Association between Metabolic Syndrome Components and Cardiac Autonomic Modulation among Children and Adolescents: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
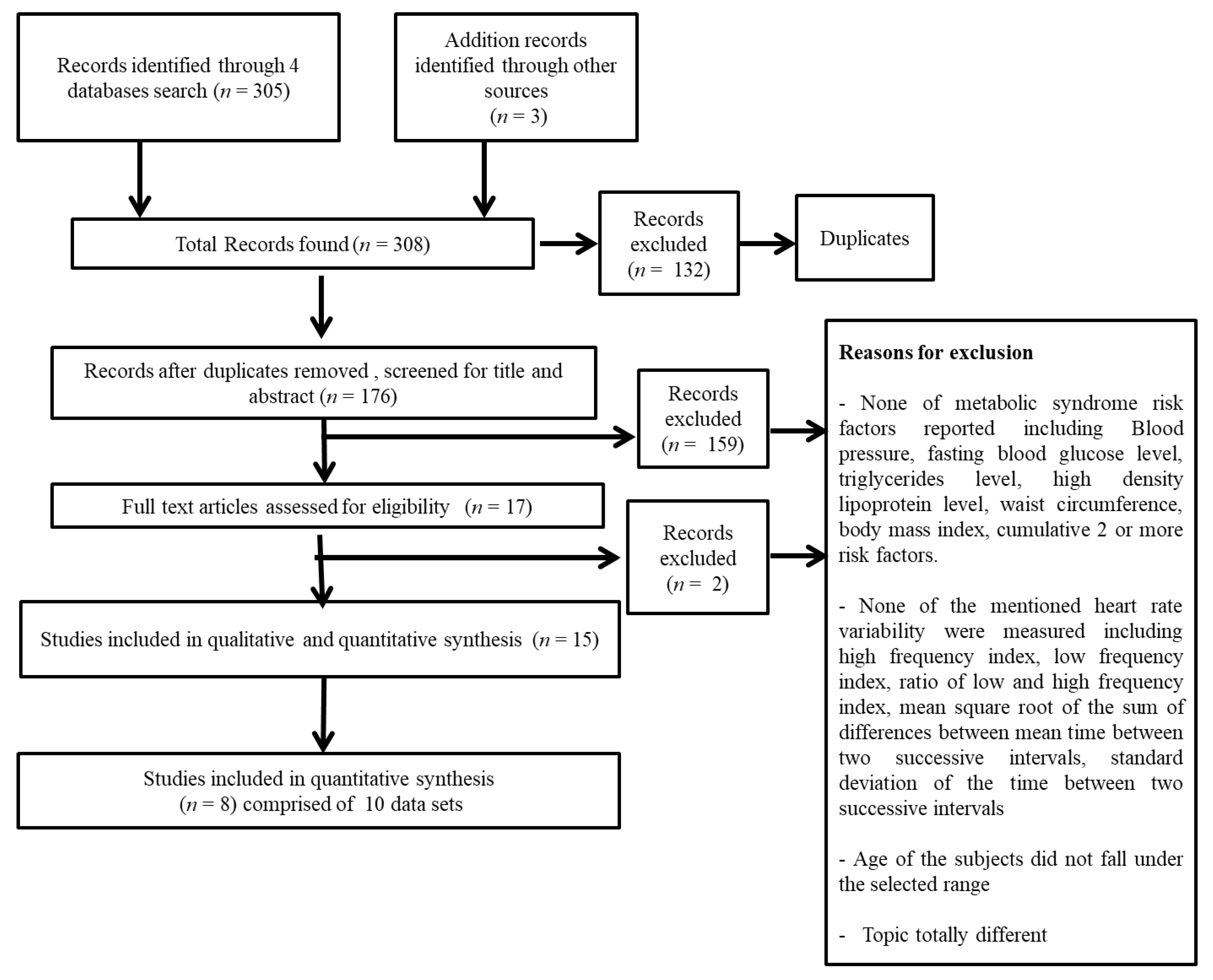
2. Methodology
2.1. Search Strategy
2.2. Exclusion and Inclusion Criteria
2.3. Quality Assessment
2.4. Data Extraction and Analysis
3. Results
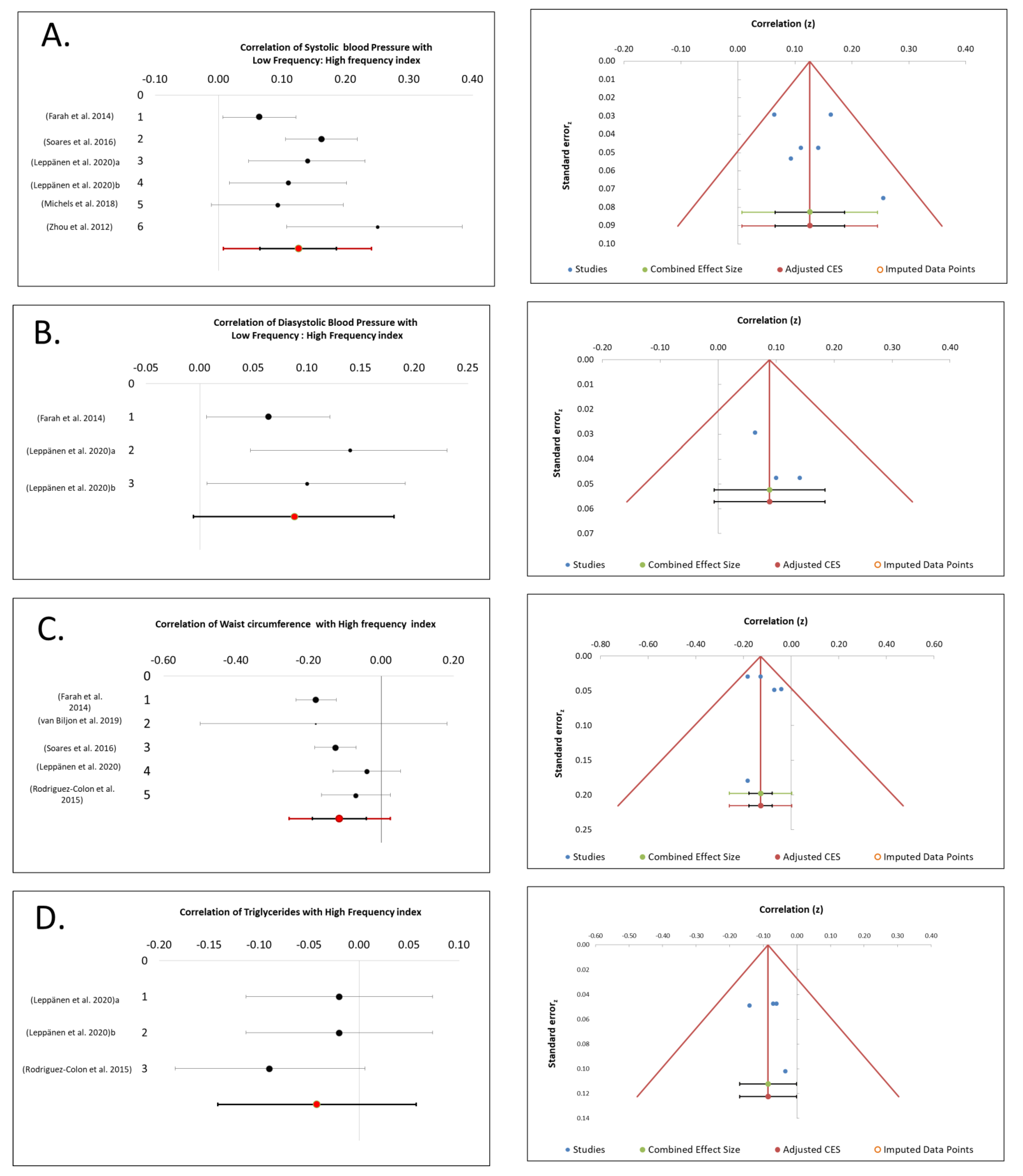
3.1. SBP Association with CAM
3.2. DBP Association with CAM
3.3. WC Association with CAM
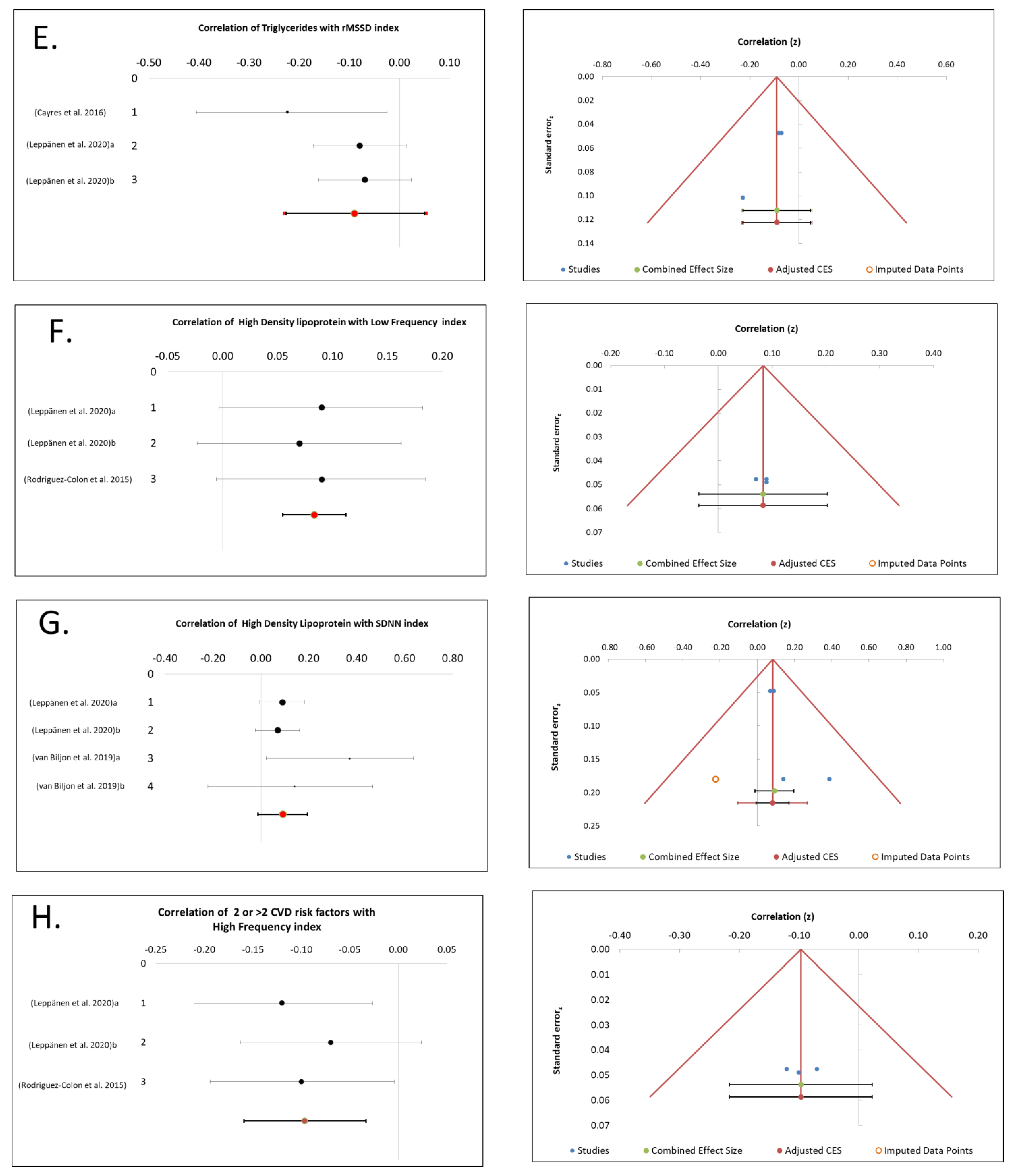
3.4. TGs Association with CAM
3.5. HDL Association with CAM
3.6. LDL Association with CAM
3.7. BMI Association with CAM
3.8. FGL Association with CAM
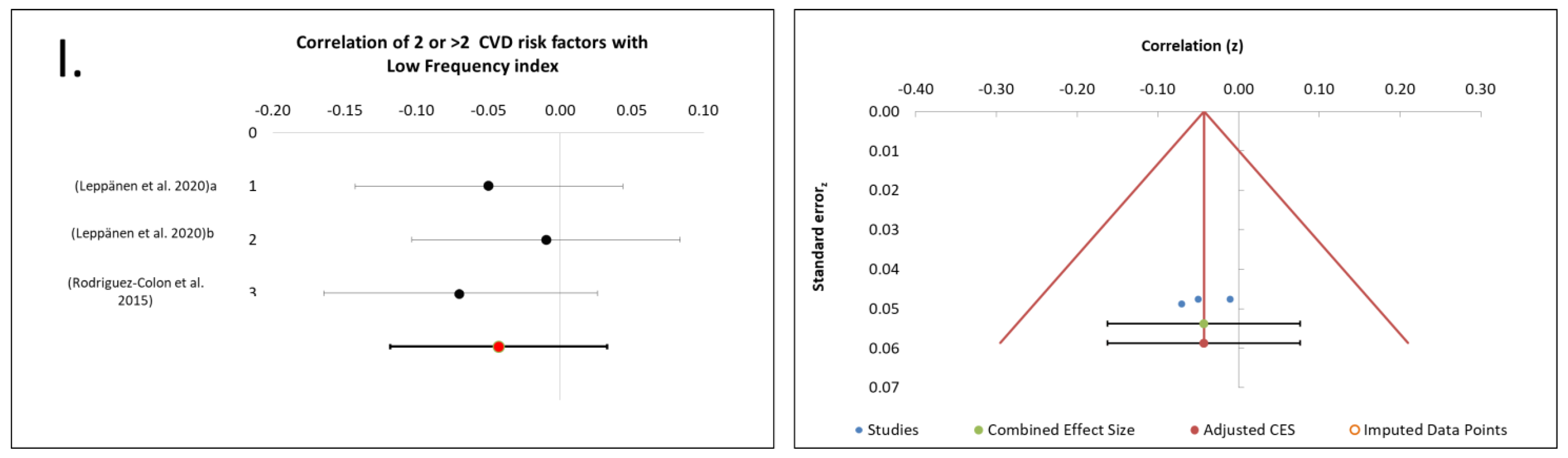
3.9. Cluster of ≥2 MetS Risk Factors Association with CAM
4. Discussion
4.1. SDNN and LF Associated Positively with HDL
4.2. rMSSD and HF Associated Negatively with TGs and WC
4.3. LF/HF Associated Positively with SBP and DBP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malpas, S.C. Sympathetic Nervous System Overactivity and Its Role in the Development of Cardiovascular Disease. Physiol. Rev. 2010, 90, 513–557. [Google Scholar] [CrossRef]
- Tsuji, H.; Larson, M.G.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of Reduced Heart Rate Variability on Risk for Cardiac Events. Circulation 1996, 94, 2850–2855. [Google Scholar] [CrossRef]
- Viljoen, M.; Claassen, N. Allostatic load and heart rate variability as health risk indicators. Afr. Health Sci. 2017, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Bond, B.; Cockcroft, E.J.; Williams, C.A.; Harris, S.; Gates, P.E.; Jackman, S.R.; Armstrong, N.; Barker, A.R.; Farah, B.Q.; Barros, M.V.G.; et al. The Georgia Cardiovascular Twin Study: Influence of genetic predisposition and chronic stress on risk for cardiovascular disease and type 2 diabetes. Diabetes Care 2012, 16, 21–28. [Google Scholar]
- Michels, N.; Matthys, D.; Thumann, B.; Marild, S.; De Henauw, S. Children’s stress-related reports and stress biomarkers interact in their association with metabolic syndrome risk. Stress Health J. Int. Soc. Investig. Stress 2018, 34, 523–533. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Magge, S.N.; Goodman, E.; Armstrong, S.C. The Metabolic Syndrome in Children and Adolescents: Shifting the Focus to Cardiometabolic Risk Factor Clustering. Pediatrics 2017, 140, e20171603. [Google Scholar] [CrossRef]
