Additional Blue LED during Cultivation Induces Cold Tolerance in Tomato Fruit but Only to an Optimum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
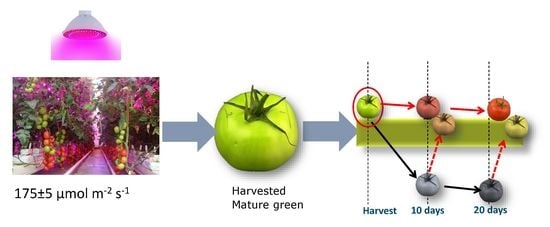
2.1. Plant Material and Growth Conditions
2.2. Light Treatments
2.3. Fruit Selection, Storage Conditions and Sample Preparation
2.4. Color and Firmness Measurements
2.5. CI Indices and Weight Loss
2.6. Total Ascorbic Acid Measurement
2.7. Catalase (CAT) Measurement
2.8. Hydrogen Peroxide (H2O2) Measurement
2.9. Malondialdehyde (MDA) Measurement
2.10. Statistical Analysis
3. Results
3.1. Effect of Light Treatments on CI in R and MG Fruit
3.2. Light Treatments Affect the Colour and Firmness at Harvest in R Tomatoes
3.3. Effect of Light Treatments and Cold Storage on Coloration and Softening of MG Fruit
3.4. Cold-Stored R Tomatoes Show Colour and Firmness Loss
3.5. AsA, CAT Activity, H2O2 and MDA Content Are Unaffected by BL Treatments
4. Discussion
4.1. Cold Tolerance Might Be Related to a Lower Lycopene Level at Harvest That Allows for More Lycopene Loss for R Tomatoes
4.2. Tomato Shows High Variation in Cold Tolerance Induction Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albornoz, K.; Cantwell, M.I.; Zhang, L.; Beckles, D.M. Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress. Sci. Rep. 2019, 9, 2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loayza, F.E.; Brecht, J.K.; Simonne, A.H.; Plotto, A.; Baldwin, E.A.; Bai, J.; Lon-Kan, E. A brief hot-water treatment alleviates chilling injury symptoms in fresh tomatoes. J. Sci. Food Agric. 2021, 101, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; East, A.R.; Brecht, J.K.; Hewett, E.W.; Heyes, J.A. Intermittent warming during low temperature storage reduces tomato chilling injury. Postharvest Biol. Technol. 2012, 74, 71–78. [Google Scholar] [CrossRef]
- Farneti, B.; Schouten, R.E.; Woltering, E.J. Low temperature-induced lycopene degradation in red ripe tomato evaluated by remittance spectroscopy. Postharvest Biol. Technol. 2012, 73, 22–27. [Google Scholar] [CrossRef]
- Sevillano, L.; Sanchez-Ballesta, M.T.; Romojaro, F.; Flores, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 2009, 89, 555–573. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P.M. Oxidative stress: Importance for postharvest quality. Hortscience 2004, 39, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Sheng, J.; Lv, S.; Zheng, Y.; Zhang, J.; Yu, M.; Shen, L. Nitric oxide participates in the regulation of LeCBF1 gene expression and improves cold tolerance in harvested tomato fruit. Postharvest Biol. Technol. 2011, 62, 121–126. [Google Scholar] [CrossRef]
- Biswas, P.; East, A.R.; Hewett, E.W.; Heyes, J.A. Chilling injury in tomato fruit. Hortic. Rev. 2016, 44, 229–278. [Google Scholar]
- Jackman, R.L.; Gibson, H.J.; Stanley, D.W. Effects of chilling on tomato fruit texture. Physiol. Plant. 1992, 86, 600–608. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Jackman, R.L.; Stanley, D.W. Chilling-associated softening of tomato fruit is related to increased pectinmethylesterase activity. J. Food Sci. 1995, 60, 1277–1281. [Google Scholar] [CrossRef]
- Rugkong, A.; McQuinn, R.; Giovannoni, J.J.; Rose, J.K.; Watkins, C.B. Expression of ripening-related genes in cold-stored tomato fruit. Postharvest Biol. Technol. 2011, 61, 1–14. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Bodbodak, S. Postharvest heat treatment for mitigation of chilling injury in fruits and vegetables. Food Bioprocess Technol. 2014, 7, 37–53. [Google Scholar] [CrossRef]
- Imahori, Y.; Bai, J.; Baldwin, E. Antioxidative responses of ripe tomato fruit to postharvest chilling and heating treatments. Sci. Hortic. 2016, 198, 398–406. [Google Scholar] [CrossRef]
