Antiviral Activity of Rosa damascena Mill. and Rosa alba L. Essential Oils against the Multiplication of Herpes Simplex Virus Type 1 Strains Sensitive and Resistant to Acyclovir
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants’ Material and Distillation of Essential Oils
2.2. Chromatographic Conditions
2.3. Cells
2.4. Viruses
2.5. Cytotoxicity Assay
2.6. Antiviral Activity Assay
2.7. Virucidal Assay
2.8. Virus Attachment Assay
2.9. Pretreatment of MDBK Cells
2.10. Combination Effect Analysis
2.11. Statistical Analysis
3. Results
3.1. Chromatographic Profile of the Essential Oils
3.2. Study on Antiviral Properties
3.2.1. Determination of Cytotoxic Concentration
3.2.2. Effect of Essential Oils on the Replication of HSV-1
3.2.3. Effect of Essential Oils on the Viability of Virions
3.2.4. Effect of Essential Oils on the Viral Adsorption
3.2.5. Protective Effect in Pretreatment of Healthy MDBK Cells
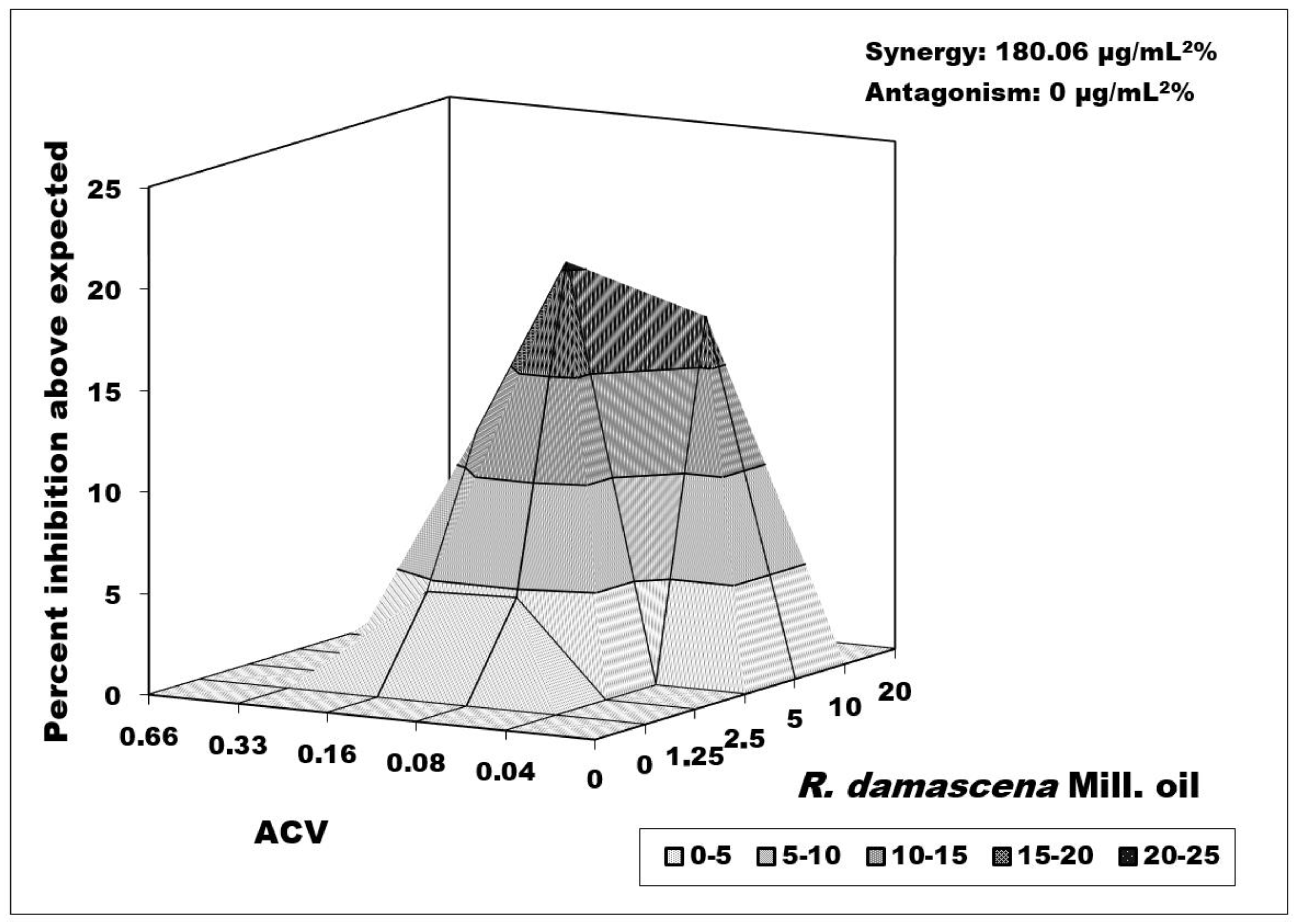
3.2.6. Combined Effect between R. damascenа Mill. Oil and ACV versus Victoria Strain Replication
3.2.7. Combined Effect between R. alba L. Oil and ACV versus Victoria Strain Replication
3.2.8. Combined Effect between Rosa damascena Mill. Oil and ACV versus R-100 Strain Replication
3.2.9. Combined Effect between Rosa alba L. Oil and ACV versus R-100 Strain—Resistant to ACV Replication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chayavichitsilp, P.; Buckwalter, J.V.; Krakowski, A.C.; Friedlander, S.F. Herpes simplex. Pediatr. Rev. 2009, 30, 119–129. [Google Scholar] [CrossRef]
- Roizman, B.; Knipe, D.M. Herpes Simplex Virus and Their Replication. In Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 2001; pp. 2399–2459. [Google Scholar]
- Walaszek, R.; Marszałek, A.; Kasperczyk, T.; Walaszek, K.; Burdacki, M. The efficacy of Aromatherapy in prevention of herpes simplex virus infections. Ind. J. Trad. Know. 2018, 17, 425–429. [Google Scholar]
- Greco, A.; Diaz, J.J.; Thouvenot, D.; Morfin, F. Novel targets for the development of anti-herpes compounds. Infect. Disord. Drug Targets 2007, 7, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Saddi, M.; Sanna, A.; Cottiglia, F.; Chisu, L.; Casu, L.; Bonsignore, L.; De Logu, A. Antiherpevirus activity of Artemisia arborescens essential oil and inhibition of lateral diffusion in Vero cells. Ann. Clin. Microbiol. Antimicrob. 2007, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jassin, S.A.A.; Naji, M.A. Novel antiviral agents: A medicinal plant perspective. J. Appl. Microbiol. 2003, 95, 412–427. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, M.; Arshad, M.; Ahmad, M.; Pomerantz, R.J.; Wigdahl, B.; Parveen, Z. Antiviral potentials of medicinal plants. Virus Res. 2008, 131, 111–120. [Google Scholar] [CrossRef]