- Laitinen, T.; Hartikainen, J.; Niskanen, L.; Geelen, G.; Länsimies, E. Sympathovagal balance is major determinant of short-term blood pressure variability in healthy subjects. Am. J. Physiol. Circ. Physiol. 1999, 276, H1245–H1252. [Google Scholar] [CrossRef]
- Rodríguez-Colón, S.M.; Bixler, E.O.; Li, X.; Vgontzas, A.N.; Liao, D. Obesity is associated with impaired cardiac autonomic modulation in children. Int. J. Pediatr. Obes. 2011, 6, 128–134. [Google Scholar] [CrossRef]
- Kaufman, C.L.; Kaiser, D.R.; Steinberger, J.; Kelly, A.S.; Dengel, D.R. Relationships of Cardiac Autonomic Function with Metabolic Abnormalities in Childhood Obesity. Obesity 2007, 15, 1164–1171. [Google Scholar] [CrossRef]
- Stefanaki, C. Prediabetes and Adolescence—Trends, Causes, Effects, and Screening. Endocrinology 2016, 12, 94. [Google Scholar] [CrossRef][Green Version]
- Bufferd, S.J.; Dougherty, L.R.; Carlson, G.A.; Rose, S.; Klein, D.N. Psychiatric Disorders in Preschoolers: Continuity from Ages 3 to 6. Am. J. Psychiatry 2012, 169, 1157–1164. [Google Scholar] [CrossRef]
- Merikangas, K.R.; Zhang, H.; Avenevoli, S.; Acharyya, S.; Neuenschwander, M.; Angst, J. Longitudinal Trajectories of Depression and Anxiety in a Prospective Community Study. Arch. Gen. Psychiatry 2003, 60, 993. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, N.; Curtis, A.J.; Heritier, S.; Gadowski, A.M.; Pavkov, M.E.; Kenealy, T.; Owens, D.R.; Thomas, R.L.; Song, S.; Wong, J.; et al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: Systematic review and meta-analyses. Diabetologia 2021, 64, 275–287. [Google Scholar] [CrossRef]
- Farah, B.Q.; Barros, M.V.G.; Balagopal, B.; Ritti-Dias, R.M. Heart rate variability and cardiovascular risk factors in adolescent boys. J. Pediatr. 2014, 165, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Colon, S.M.; He, F.; Bixler, E.O.; Fernandez-Mendoza, J.; Vgontzas, A.N.; Calhoun, S.; Zheng, Z.J.; Liao, D.; Rodríguez-Colón, S.M.; He, F.; et al. Metabolic syndrome burden in apparently healthy adolescents is adversely associated with cardiac autonomic modulation-Penn State Children. Cohort. Metab. Exp. 2015, 64, 626–632. [Google Scholar] [CrossRef] [PubMed]
