Human Blood Serum Induces p38-MAPK- and Hsp27-Dependent Migration Dynamics of Adult Human Cardiac Stem Cells: Single-Cell Analysis via a Microfluidic-Based Cultivation Platform
Abstract
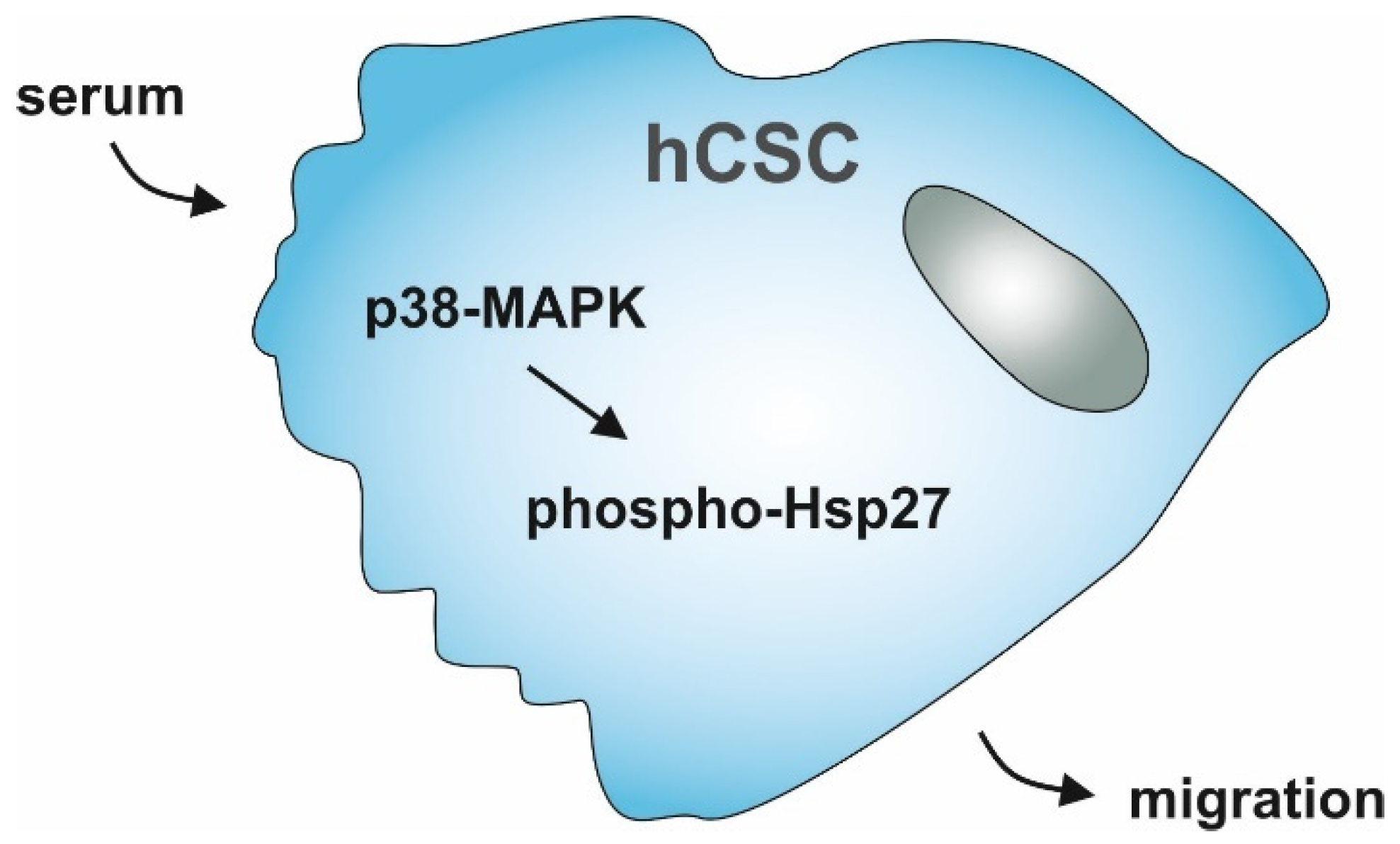
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Human Cardiac Stem Cells
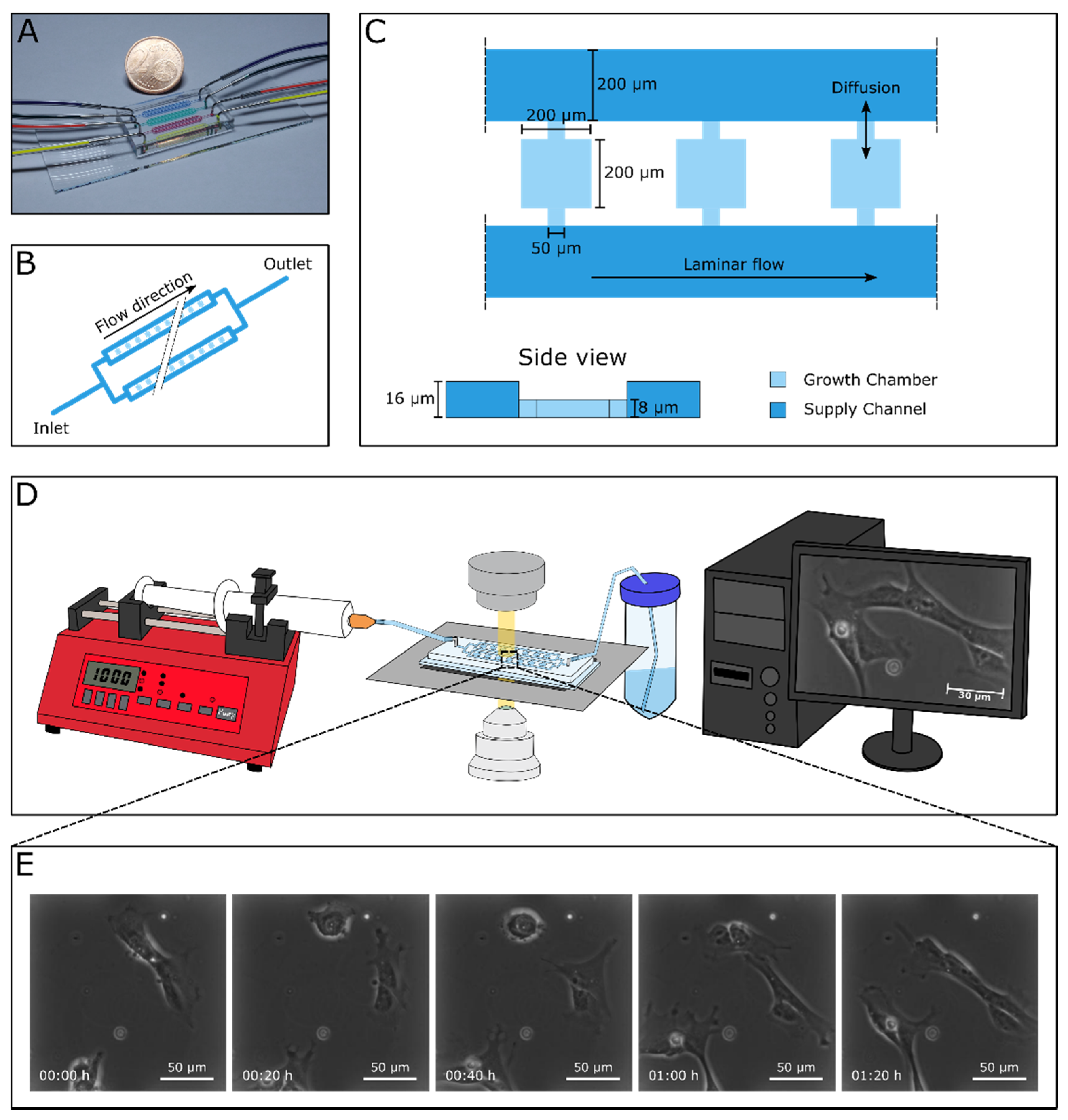
2.2. Microfluidics
2.3. Immunocytochemistry
2.4. Data Analysis
3. Results
3.1. Successful Cultivation of Human Cardiac Stem Cells in a Microfluidic Cultivation Device
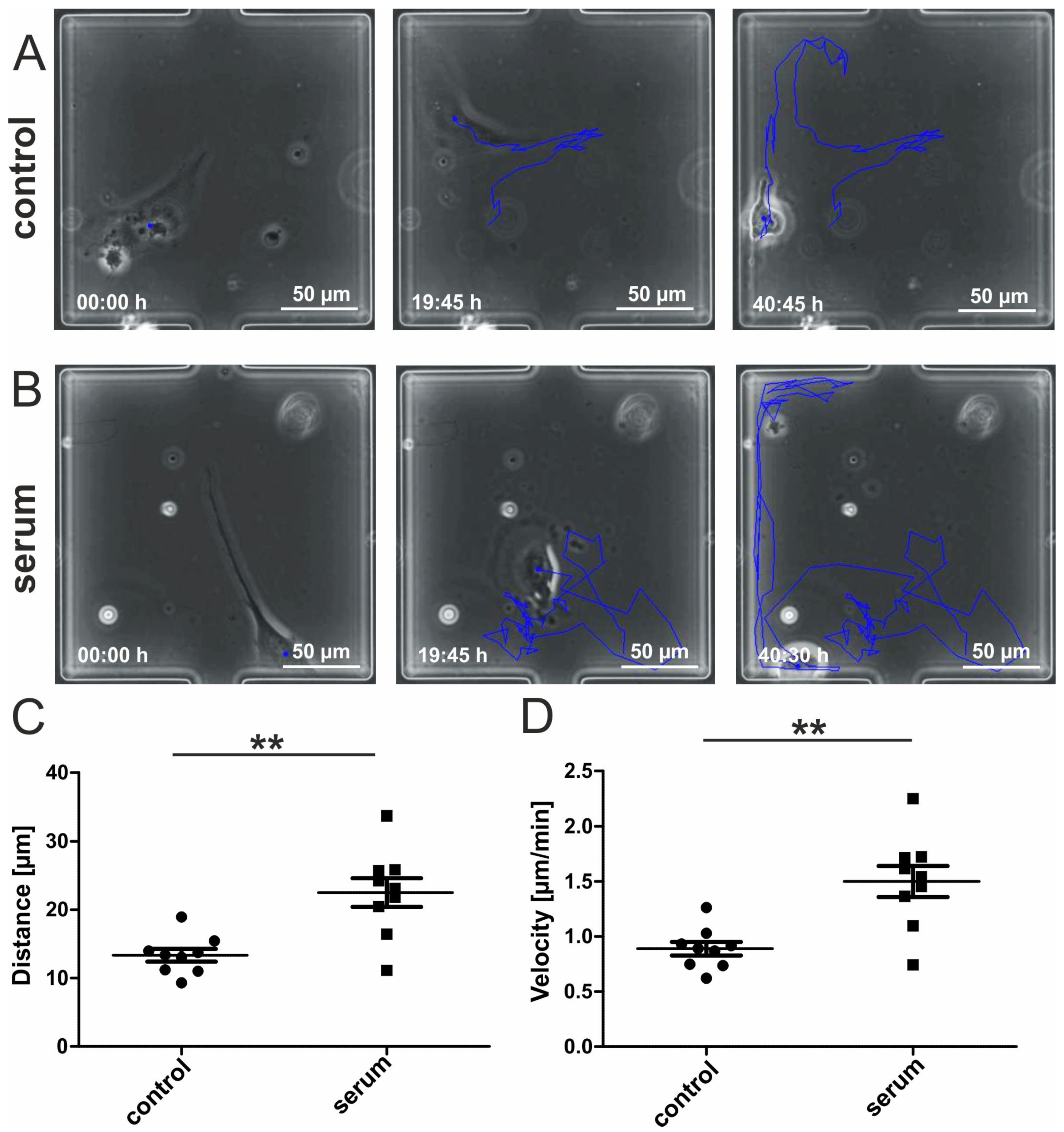
3.2. Migrating hCSCs Exhibit Diverse Morphologies and Migration Patterns
3.3. Human Blood Serum Enhances the Migration Distance and Speed of hCSCs
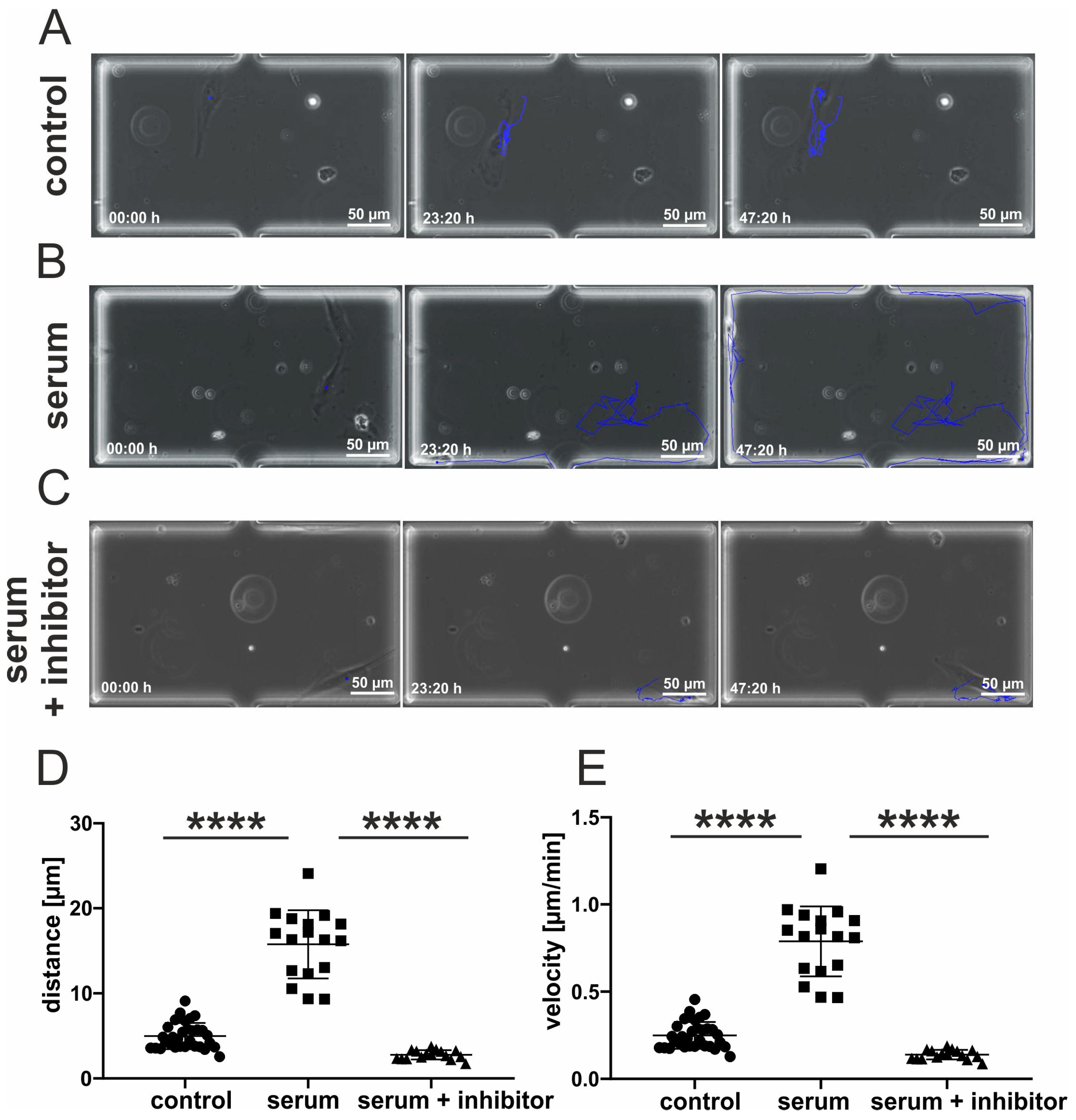
3.4. Inhibition of p38-MAPK Leads to Decreased Migration of Blood-Serum Stimulated hCSCs
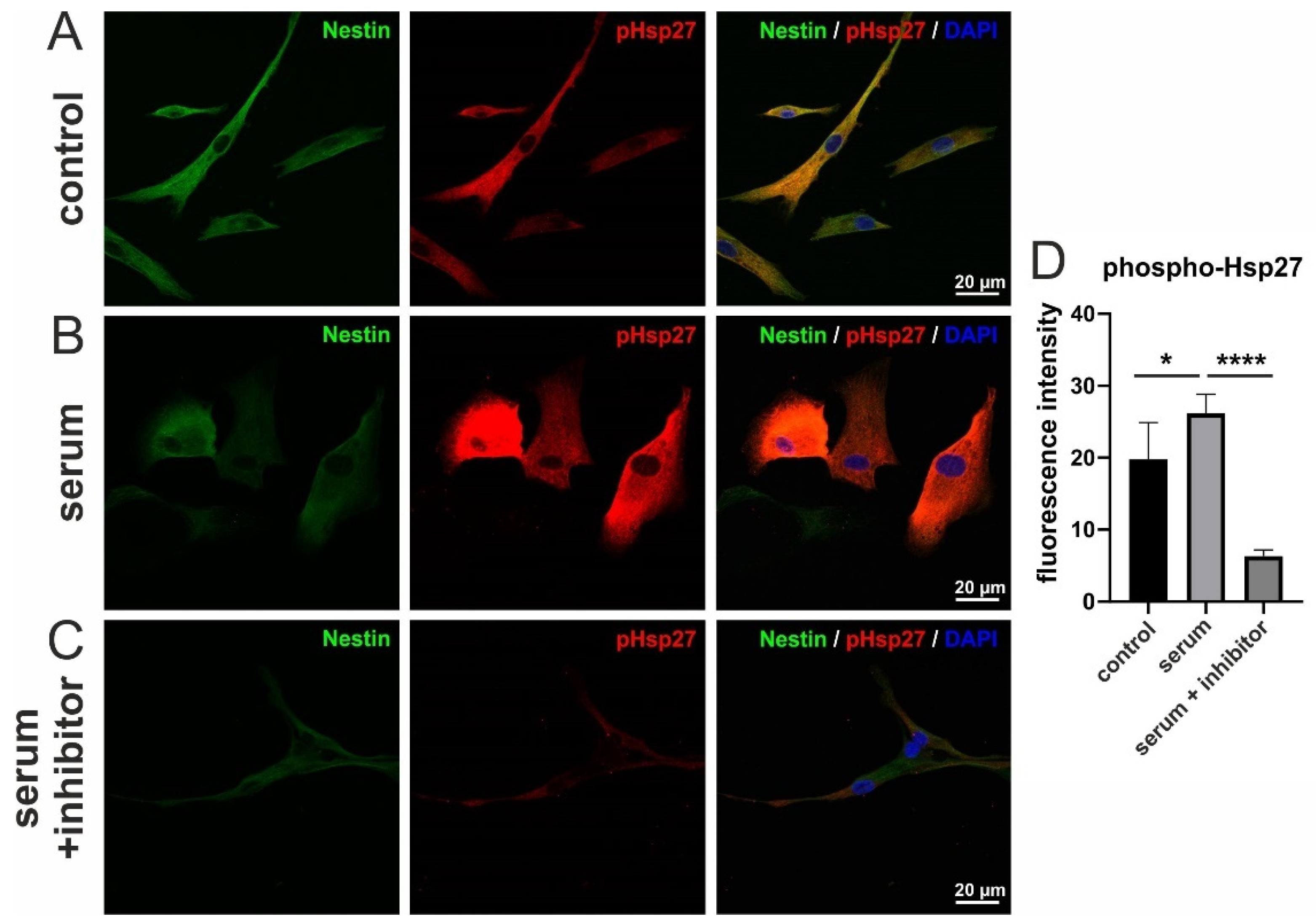
3.5. Serum-Induced Migration of hCSCs Is Regulated via p38-MAPK and Hsp27-Phosphorylation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodell, M.A.; Nguyen, H.; Shroyer, N. Somatic stem cell heterogeneity: Diversity in the blood, skin and intestinal stem cell compartments. Nat. Rev. Mol. Cell Biol. 2015, 16, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Liesveld, J.L.; Sharma, N.; Aljitawi, O.S. Stem cell homing: From physiology to therapeutics. STEM CELLS 2020, 38, 1241–1253. [Google Scholar] [CrossRef]
- Ridley, A.; Schwartz, M.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Sci. 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Raab, M.; Swift, J.; Dingal, P.C.D.P.; Shah, P.; Shin, J.-W.; Discher, D.E. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J. Cell Biol. 2012, 199, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Sakamoto, N.; Song, G.; Sato, M. Migration of Human Mesenchymal Stem Cells Under Low Shear Stress Mediated by Mitogen-Activated Protein Kinase Signaling. Stem Cells Dev. 2012, 21, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Huang, X.; Zhou, Y.; Jin, R.; Li, Q. Mechanical Stretching Promotes Skin Tissue Regeneration via Enhancing Mesenchymal Stem Cell Homing and Transdifferentiation. STEM CELLS Transl. Med. 2016, 5, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.; Mogha, P.; Dash, S.; Majumder, A.; Jadhav, S.; Sen, S. Matrix elasticity regulates mesenchymal stem cell chemotaxis. J. Cell Sci. 2018, 131, jcs.211391. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Carter, M.J.; Fylling, C.P.; Parnell, L.K. Use of Platelet Rich Plasma Gel on Wound Healing: A Systematic Review and Meta-Analysis. Eplasty 2011, 11, e38. [Google Scholar]
- Heim, M.U.; Meyer, B.; Hellstern, P. Recommendations for the use of therapeutic plasma. Curr. Vasc. Pharmacol. 2009, 7, 110–119. [Google Scholar] [CrossRef]
- Rassi, A.B.; D’Amico, E.A.; Tripodi, A.; da Rocha, T.R.F.; Migita, B.Y.; Ferreira, C.M.; Carrilho, F.J.; Farias, A.Q. Fresh frozen plasma transfusion in patients with cirrhosis and coagulopathy: Effect on conventional coagulation tests and thrombomodulin-modified thrombin generation. J. Hepatol. 2020, 72, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Krishnasamy, N.; Rangarajan, J.; Rathinam, J.; Natarajan, M.; Ramachandran, A. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J. Med. Virol. 2020, 92, 1475–1483. [Google Scholar] [CrossRef]
- Greiner, J.; Widera, D.; Müller, J.; Qunneis, F.; Zander, C.; Martin, I.; Mallah, J.; Schuetzmann, D.; Prante, C.; Schwarze, H.; et al. Efficient animal-serum free 3D cultivation method for adult human neural crest-derived stem cell therapeutics. Eur. Cells Mater. 2011, 22, 403–419. [Google Scholar] [CrossRef]