- Malacrida, C.; Valle, E.M.; Boggio, S.B. Postharvest chilling induces oxidative stress response in the dwarf tomato cultivar Micro-Tom. Physiol. Plant. 2006, 127, 10–18. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Schouten, R.E.; Huijben, T.P.; Tijskens, L.M.M.; van Kooten, O. Modelling quality attributes of truss tomatoes: Linking colour and firmness maturity. Postharvest Biol. Technol. 2007, 45, 298–306. [Google Scholar] [CrossRef]
- Heymann, T.; Heinz, P.; Glomb, M.A. Lycopene Inhibits the Isomerization of β-Carotene during Quenching of Singlet Oxygen and Free Radicals. J. Agric. Food Chem. 2015, 63, 3279–3287. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Brandt, S.; Pék, Z.; Barna, É.; Lugasi, A.; Helyes, L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Neta-Sharir, I.; Isaacson, T.; Lurie, S.; Weiss, D. Dual role for tomato heat shock protein 21: Protecting photosystem ii from oxidative stress and promoting color changes during fruit maturation. Plant Cell 2005, 17, 1829–1838. [Google Scholar] [CrossRef] [Green Version]
- Hernández, V.; Hellín, P.; Fenoll, J.; Flores, P. Increased temperature produces changes in the bioactive composition of tomato, depending on its developmental stage. J. Agric. Food Chem. 2015, 63, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Nawaz, M.A.; Wei, N.; Cheng, F.; Bie, Z. Suboptimal temperature acclimation enhances chilling tolerance by improving photosynthetic adaptability and osmoregulation ability in watermelon. Hortic. Plant J. 2020, 6, 49–60. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Zhang, J.H. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygenspecies and up-regulates the activities of antioxidant enzymes inmaize leaves. J. Expt. Bot. 2002, 379, 2401–2410. [Google Scholar] [CrossRef] [Green Version]
- Yacoubi, I.; Hamdi, K.; Fourquet, P.; Bignon, C.; Longhi, S. Structural and functional characterization of the aba-water deficit stress domain from wheat and barley: An intrinsically disordered domain behind the versatile functions of the plant abscissic acid, stress and ripening protein family. Int. J. Mol. Sci. 2021, 22, 2314. [Google Scholar] [CrossRef]
- Singh, A.P.; Mani, B.; Giri, J. OsJAZ9 is involved in water-deficit stress tolerance by regulating leaf width and stomatal density in rice. Plant Physiol. Biochem. 2021, 162, 161–170. [Google Scholar] [CrossRef]
- Hanin, M.; Brini, F.; Ebel, C.; Toda, Y.; Takeda, S.; Masmoudi, K. Plant dehydrins and stress tolerance: Versatile proteins for complex mechanisms. Plant Signal. Behav. 2011, 6, 1503–1509. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, X.; Zhang, L. Structural and functional dynamics of dehydrins: A plant protector protein under abiotic stress. Int. J. Mol. Sci. 2018, 19, 3420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, M.; Terashima, S.; Fukaya, T.; Kuboi, T. Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 2003, 217, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Liang, L.; Zhao, Y.; Guo, Z.; Lu, S. A cold responsive ethylene responsive factor from Medicago falcata confers cold tolerance by up-regulation of polyamine turnover, antioxidant protection, and proline accumulation. Plant Cell Environ. 2017, 41, 2021–2032. [Google Scholar] [CrossRef]
- Affandi, F.Y.; Verdonk, J.C.; Ouzounis, T.; Ji, Y.; Woltering, E.J.; Schouten, R.E. Far-red light during cultivation induces postharvest cold tolerance in tomato fruit. Postharvest Biol. Technol. 2020, 159, 111019. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Sun, T.; Wang, X.; Zhu, C.; Ai, Y.; Gu, H. The precise regulation of differentCORgenes by individual CBF transcription factors inArabidopsis thaliana. J. Integr. Plant Biol. 2017, 59, 118–133. [Google Scholar] [CrossRef] [Green Version]
- Rihan, H.Z.; Al-Issawi, M.; Fuller, M.P. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interact. 2017, 12, 143–157. [Google Scholar] [CrossRef]