- van Biljon, A.; McKune, A.J.; DuBose, K.D.; Kolanisi, U.; Semple, S.J. Cardiac autonomic function and its association with cardiometabolic disease risk factors in Black South African children Auton. Neurosci. Basi. Clin. 2019, 219, 1–4. [Google Scholar] [CrossRef]
- Leppänen, M.H.; Haapala, E.A.; Veijalainen, A.; Seppälä, S.; Oliveira, R.S.; Lintu, N.; Laitinen, T.; Tarvainen, M.P.; Lakka, T.A. Associations of cardiometabolic risk factors with heart rate variability in 6- to 8-year-old children: The PANIC Study. Pediatr. Diabetes 2020, 21, 251–258. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, G.; Wang, J.; Yang, S. Cardiovascular risk factors significantly correlate with autonomic nervous system activity in children. Can. J. Cardiol. 2012, 28, 477–482. [Google Scholar] [CrossRef]
- The Joanna Briggs Institute (JBI). Checklist for Prevalence Studies: The Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews. Australia 2016, 3. Available online: https://jbi.global/critical-appraisal-tools (accessed on 1 March 2021).
- Kasten, A.P.; da Rosa, B.N.; Schmit, E.F.D.; Noll, M.; Candotti, C.T. Prevalence of postural deviations in the spine in schoolchildren: A systematic review with meta-analysis. J. Hum. Growth Dev. 2017, 27, 99. [Google Scholar] [CrossRef][Green Version]
- Peterson, R.A.; Brown, S.P. On the Use of Beta Coefficients in Meta-Analysis. J. Appl. Psychol. 2005, 90, 175–181. [Google Scholar] [CrossRef]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, M.A.; Trevizan, P.F.; Scodeler, N.F. Heart rate variability, blood lipids and physical capacity of obese and non-obese children. Arq. Bras. Cardiol. 2009, 93, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Farah, B.Q.; Christofaro, D.G.D.; Cavalcante, B.R.; Andrade-Lima, A.; Germano-Soares, A.H.; Vanderlei, L.C.M.; Lanza, F.C.; Ritti-Dias, R.M. Cutoffs of Short-Term Heart Rate Variability Parameters in Brazilian Adolescents Male. Pediatr. Cardiol. 2018, 39, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.H.G.; Farah, B.Q.; Cucato, G.G.; Bastos-Filho, C.J.A.; Christofaro, D.G.D.; Vanderlei, L.C.M.; de Andrade Lima, A.H.R.; Ritti-Dias, R.M.; Germano Soares, A.H.; Farah, B.Q.; et al. Is the algorithm used to process heart rate variability data clinically relevant? Analysis in male adolescents. Einstein 2016, 14, 196–201. [Google Scholar] [CrossRef]