- Shen, J.; Gao, Q.; Zhang, Y.; He, Y. Autologous platelet-rich plasma promotes proliferation and chondrogenic differentiation of adipose-derived stem cells. Mol. Med. Rep. 2015, 11, 1298–1303. [Google Scholar] [CrossRef][Green Version]
- Pandey, S.; Hickey, D.U.; Drum, M.; Millis, D.L.; Cekanova, M. Platelet-rich plasma affects the proliferation of canine bone marrow-derived mesenchymal stromal cells in vitro. BMC Veter- Res. 2019, 15, 1–8. [Google Scholar] [CrossRef]
- Höving, A.L.; Schmidt, K.E.; Merten, M.; Hamidi, J.; Rott, A.-K.; Faust, I.; Greiner, J.F.W.; Gummert, J.; Kaltschmidt, B.; Kaltschmidt, C.; et al. Blood Serum Stimulates p38-mediated Proliferation and Changes in Global Gene Expression of Adult Human Cardiac Stem Cells. Cells 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Lotz, M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J. Orthop. Res. 2008, 26, 1407–1412. [Google Scholar] [CrossRef]
- Henry, G.; Li, W.; Garner, W.; Woodley, D.T. Migration of human keratinocytes in plasma and serum and wound re-epithelialisation. Lancet 2003, 361, 574–576. [Google Scholar] [CrossRef]
- Kondo, H.; Nomaguchi, T.A.; Yonezawa, Y. Effects of serum from human subjects of different ages on migration in vitro of human fibroblasts. Mech. Ageing Dev. 1989, 47, 25–37. [Google Scholar] [CrossRef]
- Kondo, H.; Yonezawa, Y.; Ito, H. Inhibitory effects of human serum on human fetal skin fibroblast migration: Migration-inhibitory activity and substances in serum, and its age-related changes. In Vitro Cell Dev. Biol. Anim. 2000, 36, 256–261. [Google Scholar] [CrossRef]
- Bearzi, C.; Rota, M.; Hosoda, T.; Tillmanns, J.; Nascimbene, A.; De Angelis, A.; Yasuzawa-Amano, S.; Trofimova, I.; Siggins, R.W.; LeCapitaine, N.; et al. Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14068–14073. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T.; et al. Cardiomyocyte Regeneration: A Consensus Statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Höving, A.L.; Sielemann, K.; Greiner, J.F.W.; Kaltschmidt, B.; Knabbe, C.; Kaltschmidt, C. Transcriptome Analysis Reveals High Similarities between Adult Human Cardiac Stem Cells and Neural Crest-Derived Stem Cells. Biology 2020, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.E.; Bryant, P.W.; Glembotski, C.C.; Vincent, P.A.; Pumiglia, K.M. Activation of p38 Has Opposing Effects on the Proliferation and Migration of Endothelial Cells. J. Biol. Chem. 2005, 280, 20995–21003. [Google Scholar] [CrossRef]
- Ryu, C.H.; A Park, S.; Kim, S.M.; Lim, J.Y.; Jeong, C.H.; Jun, J.A.; Oh, J.H.; Park, S.H.; Oh, W.-I.; Jeun, S.-S. Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem. Biophys. Res. Commun. 2010, 398, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hamanoue, M.; Morioka, K.; Ohsawa, I.; Ohsawa, K.; Kobayashi, M.; Tsuburaya, K.; Akasaka, Y.; Mikami, T.; Ogata, T.; Takamatsu, K. Cell-permeable p38 MAP kinase promotes migration of adult neural stem/progenitor cells. Sci. Rep. 2016, 6, 24279. [Google Scholar] [CrossRef]
- Huth, H.W.; Santos, D.M.; Gravina, H.D.; Resende, J.; Goes, A.M.; De Lima, M.E.; Ropert, C. Upregulation of p38 pathway accelerates proliferation and migration of MDA-MB-231 breast cancer cells. Oncol. Rep. 2017, 37, 2497–2505. [Google Scholar] [CrossRef][Green Version]
- Dubon, M.J.; Yu, J.; Choi, S.; Park, K.-S. Transforming growth factor β induces bone marrow mesenchymal stem cell migration via noncanonical signals and N-cadherin. J. Cell. Physiol. 2018, 233, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-F.; Xie, L.-D.; Xu, C.-S. Role of heat shock protein 27 phosphorylation in migration of vascular smooth muscle cells. Mol. Cell. Biochem. 2009, 327, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, L.-D.; Luo, L.; Zheng, S.-L.; Wang, H.-J.; Xu, C.-S. Silencing heat shock protein 27 (HSP27) inhibits the proliferation and migration of vascular smooth muscle cells in vitro. Mol. Cell. Biochem. 2014, 390, 115–121. [Google Scholar] [CrossRef]
