Mediating Roles of hsCRP, TNF-α and Adiponectin on the Associations between Body Fat and Fatty Liver Disease among Overweight and Obese Adults
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Measurements
2.3. Laboratory Measurements
2.4. Body Fat Percentage Measurement
2.5. Diagnostic Criteria of FLD
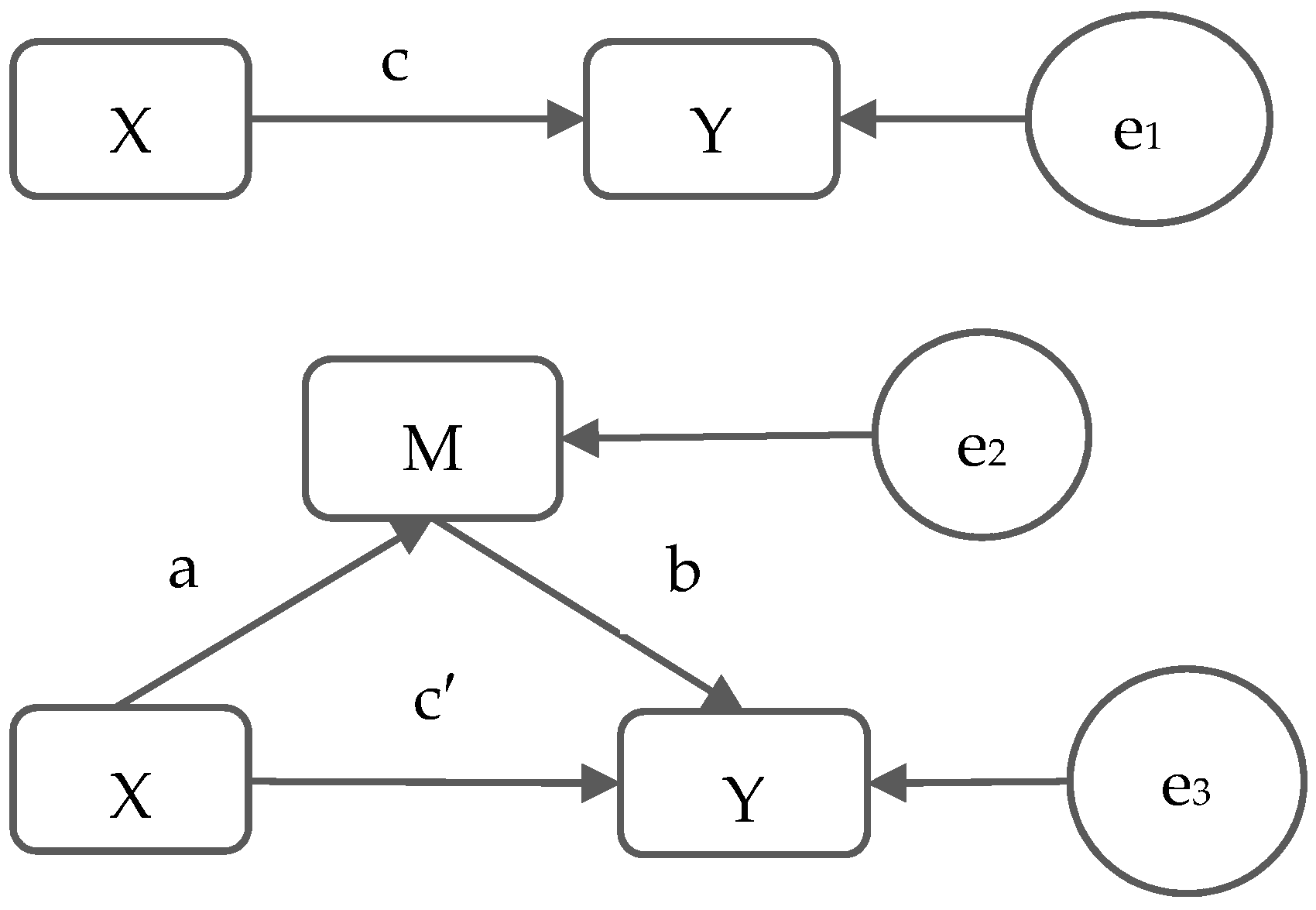
2.6. Statistical Analysis
3. Results
3.1. Basic Characteristics of Participants
3.2. Relation between Body Fat and Proposed Mediators (a)
3.3. Relation between Proposed Mediators and FLD (b)
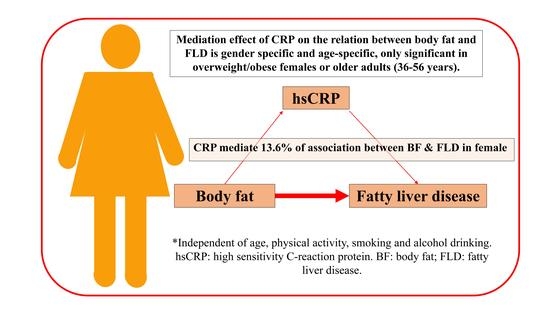
3.4. Mediation Effect of Proposed Mediators (Indirect Effect, a × b)
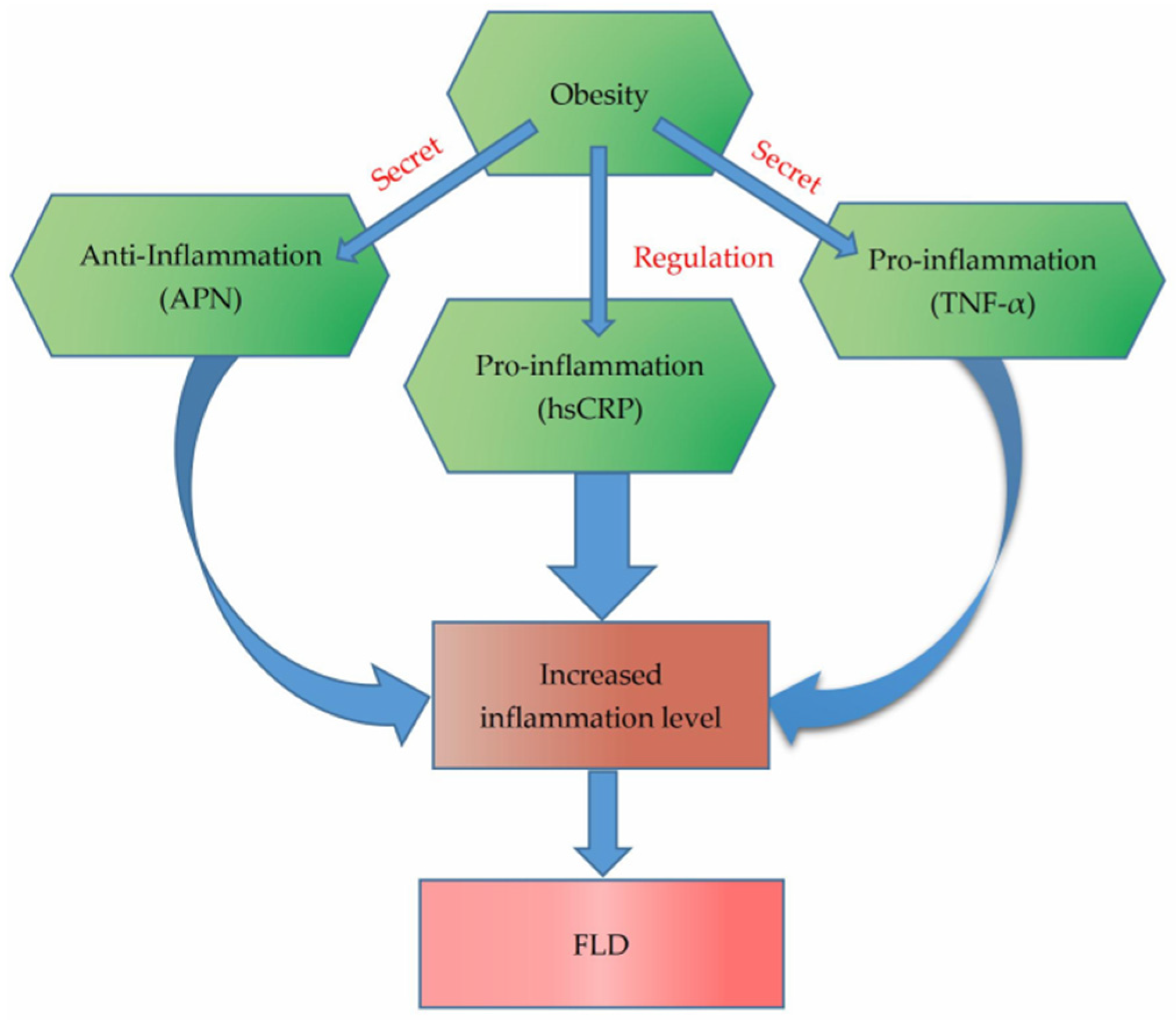
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumeister, S.E.; Völzke, H.; Marschall, P.; John, U.; Schmidt, C.O.; Flessa, S.; Alte, D. Impact of fatty liver disease on health care utilization and costs in a general population: A 5-year observation. Gastroenterology 2008, 134, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- O’Shea, R.S.; Dasarathy, S.; McCullough, A.J. Alcoholic liver disease. Hepatology 2010, 51, 307–328. [Google Scholar] [CrossRef]
- Bellentani, S.; Saccoccio, G.; Masutti, F.; Crocè, L.S.; Brandi, G.; Sasso, F.; Cristanini, G.; Tiribelli, C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann. Intern. Med. 2000, 132, 112–117. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.Z.; Zhou, Q.Y.; Wang, Y.M.; Dai, Y.N.; Zhu, J.; Yu, C.H.; Li, Y.M. Prevalence of fatty liver disease and the economy in China: A systematic review. World J. Gastroenterol. 2015, 21, 5695–5706. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, J.; Wang, W.; Zhang, X.J.; Ji, Y.X.; Zhang, P.; She, Z.G.; Zhu, L.; Cai, J.; Li, H. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology 2019, 70, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, Y.J.; Tan, K.; Zeng, L.; Liu, L.; Liu, F.J.; Zhou, T.Y.; Chen, E.Q.; Tang, H. Prevalence and risk factors of fatty liver disease in Chengdu, Southwest China. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2009, 8, 377–382. [Google Scholar]
- Wong, V.W.; Chu, W.C.; Wong, G.L.; Chan, R.S.; Chim, A.M.; Ong, A.; Yeung, D.K.; Yiu, K.K.; Chu, S.H.; Woo, J.; et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: A population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2012, 61, 409–415. [Google Scholar] [CrossRef]
- Fan, J.G. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S1), 11–17. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nier, A.; Brandt, A.; Conzelmann, I.B.; Özel, Y.; Bergheim, I. Non-Alcoholic Fatty Liver Disease in Overweight Children: Role of Fructose Intake and Dietary Pattern. Nutrients 2018, 10, 1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, N. Obesity, fatty liver disease and intestinal microbiota. World J. Gastroenterol. 2014, 20, 16452–16463. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ayada, I.; Zhang, X.; Wang, L.; Li, Y.; Wen, T.; Ma, Z.; Bruno, M.J.; de Knegt, R.J.; Cao, W.; et al. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese adults. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, in press. [Google Scholar] [CrossRef]
