Acute and Chronic Effects of Interval Training on the Immune System: A Systematic Review with Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Extraction
2.4. Study Quality
2.5. Statistical Analyses
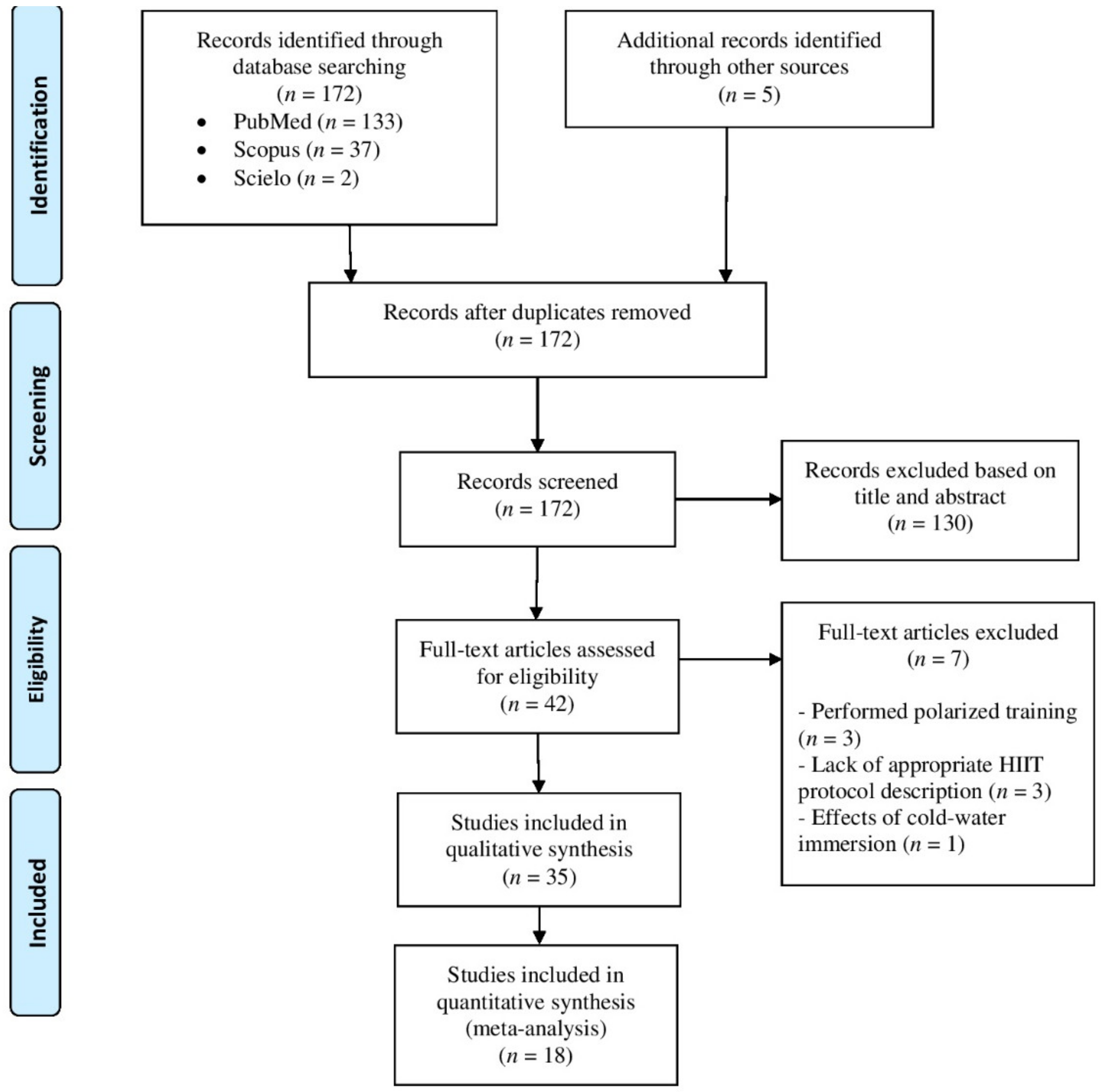
3. Results
3.1. Included Studies
3.2. Summary of Studies
3.3. Intervention Characteristics
3.4. Qualitative Analysis of Acute Effects of IT on Immune Outcomes
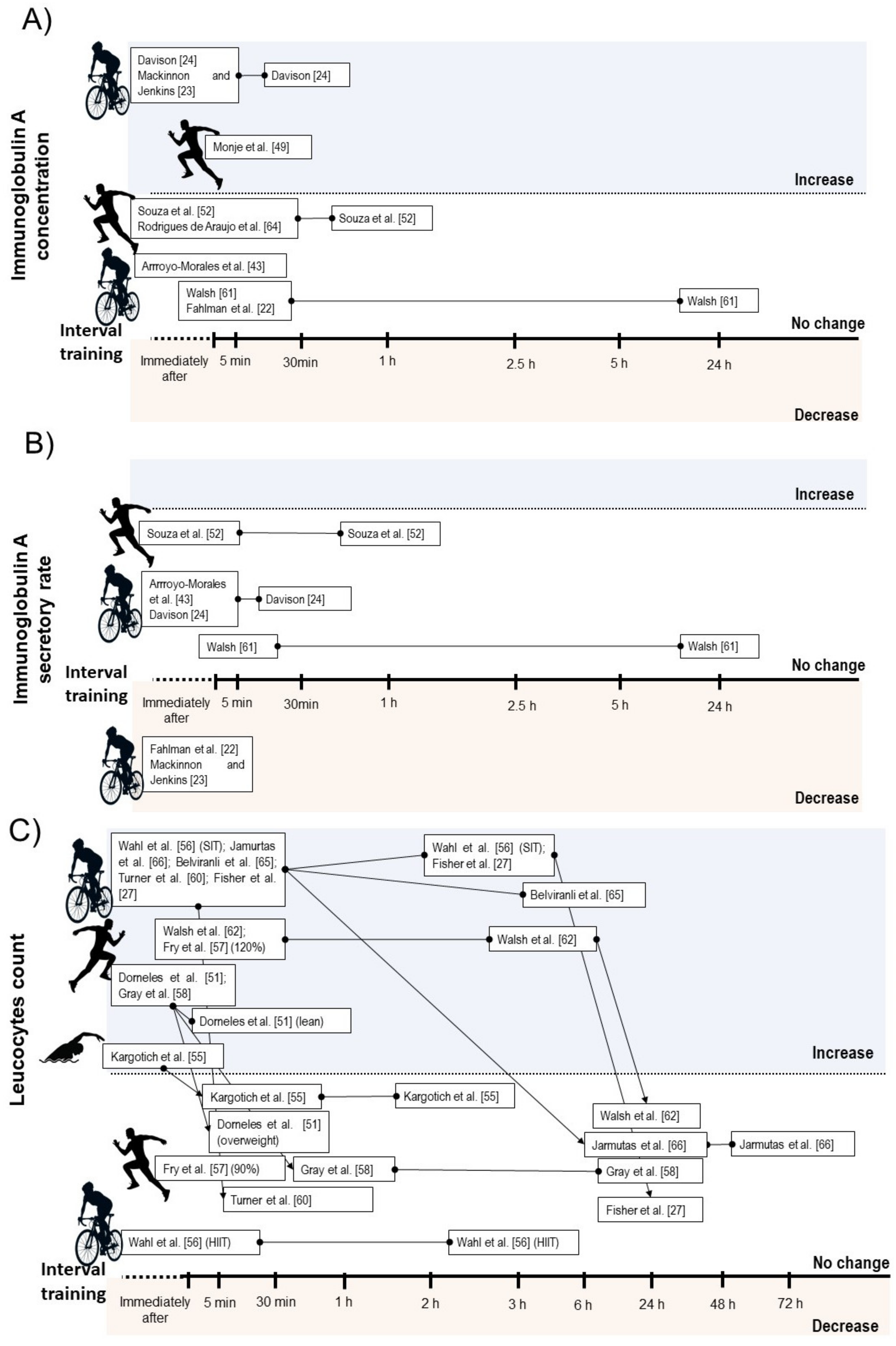
3.4.1. Salivary Immunoglobulin A
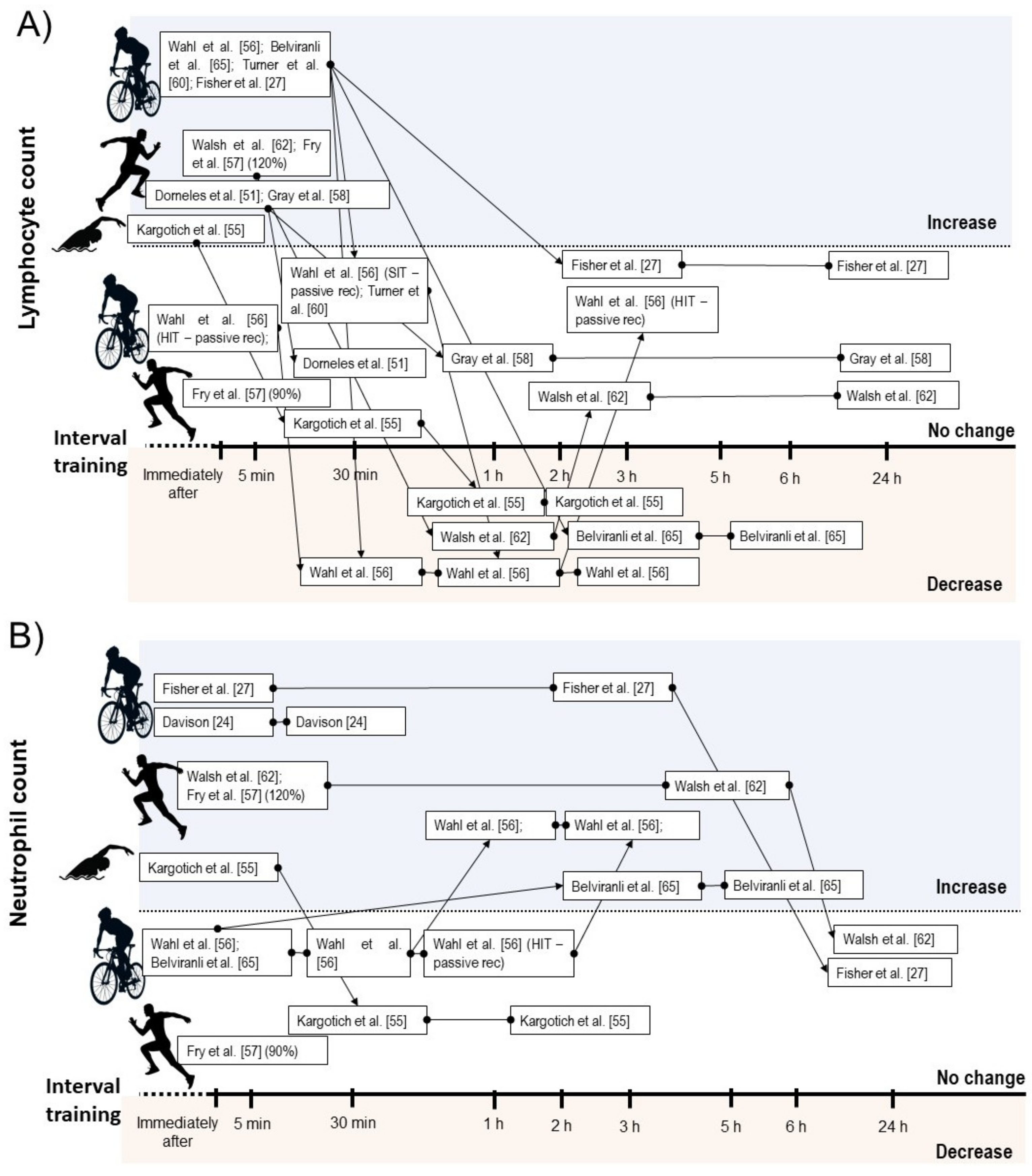
3.4.2. Leucocyte Count
3.4.3. Leucocyte Function
3.5. Qualitative Analysis of Chronic Effects of IT on Immune Outcomes
3.6. Quality Assessment
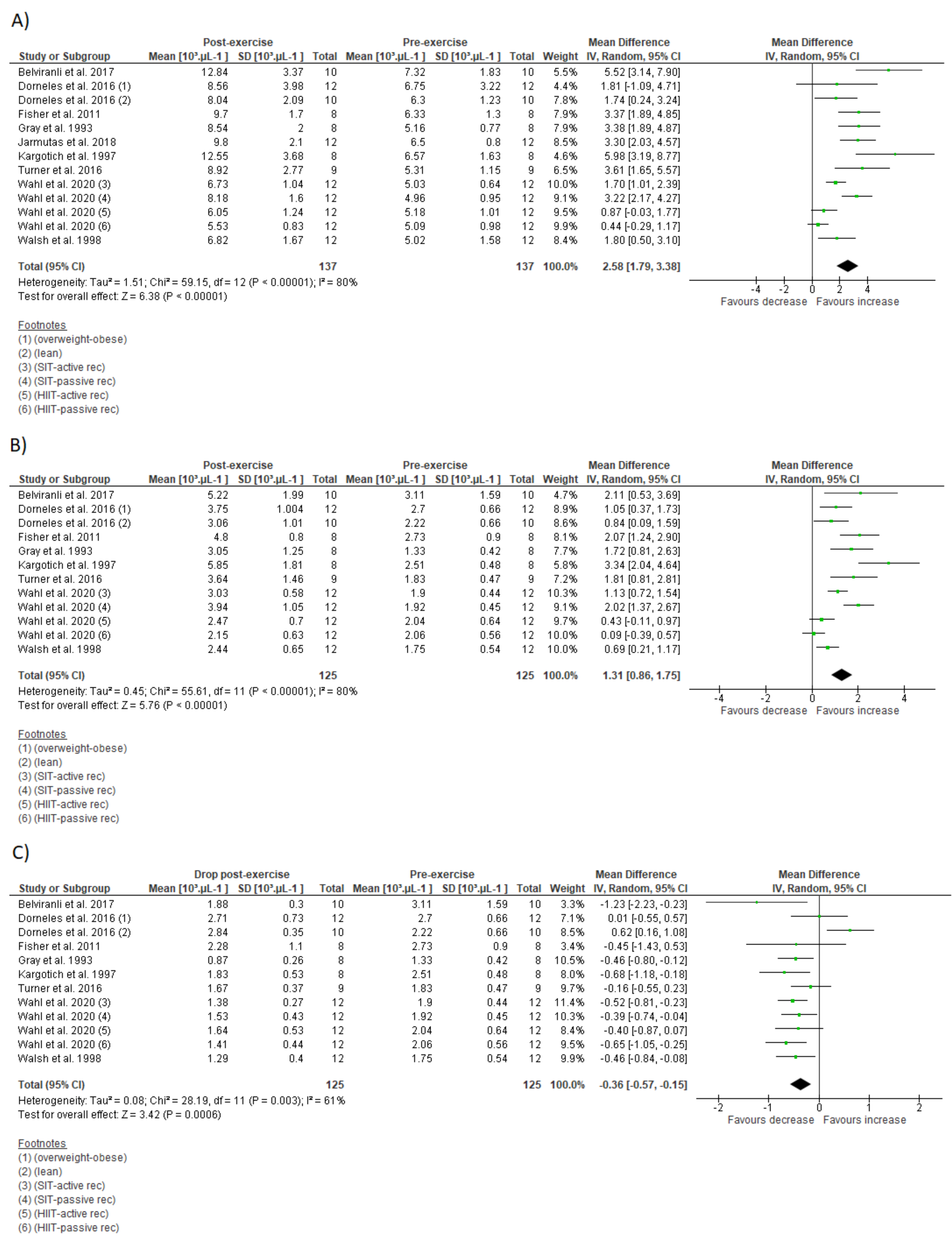
3.7. Meta-Analysis
3.8. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2018, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.A.M.; Dantas, P.M.S.; Dos Santos, I.K.; Dantas, M.P.; Da Silva, D.C.P.; Cabral, B.G.D.A.T.; Guerra, R.O.; Júnior, G.B.C. Effect of acute and chronic aerobic exercise on immunological markers: A systematic review. Front. Physiol. 2020, 10. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Rohde, T.; Ostrowski, K. Recovery of the immune system after exercise. Acta Physiol. Scand. 1998, 162, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Jeffrey, M.G.; Woods, A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, K.; Hoffman-goetz, L.; et al. Part one: Immune function and exercise. EIR 2011, 17, 6–63. [Google Scholar]
- Nieman, D.C. Exercise immunology: Future directions for research related to athletes, nutrition, and the elderly. Int. J. Sports Med. 2000, 21, 61–68. [Google Scholar] [CrossRef]
- Walsh, N.P. Recommendations to maintain immune health in athletes. Eur. J. Sport Sci. 2018, 18, 820–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viana, R.; de Lira, C.; Naves, J.P.A.; Coswig, V.S.; Del Vecchio, F.B.; Ramirez-Campillo, R.; Vieira, C.A.; Gentil, P. Can we draw general conclusions from interval training studies? Sports Med. 2018, 48, 2001–2009. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Billat, V.L.; Slawinski, J.; Bocquet, V.; Demarle, A.; Lafitte, L.; Chassaing, P.; Koralsztein, J.-P. Intermittent runs at the velocity associated with maximal oxygen uptake enables subjects to remain at maximal oxygen uptake for a longer time than intense but submaximal runs. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 81, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.; Rakobowchuck, M.; MacDonald, M.; McGee, S.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef]
- Little, J.P.; Jung, M.E.; Wright, A.E.; Wright, W.; Manders, R. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl. Physiol. Nutr. Metab. 2014, 39, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Percival, M.E.; Skelly, L.E.; Martin, B.J.; Tan, R.B.; Tarnopolsky, M.A.; Gibala, M.J. Three minutes of all-out intermittent exercise per week increases skeletal muscle oxidative capacity and improves cardiometabolic health. PLoS ONE 2014, 9, e111489. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Wilkin, G.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef]
- Peake, J.M.; Tan, S.J.; Markworth, J.F.; Broadbent, J.A.; Skinner, T.; Cameron-Smith, D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol. Metab. 2014, 307, E539–E552. [Google Scholar] [CrossRef] [Green Version]
- Billat, L.V. Interval training for performance: A scientific and empirical practice. Sports Med. 2001, 31, 13–31. [Google Scholar] [CrossRef]
- Gibala, M.J. High-intensity interval training: A time-efficient strategy for health promotion? Curr. Sports Med. Rep. 2007, 6, 211–213. [Google Scholar] [CrossRef]
- Souza, D.; Coswig, V.; De Lira, C.A.B.; Gentil, P. HIT-ing the barriers for exercising during social isolation. Biology 2020, 9, 245. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; Van Essen, M.; Wilkin, G.; Burgomaster, K.A.; Safdar, A.; Raha, S.; Tarnopolsky, M.A. Short-term sprint intervalversustraditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. J. Physiol. 2006, 575, 901–911. [Google Scholar] [CrossRef]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, O.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, J.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.; Coombes, J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Med. 2015, 45, 679–692. [Google Scholar] [CrossRef]
- Batacan, R.B.; Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2016, 51, 494–503. [Google Scholar] [CrossRef]
- Fahlman, M.M.; Engels, H.J.; Morgan, A.L.; Kolokouri, I. Mucosal IgA response to repeated Wingate tests in females. Int. J. Sports Med. 2001, 22, 127–131. [Google Scholar] [CrossRef]
- Mackinnon, L.T.; Jenkins, D.G. Decreased salivary immunoglobulins after intense interval exercise before and after training. Med. Sci. Sports Exerc. 1993, 25, 678–683. [Google Scholar] [CrossRef]