- Cayres, S.U.; Vanderlei, L.C.M.; Silva, D.R.P.; Lima, M.C.S.; Barbosa, M.F.; Fernandes, R.A. Cardiovascular and metabolic risk markers are related to parasympathetic indices in pre-pubertal adolescents. Cardiol. Young 2016, 26, 280–287. [Google Scholar] [CrossRef]
- Lee, S.; Cowan, P.A.; Wetzel, G.T.; Velasquez-Mieyer, P. Prediabetes and blood pressure effects on heart rate variability, QT-interval duration, and left ventricular hypertrophy in overweight-obese adolescents. J. Pediatr. Nurs. 2011, 26, 416–427. [Google Scholar] [CrossRef]
- Stefanaki, C.; Michos, A.; Latsios, G.; Tousoulis, D.; Peppa, M.; Zosi, P.; Boschiero, D.; Bacopoulou, F. Sexual Dimorphism of Heart Rate Variability in Adolescence: A Case-Control Study on Depression, Anxiety, Stress Levels, Body Composition, and Heart Rate Variability in Adolescents with Impaired Fasting Glucose. Int. J. Environ. Res. Public Health 2020, 17, 2688. [Google Scholar] [CrossRef]
- Plaza-Florido, A.; Alcantara, J.M.A.; Amaro-Gahete, F.J.; Sacha, J.; Ortega, F.B. Cardiovascular Risk Factors and Heart Rate Variability: Impact of the Level of the Threshold-Based Artefact Correction Used to Process the Heart Rate Variability Signal. J. Med. Syst. 2021, 45, 2. [Google Scholar] [CrossRef]
- Vrijkotte, T.G.M.; van den Born, B.J.H.; Hoekstra, C.M.C.A.; Gademan, M.G.J.; van Eijsden, M.; de Rooij, S.R.; Twickler, M.T.B. Cardiac Autonomic Nervous System Activation and Metabolic Profile in Young Children: The ABCD Study. PLoS ONE 2015, 10, e0138302. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Owolabi, E.O.; Ter Goon, D.; Adeniyi, O.V.; Ajayi, A.I. Optimal waist circumference cut-off points for predicting metabolic syndrome among low-income black South African adults. BMC Res. Notes 2018, 11, 22. [Google Scholar] [CrossRef]
- Gaillard, T.; Schuster, D.; Osei, K. Differential impact of serum glucose, triglycerides, and high-density lipoprotein cholesterol on cardiovascular risk factor burden in nondiabetic, obese African American women: Implications for the prevalence of metabolic syndrome. Metabolism 2010, 59, 1115–1123. [Google Scholar] [CrossRef]