- Suschke, U.; Sporer, F.; Schneele, J.; Geiss, H.K.; Reichling, J. Antibacterial and cytotoxic activity of Nepeta cataria L., N. cataria var. citriodora (Beck.) Balb. and Melissa officinalis L. essential oils. Nat. Prod. Commun. 2007, 2, 1934578X0700201218. [Google Scholar]
- Breer, H. Olfactory receptors: Molecular basis for recognition and discrimination of odors. Anal. Bioanal. Chem. 2003, 377, 427–433. [Google Scholar] [CrossRef]
- Handsfield, H.H. Public Health Strategies to Prevent Genital Herpes: Where Do We Stand? Curr. Infect. Dis. Rep. 2000, 2, 25–30. [Google Scholar] [CrossRef]
- García, C.C.; Talarico, L.; Almeida, N.; Colombres, S.; Duschatzky, C.; Damonte, E.B. Virucidal Activity of Essential Oils from Aromatic Plants of San Luis, Argentina. Phytother. Res. 2003, 17, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Saira, W.; Waseem, R.; Khushboo, S.H.; Muzzaffar, A.B.; Anil, P.; Aabid, H.S.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Benencia, F.; Courrege, M.C. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2000, 14, 495–500. [Google Scholar] [CrossRef]
- Mileva, M.; Ilieva, Y.; Jovtchev, G.; Gateva, S.; Zaharieva, M.M.; Georgieva, A.; Dimitrova, L.; Dobreva, A.; Angelova, T.; Vilhelmova-Ilieva, N.; et al. Rose flowers—A Delicate Perfume or a Natural Healer? Biomolecules 2021, 11, 127. [Google Scholar] [CrossRef]
- Jovtchev, G.; Stankov, A.; Georgieva, A.; Dobreva, A.; Bakalova, R.; Aoki, I.; Mileva, M. Cytotoxic and genotoxic potential of Bulgarian Rosa alba L. essential oil–in vitro model study. Biotechnol. Biotechnol. Equipment 2018, 32, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Gateva, S.; Jovtchev, G.; Chanev, C.; Georgieva, A.; Stankov, A.; Dobreva, A.; Mileva, M. Assessment of anti-cytotoxic, anti-genotoxic and antioxidant potentials of Bulgarian Rosa alba L. essential Oil. Caryol. Int. J. Cytol. Cytosyst. Cytogenet. 2020, 73, 3. [Google Scholar]
- Gateva, S.; Jovtchev, G.; Stankov, A.; Georgieva, A.; Dobreva, A.; Mileva, M. The potential of geraniol to reduce A. cytotoxic and genotoxic effects of MNNG in plant and human lymphocyte test-systems. S. Afr. J. Bot. 2019, 123, 170–179. [Google Scholar] [CrossRef]
- Available online: https://www.sis.se/api/document/preview/904024/ (accessed on 20 May 2021).
- Prichard, M.N.; Shipman, C., Jr. A three-dimensional model to analyze drug-drug interaction. Antivir. Res. 1990, 14, 181–206. [Google Scholar] [CrossRef] [Green Version]