- Davison, G. Innate immune responses to a single session of sprint interval training. Appl. Physiol. Nutr. Metab. 2011, 36, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottone, V.D.O.; Costa, K.B.; Tossige-Gomes, R.; De Matos, M.A.; Brito-Melo, G.; Magalhaes, F.D.C.; Esteves, E.A.; Amorim, F.; Rocha-Vieira, E. Late neutrophil priming following a single session of high-intensity interval exercise. Laryngo-Rhino-Otol. 2019, 40, 171–179. [Google Scholar] [CrossRef]
- Tossige-Gomes, R.; Costa, K.B.; Ottone, V.D.O.; Magalhães, F.D.C.; Amorim, F.T.; Rocha-Vieira, E. Lymphocyte redox imbalance and reduced proliferation after a single session of high intensity interval exercise. PLoS ONE 2016, 11, e0153647. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.; Schwartz, D.D.; Quindry, J.; Barberio, M.D.; Foster, E.B.; Jones, K.W.; Pascoe, D.D. Lymphocyte enzymatic antioxidant responses to oxidative stress following high-intensity interval exercise. J. Appl. Physiol. 2011, 110, 730–737. [Google Scholar] [CrossRef] [Green Version]
- Born, D.-P.; Zinner, C.; Sperlich, B. The mucosal immune function is not compromised during a period of high-intensity interval training. Is it time to reconsider an old assumption? Front. Physiol. 2017, 8, 485. [Google Scholar] [CrossRef] [Green Version]
- Navalta, J.W.; Tibana, R.A.; Fedor, E.A.; Vieira, A.; Prestes, J. Three consecutive days of interval runs to exhaustion affects lymphocyte subset apoptosis and migration. BioMed Res. Int. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Khammassi, M.; Ouerghi, N.; Said, M.; Feki, M.; Khammassi, Y.; Pereira, B.; Thivel, D.; Bouassida, A. Continuous moderate-intensity but not high-intensity interval training improves immune function biomarkers in healthy young men. J. Strength Cond. Res. 2020, 34, 249–256. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weston, K.S.; Wisløff, U.; Coombes, J. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2013, 48, 1227–1234. [Google Scholar] [CrossRef]
- Smart, N.; Waldron, M.; Ismail, H.; Giallauria, F.; Vigorito, C.; Cornelissen, V.; Dieberg, G. Validation of a new tool for the assessment of study quality and reporting in exercise training studies. Int. J. Evid. Heal. 2015, 13, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Born, D.-P.; Faiss, R.; Willis, S.J.; Strahler, J.; Millet, G.; Holmberg, H.-C.; Sperlich, B. Circadian variation of salivary immunoglobin A, alpha-amylase activity and mood in response to repeated double-poling sprints in hypoxia. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McGawley, K.; Juudas, E.; Kazior, Z.; Ström, K.; Blomstrand, E.; Hansson, O.; Holmberg, H.-C. No additional benefits of block over evenly-distributed high-intensity interval training within a polarized microcycle. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef]
- Ide, B.N.; Souza-Junior, T.P.; McAnulty, S.R.; de Faria, M.A.C.; Costa, K.A.; Nunes, L.A.S. Immunological responses to a Brazilian Jiu-Jitsu high-intensity interval training session. J. Hum. Kinet. 2019, 70, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Owen, A.L.; Wong, D.P.; Dunlop, G.; Groussard, C.; Kebsi, W.; Dellal, A.; Morgans, R.; Zouhal, H. High-intensity training and salivary immunoglobulin a responses in professional top-level soccer players: Effect of training intensity. J. Strength Cond. Res. 2016, 30, 2460–2469. [Google Scholar] [CrossRef]
- Chinda, D.; Umeda, T.; Shimoyama, T.; Kojima, A.; Tanabe, M.; Nakaji, S.; Sugawara, K. The acute response of neutrophil function to a bout of judo training. Luminescence 2003, 18, 278–282. [Google Scholar] [CrossRef]
- Broatch, J.; Petersen, A.; Bishop, D. Postexercise cold water immersion benefits are not greater than the placebo effect. Med. Sci. Sports Exerc. 2014, 46, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, G.P.; Da Silva, I.; Boeira, M.C.; Valentini, D.; Fonseca, S.G.; Lago, P.D.; Peres, A.; Romão, P.R.T. Cardiorespiratory fitness modulates the proportions of monocytes and T helper subsets in lean and obese men. Scand. J. Med. Sci. Sports 2019, 29, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.M.; Hecksteden, A.; Morsch, A.; Zundler, J.; Wegmann, M.; Kratzsch, J.; Thiery, J.; Hohl, M.; Bittenbring, J.T.; Neumann, F.; et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur. Heart J. 2018, 40, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Monje, C.; Rada, I.; Castro-Sepulveda, M.; Peñailillo, L.; Deldicque, L.; Zbinden-Foncea, H. Effects of a high intensity interval session on mucosal immune function and salivary hormones in male and female endurance athletes. J. Sports Sci. Med. 2020, 19, 436–443. [Google Scholar] [PubMed]
- Wahl, P.; Mathes, S.; Bloch, W.; Zimmer, P. Acute impact of recovery on the restoration of cellular immunological homeostasis. Laryngo-Rhino-Otol. 2019, 41, 12–20. [Google Scholar] [CrossRef]