- Kalk, W.J.; Joffe, B.I.; Sumner, A.E. The Waist Circumference of Risk in Black South African Men Is Lower Than in Men of European Ancestry. Metab. Syndr. Relat. Disord. 2011, 9, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, E.A.; Cizza, G. The Hypothalamic-Pituitary-Adrenal Axis, Obesity, and Chronic Stress Exposure: Sleep and the HPA Axis in Obesity. Curr. Obes. Rep. 2012, 1, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.I.; Smyth, N.; Hall, S.J.; Torres, S.J.; Hussein, M.; Jayasinghe, S.U.; Ball, K.; Clow, A.J. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology 2020, 114, 104599. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Chrousos, G.P.; Tsigos, C. Stress, Visceral Obesity, and Metabolic Complications. Ann. N. Y. Acad. Sci. 2006, 1083, 77–110. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Pervanidou, P.; Boschiero, D.; Chrousos, G.P. Chronic stress and body composition disorders: Implications for health and disease. Hormones 2018, 17, 33–43. [Google Scholar] [CrossRef]
- Zhao, S.; Chi, A.; Yan, J.; Yao, C. Feature of Heart Rate Variability and Metabolic Mechanism in Female College Students with Depression. Biomed. Res. Int. 2020, 2020, 5246350. [Google Scholar] [CrossRef] [PubMed]
- Deichmann, R.E.; Lavie, C.J.; Dornelles, A.C. Impact of Coenzyme Q-10 on Parameters of Cardiorespiratory Fitness and Muscle Performance in Older Athletes Taking Statins. Phys. Sportsmed. 2012, 40, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr. Metab. 2005, 2, 3. [Google Scholar] [CrossRef]
- Felger, J.C.; Haroon, E.; Woolwine, B.J.; Raison, C.L.; Miller, A.H. Interferon-alpha-induced inflammation is associated with reduced glucocorticoid negative feedback sensitivity and depression in patients with hepatitis C virus. Physiol. Behav. 2016, 166, 14–21. [Google Scholar] [CrossRef]
- Duley, J.A.; Ni, M.; Shannon, C.; Norris, R.L.; Sheffield, L.; Harris, M.; van Kuilenburg, A.B.P.; Mead, S.; Cameron, A.; Helsby, N.; et al. Towards a test to predict 5-fluorouracil toxicity: Pharmacokinetic data for thymine and two sequential metabolites following oral thymine administration to healthy adult males. Eur. J. Pharm. Sci. 2016, 81, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.X.; Xia, J.J.; Deng, F.L.; Liang, W.W.; Wu, J.; Yin, B.M.; Dong, M.X.; Chen, J.J.; Ye, F.; Wang, H.Y.; et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: A targeted metabolomics study. Transl. Psychiatry 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xie, G.; Wang, J.; Yang, S.; Leppänen, M.H.; Haapala, E.A.; Veijalainen, A.; Seppälä, S.; Oliveira, R.S.; Lintu, N.; et al. Heart rate variability and cardiovascular risk factors in adolescent boys. J. Pediatr. 2020, 14, 196–201. [Google Scholar]
