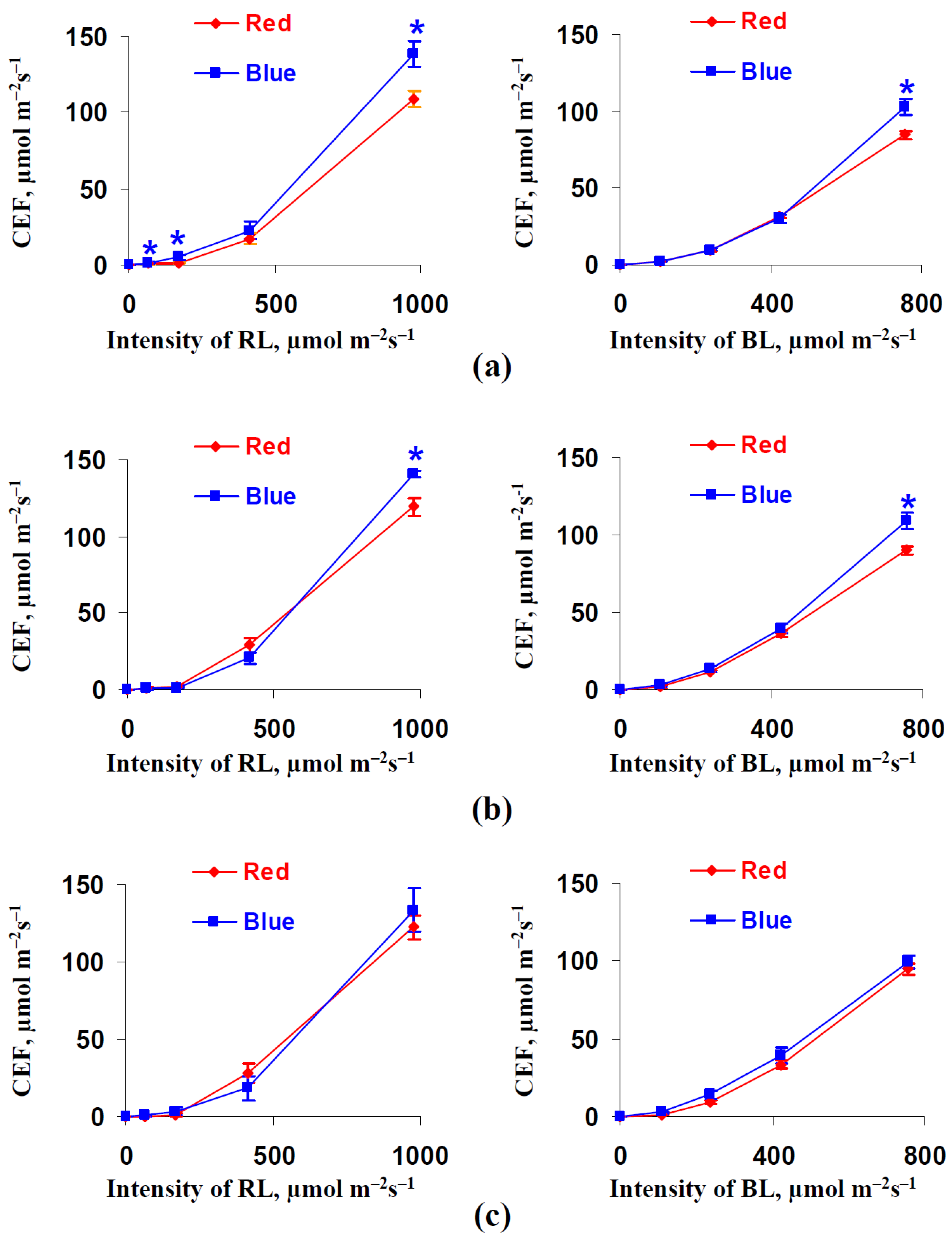
3.2. Influence of Red and Blue Variants of Illumination on Parameters of Photosynthetic Light Reactions in Leaves of Lettuce Plants
3.3. Influence of Red and Blue Variants of Illumination on Reflectance Indices in Leaves of Lettuce Plants
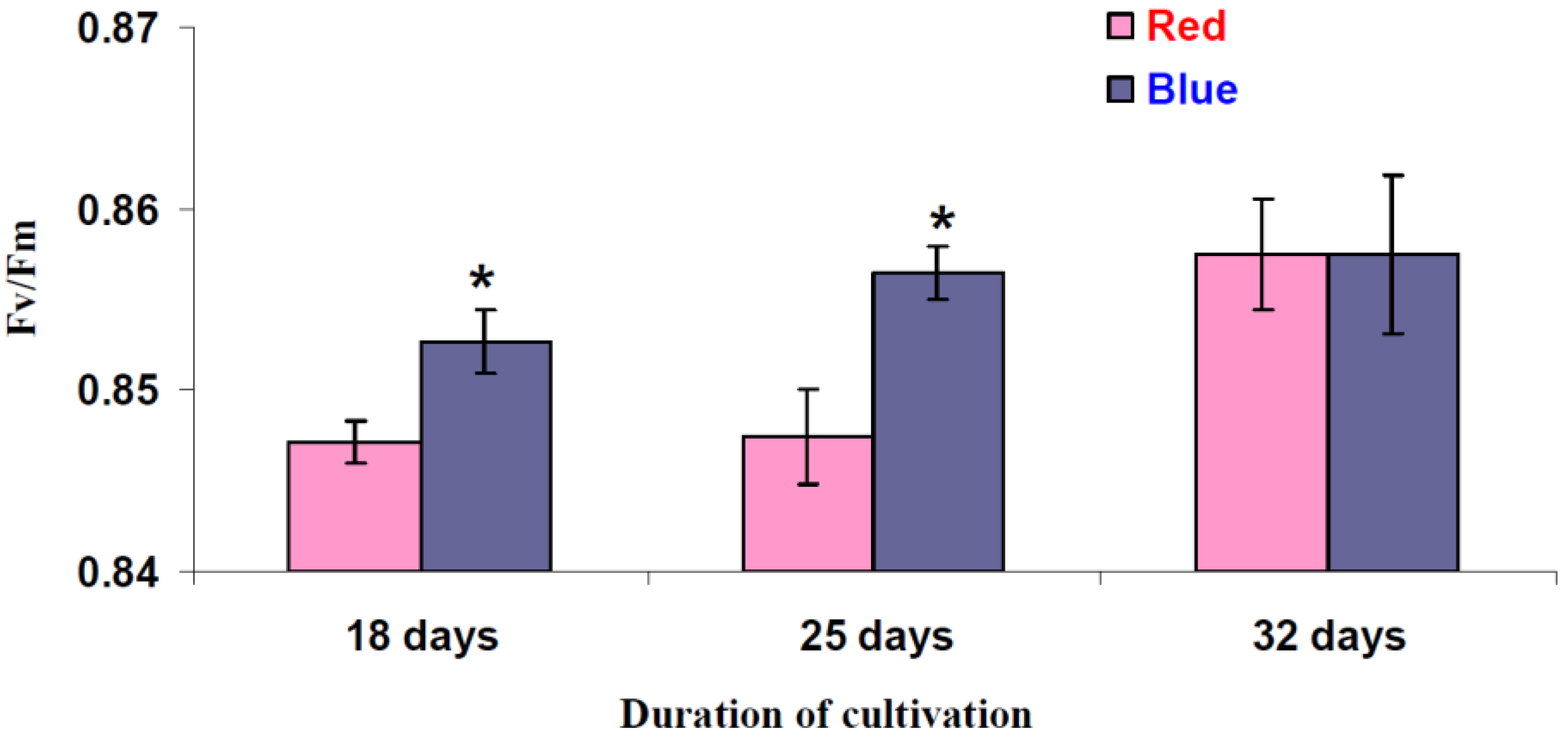
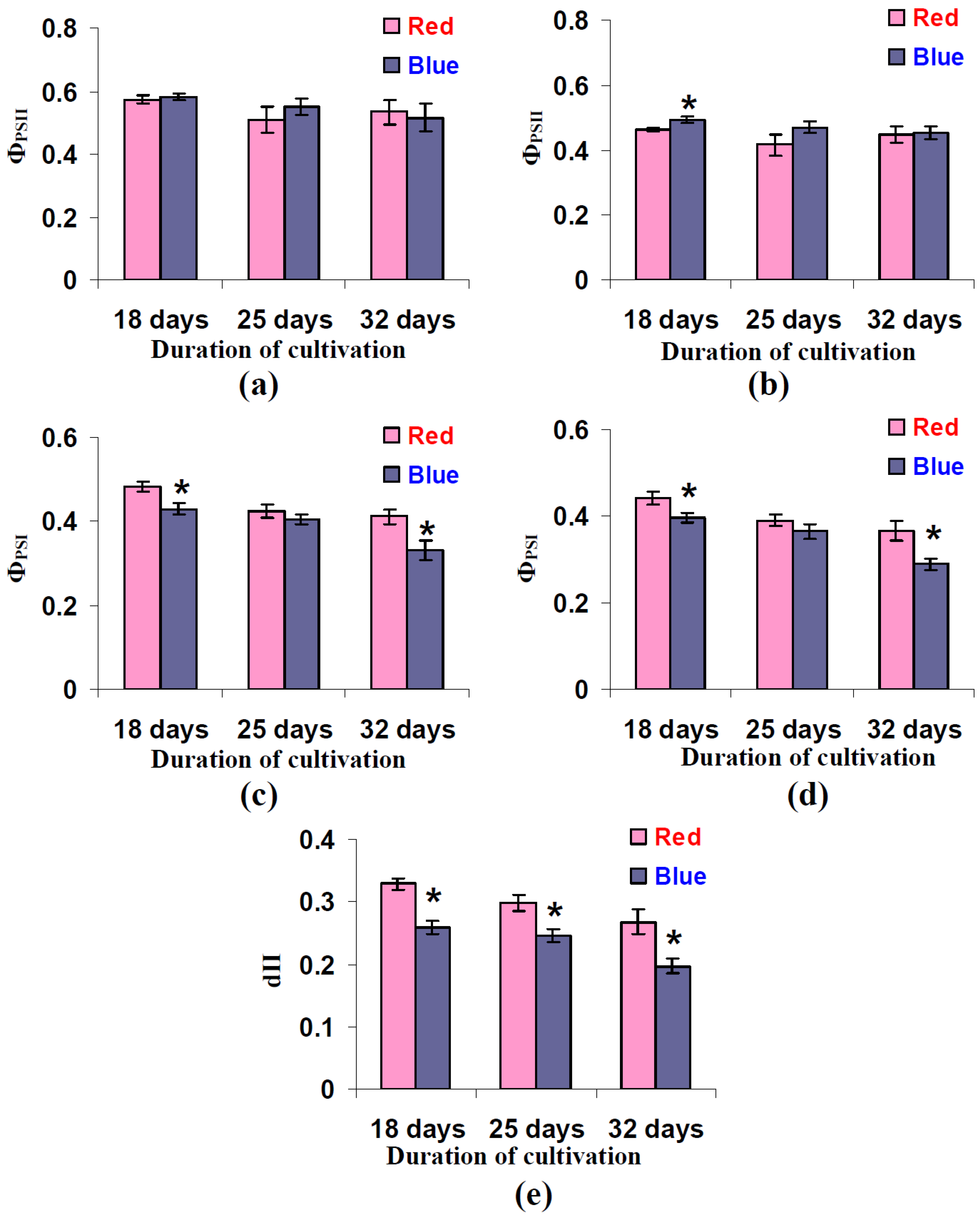
3.4. Influence of Red and Blue Variants of Illumination on Quantum Yields of Photosystems I and II and dII in Leaves of Lettuce Plants
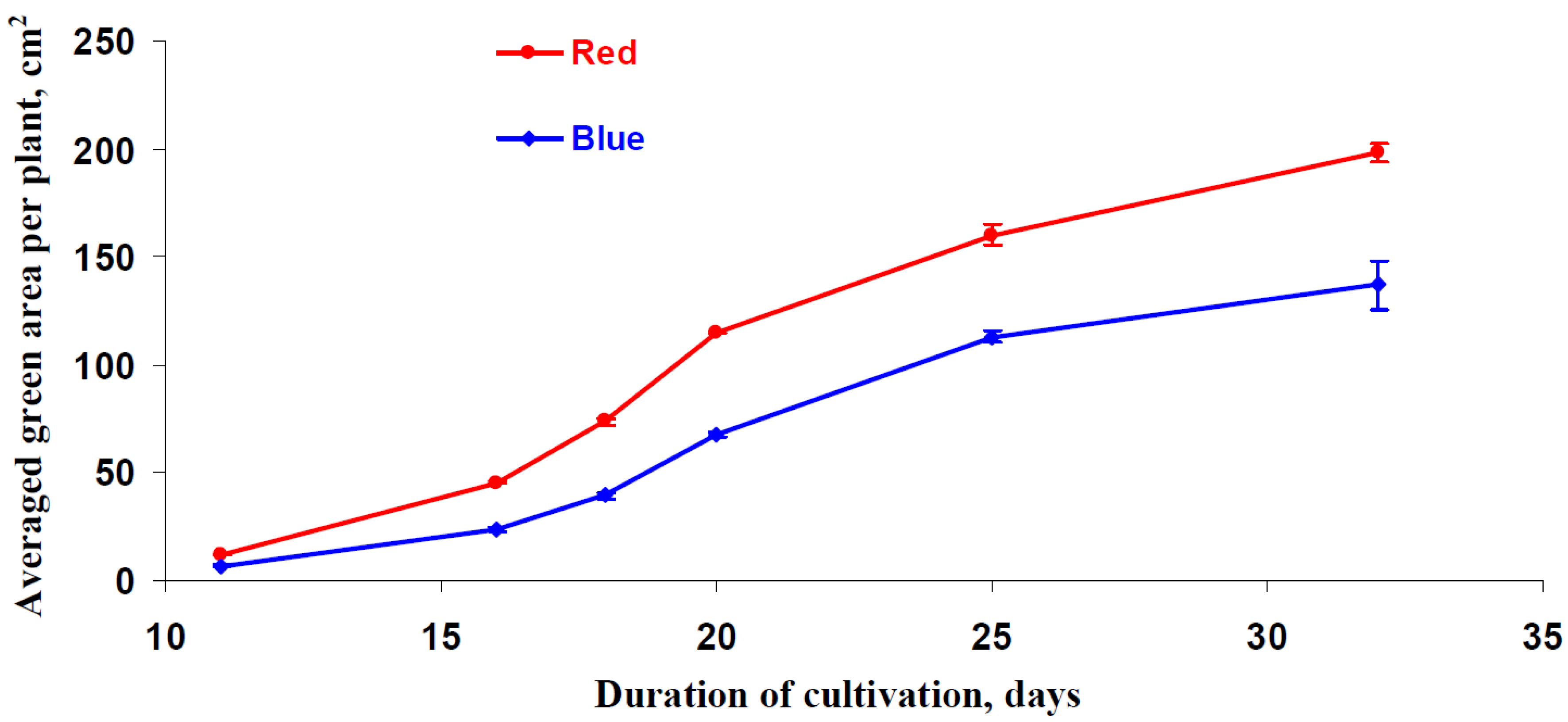
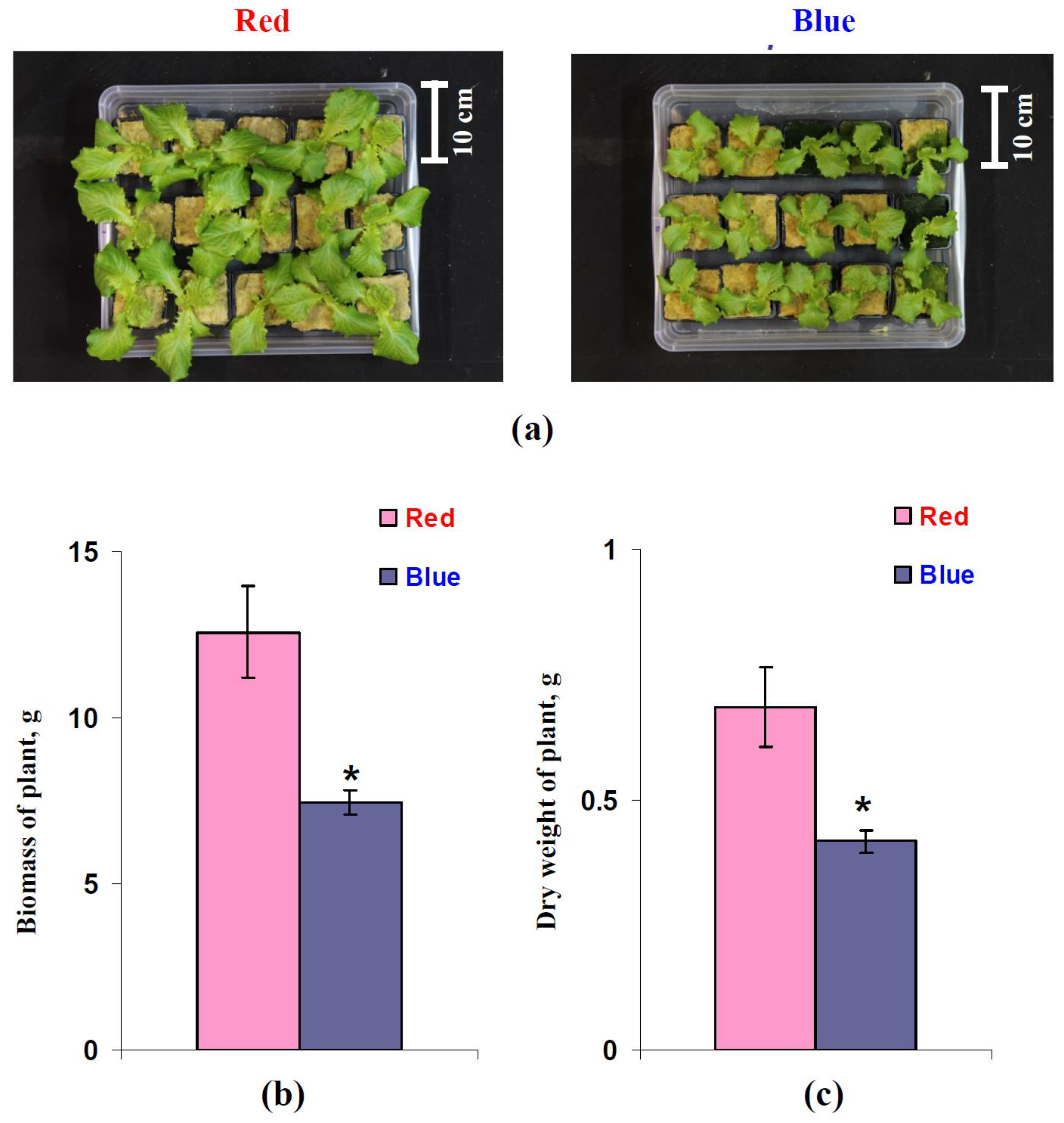
3.5. Influence of Red and Blue Variants of Illumination on Parameters of Growth of Lettuce
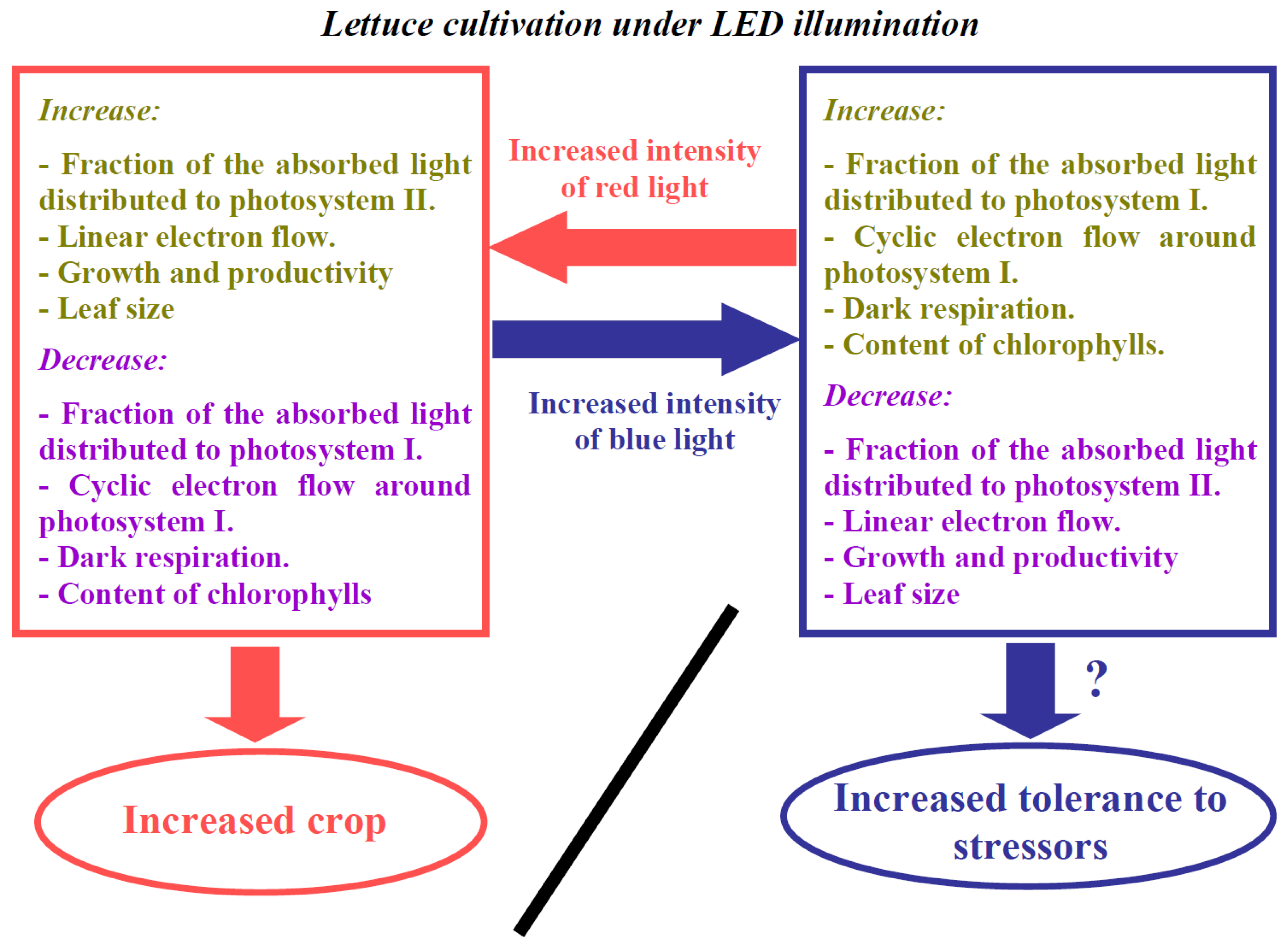
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruban, A.V. Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015, 66, 7–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef] [PubMed]
- Ptushenko, O.S.; Ptushenko, V.V.; Solovchenko, A.E. Spectrum of light as a determinant of plant functioning: A historical perspective. Life 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allakhverdiev, S.I. Recent progress in the studies of structure and function of photosystem II. J. Photochem. Photobiol. B Biol. 2011, 104, 1–8. [Google Scholar] [CrossRef]
- Johnson, M.P. Photosynthesis. Essays Biochem. 2016, 60, 255–273. [Google Scholar] [CrossRef]
- Shevela, D.; Björn, L.O. Evolution of the Z-scheme of photosynthesis: A perspective. Photosynth. Res. 2017, 133, 5–15. [Google Scholar]
- Stirbet, A.; Lazár, D.; Guo, Y.; Govindjee, G. Photosynthesis: Basics, history and modelling. Ann. Bot. 2020, 126, 511–537. [Google Scholar] [CrossRef]
- Joliot, P.; Joliot, A. Cyclic electron flow in C3 plants. Biochim. Biophys. Acta 2006, 1757, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Tikkanen, M.; Grieco, M.; Nurmi, M.; Rantala, M.; Suorsa, M.; Aro, E.M. Regulation of the photosynthetic apparatus under fluctuating growth light. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3486–3493. [Google Scholar] [CrossRef] [Green Version]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Morales, A.; Harbinson, J. Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiol. 2018, 176, 977–989. [Google Scholar] [CrossRef] [Green Version]
- Sukhova, E.; Khlopkov, A.; Vodeneev, V.; Sukhov, V. Simulation of a nonphotochemical quenching in plant leaf under different light intensities. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148138. [Google Scholar] [CrossRef]