- Jamurtas, A.Z.; Fatouros, I.G.; Deli, C.K.; Georgakouli, K.; Poulios, A.; Draganidis, D.; Papanikolaou, K.; Tsimeas, P.; Chatzinikolaou, A.; Avloniti, A.; et al. The effects of acute low-volume HIIT and aerobic exercise on leukocyte count and redox status. J. Sports Sci. Med. 2018, 17, 501–508. [Google Scholar]
- De Souza, D.C.; Matos, V.; Dos Santos, V.O.A.; Medeiros, I.F.; Marinho, C.S.R.; Nascimento, P.R.P.; Dorneles, G.P.; Peres, A.; Müller, C.H.; Krause, M.; et al. Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Front. Physiol. 2018, 9, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Araujo, V.R.; Lisboa, P.; Boaventura, G.; Caramez, F.; Pires, L.; Oliveira, E.; Moura, E.; Casimiro-Lopes, G. Acute high-intensity exercise test in soccer athletes affects salivary biochemical markers. Free Radic. Res. 2018, 52, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Belviranli, M.; Okudan, N.; Kabak, B. The effects of acute high-intensity interval training on hematological parameters in sedentary subjects. Med. Sci. 2017, 5, 15. [Google Scholar] [CrossRef]
- Krüger, K.; Alack, K.; Ringseis, R.; Mink, L.; Pfeifer, E.; Schinle, M.; Gindler, K.; Kimmelmann, L.; Walscheid, R.; Muders, K.; et al. Apoptosis of T-cell subsets after acute high-intensity interval exercise. Med. Sci. Sports Exerc. 2016, 48, 2021–2029. [Google Scholar] [CrossRef]
- Turner, J.E.; Wadley, A.J.; Aldred, S.; Fisher, J.P.; Bosch, J.A.; Campbell, J.P. Intensive exercise does not preferentially mobilize skin-homing T cells and NK cells. Med. Sci. Sports Exerc. 2016, 48, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Dorneles, G.; Haddad, D.O.; Fagundes, V.O.; Vargas, B.K.; Kloecker, A.; Romão, P.R.; Peres, A. High intensity interval exercise decreases IL-8 and enhances the immunomodulatory cytokine interleukin-10 in lean and overweight–obese individuals. Cytokine 2016, 77, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Morales, M.; Rodriguez, L.D.; Rubio-Ruiz, B.; Olea, N. Influence of gender in the psychoneuroimmunological response to therapeutic interval exercise. Biol. Res. Nurs. 2012, 14, 357–363. [Google Scholar] [CrossRef]
- Friedman, R.A.; Navalta, J.W.; Fedor, E.A.; Kell, H.B.; Lyons, T.S.; Arnett, S.W.; Schafer, M.A. Repeated high-intensity Wingate cycle bouts influence markers of lymphocyte migration but not apoptosis. Appl. Physiol. Nutr. Metab. 2012, 37, 241–246. [Google Scholar] [CrossRef]
- Thomas, N.E.; Leyshon, A.; Hughes, M.G.; Jasper, M.A.; Davies, B.; Graham, M.R.; Bulloch, J.M.; Baker, J.S. Concentrations of salivary testosterone, cortisol, and immunoglobulin A after supra-maximal exercise in female adolescents. J. Sports Sci. 2010, 28, 1361–1368. [Google Scholar] [CrossRef]
- Walsh, N. The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J. Sports Sci. 1999, 17, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Blannin, A.K.; Clark, A.M.; Cook, L.; Robson, P.; Gleeson, M. The effects of high-intensity intermittent exercise on the plasma concentrations of glutamine and organic acids. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 77, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Hinton, J.R.; Rowbottom, D.G.; Keast, D.; Morton, A.R. Acute intensive interval training and in vitroT-lymphocyte function. Int. J. Sports Med. 1997, 18, 130–135. [Google Scholar] [CrossRef]
- Kargotich, S.; Keast, D.; Goodman, C.; Crawford, G.P.; Morton, A.R. The influence of blood volume changes on leucocyte and lymphocyte subpopulations in elite swimmers following interval training of varying intensities. Int. J. Sports Med. 1997, 18, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.B.; Telford, R.D.; Collins, M.; Weidemann, M.J. The response of leukocyte subsets and plasma hormones to interval exercise—PubMed. Med. Sci. Sport Exerc. 1993, 25, 1252–1258. [Google Scholar] [CrossRef]
- Fry, R.W.; Morton, A.R.; Keast, D. Acute intensive interval training and T-lymphocyte function. Med. Sci. Sports Exerc. 1992, 24, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Fry, R.W.; Morton, A.R.; Crawford, G.P.M.; Keast, D. Cell numbers and in vitro responses of leucocytes and lymphocyte subpopulations following maximal exercise and interval training sessions of different intensities. Graefe’s Arch. Clin. Exp. Ophthalmol. 1992, 64, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.B.; Slentz, C.A.; Willis, L.H.; Hoselton, A.; Huebner, J.L.; Kraus, V.B.; Moss, J.; Muehlbauer, M.J.; Spielmann, G.; Muoio, D.M.; et al. Rejuvenation of neutrophil functions in association with reduced diabetes risk following ten weeks of low-volume high intensity interval walking in older adults with prediabetes—A pilot study. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Toohey, K.; Pumpa, K.; McKune, A.; Cooke, J.; Welvaert, M.; Northey, J.; Quinlan, C.; Semple, S. The impact of high-intensity interval training exercise on breast cancer survivors: A pilot study to explore fitness, cardiac regulation and biomarkers of the stress systems. BMC Cancer 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.B.; Willis, L.H.; Slentz, C.A.; Hoselton, A.; Kelly, L.; Huebner, J.L.; Kraus, V.B.; Moss, J.; Muehlbauer, M.J.; Spielmann, G.; et al. Ten weeks of high-intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: A pilot study. Arthritis Res. 2018, 20, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sheykhlouvand, M.; Gharaat, M.; Khalili, E.; Agha-Alinejad, H.; Rahmaninia, F.; Arazi, H. Low-volume high-intensity interval versus continuous endurance training: Effects on hematological and cardiorespiratory system adaptations in professional canoe polo athletes. J. Strength Cond. Res. 2018, 32, 1852–1860. [Google Scholar] [CrossRef]
- Bartlett, D.B.; Shepherd, S.O.; Wilson, O.J.; Adlan, A.; Wagenmakers, A.; Shaw, C.S.; Lord, J. Neutrophil and monocyte bactericidal responses to 10 weeks of low-volume high-intensity interval or moderate-intensity continuous training in sedentary adults. Oxidative Med. Cell. Longev. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-H.; Chang, S.-C.; Chou, C.-H.; Weng, T.-P.; Hsu, C.-C.; Wang, J.-S. Exercise training alleviates hypoxia-induced mitochondrial dysfunction in the lymphocytes of sedentary males. Sci. Rep. 2016, 6, 35170. [Google Scholar] [CrossRef] [Green Version]
- Trochimiak, T.; Hübner-Woźniak, E. Effect of exercise on the level of immunoglobulin a in saliva. Biol. Sport 2012, 29, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Ihalainen, J.K.; Schumann, M.; Häkkinen, K.; Mero, A.A. Mucosal immunity and upper respiratory tract symptoms in recreational endurance runners. Appl. Physiol. Nutr. Metab. 2016, 41, 96–102. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.; Oliveira, M.; McCauley, T.; Tauler, P. Sex differences in immune variables and respiratory infection incidence in an athletic population. Exerc. Immunol. Rev. 2011, 17, 122–135. [Google Scholar] [PubMed]
- Mendonca, G.V.; Heffernan, K.S.; Rossow, L.; Guerra, M.; Pereira, F.M.D.C.D.; Fernhall, B. Sex differences in linear and nonlinear heart rate variability during early recovery from supramaximal exercise. Appl. Physiol. Nutr. Metab. 2010, 35, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Turner, J.E. Debunking the myth of exercise-induced immune suppression: Redefining the impact of exercise on immunological health across the lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef]
- Tucker, W.J.; Sawyer, B.J.; Jarrett, C.L.; Bhammar, D.M.; Gaesser, G.A. Physiological responses to high-intensity interval exercise differing in interval duration. J. Strength Cond. Res. 2015, 29, 3326–3335. [Google Scholar] [CrossRef] [PubMed]
- Naves, J.P.A.; Rebelo, A.C.S.; Silva, L.R.B.; Silva, M.S.; Ramirez-Campillo, R.; Ramírez-Vélez, R.; Gentil, P. Cardiorespiratory and perceptual responses of two interval training and a continuous training protocol in healthy young men. Eur. J. Sport Sci. 2018, 19, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Mathes, S.; Achtzehn, S.; Bloch, W.; Mester, J. Active vs. passive recovery during high-intensity training influences hormonal response. Int. J. Sports Med. 2013, 35, 583–589. [Google Scholar] [CrossRef]
- Urhausen, A.; Gabriel, H.; Kindermann, W. Blood hormones as markers of training stress and overtraining. Sports Med. 1995, 20, 251–276. [Google Scholar] [CrossRef]
- Liston, A.; Gray, D. Homeostatic control of regulatory T cell diversity. Nat. Rev. Immunol. 2014, 14, 154–165. [Google Scholar] [CrossRef]
- Dressendorfer, R.H.; Petersen, S.R.; Lovshin, S.E.M.; Hannon, J.L.; Lee, S.F.; Bell, G.J. Performance enhancement with maintenance of resting immune status after intensified cycle training. Clin. J. Sport Med. 2002, 12, 301–307. [Google Scholar] [CrossRef]
- Akimoto, T.; Kumai, Y.; Akama, T.; Hayashi, E.; Murakami, H.; Soma, R.; Kuno, S.; Kono, I. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br. J. Sports Med. 2003, 37, 76–79. [Google Scholar] [CrossRef]
- Kunz, H.; Bishop, N.C.; Spielmann, G.; Pistillo, M.; Reed, J.; Ograjsek, T.; Park, Y.; Mehta, S.K.; Pierson, D.L.; Simpson, R.J.; et al. Fitness level impacts salivary antimicrobial protein responses to a single bout of cycling exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 115, 1015–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starzak, D.E.; Konkol, K.F.; McKune, A.J. Effects of cardiorespiratory fitness and obesity on salivary secretory IgA and alpha-amylase in south African children. Children 2016, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C.; Henson, D.A.; Gusewitch, G.; Warren, B.J.; Dotson, R.C.; Butterworth, D.E.; Nehlsen-Cannarella, S.L. Physical activity and immune function in elderly women. Med. Sci. Sports Exerc. 1993, 25, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Austin, M.D.; Sha, W. Upper respiratory tract infection is reduced in physically fit and active adults. Br. J. Sports Med. 2010, 45, 987–992. [Google Scholar] [CrossRef] [PubMed]