- Prichard, M.N.; Aseltine, K.R.; Shipman, C., Jr. MacSynergyTM II (version 1.0). In Users Manual; University of Michigan: Ann Arbor, MI, USA, 1993. [Google Scholar]
- Nedkov, N.; Dobreva, A.; Kovacheva, N.; Bardarov, V.; Velcheva, A. Bulgarian rose oil of white oil-bearing rose. Bulg. J. Agric. Sci. 2009, 15, 318–322. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Zhiri, A.; Idaomar, M. Cytotoxicity and gene induction by some essential oils in the yeast Saccharomyces cerevisiae. Mutat. Res. 2005, 585, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P. Essential oils for the treatment of herpes simplex virus infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.; Stashenko, E.; Ocazionez, R. Comparative study on in vitro activities of citral, limonene and essential oils from Lippia citriodora and L. alba on yellow fever virus. Nat. Prod. Commun. 2013, 8, 249–252. [Google Scholar]
- Orhan, I.E.; Ozcelik, B.; Kartal, M.; Kan, Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar]
- Schnitzler, P.; Schön, K.; Reichling, J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Die Pharm. 2001, 56, 343–347. [Google Scholar] [PubMed]
- Schnitzler, P.; Schuhmacher, A.; Astani, A.; Reichling, J. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomedicine 2008, 15, 734. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Kita, M.; Nakaya, T.; Yamamoto, T.; Kuriyama, H.; Imanishi, J. The Inhibitory Effect of Essential Oils on Herpes Simplex Virus Type-1 Replication In Vitro. Microbiol. Immunol. 2003, 47, 681–684. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative Study on the Antiviral Activity of Selected Monoterpenes Derived from Essential Oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; El-Khadragy, M.F.; Hussein, M.H.; Mahgoub, S.; Abdel-Mohsen, D.M.; Taha, H.; Bakkar, A.A.A.; Abdel Moneim, A.E.; Amin, H.K. Green Coffea arabica Extract Ameliorates Testicular Injury in High-Fat Diet/Streptozotocin-Induced Diabetes in Rats. J. Diabet. Res. 2020, 2020, 6762709. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Wei, A.; Shibamoto, T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food. Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Checconi, P.; De Angelis, M.; Marcocci, M.E.; Fraternale, A.; Magnani, M.; Palamara, A.T.; Nencioni, L. Redox-modulating agents in the treatment of viral infections. Int. J. Mol. Sci. 2020, 21, 4084. [Google Scholar] [CrossRef]
- Koch, C.; Reichling, J.; Schneele, J.; Schnitzler, P. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine 2008, 15, 71–78. [Google Scholar] [CrossRef] [PubMed]