- Wu, S.J.; Huang, W.C.; Yu, M.C.; Chen, Y.L.; Shen, S.C.; Yeh, K.W.; Liou, C.J. Tomatidine ameliorates obesity-induced nonalcoholic fatty liver disease in mice. J. Nutr. Biochem. 2021, 91, 108602. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; González-Ruíz, K.; González-Jiménez, E.; Schmidt-RioValle, J.; Correa-Rodríguez, M.; García-Hermoso, A.; Palomino-Echeverría, S.; Izquierdo, M. Serum leptin as a mediator of the influence of insulin resistance on hepatic steatosis in youths with excess adiposity. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Fat in the liver and insulin resistance. Ann. Med. 2005, 37, 347–356. [Google Scholar] [CrossRef]
- Soderborg, T.K.; Clark, S.E.; Mulligan, C.E.; Janssen, R.C.; Babcock, L.; Ir, D.; Young, B.; Krebs, N.; Lemas, D.J.; Johnson, L.K.; et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat. Commun. 2018, 9, 4462. [Google Scholar] [CrossRef] [Green Version]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Saponaro, C.; Gastaldelli, A. Not all fats are created equal: Adipose vs. ectopic fat, implication in cardiometabolic diseases. Horm. Mol. Biol. Clin. Investig. 2015, 22, 7–18. [Google Scholar] [CrossRef]
- Moller, D.E. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol. Metab. 2000, 11, 212–217. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, K.; Ryu, S.; Chang, Y.; Kim, H.R. High-normal levels of hs-CRP predict the development of non-alcoholic fatty liver in healthy men. PLoS ONE 2017, 12, e0172666. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Werneburg, N.W.; Canbay, A.; Guicciardi, M.E.; Bronk, S.F.; Rydzewski, R.; Burgart, L.J.; Gores, G.J. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 2004, 40, 185–194. [Google Scholar] [CrossRef]
- Hui, J.M.; Hodge, A.; Farrell, G.C.; Kench, J.G.; Kriketos, A.; George, J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004, 40, 46–54. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Guo, Q.; Chang, B.; Yu, Q.L.; Xu, S.T.; Yi, X.J.; Cao, S.C. Adiponectin treatment improves insulin resistance in mice by regulating the expression of the mitochondrial-derived peptide MOTS-c and its response to exercise via APPL1-SIRT1-PGC-1α. Diabetologia 2020, 63, 2675–2688. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.; Papatheodoridis, G.V.; Archimandritis, A.J. The evolving role of leptin and adiponectin in chronic liver diseases. Am. J. Gastroenterol. 2006, 101, 2629–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piya, M.K.; McTernan, P.G.; Kumar, S. Adipokine inflammation and insulin resistance: The role of glucose, lipids and endotoxin. J. Endocrinol. 2013, 216, T1–T15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Mantzoros, C.S. Adiponectin and leptin in the diagnosis and therapy of NAFLD. Metab. Clin. Exp. 2020, 103, 154028. [Google Scholar] [CrossRef]