| Study | Participants | Design | Modality/Interval Protocol | Results |
|---|---|---|---|---|
| Monje et al. 2020 [43] | 20 runners (10 men age: 21.9 ± 0.8 years; 10 women age: 25.8 ± 6.2 years) | Clinical trial | Running HIIT—10 bouts of 4 min at 90% of vO2max interspersed by 2 min of passive recovery | ↑ salivary IgA concentration 20 min after exercise |
| Wahl et al. 2020 [44] 12 men triathletes and cyclists (age: 24.7 ± 3.4 years) Randomized cross-over trial | Cycling HIIT—4 bouts of 4 min at 90–95% of peak power interspersed by 3 min of passive recovery | ↔ leucocyte count; ↓ lymphocyte count 30 min, and 60 min after exercise; ↑ neutrophil count 180 min after exercise; ↔ mixed cell count | ||
| Cycling HIIT—4 bouts of 4 min at 90–95% of peak power interspersed by 3 min at 45% of peak power | ↔ leucocyte count; ↑ lymphocyte count immediately after exercise followed by ↓ 30 min, 60 min and 180 min after exercise; ↑ neutrophil count 60 min and 180 min after exercise; ↔ mixed cell count | |||
| Cycling SIT—4 bouts of 30 s “all-out” effort interspersed by 7.5 min passive recovery | ↑ leucocyte count immediately, and 180 min after exercise; ↑ lymphocyte count immediately after exercise followed by ↓ 60 min and 180 min after exercise; ↑ neutrophil count 60 min and 180 min after exercise; ↑ mixed cell count immediately after exercise | |||
| Cycling SIT—4 bouts of 30 s “all-out” effort interspersed by 7.5 min at 45% of peak power | ↑ leucocyte count immediately, and 180 min after exercise; ↑ lymphocyte count immediately after exercise followed by ↓ 30 min, 60 min, and 180 min after exercise; ↑ neutrophil count 60 min, and 180 min after exercise; ↔ mixed cell count | |||
| De Oliveira Ottone et al. 2019 [25] | 12 inactive health men (age: 22.5 ± 3.9 years) | Clinical trial | Cycling HIIT—8 bouts of 60 s at 90% peak power interspersed by 75 s of active recovery (30 watts) | ↓ neutrophil oxidative burst in response to f-PMN 30 min after exercise; ↑ neutrophil phagocytic capacity, oxidative burst and redox status 24 h after exercise |
| Jamurtas et al. 2018 [45] | 12 health men (age: 22.4 ± 0.5 years) | Randomized cross-over trial | Cycling SIT—4 bouts of 30 s “all-out” effort interspersed by 4 min of active recovery | ↑ leucocyte count immediately after exercise |
| Souza et al. 2018 [46] | 10 obese men (age: 28.5 ± 2.7 years) | Randomized cross-over trial | Running HIIT—10 bouts of 1 min at 90% of Vmax interspersed by 1 min at 30% of Vmax | ↔ secretory IgA and IgA concentration |
| Rodrigues de Araujo et al. 2018 [47] | 32 men soccer players (age: 21.2 ± 4.2 years) | Clinical trial | Running SIT—7 bouts of 40 m “all-out” effort with direction changes interspersed by 25 s of active recovery (light jogging) | ↔ IgA concentration |
| Belviranli et al. 2017 [48] | 10 inactive health men (age: 20.0 ± 1.33 years) | Clinical trial | Cycling SIT—4 bouts of 30 s “all-out” effort interspersed by 4 min of active recovery (the load was determined according with the Monark Anaerobic Test Software) | ↑ leucocyte count immediately, 3h, and 6 h after exercise; ↑ lymphocyte count immediately after exercise followed by ↓ lymphocyte count 3 h, and 6 h after exercise; ↑ neutrophil count 3 h, and 6 h after exercise; ↔ monocyte count; ↑ eosinophil count immediately after exercise followed by ↓ 3 h, and 6 h after exercise; ↑ basophil count immediately after exercise |
| Krüger et al. 2016 [49] | 23 untrained health men (age: 25.7 ± 3.2 years) | Randomized cross-over trial | Cycling HIIT—5 bouts of 3 min at 90% peak power output interspersed by 3 min of active recovery (without resistance) | ↑ lymphocyte CD3+, CD4+ and CD8+ count immediately, and 3 h after exercise; ↑ mobilization of low differentiated T cells, regulatory T cells and progenitor cells; ↑ apoptosis in high differentiated T cells |
| Tossige-Gomes et al. 2016 [26] | 10 inactive health men (age: 23.7 ± 1.1) | Clinical trial | Cycling HIIT—8 bouts of 1 min at 100% of peak power interspersed by 75 s of active recovery at 30 W | ↑ lymphocyte redox imbalance 30 min after exercise; ↓ lymphocyte proliferation in response to antigenic, but not to mitogenic stimulation immediately and 30 min after exercise |
| 6 inactive health men (age: 21.3 ± 1.8 years) | Cycling HIIT—8 bouts of 1 min at 100% of peak power interspersed by 75 s of active recovery at 30 W | ↔ lymphocyte viability | ||
| Turner et al. 2016 [50] | 9 health men (age: 22.1 ± 3.4 years) | Randomized cross-over trial | Cycling HIIT—10 bouts of 1 min at 90% of O2max interspersed by 1 min at 40% of O2max | ↑ leucocyte, lymphocyte count immediately after exercise; mobilization of cutaneous lymphocyte natural killer and lymphocyte CD8+ to blood |