- Yao, Y.; Cui, L.; Ye, J.; Yang, G.; Lu, G.; Fang, X.; Zeng, Z.; Zhou, J. Dioscin facilitates ROS-induced apoptosis via the p38-MAPK/HSP27-mediated pathways in lung squamous cell carcinoma. Int. J. Biol. Sci. 2020, 16, 2883–2894. [Google Scholar] [CrossRef]
- Van Noort, D.; Ong, S.M.; Zhang, C.; Zhang, S.; Arooz, T.; Yu, H. Stem cells in microfluidics. Biotechnol. Prog. 2009, 25, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Lindström, S.; Andersson-Svahn, H. Overview of single-cell analyses: Microdevices and applications. Lab a Chip 2010, 10, 3363–3372. [Google Scholar] [CrossRef]
- Zhang, Q.; Austin, R.H. Applications of Microfluidics in Stem Cell Biology. BioNanoScience 2012, 2, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Lucumi, E.; Gomez-Sjoberg, R.; Fleming, R.M. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-W.; Lin, C.-C.; Lee, G.-B. Stem cells in microfluidics. Biomicrofluidics 2011, 5, 013401. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Noll, T.; Grünberger, A. Heterogeneity Studies of Mammalian Cells for Bioproduction: From Tools to Application. Trends Biotechnol. 2019, 37, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, H.; Ma, H.; Qin, J. A simple microfluidic strategy for cell migration assay in an in vitro wound-healing model. Wound Repair Regen. 2013, 21, 897–903. [Google Scholar] [CrossRef]
- Sticker, D.; Lechner, S.; Jungreuthmayer, C.; Zanghellini, J.; Ertl, P. Microfluidic Migration and Wound Healing Assay Based on Mechanically Induced Injuries of Defined and Highly Reproducible Areas. Anal. Chem. 2017, 89, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.-Q.; Yamada, M.; Kobayashi, J.; Yamato, M.; Kikuchi, A.; Okano, T. On-chip cell migration assay using microfluidic channels. Biomater. 2007, 28, 4017–4022. [Google Scholar] [CrossRef]