- Ezquerro, S.; Mocha, F.; Frühbeck, G.; Guzmán-Ruiz, R.; Valentí, V.; Mugueta, C.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Silva, C.; et al. Ghrelin Reduces TNF-α-Induced Human Hepatocyte Apoptosis, Autophagy, and Pyroptosis: Role in Obesity-Associated NAFLD. J. Clin. Endocrinol. Metab. 2019, 104, 21–37. [Google Scholar] [CrossRef]
- Liu, H.K.; Yang, M.C.; Su, Y.T.; Tai, C.M.; Wei, Y.F.; Lin, I.C.; Tsai, C.C. Novel Ultrasonographic Fatty Liver Indicator Can Predict Hepatitis in Children With Non-alcoholic Fatty Liver Disease. Front. Pediatr. 2018, 6, 416. [Google Scholar] [CrossRef]
- Oruc, N.; Ozutemiz, O.; Yuce, G.; Akarca, U.S.; Ersoz, G.; Gunsar, F.; Batur, Y. Serum procalcitonin and CRP levels in non-alcoholic fatty liver disease: A case control study. BMC Gastroenterol. 2009, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Wen, S.W.; Kaminga, A.C.; Liu, A. Gut metabolites and inflammation factors in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 8848. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, M.; Yuan, S.; Zeng, Y.; Dong, Y.; Wang, Z.; Xiao, Q.; Dong, B.; Ma, J.; Hu, J. Sex differences in the associations between adiposity distribution and cardiometabolic risk factors in overweight or obese individuals: A cross-sectional study. BMC Public Health 2021, 21, 1232. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Maylor, B.D.; Zakrzewski-Fruer, J.K.; Orton, C.J.; Bailey, D.P. Beneficial postprandial lipaemic effects of interrupting sedentary time with high-intensity physical activity versus a continuous moderate-intensity physical activity bout: A randomised crossover trial. J. Sci. Med. Sport 2018, 21, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.D.; Zheng, C.J.; Dong, Y.H.; Zou, Z.Y.; Lv, Y.; Wang, Z.H.; Yang, Z.G.; Wang, S.; Dong, B.; Ma, J. Sex difference in the mediation roles of an inflammatory factor (hsCRP) and adipokines on the relationship between adiposity and blood pressure. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2019, 42, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fan, J.G. Diagnosis and management of non-alcoholic fatty liver disease and related metabolic disorders: Consensus statement from the Study Group of Liver and Metabolism, Chinese Society of Endocrinology. J. Diabetes 2013, 5, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Joseph, R.; Lopez, R.; McCullough, A.J. Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. J. Hepatol. 2009, 51, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef] [Green Version]
- Baron, R.M.; Kenny, D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Personal. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Gilbert-Diamond, D.; Baylin, A.; Mora-Plazas, M.; Villamor, E. Chronic inflammation is associated with overweight in Colombian school children. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 244–251. [Google Scholar] [CrossRef]