| Dorneles et al. 2016 [51] | 12 overweight-obese men (age: 27.41 ± 9.20 years) | Randomized cross-over trial | Running HIIT—10 bouts of 1 min at 85–90% maximum power output interspersed by 75 s at 50% maximum power output | ↑ leucocyte, lymphocyte, and monocyte count immediately after exercise |
| 10 lean men (age: 26.5 ± 6.11 years) | Running HIIT—10 bouts of 1 min at 85–90% maximum power output interspersed by 75 s at 50% maximum power output | ↑ leucocyte immediately and 30 min after exercise; ↑lymphocyte and monocyte immediately after exercise | ||
| Arroyo-Morales et al. 2012 [52] | 50 active health subjects, 25 men (age: 22.4 ± 3.42 years) | Clinical trial | Arm-cycling SIT—3 bouts of 30 s “all-out” effort interspersed by 3 min (90 s of active recovery at 50% W work rate and 90 s of passive recovery) | ↔ secretory IgA |
| Friedman et al. 2012 [53] | 8 health subjects, 4 men (age: 24) | Clinical trial | SIT—2 sets of 3 bouts of 30 s “all-out” effort interspersed by 2 min of active recovery. Sets were separated by 6.75 min | ↑ lymphocyte CD8+, and CD8+/CD45RA+ count and ↑ lymphocyte CD8+, and CD8+/CD45RA+ migration immediately after exercise. ↑ lymphocyte CD8+, and CD8+/CD45RA+ count and ↔ lymphocyte CD4+, and CD4+/CD45RA+ migration immediately after exercise |
| Fisher et al. 2011 [27] | 8 active health men (age: 22 ± 2 years) | Clinical trial | Cycling HIIT—4 bouts with 30 s at 90% of maximum anaerobic power interspersed by 4 min of active recovery at 15% of maximum anaerobic power | ↑ leucocyte and neutrophil counts immediately and 3 h after exercise; ↑ lymphocyte count immediately after exercise; ↓ lymphocyte cell viability 3 h after exercise |
| Davison 2011 [24] | 9 active health men (age: 27 ± 5 years) | Randomized cross-over trial | Cycling SIT—4 bouts of 30 s “all-out” effort interspersed by 4 min of active recovery with light loads | ↔ secretory IgA and ↑ IgA concentration; ↑ neutrophil count immediately and 30 min after exercise; ↓ neutrophil oxidative burst in response to fMLP 30 min after exercise |
| Thomas et al. 2010 [54] | 10 health adolescent women (age 15.5 ± 0.6 years) | Clinical trial | Cycling SIT—8 bouts of 8 s “all-out” effort interspersed by 30 s of passive recovery | ↔ IgA concentration 5 min after exercise |
| Fahlman et al. 2001 [22] | 26 active health women (age: 24.2 ± 5.8 years) | Clinical trial | Cycling SIT—3 bouts of 30 s “all out” effort interspersed by 3 min (90 s of active recovery pedaling against light load and 90 s of passive recovery) | ↓ secretory IgA and ↔ IgA concentration 5 min after exercise |
| Walsh 1999 [55] | 8 trained men (age: 25 ± 1 years) | Clinical trial | Cycling HIIT –20 bouts of 1 min at 100% of O2max interspersed by 2 min at 30% of O2max | ↔ secretory IgA and IgA concentration after exercise |
| Walsh et al. 1998 [56] | 8 trained men (age: 25 ± 3 years) | Clinical trial | Cycling HIIT—20 bouts of 1 min at 100% of O2max interspersed by 2 min at 30% of O2max | ↑ leucocytes and neutrophil count 5 min, 1 h, 2.5 h, and 5 h after exercise; ↑ lymphocyte count immediately after exercise followed by ↓ 1 h after exercise |
| Hinton et al. 1997 [57] | 5 men runners (age: 23.0 ± 2.5 years) | Clinical trial | Running HIIT—15 bouts of 1 min at 90% of O2max interspersed by 2 min of passive recovery | ↓ lymphocyte function immediately after exercise |
| Kargotich et al. 1997 [58] | 8 high performance men swimmers (age: 19.9 ± 2.2 years) | Clinical trial | Swimming HIIT—15 bouts of 100 m freestyle swimming interspersed by 2 min 25 m recovery swim | ↑ leucocyte and neutrophil count immediately after exercise; ↑ lymphocyte count immediately after exercise followed by ↓ 1 h, 2 h, and 2.5 h after exercise; ↑ monocyte count immediately and 30 min after exercise; ↔ eosinophil count |
| Gray et al. 1993 [59] | 8 men triathletes (age: 31.5 ± 4.5 years) | Clinical trial | Running HIIT—1 min at 100% of vO2max interspersed by 1 min of active recovery until the exhaustion | ↑ leucocyte and lymphocyte count immediately after exercise; ↑ granulocyte and monocyte count 6 h after exercise |
| Mackinnon & Jerkin, 1993 [23] | 12 active health men (age: 17 to 25 years) | Clinical trial | Cycling SIT—5 bouts of 1 min “all out” effort interspersed by 5 min of passive recovery | ↓ secretory IgA and ↑ IgA concentration immediately after exercise |
| Fry et al. 1992 [60] | 14 men runners (age: 18–25 years) | Clinical trial | Running Treadmill HIIT—25 bouts of 1 min at one stage before that which the subject failed in the preliminary test) followed by 2 min active recovery | ↓ lymphocyte proliferative response immediately after exercise |
| 18 men kayakists (age: 18–25 years) | Paddling HIIT—25 bouts of 1 min at one stage before that which the subject failed in the preliminary test interspersed by 2 min of active recovery | ↓ lymphocyte proliferative response immediately after exercise | ||