- Sukhova, E.M.; Vodeneev, V.A.; Sukhov, V.S. Mathematical modeling of photosynthesis and analysis of plant productivity. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2021, 15, 52–72. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Goh, C.-H.; Ko, S.-M.; Koh, S.; Kim, Y.-J.; Bae, H.-J. Photosynthesis and environments: Photoinhibition and repair mechanisms in plants. J. Plant Biol. 2012, 55, 93–101. [Google Scholar] [CrossRef]
- Tikkanen, M.; Aro, E.M. Integrative regulatory network of plant thylakoid energy transduction. Trends Plant Sci. 2014, 19, 10–17. [Google Scholar] [CrossRef]
- Murata, N.; Nishiyama, Y. ATP is a driving force in the repair of photosystem II during photoinhibition. Plant Cell Environ. 2018, 41, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-S.; Chung, I.-M.; Hwang, M.H.; Kim, S.-H.; Yu, C.Y.; Ghimire, B.K. Application of light-emitting diodes for improving the nutritional quality and bioactive compound levels of some crops and medicinal plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W., III. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Miyake, C. Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: Molecular mechanisms and physiological functions. Plant Cell Physiol. 2010, 51, 1951–1963. [Google Scholar] [CrossRef] [Green Version]
- Messant, M.; Krieger-Liszkay, A.; Shimakawa, G. Dynamic changes in protein-membrane association for regulating photosynthetic electron transport. Cells 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Quail, P.H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 2010, 61, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Liu, H.; Klejnot, J.; Lin, C. The cryptochrome blue light receptors. Arab. Book 2010, 8, e0135. [Google Scholar] [CrossRef] [Green Version]
- Hart, J.E.; Gardner, K.H. Lighting the way: Recent insights into the structure and regulation of phototropin blue light receptors. J. Biol. Chem. 2021, 296, 100594. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, Y.G.; Li, X.; Ni, M. Light-regulated stomatal aperture in Arabidopsis. Mol. Plant. 2012, 5, 566–572. [Google Scholar] [CrossRef] [Green Version]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The impact of the phytochromes on photosynthetic processes. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B. Regulation of carotenoid biosynthesis in photosynthetic organs. In Carotenoids in Nature. Biosynthesis, Regulation and Function; Stange, C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 141–160. [Google Scholar]