- Cohen, E.; Margalit, I.; Shochat, T.; Goldberg, E.; Krause, I. Markers of Chronic Inflammation in Overweight and Obese Individuals and the Role of Gender: A Cross-Sectional Study of a Large Cohort. J. Inflamm. Res. 2021, 14, 567–573. [Google Scholar] [CrossRef]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013, 14, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Adair, L.; Gordon-Larsen, P.; Zhang, B.; Popkin, B. Environmental, Dietary, and Behavioral Factors Distinguish Chinese Adults with High Waist-to-Height Ratio with and without Inflammation. J. Nutr. 2015, 145, 1335–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendall, M.A.; Patel, P.; Ballam, L.; Strachan, D.; Northfield, T.C. C reactive protein and its relation to cardiovascular risk factors: A population based cross sectional study. BMJ 1996, 312, 1061–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaki, M.E.; El-Bassyouni, H.T.; El-Gammal, M.; Kamal, S. Indicators of the metabolic syndrome in obese adolescents. Arch. Med. Sci. 2015, 11, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Christ, A.; Günther, P.; Lauterbach, M.A.R.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175.e14. [Google Scholar] [CrossRef] [Green Version]
- Piening, B.D.; Zhou, W.; Contrepois, K.; Röst, H.; Gu Urban, G.J.; Mishra, T.; Hanson, B.M.; Bautista, E.J.; Leopold, S.; Yeh, C.Y.; et al. Integrative Personal Omics Profiles during Periods of Weight Gain and Loss. Cell Syst. 2018, 6, 157–170.e8. [Google Scholar] [CrossRef]
- Timpson, N.J.; Nordestgaard, B.G.; Harbord, R.M.; Zacho, J.; Frayling, T.M.; Tybjærg-Hansen, A.; Smith, G.D. C-reactive protein levels and body mass index: Elucidating direction of causation through reciprocal Mendelian randomization. Int. J. Obes. 2011, 35, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, K.; Pontillo, A.; Di Palo, C.; Giugliano, G.; Masella, M.; Marfella, R.; Giugliano, D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA 2003, 289, 1799–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, A.L.; Koehler, E.; Herring, A.H.; Paynter, L.; Du, S.; Zhang, B.; Popkin, B.; Gordon-Larsen, P. Weight Gain Trajectories Associated With Elevated C-Reactive Protein Levels in Chinese Adults. J. Am. Heart Assoc. 2016, 5, e003262. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Alvarez-Blasco, F.; Botella-Carretero, J.I.; Luque-Ramírez, M. The striking similarities in the metabolic associations of female androgen excess and male androgen deficiency. Hum. Reprod. 2014, 29, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wu, S.; Wang, Y.; Chen, S.; Liao, W.; Zhang, X.; Pan, L.; Jiang, X.; Zhang, Y.; Wang, L. Association between blood copper and nonalcoholic fatty liver disease according to sex. Clin. Nutr. 2021, 40, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Rodella, S.; Lippi, G.; Franchini, M.; Zoppini, G.; Muggeo, M.; Day, C.P. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity 2008, 16, 1394–1399. [Google Scholar] [CrossRef]
- Lizardi-Cervera, J.; Chavez-Tapia, N.C.; Pérez-Bautista, O.; Ramos, M.H.; Uribe, M. Association among C-reactive protein, Fatty liver disease, and cardiovascular risk. Dig. Dis. Sci. 2007, 52, 2375–2379. [Google Scholar] [CrossRef] [PubMed]
- de Maat, M.P.; Trion, A. C-reactive protein as a risk factor versus risk marker. Curr. Opin. Lipidol. 2004, 15, 651–657. [Google Scholar] [CrossRef]