| Fry et al. 1992 [61] | 7 men runners (age: 22.9 ± 5.6 years) | Cross-over clinical trial | Running HIIT—15 bouts of 1 min at 90% of Vmax interspersed by 2 min of active recovery | ↔ leucocytes, lymphocyte, neutrophil and monocyte count 5 min after exercise. ↔ the CD4+:CD8+ ratio and responsiveness of T cells to T cells mitogens |
| Running HIIT—15 bouts of 1 min at 120% of Vmax interspersed by 2 min of active recovery | ↑ leucocytes count, lymphocyte, neutrophil, monocyte count 5 min after exercise. ↓ the CD4+:CD8+ ratio and responsiveness of T cells to mitogens immediately after exercise | |||
| Study | Participants | Duration/Design | Modality/Interval Protocol | Results |
|---|---|---|---|---|
| Bartlett et al. 2020 [62] | 10 subjects with prediabetes, 4 men (age: 71 ± 5 years) | Ten weeks clinical trial | Walking HIIT—60–90 s at 80–90% of O2 reserve interspersed by 60–90 s of active recovery at 50–60% of VO2 reserve until complete 20 min. Frequency: 3 times per week. Supervised: Yes | ↑ neutrophil chemotaxis, mitogen stimulated ROS production and ↓ basal ROS production. ↔ neutrophil count |
| Toohey et al. 2020 [63] | 6 breast cancer survivors (age: 60 ± 8.12 years) | Twelve weeks randomized clinical trial | Cycling SIT—4 to 7 bouts of 30 s “all-out” effort interspersed by 2 min of active recovery. Frequency: 3 times per week. Supervised: Yes | ↔ IgA concentration |
| Dorneles et al. 2019 [41] | 7 sedentary obese men (age: 20 to 40 years) | One-week clinical trial | Running HIIT—10 bouts of 1 min at 85–90% maximum heart rate interspersed by 75 s at 50% maximum heart rate. Frequency: 3 times per week. Supervised: No reported | ↑ circulating of memory regulatory T cells and regulatory T cells |
| Werner et al. 2019 [42] | 29 inactive health subjects, 10 men (age: 48.4 ± 6.5 years) | Twenty-six weeks randomized controlled trial | Running HIIT—4 bouts of 4 min at 80–90% of heart rate reserve interspersed by 3 min at 65–70% of heart rate reserve. Frequency: 3 times per week. Supervised: No reported | ↔ total leucocyte counts (lymphocyte, neutrophil and monocyte); ↑ leucocyte telomerase length (lymphocyte, granulocyte) |
| Khammassi et al. 2020 [30] | 8 active health young adults (age: 18.9 ± 1.0 years) | Nine weeks randomized clinical trial | Running HIIT—3 sets of 6 to 8 30-s bouts at 100 to 110% of Vmax and 30 s of active recovery at 50% of Vmax. Frequency: 3 times per week. Supervised: No reported | ↔ total leucocyte counts (lymphocyte, neutrophil and monocyte) |
| Bartlett et al. 2018 [64] | 12 inactive elderly subjects with rheumatoid arthritis (age: 64 ± 7 years) | Ten weeks clinical trial | Walking HIIT—60–90 s at 80–90% of O2 reserve interspersed by active recovery with similar duration at 50–60% of VO2 reserve until complete 20 min of session. Frequency: 3 times per week. Supervised: Yes | ↑ neutrophil function |
| Sheykhlouvand et al. 2018 [65] | 7 men canoe polo athletes (age: 24 ± 3 years) | Three weeks randomized clinical trial | Paddling HIIT—6 bouts of 1 min at 100 to 130% vO2peak with 1:3 work to recovery ratio. Frequency: 3 times per week. Supervised: No reported | ↔ leucocyte counts |
| 7 men canoe polo athletes (age: 24 ± 3 years) | Paddling HIIT—6 to 9 bouts of 1 min at 100% vO2peak with 1:3 work to recovery ratio. Frequency: 3 times per week. Supervised: No reported | ↔ leucocyte counts | ||
| Bartlett et al. 2017 [66] | 14 inactive health adults (age: 43 ± 11 years) | Ten weeks randomized clinical trial | Cycling HIIT—15 to 60 s above 90% of maximum heart rate interspersed by 45–120 s of active recovery until complete 18–25 min. Frequency: 3 times per week. Supervised: Yes | ↑ neutrophil and monocyte function |
| Tsai et al. 2016 [67] | 20 inactive health men (age: 23.0 ± 1.7 years) | Six weeks randomized clinical trial | Cycling HIIT—5 bouts of 3 min at 80% of O2max interspersed by 3 min of active recovery at 40% of O2max. Frequency: 5 times per week. Supervised: No reported | ↑ lymphocyte function |
| Navalta et al. 2014 [29] | 12 subjects, 8 men (age: 26 ± 4 years) | Three consecutive days clinical trial | Running HIIT—30 s at 100% of Vmax interspersed by active recovery with similar duration at 50% of Vmax until exhaustion. Frequency: 3 times per week. Supervised: No reported | ↑ lymphocyte apoptosis |
| Fisher et al. 2011 [27] | 8 active health men (age: 22 ± 2 years) | One-week clinical trial | Cycling HIIT—4 bouts with 30 s at 90% of maximum anaerobic power interspersed by 4 min of active recovery at 15% of maximum anaerobic power. Frequency: 3 times per week. Supervised: No reported | ↑ lymphocyte function |
| Mackinnon & Jerkin, 1993 [23] | 12 active health men (age: 17 to 25 years) | Eight weeks clinical trial | Cycling SIT—5 bouts of 1 min “all out” effort interspersed by 5 min of passive recovery. Frequency: 3 times per week. Supervised: Yes | ↔ secretory IgA and IgA concentration |