- Quian-Ulloa, R.; Stange, C. Carotenoid biosynthesis and plastid development in plants: The role of light. Int. J. Mol. Sci. 2021, 22, 1184. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Belyaeva, O.B. Regulation of chlorophyll biogenesis by phytochrome A. Biochemistry 2019, 84, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Kong, S.G. Actin-mediated movement of chloroplasts. J. Cell Sci. 2018, 131, jcs210310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, T.; Shimazaki, K. Biochemical evidence for the requirement of 14-3-3 protein binding in activation of the guard-cell plasma membrane H+-ATPase by blue light. Plant Cell Physiol. 2002, 43, 1359–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhov, V. Electrical signals as mechanism of photosynthesis regulation in plants. Photosynth. Res. 2016, 130, 373–387. [Google Scholar] [CrossRef]
- Sukhov, V.; Sukhova, E.; Vodeneev, V. Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Prog. Biophys. Mol. Biol. 2019, 146, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Yudina, L.; Sherstneva, O.; Sukhova, E.; Grinberg, M.; Mysyagin, S.; Vodeneev, V.; Sukhov, V. Inactivation of H+-ATPase participates in the influence of variation potential on photosynthesis and respiration in peas. Plants 2020, 9, 1585. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, E.; Sukhov, V. Electrical signals, plant tolerance to actions of stressors, and programmed cell death: Is interaction possible? Plants 2021, 10, 1704. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Eprintsev, A.T.; Fedorin, D.N.; Popov, V.N. Phytochrome-mediated regulation of plant respiration and photorespiration. Plant Cell Environ. 2014, 37, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.G.; Arena, C.; De Micco, V.; Capozzi, F.; Aronne, G. Light quality shapes morpho-functional traits and pigment content of green and red leaf cultivars of Atriplex hortensis. Sci. Hort. 2019, 246, 942–950. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mickens, M.A.; Aronne, G.; Gómez, C. Spectral effects of blue and red light on growth, anatomy, and physiology of lettuce. Physiol. Plant. 2021, 172, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Q.; Xin, Y.; Mei, Z.; Gao, A.; Liu, W.; Yu, L.; Chen, X.; Chen, Z.; Wang, N. Analyses of the photosynthetic characteristics, chloroplast ultrastructure, and transcriptome of apple (Malus domestica) grown under red and blue lights. BMC Plant Biol. 2021, 21, 483. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jiang, C.; Gao, L. Polychromatic supplemental lighting from underneath canopy is more effective to enhance tomato plant development by improving leaf photosynthesis and stomatal regulation. Front. Plant Sci. 2016, 7, 1832. [Google Scholar] [CrossRef] [Green Version]