- Chen, B.; Feder, M.E.; Kang, L. Evolution of heat-shock protein expression underlying adaptive responses to envi-ronmental stress. Mol. Ecol. 2018, 27, 3040–3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Shi, L.; Chen, W.; Cao, S.; Su, X.; Yang, Z. Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Sci. Hortic. 2014, 175, 181–186. [Google Scholar] [CrossRef]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding Blue to Red Supplemental Light Increases Biomass and Yield of Greenhouse-Grown Tomatoes, but Only to an Optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef] [Green Version]
- Schouten, R.E.; Farneti, B.; Tijskens, P.; Alarcón, A.A.; Woltering, E.J. Quantifying lycopene synthesis and chlorophyll breakdown in tomato fruit using remittance VIS spectroscopy. Postharvest Biol. Technol. 2014, 96, 53–63. [Google Scholar] [CrossRef]
- Schouten, R.E.; Fan, S.; Verdonk, J.C.; Wang, Y.; Kasim, N.F.M.; Woltering, E.J.; Tijskens, L.M.M. Mango firmness modeling as affected by transport and ethylene treatments. Front. Plant Sci. 2018, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Vega-García, M.O.; López-Espinoza, G.; Ontiveros, J.C.; Caro-Corrales, J.J.; Vargas, F.D.; López-Valenzuela, J.A. Changes in Protein Expression Associated with Chilling Injury in Tomato Fruit. J. Am. Soc. Hortic. Sci. 2010, 135, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Davey, M.W.; Dekempeneer, E.; Keulemans, J. Rocket-powered high-performance liquid chromatographic analysis of plant ascorbate and glutathione. Anal. Biochem. 2003, 316, 74–81. [Google Scholar] [CrossRef]
- Nukuntornprakit, O.-A.; Chanjirakul, K.; van Doorn, W.G.; Siriphanich, J. Chilling injury in pineapple fruit: Fatty acid composition and antioxidant metabolism. Postharvest Biol. Technol. 2015, 99, 20–26. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.Y.; Shen, L.; Fan, B.; Yu, M.M.; Zheng, Y.; Lv, S.N.; Sheng, J.P. Ethylene and cold participate in the regulation ofLeCBF1gene expression in postharvest tomato fruits. FEBS Lett. 2009, 583, 3329–3334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edge, R.; Truscott, T.G. Singlet Oxygen and free radical reactions of retinoids and carotenoids—A review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Min, D.B.; Boff, J.M. Chemistry and reaction of singlet oxygen in foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef]
- Wang, L.; Baldwin, E.A.; Zhao, W.; Plotto, A.; Sun, X.; Wang, Z.; Brecht, J.K.; Bai, J.; Yu, Z. Suppression of volatile production in tomato fruit exposed to chilling temperature and alleviation of chilling injury by a pre-chilling heat treatment. LWT-Food Sci. Technol. 2015, 62, 115–121. [Google Scholar] [CrossRef]
- Carvalho, G.C.; de Camargo, B.A.F.; de Araújo, J.T.C.; Chorilli, M. Lycopene: From tomato to its nutraceutical use and its association with nanotechnology. Trends Food Sci. Technol. 2021, 118, 447–458. [Google Scholar] [CrossRef]
- Liu, D.; Shi, J.; Ibarra, A.C.; Kakuda, Y.; Xue, S.J. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT-Food Sci. Technol. 2008, 41, 1344–1349. [Google Scholar] [CrossRef]
- Kotikova, Z.; Lachman, J.; Hejtmánková, A.; Hejtmánková, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT-Food Sci. Technol. 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Farjadian, F.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Cohen, E.; Shapiro, B.; Shalom, Y.; Klein, J.D. Water Loss: A Nondestructive Indicator of Enhanced Cell Membrane Permeability of Chilling-injured Citrus Fruit. J. Am. Soc. Hortic. Sci. 1994, 119, 983–986. [Google Scholar] [CrossRef] [Green Version]
- Bae, G.; Choi, G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 2008, 59, 281–311. [Google Scholar] [CrossRef] [Green Version]