| Reference | Study Quality | Score (0–5) | Study Reporting | Score (0–10) | Total Score (0–15) | Study Quality Classification | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6a | 6b | 6c | 7 | 8a | 8b | 9 | 10 | 11 | 12 | |||||
| Khammassi et al. [30] | + | − | + | + | − | 3 | − | − | − | − | − | − | + | NA | − | + | 2 | 5 | Low |
| Toohey et al. [63] | + | + | + | − | + | 4 | + | − | + | − | + | + | + | − | + | + | 7 | 11 | High |
| Wahl et al. [44] | − | − | − | − | − | 0 | − | − | − | − | + | + | + | NA | − | + | 4 | 4 | Low |
| Dorneles et al. [41] | + | − | − | − | − | 1 | − | − | − | − | − | − | + | NA | + | + | 3 | 4 | Low |
| Werner et al. [42] | + | − | + | + | − | 3 | − | − | − | − | + | + | + | − | − | + | 4 | 7 | Low |
| de Souza et al. [46] | + | + | − | − | − | 2 | − | − | − | − | + | + | + | − | − | + | 4 | 6 | Low |
| Jamurtas et al. [45] | − | − | − | − | − | 0 | − | − | − | − | − | − | + | NA | − | + | 2 | 2 | Low |
| Sheykhlouvand et al. [65] | + | − | + | − | − | 2 | − | − | − | − | − | − | + | NA | − | + | 2 | 4 | Low |
| Bartlett et al. [66] | − | − | + | + | − | 2 | − | − | − | − | − | − | + | NA | − | + | 2 | 4 | Low |
| Krüger et al. [49] | + | − | − | − | − | 1 | − | − | − | − | − | − | − | NA | − | + | 1 | 2 | Low |
| Tsai et al. [67] | + | − | − | − | − | 1 | + | − | + | − | + | + | + | + | + | + | 8 | 9 | Fair |
| Turner et al. [50] | − | − | − | − | − | 0 | − | − | + | − | − | − | + | NA | − | + | 3 | 3 | Low |
| Davison. [24] | − | − | − | − | − | 0 | − | − | − | − | + | + | + | − | − | − | 3 | 3 | Low |
| Outcome (Subgroup) | N° of Studies | MD (95% CI) | p-Value | Heterogeneity | |
|---|---|---|---|---|---|
| I2 (%) | p-Value | ||||
| IgA concentration (µg·mL−1) | |||||
| IT type: SIT | 5 | 46.98 (56.73 to 150.68) | 0.37 | 94 | <0.001 |
| IT type: HIIT | 4 | 39.54 (19.92 to 59.16) | <0.001 | 0 | 1 |
| Sex: men | 6 | 65.62 (−6.43 to 137.66) | 0.07 | 91 | <0.001 |
| Sex: women | 3 | −18.91 (−66.24 to 28.42) | 0.43 | 0 | 0.59 |
| Modality: cycling | 5 | 53.22 (−33.53 to 139.96) | 0.23 | 94 | <0.001 |
| Modality: running | 4 | 22.07 (−17.34 to 61.47) | 0.27 | 0 | 0.92 |
| IgA secretory rate (µg·min−1) | |||||
| IT type: SIT | 6 | −17.33 (−33.68 to −0.98) | 0.03 | 68 | 0.007 |
| IT type: HIIT | 2 | −7.29 (−23.95 to 9.36) | 0.39 | 0 | 0.73 |
| Sex: men | 5 | −13.17 (−35.03 to 8.70) | 0.24 | 74 | 0.004 |
| Sex: women | 2 | −19.34 (−29.11 to −9.58) | <0.001 | 0 | 0.74 |
| Modality: cycling | - | - | - | - | - |
| Modality: running | - | - | - | - | - |
| Leucocyte count (103 µL−1) | |||||
| IT type: SIT | 5 | 3.14 (1.83 to 4.44) | <0.01 | 80 | <0.001 |
| IT type: HIIT | 9 | 2.31 (1.30 to 3.32) | <0.001 | 78 | <0.001 |
| Sex: men | - | - | - | - | - |
| Sex: women | - | - | - | - | - |
| Modality: cycling | 9 | 2.40 (1.47 to 3.33) | <0.001 | 84 | <0.001 |
| Modality: running | 3 | 2.46 (1.30 to 3.62) | <0.001 | 21 | 0.28 |
| Lymphocyte count (103 µL−1) | |||||
| IT type: SIT | 3 | 1.62 (0.89 to 2.35) | <0.001 | 66 | 0.05 |
| IT type: HIIT | 9 | 1.21 (0.67 to 1.74) | <0.001 | 81 | <0.001 |
| Sex: men | - | - | - | - | - |
| Sex: women | - | - | - | - | |
| Modality: cycling | 8 | 1.17 (0.65 to 1.70) | <0.001 | 82 | <0.001 |
| Modality: running | 3 | 1.14 (0.67 to 1.61) | <0.001 | 10 | 0.33 |
| Lymphocyte count (103 µL−1) recovery | |||||
| IT type: SIT | 3 | −0.51 (−0.77 to −0.26) | <0.001 | 18 | 0.30 |
| IT type: HIIT | 9 | −0.29 (−0.56 to 0.03) | 0.03 | 66 | 0.003 |
| Sex: men | - | - | - | - | - |
| Sex: women | - | - | - | - | - |
| Modality: cycling | 8 | −0.47 (−0.62 to −0.32) | <0.001 | 0 | 0.55 |
| Modality: running | 3 | 0.04 (−0.63, 0.72) | 0.9 | 85 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, D.; Vale, A.F.; Silva, A.; Araújo, M.A.S.; de Paula Júnior, C.A.; de Lira, C.A.B.; Ramirez-Campillo, R.; Martins, W.; Gentil, P. Acute and Chronic Effects of Interval Training on the Immune System: A Systematic Review with Meta-Analysis. Biology 2021, 10, 868. https://doi.org/10.3390/biology10090868
Souza D, Vale AF, Silva A, Araújo MAS, de Paula Júnior CA, de Lira CAB, Ramirez-Campillo R, Martins W, Gentil P. Acute and Chronic Effects of Interval Training on the Immune System: A Systematic Review with Meta-Analysis. Biology. 2021; 10(9):868. https://doi.org/10.3390/biology10090868
Chicago/Turabian StyleSouza, Daniel, Arthur F. Vale, Anderson Silva, Murilo A. S. Araújo, Célio A. de Paula Júnior, Claudio A. B. de Lira, Rodrigo Ramirez-Campillo, Wagner Martins, and Paulo Gentil. 2021. "Acute and Chronic Effects of Interval Training on the Immune System: A Systematic Review with Meta-Analysis" Biology 10, no. 9: 868. https://doi.org/10.3390/biology10090868
APA StyleSouza, D., Vale, A. F., Silva, A., Araújo, M. A. S., de Paula Júnior, C. A., de Lira, C. A. B., Ramirez-Campillo, R., Martins, W., & Gentil, P. (2021). Acute and Chronic Effects of Interval Training on the Immune System: A Systematic Review with Meta-Analysis. Biology, 10(9), 868. https://doi.org/10.3390/biology10090868