- Cammarisano, L.; Donnison, I.S.; Robson, P.R.H. The effect of red & blue rich LEDs vs. fluorescent light on Lollo Rosso lettuce morphology and physiology. Front. Plant Sci. 2021, 12, 603411. [Google Scholar]
- Cioć, M.; Tokarz, K.; Dziurka, M.; Pawłowska, B. Energy-saving LED light affects the efficiency of the photosynthetic apparatus and carbohydrate content in Gerbera jamesonii Bolus ex Hook. f. axillary shoots multiplied in vitro. Biology 2021, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. Hort. Sci. 2001, 36, 380–383. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Goto, E.; Kurata, K. Photosynthetic characteristics of rice leaves grown under red light with or without supplemental blue light. Plant Cell Physiol. 2004, 45, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, P.; Zhang, W.J.; Fu, W. Growth, photosynthesis, and nutrient uptake at different light intensities and temperatures in lettuce. Hort. Sci. 2019, 54, 1925–1933. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of ascorbate accumulation and metabolism in lettuce by the red:blue ratio of continuous light using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Li, Y.L.; Wang, L.C.; Guo, W.Z. Red and blue wavelengths affect the morphology, energy use efficiency and nutritional content of lettuce (Lactuca sativa L.). Sci. Rep. 2021, 11, 8374. [Google Scholar] [CrossRef] [PubMed]
- Grishin, A.A.; Smirnov, A.A.; Grishin, V.A.; Dorokhov, A.A.; Chilingaryan, N.O. Climatic chambers with a system of controlled phytoirradiation for growing plants. Bull. Cent. Bot. Gard. 2021, 1, 56–60. (In Russian) [Google Scholar]
- POLYPEN RP400 & RP410. Manual and User Guide. Available online: https://handheld.psi.cz/documents/PolyPen%20RP400_RP410_Manual_04_2021.pdf (accessed on 25 November 2021).
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; Type III Final Rep; The National Aeronautics and Space Administration (NASA)/Goddard Space Flight Center (GSFC): Greenbelt, MD, USA, 1974.
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.R.; de Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Haboudanea, D.; Miller, J.R.; Patteyc, E.; Zarco-Tejadad, P.J.; Strachane, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Lloret, P.; Muñoz, F.; Vilajeliu, M. Reflectance assessment of mite effects on apple trees. Int. J. Remote Sens. 1995, 16, 2727–2733. [Google Scholar] [CrossRef]