- Haukeland, J.W.; Damås, J.K.; Konopski, Z.; Løberg, E.M.; Haaland, T.; Goverud, I.; Torjesen, P.A.; Birkeland, K.; Bjøro, K.; Aukrust, P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J. Hepatol. 2006, 44, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Battat, R.; Al Khoury, A.; Restellini, S.; Sebastiani, G.; Bessissow, T. Co-existence of non-alcoholic fatty liver disease and inflammatory bowel disease: A review article. World J. Gastroenterol. 2016, 22, 7727–7734. [Google Scholar] [CrossRef]
- Ajmal, M.R.; Yaccha, M.; Malik, M.A.; Rabbani, M.U.; Ahmad, I.; Isalm, N.; Abdali, N. Prevalence of nonalcoholic fatty liver disease (NAFLD) in patients of cardiovascular diseases and its association with hs-CRP and TNF-α. Indian Heart J. 2014, 66, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lau, P.W.; Wang, J.; Dong, B.; Wu, L.; Quach, B.; Wong, D.P.; Fu, L.; Ma, J.; Wang, H. Associations among cardiorespiratory endurance, body mass index and blood pressure in Han Chinese children: Results from the 2010 Chinese National Survey On Students’ Constitution and Health. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2016, 39, 799–804. [Google Scholar] [CrossRef]
- Krasnoff, J.B.; Painter, P.L.; Wallace, J.P.; Bass, N.M.; Merriman, R.B. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology 2008, 47, 1158–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pälve, K.S.; Pahkala, K.; Suomela, E.; Aatola, H.; Hulkkonen, J.; Juonala, M.; Lehtimäki, T.; Rönnemaa, T.; Viikari, J.S.A.; Kähönen, M.; et al. Cardiorespiratory Fitness and Risk of Fatty Liver: The Young Finns Study. Med. Sci. Sports Exerc. 2017, 49, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, F.; He, Y.; Pan, X.; Wu, Y.; Hu, Z.; Lin, X.; Xu, S.; Peng, X.E. Dose-response association between physical activity and non-alcoholic fatty liver disease: A case-control study in a Chinese population. BMJ Open 2019, 9, e026854. [Google Scholar] [CrossRef]
- El Amrousy, D.; El-Afify, D. Osteocalcin and osteoprotegerin levels and their relationship with adipokines and proinflammatory cytokines in children with nonalcoholic fatty liver disease. Cytokine 2020, 135, 155215. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total | Male | Female | 19–35 Years | 36–56 Years | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Median (IQR)/% | n | Median (IQR)/% | n | Median (IQR)/% | n | Median (IQR)/% | n | Median (IQR)/% | |
| Age (yrs) | 1221 | 35.0 (30.0, 43.0) | 455 | 33.0 (29.0, 39.0) * | 766 | 37.0 (30.0, 45.0) * | 645 | 30.0 (27.0, 32.0) # | 576 | 44.0 (40.0, 50.0) # |
| Height (cm) | 1221 | 163.7 (158.1, 171.1) | 455 | 173.0 (169.1, 177.6) * | 766 | 159.4 (155.8, 163.5) * | 645 | 166.3 (160.0, 173.2) # | 576 | 161.2 (156.4, 168.0) # |
| Weight (kg) | 1221 | 77.0 (68.9, 88.8) | 455 | 88.4 (78.7, 99.6) * | 766 | 71.9 (65.9, 79.8) * | 645 | 80.8 (72.1, 93.5) # | 576 | 73.5 (66.5, 82.2) # |
| BMI (kg/m2) | 1221 | 28.5 (26.3, 31.4) | 455 | 29.4 (26.9, 32.3) * | 766 | 28.0 (26.1, 30.9) * | 645 | 29.2 (26.6, 32.1) # | 576 | 27.9 (26.1, 30.6) # |