| Compound Tested | Cytotoxicity (µg/mL) | Antiviral Activity | ||||
|---|---|---|---|---|---|---|
| Victoria Strain | R-100 Strain | |||||
| CC50 | MTC | IC50 (µg/mL) | SI | IC50 (µg/mL) | SI | |
| R. damascena Mill. oil | 530.0 ± 5.25 *** | 10.0 | - | - | - | - |
| R. alba L. oil | 570.0 ± 5.83 *** | 10.0 | - | - | - | - |
| ACV | 291.0 ± 9.4 | - | 0.33 ± 0.03 | 881.8 | 11.6 ± 1.2 | 25.3 |
| Compounds | Δlg of Infectivity Reduction | |||||
|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 60 min | 90 min | 120 min | |
| R. damascena Mill. oil | 1.0 | 1.75 | 1.75 | 2.0 | 2.25 | 3.0 |
| R. alba L. oil | 1.0 | 1.75 | 2.0 | 2.25 | 2.5 | 3.0 |
| Compound | Δlg of Virus Yield Reduction | |||
|---|---|---|---|---|
| 15 min | 30 min | 45 min | 60 min | |
| R. damascena Mill. oil | 1.5 | 2.0 | 2.75 | 4.0 |
| R. alba L. oil | 1.5 | 1.75 | 2.5 | 3.5 |
| Compound | Δlg of Virus Yield Reduction | |||||
|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 60 min | 90 min | 1120 min | |
| R. damascena Mill. oil | 1.0 | 1.5 | 2.75 | 2.75 | 3.25 | 4.25 |
| R. alba L. oil | 1.0 | 1.25 | 2.0 | 2.5 | 3.50 | 3.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilhelmova-Ilieva, N.; Dobreva, A.; Doynovska, R.; Krastev, D.; Mileva, M. Antiviral Activity of Rosa damascena Mill. and Rosa alba L. Essential Oils against the Multiplication of Herpes Simplex Virus Type 1 Strains Sensitive and Resistant to Acyclovir. Biology 2021, 10, 746. https://doi.org/10.3390/biology10080746
Vilhelmova-Ilieva N, Dobreva A, Doynovska R, Krastev D, Mileva M. Antiviral Activity of Rosa damascena Mill. and Rosa alba L. Essential Oils against the Multiplication of Herpes Simplex Virus Type 1 Strains Sensitive and Resistant to Acyclovir. Biology. 2021; 10(8):746. https://doi.org/10.3390/biology10080746
Chicago/Turabian StyleVilhelmova-Ilieva, Neli, Ana Dobreva, Rositsa Doynovska, Dimo Krastev, and Milka Mileva. 2021. "Antiviral Activity of Rosa damascena Mill. and Rosa alba L. Essential Oils against the Multiplication of Herpes Simplex Virus Type 1 Strains Sensitive and Resistant to Acyclovir" Biology 10, no. 8: 746. https://doi.org/10.3390/biology10080746
APA StyleVilhelmova-Ilieva, N., Dobreva, A., Doynovska, R., Krastev, D., & Mileva, M. (2021). Antiviral Activity of Rosa damascena Mill. and Rosa alba L. Essential Oils against the Multiplication of Herpes Simplex Virus Type 1 Strains Sensitive and Resistant to Acyclovir. Biology, 10(8), 746. https://doi.org/10.3390/biology10080746