- Gamon, J.; Peñuelas, J.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies. A review and meta-analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Zhang, C.; Filella, I.; Garbulsky, M.F.; Peñuelas, J. Affecting factors and recent improvements of the photochemical reflectance index (PRI) for remotely sensing foliar, canopy and ecosystemic radiation-use efficiencies. Remote Sens. 2016, 8, 677. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance indices associated with physiological changes in nitrogen and water-limited sunflower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Peñuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll-a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Saturation pulse method for assessment of energy conversion in PS I. PAM Appl. Notes. 2008, 1, 11–14. [Google Scholar]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [Green Version]
- Sukhov, V.; Surova, L.; Sherstneva, O.; Katicheva, L.; Vodeneev, V. Variation potential influence on photosynthetic cyclic electron flow in pea. Front. Plant Sci. 2015, 5, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhova, E.; Mudrilov, M.; Vodeneev, V.; Sukhov, V. Influence of the variation potential on photosynthetic flows of light energy and electrons in pea. Photosynth. Res. 2018, 136, 215–228. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Sherstneva, O.; Grinberg, M.; Ladeynova, M.; Vodeneev, V.; Sukhov, V. Exogenous abscisic acid can influence photosynthetic processes in peas through a decrease in activity of H+-ATP-ase in the plasma membrane. Biology 2020, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E., Jr.; Doraiswamya, P.C.; McMurtreya, J.E.; Daughtrya, C.S.T.; Perry, E.M.; Akhmedova, B. A visible band index for remote sensing leaf chlorophyll content at the canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Miliauskienė, J.; Haimi, P.J.; Laužikė, K.; Brazaitytė, A.; Duchovskis, P. The physiological response of lettuce to red and blue light dynamics over different photoperiods. Front. Plant Sci. 2021, 11, 610174. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, T.; Razi Parjikolaei, B.; Fretté, X.; Rosenqvist, E.; Ottosen, C.O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Nemali, K. Blue and far-red light affect area and number of individual leaves to influence vegetative growth and pigment synthesis in lettuce. Front. Plant Sci. 2021, 12, 667407. [Google Scholar] [CrossRef]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why is chlorophyll b only used in light-harvesting systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef] [Green Version]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic quantum yield dynamics: From photosystems to leaves. Plant Cell. 2012, 24, 1921–1935. [Google Scholar] [CrossRef] [Green Version]
- Walters, R.G. Towards an understanding of photosynthetic acclimation. J. Exp. Bot. 2005, 56, 435–447. [Google Scholar] [CrossRef] [PubMed]
| Index | 18 Days | 25 Days | 32 Days | ||||
|---|---|---|---|---|---|---|---|
| Red | Blue | Red | Blue | Red | Blue | ||
| Normalized Difference Vegetation Index (NDVI) | M | 0.647 | 0.654 | 0.646 | 0.692 | 0.648 | 0.695 |
| SE | 0.007 | 0.006 | 0.011 | 0.007 | 0.010 | 0.008 | |
| Simple Ratio Index (SR) | M | 4.732 | 4.832 | 4.814 | 5.606 | 4.808 | 5.674 |
| SE | 0.114 | 0.103 | 0.196 | 0.155 | 0.155 | 0.176 | |
| Optimized Soil-Adjusted Vegetation Index (OSAVI) | M | 0.720 | 0.712 | 0.700 | 0.707 | 0.691 | 0.693 |
| SE | 0.004 | 0.004 | 0.010 | 0.008 | 0.009 | 0.007 | |
| Simple Ratio 554/677 Greenness Index (G) | M | 3.696 | 3.364 | 3.726 | 3.327 | 3.226 | 3.113 |
| SE | 0.051 | 0.050 | 0.059 | 0.049 | 0.075 | 0.069 | |
| Modified Chlorophyll Absorption in Reflectance Index (MCARI) | M | 0.542 | 0.455 | 0.488 | 0.354 | 0.394 | 0.292 |
| SE | 0.019 | 0.016 | 0.024 | 0.015 | 0.020 | 0.015 | |
| Modified Chlorophyll Absorption in Reflectance Index 1 (MCARI1) | M | 0.967 | 0.941 | 0.867 | 0.830 | 0.871 | 0.775 |
| SE | 0.016 | 0.010 | 0.028 | 0.030 | 0.027 | 0.026 | |
| Transformed Chlorophyll Absorption Ratio Index (TCARI) | M | −0.476 | −0.394 | −0.430 | −0.308 | −0.344 | −0.256 |
| SE | 0.017 | 0.015 | 0.021 | 0.013 | 0.017 | 0.013 | |
| Triangular Vegetation Index (TVI) | M | 35.674 | 34.899 | 32.037 | 31.103 | 32.369 | 29.184 |
| SE | 0.590 | 0.376 | 1.048 | 1.120 | 1.000 | 0.995 | |
| Zarco-Tejada and Miller Index (ZMI) | M | 1.559 | 1.604 | 1.571 | 1.725 | 1.617 | 1.780 |
| SE | 0.016 | 0.015 | 0.026 | 0.019 | 0.023 | 0.023 | |
| Simple Ratio Pigment Index (SRPI) | M | 1.158 | 1.168 | 1.118 | 1.139 | 1.129 | 1.154 |
| SE | 0.012 | 0.012 | 0.014 | 0.016 | 0.013 | 0.027 | |
| Normalized Phaeophytinization Index (NPQI) | M | 0.046 | 0.032 | 0.043 | 0.061 | 0.040 | 0.039 |
| SE | 0.004 | 0.006 | 0.004 | 0.009 | 0.004 | 0.015 | |