- Casal, J.J. Photoreceptor Signaling Networks in Plant Responses to Shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E. PIFs: Systems Integrators in Plant Development. Plant Cell 2014, 26, 56–78. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, Q.; Liu, Y.; Zhao, X.; Imaizumi, T.; Somers, D.E.; Tobin, E.M.; Lin, C. Arabidopsis CRY2 and ZTL mediate blue-light regulation of the transcription factor CIB1 by distinct mechanisms. Proc. Natl. Acad. Sci. USA 2013, 110, 17582–17587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Z.; Liu, H.; Liu, B.; Liu, X.; Lin, C. Blue Light-Dependent Interaction of CRY2 with SPA1 Regulates COP1 activity and Floral Initiation in Arabidopsis. Curr. Biol. 2011, 21, 841–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, B.X.; Wei, J.J.; Zhang, Y.T.; Song, S.W.; Wei, S.U.; Sun, G.W.; Hao, Y.W.; Liu, H.C. Supplemental blue and red light promote lycopene synthesis in tomato fruits. J. Integr. Agric. 2019, 18, 590–598. [Google Scholar] [CrossRef] [Green Version]
- Alba, R.; Cordonnier-Pratt, M.-M.; Pratt, L.H. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant Physiol. 2000, 123, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Fiore, A.; Tavazza, M.; Giuliano, G. Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Sheng, J.; Li, S.; Nie, Y.; Zhao, J.; Zhu, Z.; Wang, Z.; Tang, X. The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2015, 101, 88–95. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.; Pan, Q.; Liu, Y. Improvement of chilling tolerance and accumulation of heat shock proteins in grape berries (Vitis vinifera cv. Jingxiu) by heat pretreatment. Postharvest Biol. Technol. 2005, 38, 80–90. [Google Scholar] [CrossRef]
- Luengwilai, K.; Beckles, D.M.; Saltveit, M.E. Chilling-injury of harvested tomato (Solanum lycopersicum L.) cv. Micro-Tom fruit is reduced by temperature pre-treatments. Postharvest Biol. Technol. 2012, 63, 123–128. [Google Scholar] [CrossRef]
- Cruz-Mendívil, A.; López-Valenzuela, J.A.; Calderón-Vázquez, C.L.; Vega-García, M.O.; Reyes-Moreno, C.; Valdez-Ortiz, A. Transcriptional changes associated with chilling tolerance and susceptibility in ‘Micro-Tom’ tomato fruit using RNA-Seq. Postharvest Biol. Technol. 2015, 99, 141–151. [Google Scholar] [CrossRef]
- Dhakal, R.; Baek, K.-H. Metabolic alternation in the accumulation of free amino acids and γ-aminobutyric acid in postharvest mature green tomatoes following irradiation with blue light. Hortic. Environ. Biotechnol. 2014, 55, 36–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, P.; Huang, Y.; Shan, T.; Wang, L.; Li, Y.; Zheng, Y. Effect of hot water combined with glycine betaine alleviates chilling injury in cold-stored loquat fruit. Postharvest Biol. Technol. 2016, 118, 141–147. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Naderi, R.; Sarcheshmeh, M.A.A.; Babalar, M. Amelioration of postharvest chilling injury in anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Postharvest Biol. Technol. 2015, 110, 70–76. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Huang, X.; Yang, K.; Gao, S.; Du, R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affandi, F.Y.; Prayoga, T.; Ouzounis, T.; Giday, H.; Verdonk, J.C.; Woltering, E.J.; Schouten, R.E. Additional Blue LED during Cultivation Induces Cold Tolerance in Tomato Fruit but Only to an Optimum. Biology 2022, 11, 101. https://doi.org/10.3390/biology11010101
Affandi FY, Prayoga T, Ouzounis T, Giday H, Verdonk JC, Woltering EJ, Schouten RE. Additional Blue LED during Cultivation Induces Cold Tolerance in Tomato Fruit but Only to an Optimum. Biology. 2022; 11(1):101. https://doi.org/10.3390/biology11010101
Chicago/Turabian StyleAffandi, Fahrizal Yusuf, Teddy Prayoga, Theoharis Ouzounis, Habtamu Giday, Julian C. Verdonk, Ernst J. Woltering, and Rob E. Schouten. 2022. "Additional Blue LED during Cultivation Induces Cold Tolerance in Tomato Fruit but Only to an Optimum" Biology 11, no. 1: 101. https://doi.org/10.3390/biology11010101
APA StyleAffandi, F. Y., Prayoga, T., Ouzounis, T., Giday, H., Verdonk, J. C., Woltering, E. J., & Schouten, R. E. (2022). Additional Blue LED during Cultivation Induces Cold Tolerance in Tomato Fruit but Only to an Optimum. Biology, 11(1), 101. https://doi.org/10.3390/biology11010101