| Fat mass (kg) | 1221 | 28.8 (24.8, 34.6) | 455 | 28.8 (24.0, 35.4) | 766 | 28.8 (25.1, 34.2) | 645 | 30.4 (25.7, 36.2) # | 576 | 27.4 (24.2, 32.3) # |
| Skeletal muscle mass (kg) | 1221 | 45.3 (40.2, 54.1) | 455 | 56.5 (52.2, 62.8) * | 766 | 41.4 (38.5, 44.6) * | 645 | 48.0 (41.4, 56.8) # | 576 | 43.3 (39.2, 50.8) # |
| BF (%) | 1221 | 39.3 (34.9, 42.9) | 455 | 33.8 (30.9, 36.8) * | 766 | 41.5 (38.8, 44.5) * | 645 | 39.2 (34.6, 43.3) | 576 | 39.3 (35.2, 42.6) |
| hsCRP (mg/L) a | 1215 | 1.1 (0.5, 2.2) | 454 | 1.0 (0.6, 2.1) | 761 | 1.1 (0.5, 2.3) | 641 | 1.2 (0.5, 2.40) | 574 | 1.0 (0.5, 2.0) |
| TNF-α (pg/mL) a | 896 | 16.5 (10.6, 30.0) | 312 | 16.7 (11.3, 27.8) | 584 | 16.3 (10.2, 31.0) | 421 | 17.3 (11.0, 31.0) | 475 | 16.1 (10.1, 28.9) |
| APN (ng/mL) | 896 | 2426.7 (1595.4, 3371.4) | 312 | 2037.6 (1379.9, 2949.5) | 584 | 2648.8 (1761.9, 3654.3) * | 421 | 2335.6 (1525.0, 3200.6) | 475 | 2495.5 (1676.8, 3526.3) |
| Cigarette Smoking in the past week | ||||||||||
| No | 694 | 76.7% | 147 | 46.7% * | 547 | 92.7% * | 319 | 75.2% | 375 | 78.0% |
| Yes | 211 | 23.3% | 168 | 53.3% * | 43 | 7.3% * | 105 | 24.8% | 106 | 22.0% |
| Alcohol Drinking | ||||||||||
| No | 695 | 76.8% | 163 | 51.7% * | 532 | 90.2% * | 321 | 75.7% | 374 | 77.8% |
| Yes | 210 | 23.2% | 152 | 48.3% * | 58 | 9.8% * | 103 | 24.3% | 107 | 22.2% |
| Moderate-intensity physical activity b | ||||||||||
| No | 623 | 68.8% | 208 | 66.0% | 415 | 70.3% | 286 | 67.5% | 337 | 70.1% |
| Yes | 282 | 31.2% | 107 | 34.0% | 175 | 29.7% | 138 | 32.5% | 144 | 29.9% |
| High-intensity physical activity b | ||||||||||
| No | 742 | 82.0% | 247 | 78.4% * | 495 | 83.9% * | 342 | 80.7% | 400 | 83.2% |
| Yes | 163 | 18.0% | 68 | 21.6% * | 95 | 16.1% * | 82 | 19.3% | 81 | 16.8% |
| FLD | ||||||||||
| No | 299 | 24.8% | 69 | 15.4% * | 230 | 30.3% * | 178 | 27.8% # | 121 | 21.4% # |
| Yes | 908 | 75.2% | 378 | 84.6% * | 530 | 69.7% * | 463 | 72.2% # | 445 | 78.6% # |
| Model | Effect | Estimate | SE | Z | p |
|---|---|---|---|---|---|
| Total | |||||
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2405 | 0.0250 | 9.6268 | <0.0001 |
| Mediator: hsCRP | BF (%) on hsCRP b | 0.1847 | 0.0245 | 7.5300 | <0.0001 |
| Outcome: FLD | hsCRP on FLD given BF (%) c | 0.1812 | 0.0625 | 2.9000 | 0.0037 |
| Indirect: BF (%) on FLD d | 0.0335 | 0.0125 | 2.6857 | 0.0072 | |
| Direct: BF (%) on FLD given hsCRP e | 0.2220 | 0.0257 | 8.6263 | <0.0001 | |
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2429 | 0.0251 | 9.6815 | <0.0001 |
| Mediator: TNF-α | BF (%) on TNF-α b | −0.5442 | 1.1595 | −0.4693 | 0.6390 |
| Outcome: FLD | TNF-α on FLD given BF (%) c | 0.0009 | 0.0008 | 1.1321 | 0.2576 |
| Indirect: BF (%) on FLD d | −0.0005 | 0.0015 | −0.3359 | 0.7370 | |
| Direct: BF (%) on FLD given TNF-α e | 0.2438 | 0.0252 | 9.6845 | <0.0001 | |
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2429 | 0.0251 | 9.6815 | <0.0001 |
| Mediator: APN | BF (%) on APN b | 7.3130 | 11.6965 | 0.6252 | 0.5320 |
| Outcome: FLD | APN on FLD given BF (%) c | −0.0003 | 0.0001 | −4.3838 | <0.0001 |
| Indirect: BF (%) on FLD d | −0.0019 | 0.0031 | −0.6038 | 0.5460 | |