| Photochemical Reflectance Index (PRI) | M | 0.021 | 0.021 | 0.022 | 0.027 | 0.022 | 0.028 |
| SE | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Normalized Pigment Chlorophyll Index (NPCI) | M | −0.072 | −0.077 | −0.055 | −0.063 | −0.060 | −0.068 |
| SE | 0.005 | 0.005 | 0.006 | 0.007 | 0.006 | 0.011 | |
| Carter Index 1 (Ctr1) | M | 1.871 | 1.746 | 1.970 | 1.675 | 1.707 | 1.578 |
| SE | 0.038 | 0.034 | 0.049 | 0.031 | 0.042 | 0.039 | |
| Carter Index 2 (Ctr2) | M | 0.280 | 0.272 | 0.281 | 0.237 | 0.272 | 0.231 |
| SE | 0.006 | 0.005 | 0.009 | 0.006 | 0.008 | 0.006 | |
| Lichtenthaler Index 1 (Lic1) | M | 0.778 | 0.770 | 0.781 | 0.789 | 0.760 | 0.781 |
| SE | 0.004 | 0.003 | 0.006 | 0.005 | 0.007 | 0.006 | |
| Lichtenthaler Index 2 (Lic2) | M | 0.765 | 0.825 | 0.738 | 0.804 | 0.818 | 0.824 |
| SE | 0.015 | 0.017 | 0.018 | 0.022 | 0.016 | 0.024 | |
| Structure Intensive Pigment Index (SIPI) | M | 0.739 | 0.731 | 0.742 | 0.761 | 0.726 | 0.750 |
| SE | 0.005 | 0.004 | 0.007 | 0.005 | 0.007 | 0.007 | |
| Gitelson and Merzlyak Index 1 (GM1) | M | 2.241 | 2.343 | 2.270 | 2.610 | 2.352 | 2.709 |
| SE | 0.036 | 0.035 | 0.058 | 0.046 | 0.052 | 0.054 | |
| Gitelson and Merzlyak Index 2 (GM2) | M | 2.469 | 2.570 | 2.515 | 2.895 | 2.600 | 3.015 |
| SE | 0.045 | 0.042 | 0.073 | 0.058 | 0.062 | 0.070 | |
| Anthocyanin Reflectance Index 1 (ARI1) | M | −0.428 | −0.418 | −0.544 | −0.605 | −0.493 | −0.690 |
| SE | 0.022 | 0.015 | 0.060 | 0.038 | 0.018 | 0.055 | |
| Anthocyanin Reflectance Index 2 (ARI2) | M | −0.231 | −0.229 | −0.247 | −0.289 | −0.251 | −0.309 |
| SE | 0.010 | 0.008 | 0.016 | 0.014 | 0.012 | 0.018 | |
| Carotenoid Reflectance Index 1 (CRI1) | M | 4.825 | 4.683 | 5.757 | 6.276 | 5.006 | 6.493 |
| SE | 0.174 | 0.115 | 0.389 | 0.365 | 0.220 | 0.432 | |
| Carotenoid Reflectance Index 1 (CRI2) | M | 4.397 | 4.265 | 5.213 | 5.670 | 4.513 | 5.803 |
| SE | 0.157 | 0.107 | 0.333 | 0.334 | 0.210 | 0.387 | |
| Ratio Difference Vegetation Index (RDVI) | M | 0.612 | 0.607 | 0.578 | 0.584 | 0.579 | 0.566 |
| SE | 0.005 | 0.004 | 0.012 | 0.012 | 0.011 | 0.010 | |
| Index | Fv/Fm | LEF(RL)max | LEF(BL)max | CEF(RL)max | CEF(BL)max |
|---|---|---|---|---|---|
| NDVI | 0.656 | −0.742 | −0.777 | 0.692 | 0.716 |
| SR | 0.648 | −0.767 | −0.802 | 0.662 | 0.688 |
| OSAVI | −0.618 | 0.755 | 0.744 | −0.184 | −0.177 |
| G | −0.967 | 0.856 | 0.859 | −0.677 | −0.664 |
| MCARI | −0.910 | 0.956 | 0.971 | −0.702 | −0.709 |
| MCARI1 | −0.664 | 0.893 | 0.910 | −0.482 | −0.490 |
| TCARI | 0.918 | −0.955 | −0.969 | 0.718 | 0.724 |