- Huang, X.; Li, L.; Tu, Q.; Wang, J.; Liu, W.; Wang, X.; Ren, L.; Wang, J. On-chip cell migration assay for quantifying the effect of ethanol on MCF-7 human breast cancer cells. Microfluid. Nanofluid. 2011, 10, 1333–1341. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, F.; Zhang, T.; Chen, D.; Jia, X.; Wang, J.; Guo, W.; Chen, J. A Tubing-Free Microfluidic Wound Healing Assay Enabling the Quantification of Vascular Smooth Muscle Cell Migration. Sci. Rep. 2015, 5, 14049. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-C.; Lee, T.-A.; Wu, H.-M.; Ko, P.-L.; Liao, W.-H.; Tung, Y.-C. Microfluidic Collective Cell Migration Assay for Study of Endothelial Cell Proliferation and Migration under Combinations of Oxygen Gradients, Tensions, and Drug Treatments. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boneschansker, L.; Yan, J.; Wong, E.; Briscoe, D.M.; Irimia, D. Microfluidic platform for the quantitative analysis of leukocyte migration signatures. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Wong, B.S.; Shah, S.R.; Yankaskas, C.L.; Bajpai, V.K.; Wu, P.-H.; Chin, D.; Ifemembi, B.; ReFaey, K.; Schiapparelli, P.; Zheng, X.; et al. A microfluidic cell-migration assay for the prediction of progression-free survival and recurrence time of patients with glioblastoma. Nat. Biomed. Eng. 2021, 5, 26–40. [Google Scholar] [CrossRef]
- Schmitz, J.; Täuber, S.; Westerwalbesloh, C.; Von Lieres, E.; Noll, T.; Grünberger, A. Development and application of a cultivation platform for mammalian suspension cell lines with single-cell resolution. Biotechnol. Bioeng. 2021, 118, 992–1005. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2009, 188, 11–19. [Google Scholar] [CrossRef]