- Ciccone, M.M.; Scicchitano, P.; Zito, A.; Gesualdo, M.; Sassara, M.; Calderoni, G.; Di Mauro, F.; Ladisa, G.; Di Mauro, A.; Laforgia, N. Different functional cardiac characteristics observed in term/preterm neonates by echocardiography and tissue doppler imaging. Early Hum. Dev. 2011, 87, 555–558. [Google Scholar] [CrossRef]
- World Health Organisation. Global Status Report on Noncommunicable Diseases 2014. Presented at the 2015. Available online: https://www.who.int/nmh/publications/ncd-status-report-2014/en (accessed on 1 March 2021).
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P.; Tracy, R.E.; Wattigney, W.A. Association between Multiple Cardiovascular Risk Factors and Atherosclerosis in Children and Young Adults. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef]
- McGill, H.C.; McMahan, C.A.; Herderick, E.E.; Malcom, G.T.; Tracy, R.E.; Strong, J.P. Origin of atherosclerosis in childhood and adolescence. Am. J. Clin. Nutr. 2000, 72, 1307s–1315s. [Google Scholar]
- Gotto, A.M. Lipid Lowering, Regression, and Coronary Events. Circulation 1995, 92, 646–656. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Jokinen, V.; Syvänne, M.; Nieminen, M.S.; Airaksinen, K.E.J.; Ikäheimo, M.J.; Koistinen, J.M.; Kauma, H.; Kesäniemi, A.Y.; Majahalme, S.; et al. Heart Rate Variability and Progression of Coronary Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lee, I.H.; Tsai, H.C.; Chi, M.H.; Chang, W.H.; Chen, P.S.; Chen, K.C.; Yang, Y.K. The association between plasma cholesterol and the effect of tryptophan depletion on heart rate variability. Kaohsiung J. Med. Sci. 2019, 35, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Assoumou, H.G.N.; Pichot, V.; Barthelemy, J.C.; Dauphinot, V.; Celle, S.; Gosse, P.; Kossovsky, M.; Gaspoz, J.M.; Roche, F. Metabolic Syndrome and Short-Term and Long-Term Heart Rate Variability in Elderly Free of Clinical Cardiovascular Disease: The PROOF Study. Rejuvenation Res. 2010, 13, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Gadegbeku, C.A.; Dhandayuthapani, A.; Sadler, Z.E.; Egan, B.M. Raising lipids acutely reduces baroreflex sensitivity. Am. J. Hypertens. 2002, 15, 479–485. [Google Scholar] [CrossRef]
- 28TH International symposium on the autonomic nervous system. Clin. Auton. Res. 2017, 27, 295–353. [CrossRef]