| TVI | −0.634 | 0.878 | 0.894 | −0.449 | −0.457 |
| ZMI | 0.779 | −0.875 | −0.902 | 0.687 | 0.691 |
| SRPI | 0.034 | 0.045 | 0.044 | 0.239 | 0.134 |
| NPQI | 0.071 | 0.099 | 0.044 | 0.090 | 0.290 |
| PRI | 0.666 | −0.805 | −0.839 | 0.616 | 0.636 |
| NPCI | 0.028 | −0.153 | −0.155 | −0.197 | −0.103 |
| Ctr1 | −0.924 | 0.797 | 0.814 | −0.683 | −0.685 |
| Ctr2 | −0.734 | 0.804 | 0.837 | −0.697 | −0.718 |
| Lic1 | −0.178 | −0.063 | −0.101 | 0.217 | 0.254 |
| Lic2 | 0.853 | −0.636 | −0.630 | 0.693 | 0.662 |
| SIPI | 0.171 | −0.334 | −0.379 | 0.399 | 0.470 |
| GM1 | 0.763 | −0.861 | −0.889 | 0.703 | 0.709 |
| GM2 | 0.755 | −0.862 | −0.891 | 0.680 | 0.689 |
| ARI1 | −0.535 | 0.810 | 0.836 | −0.417 | −0.424 |
| ARI2 | −0.674 | 0.834 | 0.867 | −0.515 | −0.541 |
| CRI1 | 0.408 | −0.696 | −0.726 | 0.421 | 0.445 |
| CRI2 | 0.386 | −0.675 | −0.705 | 0.419 | 0.446 |
| RDVI | −0.559 | 0.811 | 0.817 | −0.270 | −0.273 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudina, L.; Sukhova, E.; Mudrilov, M.; Nerush, V.; Pecherina, A.; Smirnov, A.A.; Dorokhov, A.S.; Chilingaryan, N.O.; Vodeneev, V.; Sukhov, V. Ratio of Intensities of Blue and Red Light at Cultivation Influences Photosynthetic Light Reactions, Respiration, Growth, and Reflectance Indices in Lettuce. Biology 2022, 11, 60. https://doi.org/10.3390/biology11010060
Yudina L, Sukhova E, Mudrilov M, Nerush V, Pecherina A, Smirnov AA, Dorokhov AS, Chilingaryan NO, Vodeneev V, Sukhov V. Ratio of Intensities of Blue and Red Light at Cultivation Influences Photosynthetic Light Reactions, Respiration, Growth, and Reflectance Indices in Lettuce. Biology. 2022; 11(1):60. https://doi.org/10.3390/biology11010060
Chicago/Turabian StyleYudina, Lyubov, Ekaterina Sukhova, Maxim Mudrilov, Vladimir Nerush, Anna Pecherina, Alexandr A. Smirnov, Alexey S. Dorokhov, Narek O. Chilingaryan, Vladimir Vodeneev, and Vladimir Sukhov. 2022. "Ratio of Intensities of Blue and Red Light at Cultivation Influences Photosynthetic Light Reactions, Respiration, Growth, and Reflectance Indices in Lettuce" Biology 11, no. 1: 60. https://doi.org/10.3390/biology11010060
APA StyleYudina, L., Sukhova, E., Mudrilov, M., Nerush, V., Pecherina, A., Smirnov, A. A., Dorokhov, A. S., Chilingaryan, N. O., Vodeneev, V., & Sukhov, V. (2022). Ratio of Intensities of Blue and Red Light at Cultivation Influences Photosynthetic Light Reactions, Respiration, Growth, and Reflectance Indices in Lettuce. Biology, 11(1), 60. https://doi.org/10.3390/biology11010060