| Direct: BF (%) on FLD given APN e | 0.2501 | 0.0255 | 9.7900 | <0.0001 | |
| Male | |||||
| Initial: BF (%) | Total: BF (%) on FLD a | 0.3297 | 0.0561 | 5.8802 | <0.0001 |
| Mediator: hsCRP | BF (%) on hsCRP b | 0.2014 | 0.0439 | 4.5844 | <0.0001 |
| Outcome: FLD | hsCRP on FLD given BF (%) c | 0.4800 | 0.2556 | 1.8783 | 0.0603 |
| Indirect: BF (%) on FLD d | 0.0967 | 0.0567 | 1.7037 | 0.0884 | |
| Direct: BF (%) on FLD given hsCRP e | 0.2985 | 0.0565 | 5.2836 | <0.0001 | |
| Initial: BF (%) | Total: BF (%) on FLD a | 0.3287 | 0.0561 | 5.8589 | <0.0001 |
| Mediator: TNF-α | BF (%) on TNF-α b | −1.2564 | 1.2003 | −1.0467 | 0.2961 |
| Outcome: FLD | TNF-α on FLD given BF (%) c | 0.0006 | 0.0018 | 0.3546 | 0.7229 |
| Indirect: BF (%) on FLD d | −0.0008 | 0.0032 | −0.2490 | 0.8033 | |
| Direct: BF (%) on FLD given TNF-α e | 0.3291 | 0.0561 | 5.8677 | <0.0001 | |
| Initial: BF (%) | Total: BF (%) on FLD a | 0.3287 | 0.0561 | 5.8589 | <0.0001 |
| Mediator: APN | BF (%) on APN b | 13.7954 | 16.3957 | 0.8414 | 0.4008 |
| Outcome: FLD | APN on FLD given BF (%) c | −0.0001 | 0.0001 | −0.8838 | 0.3768 |
| Indirect: BF (%) on FLD d | −0.0016 | 0.0034 | −0.4714 | 0.6347 | |
| Direct: BF (%) on FLD given APN e | 0.3336 | 0.0569 | 5.8662 | <0.0001 | |
| Female | |||||
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2130 | 0.0284 | 7.4941 | <0.0001 |
| Mediator: hsCRP | BF (%) on hsCRP b | 0.1804 | 0.0295 | 6.1109 | <0.0001 |
| Outcome: FLD | hsCRP on FLD given BF (%) c | 0.1609 | 0.0632 | 2.5464 | 0.0109 |
| Indirect: BF (%) on FLD d | 0.0290 | 0.0125 | 2.3241 | 0.0201 | |
| Direct: BF (%) on FLD given hsCRP e | 0.1939 | 0.0294 | 6.5876 | <0.0001 | |
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2165 | 0.0285 | 7.5927 | <0.0001 |
| Mediator: TNF-α | BF (%) on TNF-α b | −0.0605 | 1.7425 | −0.0347 | 0.9723 |
| Outcome: FLD | TNF-α on FLD given BF (%) c | 0.0010 | 0.0010 | 1.0473 | 0.2949 |
| Indirect: BF (%) on FLD d | −0.0001 | 0.0025 | −0.0251 | 0.9800 | |
| Direct: BF (%) on FLD given TNF-α e | 0.2174 | 0.0287 | 7.5870 | <0.0001 | |
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2165 | 0.0285 | 7.5927 | <0.0001 |
| Mediator: APN | BF (%) on APN b | 0.3482 | 16.215 | 0.0215 | 0.9829 |
| Outcome: FLD | APN on FLD given BF (%) c | −0.0003 | 0.0001 | −4.4527 | <0.0001 |
| Indirect: BF (%) on FLD d | −0.0001 | 0.0050 | −0.0210 | 0.9833 | |
| Direct: BF (%) on FLD given APN e | 0.2230 | 0.0290 | 7.6782 | <0.0001 |
| Model | Effect | Estimate | SE | Z | p |
|---|---|---|---|---|---|
| 19–35 years | |||||
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2795 | 0.0370 | 7.5600 | <0.0001 |
| Mediator: hsCRP | BF (%) on hsCRP b | 0.2264 | 0.0380 | 5.9559 | <0.0001 |
| Outcome: FLD | hsCRP on FLD given BF (%) c | 0.0581 | 0.0606 | 0.9602 | 0.3369 |
| Indirect: BF (%) on FLD d | 0.0132 | 0.0141 | 0.9352 | 0.3497 | |
| Direct: BF (%) on FLD given hsCRP e | 0.2707 | 0.0379 | 7.1500 | <0.0001 | |
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2743 | 0.0365 | 7.5214 | <0.0001 |