- Eriksson, K.F.; Nilsson, H.; Lindgärde, F.; Österlin, S.; Dahlin, L.B.; Lilja, B.; Rosén, I.; Sundkvist, G. Diabetes Mellitus but not Impaired Glucose Tolerance is Associated with Dysfunction in Peripheral. Nerves. Diabet. Med. 1994, 11, 279–285. [Google Scholar] [CrossRef]
- Balikai, F.; Deshpande, N.; Javali, S.; Shetty, D.; Benni, J.; Shindhe, V.; Jaalam, K.; Kapoor, N. The relationship between serum triglyceride level and heart rate variability in type 2 diabetes mellitus patients of North Karnataka. J. Diabetol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Valensi, P.; Thi, B.N.; Lormeau, B.; Pariès, J.; Attali, J.R. Cardiac autonomic function in obese patients. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 113–118. [Google Scholar]
- Fidan-Yaylali, G.; Yaylali, Y.T.; Erdogan, Ç.; Can, B.; Senol, H.; Gedik-Topçu, B.; Topsakal, S. The Association between Central Adiposity and Autonomic Dysfunction in Obesity. Med. Princ. Pract. 2016, 25, 442–448. [Google Scholar] [CrossRef]
- Windham, B.G.; Fumagalli, S.; Ble, A.; Sollers, J.J.; Thayer, J.F.; Najjar, S.S.; Griswold, M.E.; Ferrucci, L. The Relationship between Heart Rate Variability and Adiposity Differs for Central and Overall Adiposity. J. Obes. 2012, 2012, 149516. [Google Scholar] [CrossRef]
- Altuncu, M.E.; Baspinar, O.; Keskin, M. The use of short-term analysis of heart rate variability to assess autonomic function in obese children and its relationship with metabolic syndrome. Cardiol. J. 2012, 19, 501–506. [Google Scholar] [CrossRef][Green Version]
- Zouhal, H.; Lemoine-Morel, S.; Mathieu, M.-E.; Casazza, G.A.; Jabbour, G. Catecholamines and Obesity: Effects of Exercise and Training. Sport. Med. 2013, 43, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Eckberg, D.L. Sympathovagal Balance. Circulation 1997, 96, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Pal, P.; Lalitha, V.; Dutta, T.K.; Adithan, C.; Nanda, N. Sympathovagal Imbalance in Young Prehypertensives: Importance of Male-Female Difference. Am. J. Med. Sci. 2013, 345, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Borres, M.; Thulesius, O.; Tamai, H.; Ericson, M.O.; Lindblad, L.-E. Blood pressure and cardiovascular autonomic function in healthy children and adolescents. J. Pediatr. 2000, 137, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Adithan, C.; Ananthanarayanan, P.H.; Pal, P.; Nanda, N.; Durgadevi, T.; Lalitha, V.; Syamsunder, A.N.; Dutta, T.K. Sympathovagal Imbalance Contributes to Prehypertension Status and Cardiovascular Risks Attributed by Insulin Resistance, Inflammation, Dyslipidemia and Oxidative Stress in First Degree Relatives of Type 2 Diabetics. PLoS ONE 2013, 8, e78072. [Google Scholar] [CrossRef]