- Petrie, R.J.; Gavara, N.; Chadwick, R.S.; Yamada, K.M. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J. Cell Biol. 2012, 197, 439–455. [Google Scholar] [CrossRef]
- Petrie, R.J.; Yamada, K.M. At the leading edge of three-dimensional cell migration. J. Cell Sci. 2012, 125, 5917–5926. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Humphries, B.; Brien, R.; Wicha, M.S.; Luker, K.E.; Luker, G.D.; Chen, Y.-C.; Yoon, E. Morphology-based prediction of cancer cell migration using an artificial neural network and a random decision forest. Integr. Biol. 2018, 10, 758–767. [Google Scholar] [CrossRef]
- Hedges, J.C.; Dechert, M.A.; Yamboliev, I.A.; Martin, J.L.; Hickey, E.; Weber, L.A.; Gerthoffer, W.T. A Role for p38MAPK/HSP27 Pathway in Smooth Muscle Cell Migration. J. Biol. Chem. 1999, 274, 24211–24219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, J.; Zhou, L.; Qin, L. Utilizing a high-throughput microfluidic platform to study hypoxia-driven mesenchymal-mode cell migration. Integr. Biol. 2015, 7, 672–680. [Google Scholar] [CrossRef]
- Suslov, O.N.; Kukekov, V.G.; Ignatova, T.N.; Steindler, D.A. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc. Natl. Acad. Sci. USA 2002, 99, 14506–14511. [Google Scholar] [CrossRef]
- Bryder, D.; Rossi, D.J.; Weissman, I.L. Hematopoietic Stem Cells: The Paradigmatic Tissue-Specific Stem Cell. Am. J. Pathol. 2006, 169, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Dulken, B.W.; Leeman, D.S.; Boutet, S.C.; Hebestreit, K.; Brunet, A. Single-Cell Transcriptomic Analysis Defines Heterogeneity and Transcriptional Dynamics in the Adult Neural Stem Cell Lineage. Cell Rep. 2017, 18, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Höving, A.L.; Windmöller, B.A.; Knabbe, C.; Kaltschmidt, B.; Kaltschmidt, C.; Greiner, J.F.W. Between Fate Choice and Self-Renewal—Heterogeneity of Adult Neural Crest-Derived Stem Cells. Front. Cell Dev. Biol. 2021, 9, 662754. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.G. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012, 22, 457–472. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höving, A.L.; Schmitz, J.; Schmidt, K.E.; Greiner, J.F.W.; Knabbe, C.; Kaltschmidt, B.; Grünberger, A.; Kaltschmidt, C. Human Blood Serum Induces p38-MAPK- and Hsp27-Dependent Migration Dynamics of Adult Human Cardiac Stem Cells: Single-Cell Analysis via a Microfluidic-Based Cultivation Platform. Biology 2021, 10, 708. https://doi.org/10.3390/biology10080708
Höving AL, Schmitz J, Schmidt KE, Greiner JFW, Knabbe C, Kaltschmidt B, Grünberger A, Kaltschmidt C. Human Blood Serum Induces p38-MAPK- and Hsp27-Dependent Migration Dynamics of Adult Human Cardiac Stem Cells: Single-Cell Analysis via a Microfluidic-Based Cultivation Platform. Biology. 2021; 10(8):708. https://doi.org/10.3390/biology10080708
Chicago/Turabian StyleHöving, Anna L., Julian Schmitz, Kazuko E. Schmidt, Johannes F. W. Greiner, Cornelius Knabbe, Barbara Kaltschmidt, Alexander Grünberger, and Christian Kaltschmidt. 2021. "Human Blood Serum Induces p38-MAPK- and Hsp27-Dependent Migration Dynamics of Adult Human Cardiac Stem Cells: Single-Cell Analysis via a Microfluidic-Based Cultivation Platform" Biology 10, no. 8: 708. https://doi.org/10.3390/biology10080708
APA StyleHöving, A. L., Schmitz, J., Schmidt, K. E., Greiner, J. F. W., Knabbe, C., Kaltschmidt, B., Grünberger, A., & Kaltschmidt, C. (2021). Human Blood Serum Induces p38-MAPK- and Hsp27-Dependent Migration Dynamics of Adult Human Cardiac Stem Cells: Single-Cell Analysis via a Microfluidic-Based Cultivation Platform. Biology, 10(8), 708. https://doi.org/10.3390/biology10080708