| Mediator: TNF-α | BF (%) on TNF-α b | −0.6699 | 2.0690 | −0.3238 | 0.7463 |
| Outcome: FLD | TNF-α on FLD given BF (%) c | 0.0008 | 0.0008 | 1.0200 | 0.3077 |
| Indirect: BF (%) on FLD d | −0.0006 | 0.0024 | −0.2255 | 0.8216 | |
| Direct: BF (%) on FLD given TNF-α e | 0.2764 | 0.0367 | 7.5226 | <0.0001 | |
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2743 | 0.0365 | 7.5214 | <0.0001 |
| Mediator: APN | BF (%) on APN b | 0.5105 | 15.1970 | 0.3626 | 0.7171 |
| Outcome: FLD | APN on FLD given BF (%) c | −0.0002 | 0.0001 | −1.9225 | 0.0545 |
| Indirect: BF (%) on FLD d | −0.0009 | 0.0029 | −0.3173 | 0.7510 | |
| Direct: BF (%) on FLD given APN e | 0.2782 | 0.0368 | 7.5512 | <0.0001 | |
| 36–56 years | |||||
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2030 | 0.0349 | 5.8166 | <0.0001 |
| Mediator: hsCRP | BF (%) on hsCRP b | 0.1222 | 0.0315 | 3.8813 | 0.0001 |
| Outcome: FLD | hsCRP on FLD given BF (%) c | 0.6370 | 0.1639 | 3.8876 | 0.0001 |
| Indirect: BF (%) on FLD d | 0.0778 | 0.0288 | 2.7023 | 0.0069 | |
| Direct: BF (%) on FLD given hsCRP e | 0.1733 | 0.0363 | 4.7771 | <0.0001 | |
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2128 | 0.0355 | 5.9919 | <0.0001 |
| Mediator: TNF-α | BF (%) on TNF-α b | −0.5210 | 0.9814 | −0.5308 | 0.5958 |
| Outcome: FLD | TNF-α on FLD given BF (%) c | 0.0012 | 0.0023 | 0.5281 | 0.5975 |
| Indirect: BF (%) on FLD d | −0.0006 | 0.0028 | −0.2244 | 0.8225 | |
| Direct: BF (%) on FLD given TNF-α e | 0.2129 | 0.0355 | 5.9929 | <0.0001 | |
| Initial: BF (%) | Total: BF (%) on FLD a | 0.2128 | 0.0355 | 5.9919 | <0.0001 |
| Mediator: APN | BF (%) on APN b | 11.9545 | 18.4330 | 0.6485 | 0.5170 |
| Outcome: FLD | APN on FLD given BF (%) c | −0.0003 | 0.0001 | −3.8816 | 0.0001 |
| Indirect: BF (%) on FLD d | −0.0037 | 0.0060 | −0.6200 | 0.5353 | |
| Direct: BF (%) on FLD given APN e | 0.2273 | 0.0369 | 6.1688 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Tang, H.; Li, F.; Wu, S.; Dong, Y.; Yang, Y.; Baker, J.S.; Ma, J. Mediating Roles of hsCRP, TNF-α and Adiponectin on the Associations between Body Fat and Fatty Liver Disease among Overweight and Obese Adults. Biology 2021, 10, 895. https://doi.org/10.3390/biology10090895
Xie M, Tang H, Li F, Wu S, Dong Y, Yang Y, Baker JS, Ma J. Mediating Roles of hsCRP, TNF-α and Adiponectin on the Associations between Body Fat and Fatty Liver Disease among Overweight and Obese Adults. Biology. 2021; 10(9):895. https://doi.org/10.3390/biology10090895
Chicago/Turabian StyleXie, Ming, Haokai Tang, Feifei Li, Si Wu, Yanhui Dong, Yide Yang, Julien Steven Baker, and Jun Ma. 2021. "Mediating Roles of hsCRP, TNF-α and Adiponectin on the Associations between Body Fat and Fatty Liver Disease among Overweight and Obese Adults" Biology 10, no. 9: 895. https://doi.org/10.3390/biology10090895
APA StyleXie, M., Tang, H., Li, F., Wu, S., Dong, Y., Yang, Y., Baker, J. S., & Ma, J. (2021). Mediating Roles of hsCRP, TNF-α and Adiponectin on the Associations between Body Fat and Fatty Liver Disease among Overweight and Obese Adults. Biology, 10(9), 895. https://doi.org/10.3390/biology10090895