- Kirkman, E.; Sawdon, M. Neurological and humoral control of blood pressure. Anaesth. Intensive Care Med. 2010, 11, 159–164. [Google Scholar] [CrossRef]
- Tonhajzerova, I.; Mestanik, M.; Mestanikova, A.; Jurko, A. Respiratory sinus arrhythmia as a non-invasive index of ′brain-heart′ interaction in stress. Indian J. Med. Res. 2016, 144, 815. [Google Scholar]
- Alderman, B.L.; Olson, R.L. The relation of aerobic fitness to cognitive control and heart rate variability: A neurovisceral integration study. Biol. Psychol. 2014, 99, 26–33. [Google Scholar] [CrossRef]
- Wadden, K.P.; Snow, N.J.; Sande, P.; Slawson, S.; Waller, T.; Boyd, L.A. Yoga Practitioners Uniquely Activate the Superior Parietal Lobule and Supramarginal Gyrus During Emotion Regulation. Front. Integr. Neurosci. 2018, 1, 60. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supriya, R.; Li, F.-F.; Yang, Y.-D.; Liang, W.; Baker, J.S. Association between Metabolic Syndrome Components and Cardiac Autonomic Modulation among Children and Adolescents: A Systematic Review and Meta-Analysis. Biology 2021, 10, 699. https://doi.org/10.3390/biology10080699
Supriya R, Li F-F, Yang Y-D, Liang W, Baker JS. Association between Metabolic Syndrome Components and Cardiac Autonomic Modulation among Children and Adolescents: A Systematic Review and Meta-Analysis. Biology. 2021; 10(8):699. https://doi.org/10.3390/biology10080699
Chicago/Turabian StyleSupriya, Rashmi, Fei-Fei Li, Yi-De Yang, Wei Liang, and Julien S. Baker. 2021. "Association between Metabolic Syndrome Components and Cardiac Autonomic Modulation among Children and Adolescents: A Systematic Review and Meta-Analysis" Biology 10, no. 8: 699. https://doi.org/10.3390/biology10080699
APA StyleSupriya, R., Li, F.-F., Yang, Y.-D., Liang, W., & Baker, J. S. (2021). Association between Metabolic Syndrome Components and Cardiac Autonomic Modulation among Children and Adolescents: A Systematic Review and Meta-Analysis. Biology, 10(8), 699. https://doi.org/10.3390/biology10080699








